Abstract
Methylene blue is used primarily in the treatment of patients with methemoglobinemia. Most recently, methylene blue has been used as a treatment for refractory distributive shock from a variety of causes such as sepsis and anaphylaxis. Many studies suggest that the nitric oxide–cyclic guanosine monophosphate (NO–cGMP) pathway plays a significant role in the pathophysiology of distributive shock. There are some experimental and clinical experiences with the use of methylene blue as a selective inhibitor of the NO–cGMP pathway. Methylene blue may play a role in the treatment of distributive shock when standard treatment fails.
Keywords: Methylene blue, Refractory shock, Cardiovascular drug overdoses, Calcium channel blockers
Introduction
Methylene blue is a phenothiazine-related heterocyclic aromatic molecule (C16H18N3SCl). It is a solid, odorless, dark green powder at room temperature that yields a blue solution when it is dissolved in water. Methylene blue is used in a wide variety of settings and for many purposes; for example, as a redox indicator or as a dye/stain [1, 2].  The medicinal use of methylene blue dates back to the late 1800s as a treatment for malaria, a urinary analgesic, and in the treatment for cyanide and carbon monoxide poisoning [3–6]. Methylene blue is currently utilized as a treatment for ifosfamide neurotoxicity [7, 8]. Methylene blue is most commonly used as a reducing agent in the treatment of patients with methemoglobinemia [9–11]. Most recently, methylene blue has been used as a treatment for refractory distributive shock through a lesser known property: inhibition of the downstream effect of nitric oxide (NO). The objective of this review is to explain the pathophysiological basis of methylene blue for the use in distributive shock and review the current literature.
The medicinal use of methylene blue dates back to the late 1800s as a treatment for malaria, a urinary analgesic, and in the treatment for cyanide and carbon monoxide poisoning [3–6]. Methylene blue is currently utilized as a treatment for ifosfamide neurotoxicity [7, 8]. Methylene blue is most commonly used as a reducing agent in the treatment of patients with methemoglobinemia [9–11]. Most recently, methylene blue has been used as a treatment for refractory distributive shock through a lesser known property: inhibition of the downstream effect of nitric oxide (NO). The objective of this review is to explain the pathophysiological basis of methylene blue for the use in distributive shock and review the current literature.
Methods
The authors conducted a scientific review of all available literature published over the last 20 years. Our primary objective was to evaluate the use of methylene blue as a treatment for distributive shock from causes such as sepsis and anaphylaxis. Our secondary objective was to summarize the proposed mechanisms for the use of methylene blue based on existing human, animal, and in vitro studies. We initiated a PubMed database search using the MESH terms “methylene blue,” “sepsis,” “anaphylaxis,” “nitric oxide,” “nitric oxide synthase,” and “cGMP.” Articles were selected and agreed upon by the authors based on relevance and impact. Effort was made to include both positive and negative studies where appropriate. Emphasis was placed on well-conducted experimental data, case studies, and controlled trials when possible. Studies were only excluded due to redundancy. After analysis of the available data, this paper concludes with recommendations based on the existing scientific evidence.
Physiology of Vascular Tone
Nitric Oxide Synthase General Structure and Function
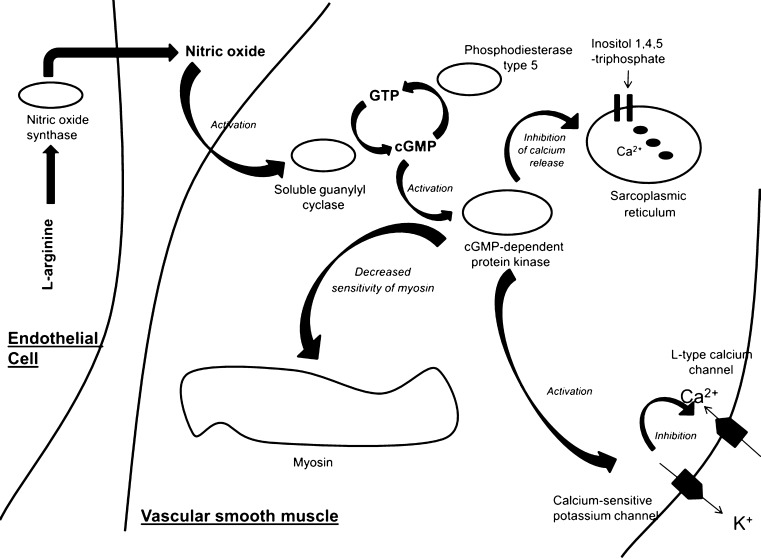
NO has many functions, such as serving as a messenger molecule, regulating gene transcription and mRNA translation, as well as assisting in neurotransmission [12, 13]. NO also plays an important role in the regulation of vascular tone (Fig. 1) and pathophysiology of shock when it is excessively produced. Initial evidence that NO plays an important role in the regulation of vascular tone was demonstrated with nitrate production in experimental models [14]. Later work revealed that l-arginine was the substrate and that l-citrulline was a by-product for the production of NO by an enzyme known as nitric oxide synthase (NOS) [15]. NOS, the enzyme that produces NO, is a homodimer with a substrate access channel which extends from the active site toward the dimer interface along with a reductase domain and an oxygenase domain (heme and H4B binding) [16]. In humans, there exist three different isoforms of NOS known as: neuronal NOS (nNOS, NOS1), inducible NOS (iNOS, NOS2), and endothelial NOS (eNOS, NOS3) [17]. All isoforms of NOS utilize l-arginine as a substrate along with oxygen and reduced nicotinamide-adenine-dinucleotide phosphate as co-substrates [18]. In addition, all isoforms of NOS bind calmodulin and calcium; activated calmodulin is important for the regulation of eNOS and nNOS activity [19]. Production of NO is a two-step process: In the first step, NOS hydroxylates l-arginine to an intermediate known as N-hydroxy-l-arginine. In a second step, NOS oxidizes this intermediate to l-citrulline and NO. Inactivation of NO occurs when it combines with a superoxide anion, O2−, to form a very potent oxidant known as peroxynitrite. Peroxynitrite causes DNA damage and oxidation, nitration, and S-nitrosylation of lipids and proteins [20, 21].
