ABSTRACT
Translating tobacco dependence treatments that are effective in research settings into real-world clinical settings remains challenging. Electronic health record (EHR) technology can facilitate this process. This paper describes the accomplishments and lessons learned from a translational team science (clinic/research) approach to the development of an EHR tool for participant recruitment and clinic engagement in tobacco cessation research. All team stakeholders—research, clinical, and IT—were engaged in the design and planning of the project. Results over the first 17 months of the study showed that over one half of all smokers, coming in for any type of clinic appointment, were offered participation in the study, a very high level of adherent use of the EHR. Study recruitment over this period was 1,071 individuals, over 12 % of smokers in the participating clinics.
KEYWORDS: Electronic health records, Recruitment, Tobacco cessation
BACKGROUND
While research advances have extended the effectiveness and reach of tobacco dependence treatment over the past two decades, the disjointed process for bringing laboratory advances into clinical practice has often resulted in interventions that yield diminished effects in real world use [1–5]. One approach to address this “translation gap” is to develop and test interventions in real world clinical settings whenever possible. However, research conducted in these settings poses challenges that can compromise internal validity (e.g., reduce the accurate assessment of outcomes) or render the intervention context unrepresentative of real world conditions (e.g., due to burdensome consent processes, confidentiality regulations, and assessment burdens). Despite these limitations, strategies are available that can foster enhanced translation. For instance, in the case of interventions designed for use in healthcare settings, research designs can enhance translation by leveraging existing clinic infrastructure and workflow resources into intervention and research methods. The electronic health record (EHR), a common and effective component of clinic operations, is one such resource.
The current paper describes how the EHR was used to achieve research and clinical goals in an ongoing tobacco cessation effectiveness study conducted in primary care. The chief goal of the translational research described in this narrative is to identify effective cessation interventions for all smokers seen in primary care settings, even those not initially willing to quit. This team science approach features collaboration between primary care practices, information technology specialists, and tobacco cessation scientists. The research enlists healthcare systems as partners to organize and deliver study-related activities. The healthcare setting was chosen because it provides an ideal opportunity to intervene; more than 70 % of smokers visit such settings each year [6]. However, current efforts to improve real-world smoking cessation outcomes within primary care settings continue to yield low success rates [7–9]. This lack of success appears to be due, in part, to conflicting duties and time constraints of clinic staff, the need for burdensome staff training, and the interference of research activities into clinic work flow [10–14].
Researchers in healthcare have attempted to overcome the above barriers through EHR's [15–19]. The rapidly expanding use of EHR's in primary care, incentivized by the recent federal health care reform initiative, has a great potential to connect smokers with evidence-based treatment [20, 21]. The potential of the EHR arises from its reach into healthcare systems, its regular use by diverse clinicians and staff, and its potential to serve as an efficient intervention and communication channel for patient care without heavy investments of time in training and knowledge acquisition or interference with daily workflow in the clinic. In 2010, 54 % of primary care practices used some form of EHR [22]; this number continues to rise steadily.
The coordination and communication tools provided by the EHR are a source of several opportunities for the more efficient translation of clinical interventions (Table 1). While some additional real-world uses of EHR's, such as integrated scheduling of research participant visits and sharing of real-time data on research progress, are likely to be limited by human research protection requirements, the four advantages listed in Table 1 still offer powerful tools for clinical translational research.
Table 1.
Four opportunities arising from the use of the EHR as a platform for translational science
| 1. EHRs provide an ideal platform for the recruitment of research participants, prompting clinic staff to refer qualifying individuals through a pop-up invitation box. |
| 2. EHRs permit researchers to communicate study participation and treatment information back to clinic staff easily and confidentially. |
| 3. EHRs provide real-time data to researchers across multiple research and health domains, e.g., allowing them to track research progress (e.g., recruitment and treatment completion), health outcomes (e.g., new diagnoses), and health care (e.g., pharmacy billings), through data automatically accrued via normal health care operations. |
| 4. EHRs are maintained for all patients within the health system, allowing researchers to compare target patients to nontarget patient groups and referred patients to those who were not asked or did not accept referral. This greatly facilitates analysis of the reach of the intervention. |
While some researchers have used EHR resources in their work [23–27], such use of the EHR is in its infancy, and relatively little has been written about how to implement it effectively. Relevant questions that need to be addressed include: What are the challenges that need to be overcome (human, technological, organizational, and financial)? How can these challenges be addressed? What work is required to coordinate clinic's EHR into a research plan? The goal of this paper is to describe how one translational, smoking cessation clinical trial was conducted in primary care clinics, using the EHR as a recruitment and communication platform.
