Abstract
Intravenous lipid emulsion (ILE) is an adjunctive antidote used in selected critically ill poisoned patients. These patients may also require administration of advanced cardiac life support (ACLS) drugs. Limited data is available to describe interactions of ILE with standard ACLS drugs, specifically epinephrine. Twenty rats with intra-arterial and intravenous access were sedated with isoflurane and split into ILE or normal saline (NS) pretreatment groups. All received epinephrine 15 μm/kg intravenously (IV). Continuous mean arterial pressure (MAP) and heart rate (HR) were monitored until both indices returned to baseline. Standardized t tests were used to compare peak MAP, time to peak MAP, maximum change in HR, time to maximum change in HR, and time to return to baseline MAP/HR. There was a significant difference (p = 0.023) in time to peak MAP in the ILE group (54 s, 95 % CI 44–64) versus the NS group (40 s, 95 % CI 32–48) and a significant difference (p = 0.004) in time to return to baseline MAP in ILE group (171 s, 95 % CI 148–194) versus NS group (130 s, 95 % CI 113–147). There were no significant differences in the peak change in MAP, peak change in HR, time to minimum HR, or time to return to baseline HR between groups. ILE-pretreated rats had a significant difference in MAP response to epinephrine; ILE delayed the peak effect and prolonged the duration of effect of epinephrine on MAP, but did not alter the peak increase in MAP or the HR response.
Keywords: Advanced cardiac life support, Epinephrine, Rats, Fat emulsions, Intravenous
Introduction
Since its introduction in 1961, intravenous lipid emulsion (ILE) has evolved from a component of total parenteral nutrition, to a drug delivery vehicle, to its most recent role as an adjunct in resuscitation of local anesthetic induced cardiovascular collapse [1]. Current literature strongly supports the administration of ILE to patients who develop cardiac arrest after receiving an overdose of a local anesthetic such as bupivacaine [2–4]. Following this trend of expanding utility, ILE has been shown to be efficacious in the emergency treatment of neurotoxicity and cardiotoxicity that results from a variety of cardiovascular and psychoactive drug classes. Animal studies have established benefit from ILE in various models of toxicity including verapamil [5, 6], propranolol [7], amiodarone [8], and clomipramine [9]. Case studies have demonstrated successful use of ILE in haloperidol induced cardiac arrest [10], lamotrigine overdose [11], and in resuscitations of patients with combination ingestions of quetiapine/sertraline [12] and buproprion/lamotrigine [13].
Existing data on ILE demonstrates a theoretical benefit in patients presenting in extremis due to various toxic ingestions, a population that is difficult to treat and often refractory to standard interventions; ILE may be an important tool in poisoning resuscitations. Several mechanisms have been proposed for ILE’s antidotal properties including the creation of an expanded lipid compartment in the blood and the modulation of myocardial electrolyte concentrations and/or energy supply [1]. How it reverses the effects of toxic agents or how it may affect subsequently administered therapeutic maneuvers is not clear. Critically ill poisoned patients who received ILE may require ALCS drugs, given the severe nature of their conditions. Studies suggest ILE may affect lidocaine [14] and amiodarone [8] administration, but minimal literature exists on ILE’s interaction with other important ACLS agents, specifically epinephrine.
A range of results have been described in the few studies that explore co-administration of epinephrine and ILE in resuscitations. Hiller et al. reported the combination of ILE and epinephrine resulted in a faster return of spontaneous circulation in a rat model of bupivacaine overdose, but that there was a more sustained recovery in animals given ILE alone [15]. Another study reported that ILE decreased the response of an isolated rat artery to endogenous and exogenous norepinephrine in vitro [16].
As ILE gains acceptance as an important pharmacologic option in a variety of resuscitation situations, any potential interactions with standard ACLS drugs should be elucidated so that therapy may be adjusted accordingly. No study has directly examined the relationship between epinephrine and ILE. The present study aimed to determine if ILE would affect the physiologic response, namely changes in mean arterial pressure (MAP) and heart rate (HR), to epinephrine in an animal model.
Methods
Study Design
This was a laboratory study using a rat model of epinephrine exposure. Approval was obtained from the Lifespan/Rhode Island Hospital Institutional Animal Care and Use Committee prior to its commencement. All care and handling of animals were in accord with National Institutes of Health guidelines for ethical animal research.