Fig. 1.
Regulation of the relaxation of vascular smooth muscle by nitric oxide. Nitric oxide is produced from l-arginine by nitric oxide synthase leading to the activation of soluble guanylyl cyclase with production of cyclic guanosine 3′,5′-monophosphate (cGMP). cGMP-dependent protein kinase is activated, leading to (1) decreased sensitivity of myosin to calcium-induced contractions, (2) activation of calcium-sensitive potassium channels which in turn decreases calcium entry through calcium channels, and (3) inhibition of release of calcium from the sarcoplasmic reticulum. This results in relaxation of smooth muscle
Certain neurons of the brain express nNOS that is regulated by calcium and calmodulin [22]. Although nNOS exists in the brain, spinal cord, adrenal glands, and sympathetic ganglia, the largest source of nNOS in mammals is found within skeletal muscle [23]. The physiologic functions of nNOS are varied and include modulation of learning, neurogenesis, and memory [24, 25]. There is strong evidence that nNOS also centrally regulates blood pressure [26, 27]. Certain neurogenerative diseases such as Parkinson’s and Alzheimer’s disease are associated with increased nNOS function. Increased nNOS function may lead to neuron cell death from large calcium influx mediated by N-methyl-d-aspartate receptors. Increased nNOS activity may also result in increased NO production with peroxynitrite formation leading to cell injury and mitochondrial dysfunction [28].
Endothelial NOS (eNOS) exists primarily in endothelial cells, but also occurs in other cell types including platelets and cardiac myocytes [29]. eNOS activity significantly increases with a rise in intracellular calcium with resultant increased NO production [30]. The physiologic functions of eNOS are varied and include inhibition of platelet aggregation and adhesion and inhibition of leukocyte aggregation, all of which help prevent atherogenesis [31, 32]. The primary role of eNOS appears to be regulation of cardiovascular functions such as blood pressure [33]. Dysfunction of eNOS activity is associated with cardiovascular disease through either inability to generate adequate amounts of NO for proper vasodilation and inhibition of platelet/leukocyte aggregation or excessive production of NO with resultant oxidative stress and endothelial cell dysfunction [34].
Inducible NOS (iNOS) expression is regulated by a variety of factors such as cytokines, interleukins, and bacterial lipopolysaccharide. iNOS was first identified in macrophages. However, further work revealed that expression of iNOS can be stimulated in almost any cell line [35, 36]. The primary physiologic function of iNOS in macrophages is to produce NO as a major cytotoxic agent. Thus, iNOS plays a role in immune defense and mediation of inflammation. NO can also inhibit enzymes that contain iron within their catalytic centers, as is often found in microorganisms. The production of NO has a cytotoxic effect on tumors and various microorganisms, such as parasites [37, 38]. While the production of NO from iNOS plays an important role in host defense, large concentrations of NO may also injure surrounding host cells. Overproduction of NO contributes to allograft rejection and is also implicated in the pathogenesis of various forms of distributive shock such as sepsis and anaphylaxis characterized by arteriolar vasodilation, hypotension, and poor response to vasopressors [39–41].
Guanylyl Cyclase and Signaling by Cyclic Guanosine Monophosphate
An important signaling pathway of NO is the activation of soluble guanylyl cyclase (sGC) resulting in the production of cyclic guanosine monophosphate (cGMP) [42]. sGC are a family of proteins composed of membrane-bound and soluble isoforms and expressed in virtually all cell types [43]. sGC catalyze conversion of the purine nucleoside guanosine-5′-triphosphate to cGMP in response to various messengers such as peptide ligands, calcium influx, and NO [44].
Intracellular cGMP gates specific ion channels, modulates nucleotide concentrations by regulating various phosphodiesterases, and activates protein kinases [42]. cGMP has a central role in many other vital processes such as retinal phototransduction, electrolyte regulation, and mediation of vascular smooth muscle tone [45, 46]. Excessive cGMP production, generally through abundant NO availability, is implicated in the pathophysiology of distributive shock such as sepsis and anaphylaxis [47]. Dysfunction within the NO–cGMP pathway has been implicated in certain disease states of distributive shock such as sepsis and anaphylaxis.
Distributive Shock
The definition of shock has evolved over centuries. The term “shock” was defined as sudden deterioration of a patient’s condition from major trauma in the 1700s. This term was translated by the English physician Clarke from the writings of a French surgeon Le Dran who is often credited with the origin of the term “shock.” While the term “shock” has taken on many meanings, the current emphasis is on tissue perfusion in relationship to cellular function. Shock is often a syndrome caused by a systemic derangement of perfusion leading to widespread cellular hypoxia and organ dysfunction. While hypovolemic shock from blood loss following trauma was the first form of shock to be recognized, other etiologies of shock are now recognized. These include cardiogenic (e.g., heart failure), obstructive (e.g., tension pneumothorax), and distributive shock. Distributive shock is characterized by loss of vasomotor tone or an overall decrease in systematic vascular resistance, and is often accompanied by an increase in cardiac output. Sepsis and anaphylaxis are the two most common etiologies of distributive shock. Also, drug-induced shock from certain medications such as calcium channel blockers may have a distributive component as well.
The NO–cGMP Pathway in Anaphylaxis and the Role of Methylene Blue
Anaphylaxis is a serious allergic reaction with a systemic response that can be life-threatening. The most common causes of anaphylaxis include allergic reaction to medications, food, and hymenoptera venom, although in up to 75 % of cases, there is no previous history of allergy [48]. The signs and symptoms of anaphylaxis manifest in all organ systems, but the life-threatening effects occur in the respiratory (e.g., bronchospasm) and cardiovascular system (e.g., refractory hypotension from peripheral vasodilation) [49].
