Abstract
Introduction
Severe anion gap (AG) acidosis associated with intravenous sodium thiosulfate (STS) administration has not been previously described in nondialysis chronic kidney disease (CKD) patients.
Case Report
We present a CKD patient with a baseline creatinine 1.8 mg/dL (eGFR 28 ml/min/1.73 m2) who developed sustained and life-threatening AG acidosis associated with intravenous STS treatment for calciphylaxis.
Discussion
Although marketed as a safe drug, STS can cause life-threatening acidosis as illustrated in this case. STS-induced AG acidosis should be considered in the differential diagnosis of severe acidosis in patients receiving STS. Dosage adjustment and close follow-up of patients’ acid–base status after STS initiation is necessary.
Keywords: Anion gap acidosis, Sodium thiosulfate (STS), Chronic kidney disease, Calciphylaxis, GOLD MARKS
Introduction
Severe anion gap (AG) acidosis is a medical emergency. Determining the etiology of acidosis is paramount to effective treatment. Medical mnemonics used to differentiate causes of an anion gap acidosis include GOLD MARK (Glycols, Oxoproline, l-lactate, d-lactate, Methanol, Aspirin, Renal Failure and Ketoacidosis) [1].
Calciphylaxis is a type of calcific arteriopathy (also known as calcific uremic arteriopathy) caused by intimal hypertrophy, medial calcification, endovascular fibrosis, and thrombosis of small arteries and arterioles, leading to ischemic infarction, necrotic ulceration, and secondary infection of the involved areas of skin and subcutaneous adipose tissue. Calciphylaxis occurs primarily in patients with end-stage renal disease, although recently, there have been an increasing number of studies showing its occurrence in nondialysis chronic kidney disease (CKD) patients [2]. The pathogenesis of calciphylaxis is not entirely clear, and the prognosis is poor, with 6-month mortality rate of 33–80 % [3]. To date, no definitive therapy has been established. The general approach has been to maximize supportive measures including meticulous wound care. Specific therapy, such as sodium thiosulfate (STS) given intravenously (IV) has been used with some success and is considered a safe treatment option [4–6].
We report a case of sustained and life-threatening AG acidosis associated with the use of STS for calciphylaxis. This case illustrates the potential danger associated with STS use in non-dialysis CKD patients and cautions clinicians to suspect STS, when appropriate, as a potential etiology of AG acidosis.
Case Report
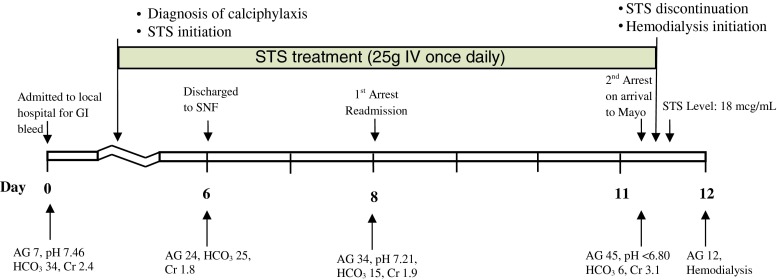
A 71-year-old Caucasian woman with a medical history of stage IV CKD (baseline serum creatinine (Cr), 1.8 mg/dL; eGFR, 28 ml/min/1.73 m2), morbid obesity (body mass index, 41 kg/m2), type II diabetes mellitus, coronary artery disease, positional obstructive sleep apnea, hypertension and hyperlipidemia was emergently readmitted to a local hospital (30 h after discharge) for loss of consciousness (Fig. 1, day 8).
Fig. 1.
Timeline of clinical events correlated with laboratory markers of acidosis. STS sodium thiosulfate, AG anion gap, HCO3 bicarbonate, SNF skilled nursing facility
Eight days prior (Fig. 1, day 0), she had been hospitalized for lower gastrointestinal bleed. Her outpatient medications included a stable regimen of amlodipine, carvedilol, clopidogrel, simvastatin, and insulin. Admission laboratory studies (Table 1, day 0) showed pH 7.46, pCO2 45.7 mmHg, pO285 mmHg, HCO3 34 mmol/L (AG7), and Cr 2.4 mg/dL. The bicarbonate elevation was attributed to mild volume contraction. While hospitalized, calciphylaxis was diagnosed (Fig. 2) and oral cinacalcet (30 mg daily) and IV STS (25 g daily) were initiated. Her gastrointestinal bleeding was controlled with conservative measures. She was subsequently discharged to a skilled nursing facility (Fig. 1, day 6) on the same dosages of daily cinacalcet and STS in addition to her prior outpatient medication regimen. Her laboratory studies on the day of discharge are shown in Table 1, notably AG was elevated to 24. Serum parathyroid hormone and phosphorus concentration were not obtained during the hospital stay. There were no interim laboratory studies or any change in medications during the period of discharge and readmission.