Animal Handling and Preparation
Twenty male Sprague–Dawley rats weighing 225–250 g were obtained with femoral arterial and femoral venous catheters placed by the vendor prior to delivery (Harlan Laboratories, Indianapolis, IN, USA). Animals were housed in single cages and allowed access to food and water ad libitum. All catheters were assessed for patency and flushed with heparin/saline solution according to the manufacturer’s recommendations.
Sedation/Analgesia
Anesthesia was induced with 5 % isoflurane and maintained with 1.5–2 % isoflurane via nose cone for the duration of the study. The animals were spontaneously breathing and not paralyzed. Adequacy of anesthesia was periodically evaluated by toe pinch or tail pinch method.
Respiratory and Hemodynamic Monitoring/Data Acquisition
Throughout the experiment, respiratory rate, ECG rhythm, HR and MAP were recorded continuously via an external respiratory probe, subcutaneous 3 three lead ECG, and a femoral arterial line, respectively. The data was collected electronically using the MP100 system from Biopac with Acknowledge Version 3.9.1 software (Goleta, CA, USA). Due to significant HR variability using the software’s standard calculation methods, HR data was screened for potential spurious values. For all measurements in question (i.e., differed significantly from previous or subsequent values or was less than 100 bpm), the HR was manually measured from the ECG tracing by the authors to ensure accuracy.
Dose–Response Protocol
As a wide range of epinephrine dosing in rat models is reported in the literature [15, 17, 18], preliminary work to determine appropriate dosing of epinephrine in this model was performed. A dose–response relationship was established by dividing six animals into three groups of two. Each group received a single intravenous dose of epinephrine (2, 15, or 30 μm/kg) and the hemodynamic response to each dose was recorded until the animals returned to baseline indices. Two micrograms per kilogram of epinephrine produced an inconsistent and brief elevation of MAP. Thirty micrograms per kilogram of epinephrine resulted in hemodynamic instability, ECG changes and rapid death in both animals. Administration of 15 μm/kg produced a reliable increase in MAP for a duration of time that was deemed appropriate for the study before they returned to baseline. This dose was used for the remainder of the study.
Drug Exposure
Twenty animals were randomized into two equal groups—ILE and normal saline (NS) groups. Each animal was sedated and both arterial and venous catheters were accessed and monitoring was initiated. After a 3-min stabilization period, each animal received either an ILE (20 % intravenous lipid emulsion, Baxter, Deerfield, IL, USA) 15 cm3/kg bolus over seven minutes or NS 15 cm3/kg bolus over 7 min. This ILE dose was selected based on previous rat models of ILE resuscitation which have successfully used doses ranging from 10 to 19 cm3/kg [4, 6, 7]. Two minutes after completion of the infusion and once MAP/HR returned to baseline, a bolus dose of intravenous epinephrine (15 μm/kg) was administered. The animals were observed until HR and MAP returned to baseline for 3 min. Upon completion of the experiment, animals were euthanized by carbon dioxide asphyxiation.
Data Analysis
The sample size was calculated to detect a difference of 1.5 times the standard deviation (power = 90 %, p = 0.05), i.e., 45 beats per minute (bpm) difference in heart rate, 7.5 mmHg difference in MAP, and 10 s difference in onset or duration of effect. Standardized two tailed t tests were used to compare both groups with respect to weight, baseline HR and baseline MAP, peak change in MAP/HR, time to peak change in MAP/HR, and time to return to baseline MAP/HR in the ILE and saline groups. Statistical analysis was performed with Graphpad Prism for Windows (Version 6.01, San Diego, CA, USA).
Results
Descriptive Statistics
Between the ILE and control groups, there were no significant differences between weight (264 vs. 269 g, p = 0.19), baseline HR (363 vs. 357 bpm, p = 0.63) and baseline MAP (86 vs. 86 mmHg, p = 0.98). There was no significant difference in MAP or HR between ILE and NS groups prior to infusion or immediately prior to epinephrine infusion (Tables 1 and 2).
Table 1.
Comparison of ILE and NS groups. HR reported in bpm, time reported in seconds. Mean values given (interquartile range in parentheses)
| ILE | NS | p value | |
|---|---|---|---|
| HR at start of ILE or NS infusion | 363.2 (343.6–380.1) | 357.4 (328.8–385.3) | 0.63 |
| HR at start of epinephrine infusion | 340.3 (325.3–358) | 344 (325.5–356.5) | 0.68 |
| Maximum decrease in HR | 231 (202–268) | 212 (159–249) | 0.38 |
| Time to minimum HR | 38 (27–39) | 26 (17–32) | 0.13 |
| Time to return to baseline HR | 168.6 (96–267) | 230 (182.5–282) | 0.09 |
Table 2.