The pathophysiology of distributive shock from anaphylaxis is complex involving many mediators such as bradykinin and other leukotrienes, but the mediator believed to have the most significant role is histamine [50]. Early experiments demonstrate that histamine-induced vasodilation is dependent on intact endothelium containing eNOS which suggests that histamine’s action is at least partly mediated through nitric oxide [51, 52]. Further evidence to support the role of the NO–cGMP pathway in anaphylaxis is demonstrated by the finding that increased histamine results in upregulation of eNOS gene expression with increased production of NO leading to vasodilation through increased activity of GC. Inhibition of GC reduces vasodilation induced by histamine [53–55]. This evidence provides support that histamine-induced vasodilation is at least partly mediated through the NO–cGMP pathway and that inhibition of this pathway reverses the vasodilation.
The treatment of anaphylaxis-induced hypotension consists of isotonic fluids, antihistamines, steroids, and in severe cases epinephrine. While sufficient in many instances, severe cases of anaphylaxis may not respond to these interventions, and patients may remain persistently hypotensive. There are some experimental and clinical experiences with the use of methylene blue as a selective inhibitor of the NO–cGMP pathway in anaphylaxis. A study of anaphylactic shock in rabbits showed that the use of methylene blue (3 mg/kg) as a single dose increased survival time compared to controls. Plasma nitrate, a marker of NO release, did not differ between groups. This finding suggests that methylene blue does not mediate its action through NOS inhibition or NO scavenging, and suggests that its primary action may be mediated through GC inhibition [56].
The use of methylene blue for the treatment of humans with refractory anaphylactic shock is limited to case reports and case series. Methylene blue was used successfully to treat three patients who experienced anaphylactic shock following radiocontrast injection during coronary angiography. All had prompt improvement and hemodynamic stabilization without obvious adverse effects [57]. The successful use of methylene blue for refractory hypotension secondary to protamine during cardiac bypass is also reported [58]. Table 1 lists reported cases of methylene blue administration for treatment of anaphylactic shock.
Table 1.
Summary of publishing cases of methylene blue use for refractory shock from anaphylaxis
| Reference | Age/gender | Cause of anaphylaxis | Clinical presentation | Interventions | IV methylene blue dose | Time of effect | Outcome |
|---|---|---|---|---|---|---|---|
| [57] | 57-year-old woman | Radiocontrast | Refractory hypotension, angioedema | Hydrocortisone, epinephrine | 1.5 mg/kg bolus | 20 min | Survival |
| [57] | 48-year-old woman | Radiocontrast | Refractory hypotension, oxygen desaturation | Intubation, Isotonic fluids, epinephrine, dopamine, hydrocortisone | 2 mg/kg bolus followed by 2 mg/kg/h for 2 h | 10 min | Survival |
| [57] | 53-year-old man | Radiocontrast | Refractory hypotension, angioedema | Hydrocortisone ×2 | 1.5 mg/kg infusion | Immediate | Survival |
| [58] | 72-year-old man | Protamine 100 mg | Refractory hypotension, urticaria | Hydrocortisone, pressure, calcium, cardiac bypass | 100 mg | 15 min | Survival |
| [58] | 72-year-old woman | Aprotinin | Refractory hypotension | Hydrocortisone, pressure, fluids, cardiac bypass | 100 mg | 10 min | Survival |
| [98] | 23-year-old woman | Unknown | Angioedema | Antihistamine, epinephrine | 1.5 mg/kg bolus with 1.5 mg/kg infusion | 20 min | Survival |
| [99] | 79-year-old man | Protamine | Urticaria, angioedema, refractory hypotension | Antihistamine pressors, fluids | 35 mg | 20 min | Survival |
| [100] | 72-year-old man | Protamine | Refractory hypotension | Pressors | 2 mg/kg bolus | 30 min | Survival |
The Role of the NO–cGMP Pathway in Septic Shock and Treatment with Methylene Blue
Sepsis is defined as a constellation of clinical findings that result from an infection and a subsequent inflammatory response from a combination of pathogenic products and the body’s own immune response. The most severe form of sepsis is “septic shock,” which is defined as hypotension with signs of tissue hypoperfusion despite fluid resuscitation in the presence of overwhelming infection [59]. There are over 700,000 cases of severe sepsis in the USA annually, and it is the leading cause of ICU mortality [60].
Sepsis is associated with a complex hemodynamic profile when compared to other causes of shock. Septic shock can have features of hypovolemia from capillary leak (i.e., hypovolemic shock) and depressed myocardial function with resultant reduction in cardiac output (i.e., cardiogenic shock). The most characteristic finding with septic shock is profound peripheral vasodilation (i.e., distributive shock). The pathogenesis of septic shock is a complex response of cellular activation that releases a multitude of proinflammatory mediators which includes excessive NO [61]. The overproduction of NO by iNOS in sepsis results in increased production of cGMP contributing to the refractory hypotension observed with septic shock. Numerous animal studies demonstrate large increases of NO after exposure to lipopolysaccharide (i.e., endotoxin) with resultant hypotension [62–67].
The current management of septic shock includes early goal-directed therapy centered on early administration of empiric antibiotics, volume resuscitation, frequent monitoring, and cardiac support with appropriate use of vasopressors. Despite these therapies, the mortality rate remains around 30 % [68]. As a result, the need for additional or alternative therapy for septic shock is clear. One such treatment modality may involve inhibition along the NO–cGMP pathway. Earlier work with nonspecific competitive NOS inhibitors, such as N-monomethyl-l-arginine for the treatment of distributive shock in sepsis noted an increase in mean arterial pressure but no mortality benefit. In fact, the use of nonspecific nitric oxide synthase inhibitors resulted in increased mortality in both animal and humans. NO has a vital, positive role in other pathways so nonspecific inhibition of nitric oxide synthase is detrimental [69]. This begs the questions of whether inhibition further along the NO–cGMP pathway might be beneficial. Later studies examined selective inhibition of guanylate cyclase. One of these selective agents is methylene blue, which may restore vascular tone and improve tissue perfusion.