Table 1.
Laboratory data of the patient
| ref range | Units | Baseline | Discharge to SNF | First arrest | Second arrest | |
|---|---|---|---|---|---|---|
| Day 0 | Day 6 | Day 8 | Day 11 | |||
| Hemoglobin | g/dL | 12–15.5 | 5.1 (GI bleed) | 10.7 | 8.9 | 7.9 |
| Hematocrit | % | 34.–44.5 | 16.3 | 32.2 | 27.5 | 24.3 |
| Sodium | mmol/L | 135–145 | 137 | 140 | 142 | 144 |
| Potassium | mmol/L | 3.6–5.2 | 4.5 | 3.5 | 3.8 | 4.9 |
| Chloride | mmol/L | 100–108 | 96 | 91 | 93 | 93 |
| Bicarbonate | mmol/L | 22–29 | 34 | 25 | 15 | 6 |
| Anion gap | 7–15 | 7 | 24 | 34 | 45 | |
| Blood urea nitrogen | mg/dL | 6–21 | 69.8 | 45.8 | 57.6 | 73 |
| Creatinine | mg/dL | 0.6–1.1 | 2.4 | 1.8 | 1.9 | 3.1 |
| Glucose | mg/dL | 70–100 | 121 | 142 | 185 | 174 |
| Lactate | mmol/L | 0.6–2.3 | 1.1 | –a | 0.5 | 0.6 |
| Calcium | mg/dL | 8.9–10.1 | 8.6 | 8.9 | 8.8 | –a |
| Ionized calcium | mg/dL | 4.65–5.3 | –a | –a | –a | 4.55 |
| Albumin | g/dL | 3.4–4.7 | 1.9 | –a | 1.7 | –a |
| Phosphorus | mg/dL | 2.5–4.5 | –a | –a | –a | 10.3 |
| Magnesium | mg/dL | 1.7–2.3 | –a | –a | 2.0 | 1.6 |
| pH | 7.350–7.450 | 7.46 | –a | 7.21 | <6.80 | |
| pO2 | 80–100 | 85 | –a | 64 | 309 | |
| pCO2 | 35.0–45.0 | 45.7 | –a | 39 | 53 |
SNF skilled nursing facility
aNo values available
Fig. 2.
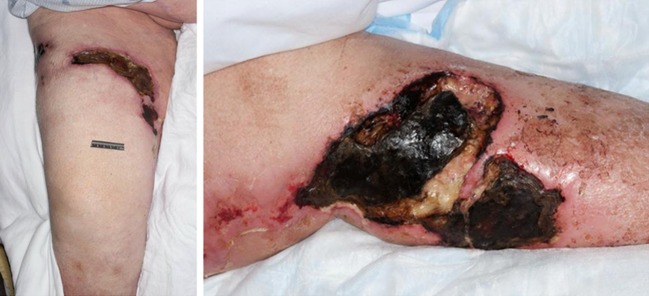
Ischemic lesions of calciphylaxis in the patient’s lower extremity
As per outside records, she required approximately 5 min of cardiopulmonary resuscitation on readmission (Fig. 1, day 8). She regained hemodynamic stability and did not require intubation. Her ECGs showed sinus rhythm of 60 per minute without arrhythmia. The etiology of the episode was unclear; a vasovagal spell was listed on her records as a potential cause. Laboratory studies during the resuscitations revealed pH 7.21, pCO2 39 mmHg, pO264 mmHg, HCO3 15 mmol/L (AG 34), and Cr 1.9 mg/dL. She received empirical IV meropenem and vancomycin in addition to her outpatient regimen including STS for the following 2 days. Her kidney function and acidosis worsened; decision was then made to transfer the patient to our institution for further management.
Upon arrival at our institution (Fig. 1, day 11), she became acutely unresponsive, obtunded, and hypoxic. She required approximately 5 min of cardiopulmonary resuscitation and intubation. Naloxone was administered without any notable effect. Electrocardiogram during resuscitation showed sinus bradycardia with a rate of 40 per minute and QTc of 506 ms. Laboratory data during resuscitation showed pH <6.80, pCO2 53 mmHg, pO2 309 mmHg, HCO3 6 mmol/L (AG45), and Cr 3.1 mg/dL. Additional data are shown in Table 1 (day 11).