Comparison of ILE and NS groups. MAP reported in mmHg, time reported in seconds. Mean values given (interquartile range in parentheses)
| ILE | NS | p value | |
|---|---|---|---|
| MAP at start of ILE or NS infusion | 85.9 (73.75–91) | 85.8 (78.25–91.5) | 0.98 |
| MAP at start of epinephrine infusion | 82.5 (76.75–89) | 77.9 (67–86.75) | 0.30 |
| Peak increase in MAP | 75.4 (68.5–86.75) | 69.9 (63.5–75.5) | 0.29 |
| Time to peak MAP | 54 (40–62.5) | 40 (30–50) | 0.02 |
| Time to return to baseline MAP | 171 (147.5–200) | 130 (117.5–150) | 0.004 |
Experimental Arm
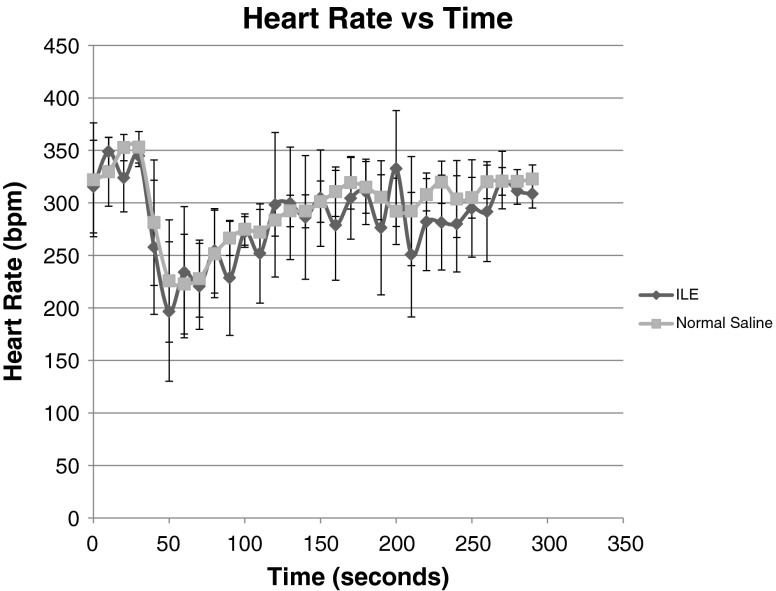
There was a marked decrease in HR after epinephrine administration, however the was no significant difference in maximum change (decrease) in HR (p = 0.38) between the ILE and NS groups (231 bpm, 95 % CI 198–264 vs 212 bpm, 95 % CI 175–249). There was no difference in time to minimum HR (p = 0.13) in ILE or NS (38 s, 95 % CI 22–54 vs 26 s, 95 % CI 19–33) or time to return of HR to baseline (p = 0.09) in either group (168.6 s, 95 %CI 103–234.2 vs 230 s, 95 % CI 185.1–275.5; Fig. 1 and Table 1).
Fig. 1.
Heart Rate variation in ILE vs NS groups, overall trend with 95 % CI
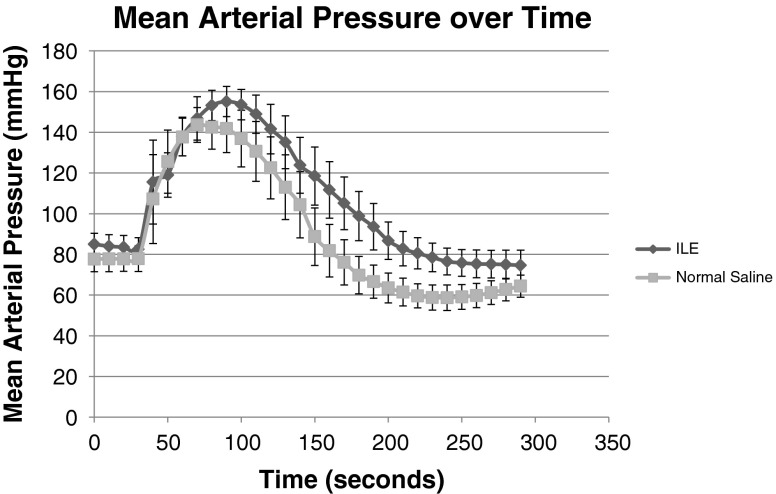
There was a marked increase in MAP immediately following epinephrine administration. There was no significant difference (p = 0.29) in the peak MAP of the ILE group (75.4 mmHg, 95 % CI 66–85) versus the saline group (69.9 mmHg, 95 % CI 64–76). There was a significant difference (p = 0.023) in time to peak MAP in the ILE group (54 s, 95 % CI 44–64) versus the saline group (40 s, 95 % CI 32–48) and a significant difference (p = 0.004) in time to return to baseline MAP in ILE group (171 s, 95 % CI 148–194) versus saline group (130 s, 95 % CI 113–147; Fig. 2 and Table 2).