There are multiple experimental models of septic shock that support the use of methylene blue in the treatment of refractory distributive shock [70–76]. The majority of clinical data on the use of methylene blue for such therapy is observational with case reports and small observational studies, but there are also small controlled clinical trials [77–82]. The majority of these studies demonstrate an increase in mean arterial pressure and/or decrease in pressor requirement. The first prospective randomized controlled study using methylene blue in the treatment of septic shock was published in 2001. Patients were randomized 1:1 to receive either methylene blue or isotonic saline as a control in addition to conventional treatment. When compared to the control group, methylene blue reduced pressor requirement. Although 5/10 patients treated with methylene blue survived as opposed to 3/10 patients who received conventional treatment, this study was not powered to find a mortality difference [83].
Another randomized control trial measured plasma concentrations of cytokines in severe sepsis. In this study, 15 patients received methylene blue and 15 patients received isotonic saline for 6 h. Various cytokines such as interleukin-2 and tumor necrosis factor alpha were measured at baseline, immediately after treatment, 24 h posttreatment, and 48 h posttreatment. When compared to the control group, methylene blue resulted in higher mean arterial pressures, but no difference in mortality or in the various cytokines measured. The primary limitation of this study was the short observation period and small sample size [84].
A prospective, randomized, double-blind, single-center study in 15 consecutive, mechanically ventilated patients with septic shock admitted to the intensive care unit compared escalating doses of methylene blue. Methylene blue was infused at 1 mg/kg (n = 4), 3 mg/kg (n = 6), or 7 mg/kg (n = 5) over 20 min. Methylene blue had a dose-dependent effect on cardiac index, mean pulmonary artery and pulmonary artery occlusion pressure as well as oxygen delivery and lactate concentrations. The data suggested that in human septic shock, methylene blue increases mean arterial blood pressure through an increase in cardiac index and systemic vascular resistance [85].
In summary, the clinical studies that evaluate the use of methylene blue for septic shock demonstrate an improvement in hemodynamics and address the mechanism by which methylene blue may work. However, no morality benefit is shown which may be due to the small sample sizes found in these studies.
The dosing regimen for methylene blue based on experimental and clinical data is not entirely clear but does seem to be similar to what is used for the treatment of methemoglobinemia: 1–2 mg/kg. The dosing used for refractory septic shock has included a single bolus, repeated bolus based on response, low-dose infusion, and infusions followed by a bolus. It is clear based on other studies that the use of high doses of methylene blue, typically doses greater than 7 mg/kg, is associated with adverse effects such as paradoxical induction of methemoglobinemia, acute hemolytic anemia, and detrimental effects on pulmonary function [86, 87].
Based on current data, it appears that the use of a single bolus improves hemodynamics for the duration of a few hours. It is unclear if there is any mortality benefit with temporary improvement of hemodynamics, and for this reason, some authors have advocated using methylene blue as an infusion [78, 79]. However, due to limited experience with this method of administration as well as the lack of data on dosing methylene blue in the setting of liver or kidney dysfunction, the use of methylene blue as an infusion cannot be recommended at this time.
The use of methylene blue has a favorable side effect profile, but it is associated with some adverse effects that are important for the clinician to note. Methylene blue rarely may cause shortness of breath, tremors, vomiting, blue discoloration of body fluids, and acute hemolytic anemia with high doses [88]. Another uncommon adverse effect for clinicians to be aware of is the precipitation of serotonin syndrome [89, 90]. Serotonin syndrome is a potentially fatal condition characterized by autonomic instability that includes agitation, tachycardia, hypertension, and hyperthermia due to excessive stimulation at the 5-HT2A receptor. Serotonin syndrome is most commonly precipitated from drug interactions of multiple serotonergic agents or agents that inhibit metabolism of serotonin such as monoamine oxidase inhibitors [91]. Methylene blue may cause serotonin syndrome because both the parent compound and metabolite, azure B, inhibit MAO-A [92]. Methylene blue causing serotonin syndrome is relatively rare occurring mostly with the use of very high doses.
The patient population that may benefit from the use of methylene blue for refractory distributive shock requires careful consideration. Cardiac support with catecholamines and volume expansion should be the initial therapy of choice. In patients who do not respond to standard treatment, methylene blue may be considered. The ideal dose in unknown, but based on the existing literature and safety profile, we recommend a dose of 1–2 mg/kg as a single bolus. Methylene blue should not be used in patients with pulmonary hypertension, underlying G6PD deficiency, and acute lung injury.
The Use of Methylene Blue for Refractory Shock from Cardiovascular Drug Poisoning
Methylene blue has also been utilized in a case of refractory distributive shock from a cardiovascular drug overdose with a dihydropyridine calcium channel blocker [93]. This case described a confirmed isolated dihydropyridine overdose resulting in profound distributive shock as demonstrated by a Swan-Ganz catheter. The patient remained hypotensive despite multiple therapeutic interventions including isotonic saline, multiple doses of calcium gluconate, glucagon, multiple pressors and high-dose insulin euglycemic (HIE) therapy for several hours. The patient then received methylene blue at 2 mg/kg and soon after showed improvement in hemodynamics with eventual discontinuation of both pressors and HIE. While this case should not be used as sole support for the use of methylene blue as treatment for cardiovascular drug poisoning, this is a potential area of research.
While the primary mechanism of calcium channel blockers involves blockade of l-type calcium channels, experimental studies have demonstrated an additional vasodilatory mechanism through the release of nitric oxide. Although the exact mechanism of NO release is not clear, one of the reasons some dihydropyridine calcium channel blockers increase nitric oxide production is from an increased endothelial nitric oxide synthase activity through phosphorylation of this enzyme [94–97]. It is not clear how much of a role the NO–cGMP pathway may play in an acute overdose with certain calcium channel blockers, but this may explain the mechanism by which methylene blue acts.