She was admitted to the medical intensive care unit. A repeat ABG (2 h later) showed pH 6.96, pCO231 mmHg, pO276 mmHg, and HCO3 7 mmol/L. Her serum osmolar gap, beta-hydroxybutyrate, salicylates, volatile alcohols, and d-lactate were all negative or within normal limits. There was no change in serial blood troponin levels. Urine drug and organic acid screen were negative for nonprescribed drugs and oxoproline. Imaging studies of the abdomen and pelvis failed to identify any abnormality that could account for the severe AG acidosis. Hemodialysis was initiated for her within 6 h of her arrival. Serum STS level obtained at approximately 3.5 h into her first hemodialysis was 18 mcg/ml (no known reference range for patients receiving IV STS). The level most likely underestimated the true initial level of STS, as STS is efficiently cleared with dialysis [9]. Repeat laboratory study the next day showed HCO3 of 23 mmol/L and AG of 12.
STS was not reinitiated in our institution. After correcting the acidosis, the patient’s cardiopulmonary status improved and she was extubated within 2 days. She continued, however, to experience pain related to the calciphylaxis. She subsequently declined further interventions. Per her wish, she was discharged to hospice.
Discussion
We investigated multiple potential etiologies for the patient’s acidosis. There was no evidence of contribution from volatile alcohols, oxoproline, lactic acid, d-lactate, salicylates, ketoacids, or other organic acids.
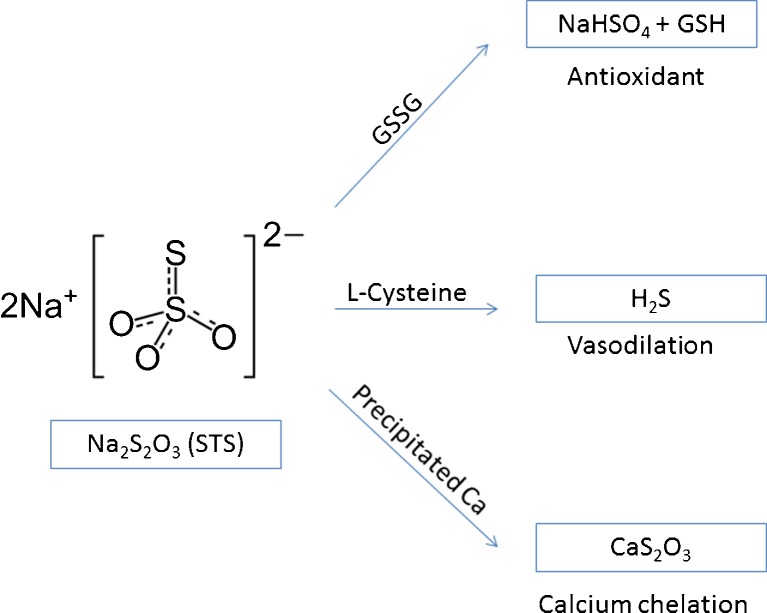
Sodium thiosulfate (Na2S2O3) was first described in the treatment of cyanide poisoning in 1933 [7]. STS was subsequently approved by the Food and Drug Administration in 1992 for the same indication. Since its approval, STS has been used off-label for many clinical conditions including cisplatin toxicity, mechlorethamine extravasation, calcium-containing nephrolithiasis, tumoral calcification, and, in recent years, for calciphylaxis. In practice, STS has gradually becoming an accepted treatment for calciphylaxis [4, 5]. It is postulated that STS dissolves the poorly soluble calcium salts into more soluble calcium thiosulfate and that STS possesses vasodilatory and antioxidant effects (Fig. 3) [4, 5]. The literature on its use for calciphylaxis, however, relates almost exclusively to dialysis patients [4–6]. There is virtually no literature to guide its safe use for nondialysis CKD patients.
Fig. 3.
Sodium thiosulfate (STS) forms thiosulfuric acid (H2S2O3) in body fluids, producing active metabolites functioning as antioxidant, vasodilator, and calcium chelator
STS is an anion with a molecular weight of 248 Da. It is fully dissolved in aqueous solution but poorly absorbed when administered orally. In the circulation, it has a volume of distribution of 47 % of body weight. STS generates a number of metabolic products, many of which are highly acidic including thiosulfuric acid and hydrogen sulfide (Fig. 3). STS and its metabolites are excreted approximately 95 % renally and 2 % via the biliary system [8]. Reported STS dosing range for treating calciphylaxis in dialysis patients has been between 5 g (four times weekly) and 25 g (three times weekly), given intravenously [4, 5, 9, 10]. STS is shown to be efficiently cleared by intermittent dialysis [5, 10].