Fig. 2.
MAP variation in ILE vs NS groups, overall trend with 95 % CI
Discussion
We observed profound bradycardia immediately following epinephrine administration in this rat model, likely secondary due to a baroreflex mechanism. ILE did not have a statistically significant effect on the magnitude, onset, or duration of this effect.
ILE delayed both the onset and resolution of epinephrine’s effect on MAP but did not change the magnitude of its effect. The mechanism responsible for this finding is unclear, but current theories of ILE’s biochemical action may provide some insight.
Three major theories are frequently cited to explain ILE’s effects. The first postulates that the concentrated lipid solution creates a compartment in the plasma, a “lipid sink”, which sequesters highly lipophilic drugs, preventing them from affecting other tissues. The tendency for bupivacaine to preferentially move into the lipid phase of plasma in the presence of ILE has been demonstrated in both in vivo and in vitro models, lending support to this theory [4, 19]. A second speculated mechanism is that ILE may increase myocardial calcium levels leading to improved contractility [20, 21]. A third possibility is that ILE may improve myocardial ATP synthesis by increasing the amount of available fatty acid substrate [22, 23]. A fourth possibility is that some combination of these factors is involved and the beneficial effect of ILE depends upon which effect predominates in a particular scenario.
One potential explanation for the observed phenomenon in our study is interference at the level of receptor binding. ILE may delay the initial delivery or binding of epinephrine to its receptors and/or delay its dissociation. Sequestration of epinephrine into a lipid compartment created by ILE would explain delayed delivery. However, given that epinephrine is primarily a water soluble compound with a partition coefficient of −1.37 [24], this should not occur to any great extent.
Another potential mechanism for interaction may be a physiologic alteration at the level of the receptor’s action on the cell. Epinephrine acts on beta-1, beta-2 and alpha 1 adrenergic receptors [25]. Epinephrine-mediated blood pressure increase occurs primarily through activation of alpha 1 adrenergic receptors, which are G protein coupled receptors distributed on the peripheral vasculature. Activation of the G-protein mechanism leads to smooth muscle contraction, and thus vasoconstriction, via calcium influx [26]. ILE has been shown to alter myocardial calcium homeostasis [20, 27], but its effect on calcium levels in vascular smooth muscle has not been well studied. ILE has also been shown to enhance alpha 1 mediated vasoconstriction [28] and reduce endothelium-mediated vasodilation after sustained infusion [29]. While the doses used for antidotal ILE therapy are insufficient to directly affect vascular tone itself, it may prolong the actions of epinephrine by elevating intracellular calcium levels and facilitating vasoconstriction or limiting subsequent vasodilation.
Another explanation would hinge on the third postulated mechanism of ILE action. Myocardial contraction is primarily driven by beta-oxidation of free fatty acids, a process which accounts for 50–70 % of myocardial ATP production [30]. ILE, which is composed primarily of long chain fatty acids, provides a potential source of energy for the myocardium. The increased energy demand imposed by epinephrine administration may be offset by the energy surplus created by ILE, prolonging the duration of epinephrine’s action.
Further research is needed to better evaluate the mechanism of this interaction, of ILE’s antidotal properties, and of ILE’s physiologic effects in general. This study does raise the question of whether this delay of action by ILE is an epinephrine specific effect, or if it occurs with other drugs. Potential interactions between ILE and other ACLS drugs and vasopressors are yet unknown.
Limitations
Our study has several limitations. As this is an animal study, the generalizability of the findings to human cardiovascular physiology is uncertain. Given the short duration of these effects, the impact of this finding on clinical practice with regard to co-administration of these two drugs is unclear. However, it is possible that the effect may be more pronounced when the two drugs are co-administered to patients that have much slower metabolic rates than rats, and have significantly depressed cardiac output (as in cardiac arrest or peri-arrest states).
Also, the study animals were hemodynamically normal without concomitant pathology or toxic ingestion, which is not true of the patient population in which these drugs will be administered simultaneously.