Conclusion
Increased NO and cGMP production in tissues is well documented in various conditions of distributive shock such as sepsis and anaphylaxis. The NO–cGMP pathway plays a central role in the pathophysiology of distributive shock. There is experimental and clinical evidence that support the use of a selective NO–cGMP pathway inhibitor, methylene blue, as a treatment in cases of refractory vasodilatory shock with no response to conventional treatment. Clinicians should be aware of potential adverse effects and drug interactions with serotonergic agents when considering the use of methylene blue.
Acknowledgments
This study was supported in part by grant 5UL1RR029893 from the National Center for Research Resources, National Institutes of Health.
Conflict of Interest
The authors have no conflict of interest to declare.
Contributor Information
David H. Jang, Phone: +1-212-4474941, FAX: +1-212-4478223, Email: Jangd01@nyumc.org
Lewis S. Nelson, Email: lnelsonmd@gmail.com
Robert S. Hoffman, FAX: +1-212-4478223, Email: bobhoffmd@gmail.com
References
- 1.Spaeth CS, Robison T, Fan JD et al. (2012) Cellular mechanisms of plasmalemmal sealing and axonal repair by polyethylene glycol and methylene blue. J Neurosci Res 90(5):955–66 doi:10.1002/jnr.23022 [DOI] [PubMed]
- 2.Sohrabnezhad SH. Study of catalytic reduction and photodegradation of methylene blue by heterogeneous catalyst. Spectrochim Acta A Mol Biomol Spectrosc. 2011;81(1):228–235. doi: 10.1016/j.saa.2011.05.109. [DOI] [PubMed] [Google Scholar]
- 3.Kasozi DM, Gromer S, Adler H, et al. The bacterial redox signaller pyocyanin as an antiplasmodial agent: comparisons with its thioanalog methylene blue. Redox Rep. 2011;16(4):154–165. doi: 10.1179/174329211X13049558293678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meissner PE, Mandi G, Coulibaly B, et al. Methylene blue for malaria in Africa: results from a dose-finding study in combination with chloroquine. Malar J. 2006;8:5–84. doi: 10.1186/1475-2875-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adjalley SH, Johnston GL, Li T, Eastman RT, et al. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci U S A. 2011;108(47):E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanzlik PJ. Subject of this letter: Methylene blue as antidote for cyanide poisoning. Cal West Med. 1933;38(3):225–226. [PMC free article] [PubMed] [Google Scholar]
- 7.Giovanis P, Garna A, Marcante M, et al. Ifosfamide encephalopathy and use of methylene blue. A case report of different sequential neurotoxicity. Tumori. 2009;95(4):545–546. doi: 10.1177/030089160909500426. [DOI] [PubMed] [Google Scholar]
- 8.Richards A, Marshall H, McQuary A. Evaluation of methylene blue, thiamine, and/or albumin in the prevention of ifosfamide-related neurotoxicity. J Oncol Pharm Pract. 2011;17(4):372–380. doi: 10.1177/1078155210385159. [DOI] [PubMed] [Google Scholar]
- 9.Barclay JA, Ziemba SE, Ibrahim RB. Dapsone-induced methemoglobinemia: a primer for clinicians. Ann Pharmacother. 2011;45(9):1103–1115. doi: 10.1345/aph.1Q139. [DOI] [PubMed] [Google Scholar]
- 10.So TY, Farrington E. Topical benzocaine-induced methemoglobinemia in the pediatric population. J Pediatr Health Care. 2008;22(6):335–339. doi: 10.1016/j.pedhc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Hahn IH, Hoffman RS, Nelson LS. EMLA-induced methemoglobinemia and systemic topical anesthetic toxicity. J Emerg Med. 2004;26(1):85–88. doi: 10.1016/j.jemermed.2003.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 14.Llyengar R, Stuehr DJ, Marietta MA. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad. 1987;84:6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hibbs JB, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role of L-argininc deiminasc and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 16.Garcin ED, Bruns CM, Lloyd SJ, et al. Structural basis for isozyme-specific regulation of electron transfer in nitric-oxide synthase. J Biol Chem. 2004;279:37918–37927. doi: 10.1074/jbc.M406204200. [DOI] [PubMed] [Google Scholar]
- 17.Daff S. NO synthase: structures and mechanisms. Nitric Oxide. 2012;23:1–11. doi: 10.1016/j.niox.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Stuehr D, Pou S, Rosen GM. Oxygen reduction by nitric-oxide synthases. J Biol Chem. 2001;276:14533–14536. doi: 10.1074/jbc.R100011200. [DOI] [PubMed] [Google Scholar]
- 19.Tatsumi R, Wuollet AL, Tabata K, et al. A role for calcium-calmodulin in regulating nitric oxide production during skeletal muscle satellite cell activation. Am J Physiol Cell Physiol. 2009;296(4):C922–C929. doi: 10.1152/ajpcell.00471.2008. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JS, Beckman TW, Chen J, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckman JS, Crow JP. Pathological implications of nitric oxide, superoxide and peroxynitrite formation. Biochem Soc Trans. 1993;21(2):330–334. doi: 10.1042/bst0210330. [DOI] [PubMed] [Google Scholar]
- 22.Ma L. Evidence for nitric oxide-generator cells in the brain. Bull Tokyo Med Dent Univ. 1993;40(3):125–134. [PubMed] [Google Scholar]
- 23.Forstermann U, Closs EI, Pollock JS, Nakane, et al. Nitric oxide synthase isozymes: characterization, purification, molecular cloning, and functions. Hypertension. 1994;23:1121–1131. doi: 10.1161/01.HYP.23.6.1121. [DOI] [PubMed] [Google Scholar]
- 24.Salemme E, Diano S, Maharajan P, Maharajan V. Nitric oxide, a neuronal messenger. Its role in the hippocampus neuronal plasticity. Riv Biol. 1996;89(1):87–107. [PubMed] [Google Scholar]