The safety and efficacy of STS in the treatment of calciphylaxis have been demonstrated in patients with normal kidney function as well as in patients with dialysis-dependent renal failure [2, 6]. Adverse effect profile of STS has been considered acceptable and mainly includes mild gastrointestinal disturbance, headaches, rhinorrhea, and transient AG acidosis without major untoward consequences [8]. A case report by Selk and Rodby describes a hemodialysis patient who developed a severe AG acidosis following STS administration (AG44 and bicarbonate, 10 mmol/L) [9]. The patient, however, suffered no major adverse consequences from STS despite the severe AG acidosis between each dialysis session. In fact, because of the lack of major consequences, she was rechallenged several times to document the coupling of AG acidosis and STS administration.
In the current case, our patient had nondialysis-dependent CKD. Despite her renal dysfunction, she received daily STS until transfer to our institution. STS given at 25 g per day hypothetically generates approximately 200 mmol of proton daily. With her marginal kidney function and without being on dialysis, severe acidosis would be expected as protons accumulate. We feel that this patient’s clinical deterioration and arrest was due to the profound acidosis resulting from STS administration that ultimately led to the patient’s cardiopulmonary arrest. Notably, even after the first episode of arrest, the relationship of AG acidosis and STS administration was unrecognized. In retrospect, her arrest episodes associated with severe AG acidosis were attributable to STS as no other causes of AG acidosis were identified. Limitations of this report include potential unaccounted factors and/or acidic agents given at outsides facilities confounding the AG acidosis. After extensive investigation, however, we could not identify any of those interfering factors.
In summary, a case of life-threatening AG acidosis directly related to STS treatment in a nondialysis CKD patient is described. With a growing nondialysis CKD population, recognizing the potential risk of severe AG acidosis associated with STS administration for calciphylaxis cautions clinicians to follow-up patients closely after STS initiation. Moreover, we propose to consider adding S (for STS) to the AG acidosis differential list and modifying the mnemonic to “GOLD MARKS” to include STS-associated AG acidosis.
Acknowledgments
Conflict of Interest
None
References
- 1.Mehta AN, Emmett JB, Emmett M. GOLD MARK: an anion gap mnemonic for the 21st century. Lancet. 2008;372:892. doi: 10.1016/S0140-6736(08)61398-7. [DOI] [PubMed] [Google Scholar]
- 2.Nigwekar SU, Wolf M, Sterns RH, Hix JK. Calciphylaxis from nonuremic causes: a systematic review. Clin J Am Soc Nephrol. 2008;3:1139–1143. doi: 10.2215/CJN.00530108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weenig RH, Sewell LD, Davis MD, McCarthy JT, Pittelkow MR. Calciphylaxis: natural history, risk factor analysis, and outcome. J Am Acad Dermatol. 2007;56:569–579. doi: 10.1016/j.jaad.2006.08.065. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MR, Tyagi SC, Kolb L, Sowers JR, Khanna R. Vascular ossification-calcification in metabolic syndrome, type 2 diabetes mellitus, chronic kidney disease, and calciphylaxis-calcific uremic arteriolopathy: the emerging role of sodium thiosulfate. Cardiovasc Diabetol. 2005;4:4. doi: 10.1186/1475-2840-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brucculeri M, Cheigh J, Bauer G, Serur D. Long-term intravenous sodium thiosulfate in the treatment of a patient with calciphylaxis. Semin Dial. 2005;18:431–434. doi: 10.1111/j.1525-139X.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin C, Farah M, Leung M, et al. Multi-intervention management of calciphylaxis: a report of 7 cases. Am J Kidney Dis. 2011;58:988–991. doi: 10.1053/j.ajkd.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Chen KK, Rose CL, Clowes GHA. Methylene blue, nitrites and sodium thiosulfate against cyanide poisoning, potentiation of antidotal action of sodium tetrathionate and sodium nitrate in cyanide poisoning. Proc Soc Exp Biol Med. 1933;31:250–253. doi: 10.3181/00379727-31-7079P. [DOI] [Google Scholar]
- 8.Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992;32:368–375. doi: 10.1002/j.1552-4604.1992.tb03849.x. [DOI] [PubMed] [Google Scholar]
- 9.Selk N, Rodby RA. Unexpectedly severe metabolic acidosis associated with sodium thiosulfate therapy in a patient with calcific uremic arteriolopathy. Semin Dial. 2011;24:85–88. doi: 10.1111/j.1525-139X.2011.00848.x. [DOI] [PubMed] [Google Scholar]
- 10.Singh RP, Derendorf H, Ross EA. Simulation-based sodium thiosulfate dosing strategies for the treatment of calciphylaxis. Clin J Am Soc Nephrol. 2011;6:1155–1159. doi: 10.2215/CJN.09671010. [DOI] [PMC free article] [PubMed] [Google Scholar]