Conclusion
ILE delayed and prolonged the MAP effect of administered epinephrine. Our results provide further evidence that lipid solubility may not be the only factor when considering ILE’s effect on other drugs. As ILE becomes an increasingly ubiquitous adjunct to the resuscitation of the critically ill intoxicated patient, additional research is imperative to better characterize the implications and interactions of ILE therapy on concomitant standard therapies.
Acknowledgments
Funding for the study was generously provided by University Emergency Medicine Foundation via a resident scholarly development grant. Support for study logistics was provided by Dr. Francesca Beaudoin. Statistical support was provided by Dr. Jason Machan. Evelyn Tolbert, Dr. Lance Dworkin, and Scott McAllister provided procedural training and technical assistance.
Funding Source
University Emergency Medicine Foundation resident scholarly development grant
Footnotes
This data was presented in abstract form at the New England Regional Society of Academic Emergency Medicine Conference, Springfield, MA, USA on March 21, 2012 and the Society of Academic Emergency Medicine 2012 Annual Meeting, Chicago, IL, USA on May 12, 2012.
References
- 1.Turner-Lawrence DE, Kerns W., II Intravenous fat emulsion: a potential novel antidote. J Med Toxicol. 2008;4:109–114. doi: 10.1007/BF03160965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Picard J, Ward SC, Zumpe R, Meek T, Barlow J, Harrop-Griffiths W. Guidelines and the adoption of ‘lipid rescue’ therapy for local anaesthetic toxicity. Anaesthesia. 2009;64(2):122–125. doi: 10.1111/j.1365-2044.2008.05816.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupivacaine-induced cardiac toxicity. Reg Anesth Pain Med. 2003;28(3):198–202. doi: 10.1053/rapm.2003.50041. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg GL, VadeBoncouer T, Ramaraju GA, Garcia-Amaro MF, Cwik MJ. Pretreatment or resuscitation with a lipid infusion shifts the dose–response to bupivacaine-induced asystole in rats. Anesthesiology. 1998;88(4):1071–1075. doi: 10.1097/00000542-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Bania TC, Chu J, Perez E, Su M, Hahn IH. Hemodynamic effects of intravenous fat emulsion in an animal model of severe verapamil toxicity resuscitated with atropine, calcium, and saline. Acad Emerg Med. 2007;4(2):105–111. doi: 10.1111/j.1553-2712.2007.tb01752.x. [DOI] [PubMed] [Google Scholar]
- 6.Perez E, Bania TC, Medlej K, Chu J. Determining the optimal dose of intravenous fat emulsion for the treatment of severe verapamil toxicity in a rodent model. Acad Emerg Med. 2008;15(12):1284–1289. doi: 10.1111/j.1553-2712.2008.00259.x. [DOI] [PubMed] [Google Scholar]
- 7.Cave G, Harvey MG, Castle CD. The role of fat emulsion therapy in a rodent model of propranolol toxicity: a preliminary study. J Med Toxicol. 2006;2(1):4–7. doi: 10.1007/BF03161005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niiya T, Litonius E, Petaja L, Neuvonen PJ, Rosenberg PH. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56(4):402–408. doi: 10.1016/j.annemergmed.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 9.Harvey M, Cave G. Intralipid outperforms sodium bicarbonate in a rabbit model of clomipramine toxicity. Ann Emerg Med. 2007;49(2):178–185. doi: 10.1016/j.annemergmed.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Weinberg G, Di Gregorio G, Hiller D, Hewett A, Sirianni AG. Reversal of haloperidol-induced cardiac arrest by using lipid emulsion. Ann Intern Med. 2009;150(10):737–738. doi: 10.7326/0003-4819-150-10-200905190-00023. [DOI] [PubMed] [Google Scholar]
- 11.Castanares-zapatero D, Wittebole X, Huberlant V, Morunglav M, Hantson P. Llipid emulsion as rescue therapy in lamotrigine overdose. J Emerg Med. 2012;42(1):48–51. doi: 10.1016/j.jemermed.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 12.Finn SD, Uncles DR, Willers J, Sable N. Early treatment of a quetiapine and sertraline overdose with intralipid. Anesthesia. 2009;64(2):191–194. doi: 10.1111/j.1365-2044.2008.05744.x. [DOI] [PubMed] [Google Scholar]