- 25.Izumi Y, Clifford DB, Zorumski CF. Inhibition of long-term potentiation by NMDA-mediated nitric oxide release. Science. 1992;257:1273–1276. doi: 10.1126/science.1519065. [DOI] [PubMed] [Google Scholar]
- 26.Handy RL, Wallace P, Moore PK. Inhibition of nitric oxide synthase by isothioureas: cardiovascular and antinociceptive effects. Pharmacol Biochem Behav. 1996;55(2):179–184. doi: 10.1016/S0091-3057(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 27.Togashi H, Sakuma I, Yoshioka M, et al. A central nervous system action of nitric oxide in blood pressure regulation. J Pharmacol Exp Ther. 1992;262:343–347. [PubMed] [Google Scholar]
- 28.Lipton SA, Choi YB, Pan ZH, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitrosocompounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 29.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 30.Venema RC, Sayegh HS, Arnal JF, et al. Role of the enzyme calmodulin-binding domain in membrane association and phospholipid inhibition of endothelial nitric oxide synthase. J Biol Chem. 1995;270(24):14705–14711. doi: 10.1074/jbc.270.24.14705. [DOI] [PubMed] [Google Scholar]
- 31.Zeiher AM, Fisslthaler B, Schray-Utz B, et al. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.RES.76.6.980. [DOI] [PubMed] [Google Scholar]
- 32.Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing factor from cultured human endothelial cells inhibits aggregation of human platelets. Thromb Res. 1987;47:561–571. doi: 10.1016/0049-3848(87)90361-6. [DOI] [PubMed] [Google Scholar]
- 33.Kurihara N, Alfie ME, Sigmon DH, et al. Role of nNOS in blood pressure regulation in eNOS null mutant mice. Hypertension. 1998;32(5):856–861. doi: 10.1161/01.HYP.32.5.856. [DOI] [PubMed] [Google Scholar]
- 34.Triggle CR, Ding H. A review of endothelial dysfunction in diabetes: a focus on the contribution of a dysfunctional eNOS. J Am Soc Hypertens. 2010;4(3):102–115. doi: 10.1016/j.jash.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-G. [DOI] [PubMed] [Google Scholar]
- 36.MacNaul KL, Hutchinson NI. Differential expression of iNOS and cNOS mRNA in human vascular smooth muscle cells and endothelial cells under normal and inflammatory conditions. Biochem Biophys Res Commun. 1993;196(3):1330–1334. doi: 10.1006/bbrc.1993.2398. [DOI] [PubMed] [Google Scholar]
- 37.Tamir S, deRojas-Walker T, Gal A, et al. Nitric oxide production in relation to spontaneous B-cell lymphoma and myositis in SJL mice. Cancer Res. 1995;55(19):4391–4397. [PubMed] [Google Scholar]
- 38.Losada AP, Bermúdez R, Faílde LD, et al. Quantitative and qualitative evaluation of iNOS expression in turbot (Psetta maxima) infected with Enteromyxum scophthalmi. Fish Shellfish Immunol. 2012;32(2):243–248. doi: 10.1016/j.fsi.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Sade K, Schwartz IF, Etkin S, et al. Expression of inducible nitric oxide synthase in a mouse model of anaphylaxis. J Investig Allergol Clin Immunol. 2007;17(6):379–385. [PubMed] [Google Scholar]
- 40.Matuschek A, Ulbrich M, Timm S, et al. Analysis of parathyroid graft rejection suggests alloantigen-specific production of nitric oxide by iNOS-positive intragraft macrophages. Transpl Immunol. 2009;21(4):183–191. doi: 10.1016/j.trim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 41.Okamoto I, Abe M, Shibata K, et al. Evaluating the role of inducible nitric oxide synthase using a novel and selective inducible nitric oxide synthase inhibitor in septic lung injury produced by cecal ligation and puncture. Am J Respir Crit Care Med. 2000;162(2 Pt 1):716–722. doi: 10.1164/ajrccm.162.2.9907039. [DOI] [PubMed] [Google Scholar]
- 42.Lucas KA, Pitari GM, Kazerounian S, et al. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–413. [PubMed] [Google Scholar]
- 43.Wedel B, Garbers D. The guanylyl cyclase family at Y2K. Annu Rev Physiol. 2001;63:215–233. doi: 10.1146/annurev.physiol.63.1.215. [DOI] [PubMed] [Google Scholar]
- 44.Mujoo K, Sharin VG, Martin E, et al. Role of soluble guanylyl cyclase-cyclic GMP signaling in tumor cell proliferation. Nitric Oxide. 2010;22(1):43–50. doi: 10.1016/j.niox.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schoeffter P, Lugnier C, Demesy-Waeldele F, et al. Role of cyclic AMP- and cyclic GMP-phosphodiesterases in the control of cyclic nucleotide levels and smooth muscle tone in rat isolated aorta. A study with selective inhibitors. Biochem Pharmacol. 1987;36(22):3965–3972. doi: 10.1016/0006-2952(87)90465-5. [DOI] [PubMed] [Google Scholar]
- 46.Bensinger RE, Podos SM. Cyclic nucleotide metabolism in the retina. Invest Ophthalmol. 1975;14(4):263–266. [PubMed] [Google Scholar]
- 47.Kumar A, Brar R, Wang P, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276:R256–R276. doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]
- 48.Winbery SL, Lieberman PL. Histamine and antihistamines in anaphylaxis. Clin Allergy Immunol. 2002;17:287–317. [PubMed] [Google Scholar]
- 49.Lieberman P. The use of antihistamines in the prevention and treatment of anaphylaxis and anaphylactoid reactions. J Allergy Clin Immunol. 1990;86(4 Pt 2):684–686. doi: 10.1016/S0091-6749(05)80241-6. [DOI] [PubMed] [Google Scholar]
- 50.Enjeti S, Bleecker ER, Smith PL, et al. Hemodynamic mechanisms in anaphylaxis. Circ Shock. 1983;11:297–309. [PubMed] [Google Scholar]