- 13.Sirianni AJ, Osterhoudt KC, Calello DP, Muller AA, Waterhouse MR, Goodkin MB, Weinberg GL, Henretig FM. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51(4):412–415. doi: 10.1016/j.annemergmed.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Lange DB, Schwartz D, Daroza G, Gair R (2012) Use of intravenous lipid emulsion to reverse central nervous system toxicity of an iatrogenic local anesthetic overdose in a patient on peritoneal dialysis. Ann Pharmacotherapy. doi:10.1345/aph.1R298 [DOI] [PubMed]
- 15.Hiller DB, Gregorio GD, Ripper R, Kelly K, Massad M, Edelman L, Edelman G, Feinstein DL, Weinberg GL. Epinephrine impairs lipid resuscitation from bupivacaine overdose: a threshold effect. Anesthesiology. 2009;111(3):498–505. doi: 10.1097/ALN.0b013e3181afde0a. [DOI] [PubMed] [Google Scholar]
- 16.Biddle NL, Gelb AW, Hamilton JT. Propofol differentially attenuates the responses to exogenous and endogenous norepinephrine in the isolated rat femoral artery in vitro. Anesth Analg. 1995;80(4):793–799. doi: 10.1097/00000539-199504000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Chen MH, Lu JY, Xie L, Zheng JH, Song FQ. What is the optimal dose of epinephrine during cardiopulmonary resuscitation in a rat model? Am J Emerg Med. 2010;28(3):284–290. doi: 10.1016/j.ajem.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 18.McCaul CL, McNamara PJ, Engelberts D, Wilson GJ, Romaschin A, Redington AN, Kavanagh BP. Epinephrine increases mortality after brief asphyxial cardiac arrest in an in vivo rat model. Anesth Analg. 2006;102(2):542–548. doi: 10.1213/01.ane.0000195231.81076.88. [DOI] [PubMed] [Google Scholar]
- 19.Mazoit JX, Le Guen R, Beloeil H, Benhamou D. Binding of long-lasting local anesthetics to lipid emulsions. Anesthesiology. 2009;110(2):380–386. doi: 10.1097/ALN.0b013e318194b252. [DOI] [PubMed] [Google Scholar]
- 20.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci USA. 1992;89(14):6452–6456. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stehr SN, Ziegeler JC, Pexa A, Oertel R, Deussen A, Koch T, Hubler M. The effects of lipid infusion on myocardial function and bioenergetics in l-bupivacaine toxicity in the isolated rat heart. Anesth Analg. 2007;104(1):186–192. doi: 10.1213/01.ane.0000248220.01320.58. [DOI] [PubMed] [Google Scholar]
- 22.Rothschild L, Bern S, Oswald S, Weinberg G. Intravenous lipid emulsion in clinical toxicology. Scand J Trauma Resusc Emerg Med. 2010;18:51. doi: 10.1186/1757-7241-18-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van de Velde M, Wouters PF, Rolf N, Van Aken H, Flameng W, Vandermeersch E. Long-chain triglycerides improve recovery from myocardial stunning in conscious dogs. Cardiovasc Res. 1996;32(6):1008–1015. doi: 10.1016/S0008-6363(96)00165-4. [DOI] [PubMed] [Google Scholar]
- 24.Epinephrine. In: Drug bank—open data drug and target data bank. Genome Alberta & Genome Canada. http://www.drugbank.ca/drugs/db00668. Accessed 14 Feb 2012
- 25.Mcevoy G, Miller J, editors. Epinephrine. AHFS drug information. Bethesda, MD: Authority of the Board of the American Society of Health-System Pharmacists; 2008. pp. 1377–1382. [Google Scholar]
- 26.Guimaraes S, And Moura D. Vascular adrenoceptors: an update. Pharmacol Rev. 2001;53(2):319–356. [PubMed] [Google Scholar]
- 27.Rahman S, Li J, Bopassa JC, Umar S, Iorga A, Partownavid P, Eghbali M. Phosphorylation of gsk-3beta mediates intralipid-induced cardioprotection against ischemia/reperfusion injury. Anesthesiology. 2011;115(2):242–253. doi: 10.1097/ALN.0b013e318223b8b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haastrup AT, Stepniakowski KT, Goodfriend TL, Egan BM. Intralipid enhances alpha1-adrenergic receptor mediated pressor sensitivity. Hypertension. 1998;32(4):693–698. doi: 10.1161/01.HYP.32.4.693. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100(5):1230–1239. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopaschuk GD, Ussher JR, Folmes CD, Jaswal JS, Stanley WC. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90(1):207–258. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]