- 51.Toda N. Endothelium-dependent relaxation induced by angiotensin II and histamine in isolated arteries of dog. Br J Pharmacol. 1984;81:301–307. doi: 10.1111/j.1476-5381.1984.tb10079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krstic MK, Stepanovic RM, Krstic SK, et al. Endothelium-dependent relaxation of the rat renal artery caused by activation of histamine H1-receptors. Pharmacology. 1989;38:113–120. doi: 10.1159/000138526. [DOI] [PubMed] [Google Scholar]
- 53.Rosenkranz-Weiss P, Sessa WC, Milstien S, et al. Regulation of nitric oxide synthesis by proinflammatory cytokines in human umbilical vein endothelial cells: elevations in tetrahydrobiopterin levels enhance endothelial nitric oxide synthase specific activity. J Clin Invest. 1994;93:2236–2243. doi: 10.1172/JCI117221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Burkhardt C, Heinrich UR, et al. Histamine upregulates gene expression of endothelial nitric oxide synthase in human vascular endothelial cells. Circulation. 2003;107:2348–2354. doi: 10.1161/01.CIR.0000066697.19571.AF. [DOI] [PubMed] [Google Scholar]
- 55.Champion HC, Kadowitz PJ. NO release and the opening of K + ATP channels mediate vasodilator responses to histamine in the cat. Am J Physiol. 1997;273(2 pt 2):H928–H937. doi: 10.1152/ajpheart.1997.273.2.H928. [DOI] [PubMed] [Google Scholar]
- 56.Buzato MA, Viaro F, Piccinato CE, et al. The use of methylene blue in the treatment of anaphylactic shock induced by compound 48/80: experimental studies in rabbits. Shock. 2005;23(6):582–587. [PubMed] [Google Scholar]
- 57.Oliveira Neto AM, Duarte NM, Vicente WV, et al. Methylene blue: an effective treatment for contrast medium-induced anaphylaxis. Med Sci Monit. 2003;9(11):CS102–CS106. [PubMed] [Google Scholar]
- 58.Del Duca D, Sheth SS, Clarke AE, et al. Use of methylene blue for catecholamine-refractory vasoplegia from protamine and aprotinin. Ann Thorac Surg. 2009;87(2):640–642. doi: 10.1016/j.athoracsur.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 59.Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 60.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 61.Dellinger RP. Inflammation and coagulation: implications for the septic patient. Clin Infect Dis. 2003;36:1259–1265. doi: 10.1086/374835. [DOI] [PubMed] [Google Scholar]
- 62.Kumar A, Brar R, Wang P, et al. Role of nitric oxide and cGMP in human septic serum-induced depression of cardiac myocyte contractility. Am J Physiol. 1999;276(1 Pt 2):R265–R276. doi: 10.1152/ajpregu.1999.276.1.R265. [DOI] [PubMed] [Google Scholar]
- 63.Symeonides S, Balk RA (1999) Nitric oxide in the pathogenesis of sepsis. Infect Dis Clin North Am 13(2):449–63 x [DOI] [PubMed]
- 64.Fleming I, Julou-Schaeffer G, Gray GA, et al. Evidence that an L-arginine/nitric oxide dependent elevation of tissue cyclic GMP content is involved in depression of vascular reactivity by endotoxin. Br J Pharmacol. 1991;103(1):1047–1052. doi: 10.1111/j.1476-5381.1991.tb12298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schott CA, Gray GA, Stoclet JC. Dependence of endotoxin-induced vascular hyporeactivity on extracellular L-arginine. Br J Pharmacol. 1993;108(1):38–43. doi: 10.1111/j.1476-5381.1993.tb13436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Julou-Schaeffer G, Gray GA, Fleming I, et al. Loss of vascular responsiveness induced by endotoxin involves L-arginine pathway. Am J Physiol. 1990;259(4 Pt 2):H1038–H1043. doi: 10.1152/ajpheart.1990.259.4.H1038. [DOI] [PubMed] [Google Scholar]
- 67.Schuller F, Fleming I, Stoclet JC, et al. Effect of endotoxin on circulating cyclic GMP in the rat. Eur J Pharmacol. 1992;212(1):93–96. doi: 10.1016/0014-2999(92)90077-H. [DOI] [PubMed] [Google Scholar]
- 68.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 69.Watson D, Grover R, Anzueto A, et al. Cardiovascular effects of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) in patients with septic shock: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144–002) Crit Care Med. 2004;32(1):13–20. doi: 10.1097/01.CCM.0000104209.07273.FC. [DOI] [PubMed] [Google Scholar]
- 70.Cheng X, Pang CC. Pressor and vasoconstrictor effects of methylene blue in endotoxaemic rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;357(6):648–653. doi: 10.1007/PL00005220. [DOI] [PubMed] [Google Scholar]
- 71.Fernandes D, Sordi R, Pacheco LK, et al. Late, but not early, inhibition of soluble guanylate cyclase decreases mortality in a rat sepsis model. J Pharmacol Exp Ther. 2009;328(3):991–999. doi: 10.1124/jpet.108.142034. [DOI] [PubMed] [Google Scholar]
- 72.Demirbilek S, Sizanli E, Karadag N, et al. The effects of methylene blue on lung injury in septic rats. Eur Surg Res. 2006;38(1):35–41. doi: 10.1159/000091525. [DOI] [PubMed] [Google Scholar]
- 73.Menardi AC, Viaro F, Vicente WV, et al. Hemodynamic and vascular endothelium function studies in healthy pigs after intravenous bolus infusion of methylene blue. Arq Bras Cardiol. 2006;87(4):525–532. doi: 10.1590/S0066-782X2006001700019. [DOI] [PubMed] [Google Scholar]
- 74.Evgenov OV, Sveinbjørnsson B, Bjertnaes LJ. Continuously infused methylene blue modulates the early cardiopulmonary response to endotoxin in awake sheep. Acta Anaesthesiol Scand. 2001;45(10):1246–1254. doi: 10.1034/j.1399-6576.2001.451013.x. [DOI] [PubMed] [Google Scholar]
- 75.Evgenov OV, Sager G, Bjertnaes LJ. Methylene blue reduces lung fluid filtration during the early phase of endotoxemia in awake sheep. Crit Care Med. 2001;29(2):374–379. doi: 10.1097/00003246-200102000-00028. [DOI] [PubMed] [Google Scholar]
- 76.Galili Y, Kluger Y, Mianski Z, et al. Methylene blue—a promising treatment modality in sepsis induced by bowel perforation. Eur Surg Res. 1997;29(5):390–395. doi: 10.1159/000129548. [DOI] [PubMed] [Google Scholar]
- 77.Dumbarton TC, Minor S, Yeung CK, et al. Prolonged methylene blue infusion in refractory septic shock: a case report. Can J Anaesth. 2011;58(4):401–405. doi: 10.1007/s12630-011-9458-x. [DOI] [PubMed] [Google Scholar]
- 78.van Haren FM, Pickkers P, Foudraine N et al. (2010) The effects of methylene blue infusion on gastric tonometry and intestinal fatty acid binding protein levels in septic shock patients. J Crit Care 25(2):358.e1-7 [DOI] [PubMed]
- 79.Brown G, Frankl D, Phang T. Continuous infusion of methylene blue for septic shock. Postgrad Med J. 1996;72(852):612–614. doi: 10.1136/pgmj.72.852.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heemskerk S, van Haren FM, Foudraine NA, et al. Short-term beneficial effects of methylene blue on kidney damage in septic shock patients. Intensive Care Med. 2008;34(2):350–354. doi: 10.1007/s00134-007-0867-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park BK, Shim TS, Lim CM, et al. The effects of methylene blue on hemodynamic parameters and cytokine levels in refractory septic shock. Korean J Intern Med. 2005;20(2):123–128. doi: 10.3904/kjim.2005.20.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donati A, Conti G, Loggi S, et al. Does methylene blue administration to septic shock patients affect vascular permeability and blood volume? Crit Care Med. 2002;30(10):2271–2277. doi: 10.1097/00003246-200210000-00015. [DOI] [PubMed] [Google Scholar]
- 83.Kirov MY, Evgenov OV, Evgenov NV, et al. Infusion of methylene blue in human septic shock: a pilot, randomized, controlled study. Crit Care Med. 2001;29(10):1860–1867. doi: 10.1097/00003246-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 84.Memis D, Karamanlioglu B, Yuksel M, et al. The influence of methylene blue infusion on cytokine levels during severe sepsis. Anaesth Intensive Care. 2002;30(6):755–762. doi: 10.1177/0310057X0203000606. [DOI] [PubMed] [Google Scholar]
- 85.Juffermans NP, Vervloet MG, Daemen-Gubbels CR, et al. A dose-finding study of methylene blue to inhibit nitric oxide actions in the hemodynamics of human septic shock. Nitric Oxide. 2010;22(4):275–280. doi: 10.1016/j.niox.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Weingartner R, Oliveira E, Oliveira ES, et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a short infusion of methylene blue. Braz J Med Biol Res. 1999;32(12):1505–1513. doi: 10.1590/S0100-879X1999001200009. [DOI] [PubMed] [Google Scholar]
- 87.Gachot B, Bedos JP, Veber B, et al. Short-term effects of methylene blue on hemodynamics and gas exchange in humans with septic shock. Intensive Care Med. 1995;21(12):1027–1031. doi: 10.1007/BF01700666. [DOI] [PubMed] [Google Scholar]
- 88.Goluboff N, Wheaton R. Methylene blue-induced cyanosis and acute hemolytic anemia complicating the treatment of methemoglobinemia. J Pediatr. 1961;58:86–89. doi: 10.1016/S0022-3476(61)80064-4. [DOI] [PubMed] [Google Scholar]
- 89.Héritier Barras AC, Walder B, Seeck M. Serotonin syndrome following methylene blue infusion: a rare complication of antidepressant therapy. J Neurol Neurosurg Psychiatry. 2010;81(12):1412–1413. doi: 10.1136/jnnp.2009.172221. [DOI] [PubMed] [Google Scholar]
- 90.McDonnell AM, Rybak I, Wadleigh M et al. (2012) Suspected serotonin syndrome in a patient being treated with methylene blue for ifosfamide encephalopathy. J Oncol Pharm Pract 18(4):436–9 [DOI] [PubMed]
- 91.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 92.Petzer A, Harvey BH, Wegener G, et al. Azure B, a metabolite of methylene blue, is a high-potency, reversible inhibitor of monoamine oxidase. Toxicol Appl Pharmacol. 2012;258(3):403–409. doi: 10.1016/j.taap.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 93.Jang DH, Nelson LS, Hoffman RS. Methylene blue in the treatment of refractory shock from an amlodipine overdose. Ann Emerg Med. 2011;58(6):565–567. doi: 10.1016/j.annemergmed.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 94.Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels, an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–580. doi: 10.1161/01.CIR.97.6.576. [DOI] [PubMed] [Google Scholar]
- 95.Zhang X, Loke KE, Mital S, et al. Paradoxical release of nitric oxide by an L-type calcium channel antagonist, the R+ enantiomer of amlodipine. J Cardiovasc Pharmacol. 2002;39:208–214. doi: 10.1097/00005344-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 96.Lensai H, et al. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser and Thr. Cardiovasc Res. 2003;59:844–853. doi: 10.1016/S0008-6363(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 97.Xu B, Xiao-hung L, Lin G, et al. Amlodipine, but not verapamil or nifedipine, dilates rabbit femoral artery largely through a nitric oxide- and kinin-dependent mechanism. Br J Pharmacol. 2002;136:375–382. doi: 10.1038/sj.bjp.0704753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodrigues JM, Pazin Filho A, Rodrigues AJ, et al. Methylene blue for clinical anaphylaxis treatment: a case report. Sao Paulo Med J. 2007;125:60–62. doi: 10.1590/S1516-31802007000100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weissgerber AJ. Methylene blue for refractory hypotension: a case report. AANA J. 2008;76(4):271–274. [PubMed] [Google Scholar]
- 100.Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg. 2003;125(2):426–427. doi: 10.1067/mtc.2003.140. [DOI] [PubMed] [Google Scholar]