RESEARCH DESIGN
This research was initiated in July 2010. This NCI-funded clinical trial aims to develop a set of treatments for health systems that are highly effective for virtually every smoker seeking healthcare, regardless of their current readiness to quit smoking. The research will recruit 1,700 smokers into three separate, well-powered (β > 0.80) factorial experiments [28] that will compare multiple smoking cessation interventions in patients recruited from 10–12 primary care or family practice clinics in southern Wisconsin. Cessation treatments will be delivered on site or in person and by telephonic means.
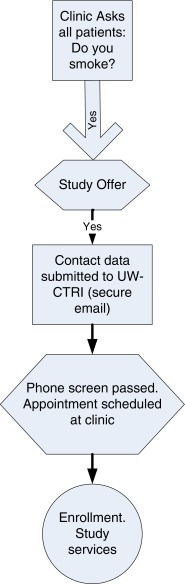
The basic EHR-based recruitment model is outlined in Fig. 1. Building on existing EHR platforms, all patients should be asked at every visit about their smoking status as part of the standard vital signs assessment [29, 30], as depicted in Fig. 2. If a patient reports current smoking, the person rooming the patient (e.g., medical assistant (MA), nurse) is prompted to read a scripted offer, presented via an EHR screen, about participating in a smoking study. The patient needs to express only a willingness to take a call to hear about the study. If the patient is willing to be contacted, a button is clicked in the EHR, and contact information is automatically sent to the study recruitment email account within the EHR (this could also be done by secure FAX or FTP). Thus, contact information from the medical record essential for recruitment is transmitted without extra work by clinic staff.
Fig 1.
Electronic record referral process
Fig 2.
Sample screen to identify tobacco use
This study design was constructed to fit well into the typical clinic workflow. The invitation script involves a small addition to the workload of the “roomer” or MA, which takes about 20 s. A brief survey of clinic staff showed that this is perceived as nonburdensome and an appropriate clinical activity for these staffs; clinic managers reported no complaints about this activity. This method of recruitment saves hundreds of hours that would have been required if recruitment was conducted by a research staff. There are two other critical advantages of this approach: (1) the individuals being recruited are more likely to say yes to persons they have probably seen before and trust as staff of their home clinic [31], and (2) more importantly, the recruitment method is easily translatable into a standard clinical practice.
Once a referral is received, a study staff member contacts the patient by phone, assesses eligibility, and schedules a visit at the referring clinic to begin study participation. By prior arrangement with the clinic management, study staff members are assigned to see participating patients in their own clinics on specific dates. In nonresearch implementation, scheduling and intervention could be arranged through the EHR and provided by clinic staff; however, as a research study done by an outside entity, HIPAA and human subjects protections did not allow this communication prior to consent. Removal of this barrier would allow immediate clinic-based scheduling of a treatment visit, reduce costs, and reduce loss of patients during the recruitment process.
Following patient consent and enrollment in the study, the study staff emails the referring clinician, providing enrollment and medication information for the EHR. Again, in nonresearch implementation, this step would be streamlined through direct EHR entry of medication, treatment, and future appointment information by the person providing the intervention. The study incurs higher than necessary staff costs as a result of this inefficiency.
Two other key EHR features, not related to individual patient care, are integrated into this research. Both relate more to overall study and clinic operation than to treatment of individual patients. First, the real-time data feature of the EHR allows for production of a feedback report showing referral patterns for each individual clinic, overall, and for each individual-referring staff member. These reports are used by the research staff to provide clinic managers and MAs with weekly individual and comparative feedback on how their clinic is doing (assessing smoking status and making referrals) as well as identifying specific staff members who may require additional training and support to perform the brief intervention. A sample of that report is illustrated in Table 2. Provider performance feedback like this has demonstrated real-world effectiveness in increasing level of staff referral of patients to tobacco cessation services [32, 33]. Second, the EHR will enable the study team to examine demographic and diagnostic information on the people who decline study participation and how they compare to those who accept it. The ability to translate research findings can be severely hampered by an inability to determine what proportion of eligible persons actually accept an intervention and how the “accepters” differ from those who do not [34–38]. Beyond providing key data on the reach of the study within the patient population presenting at each clinic (e.g., the percent of patients asked about their smoking status and the percent of smokers invited to participate), the EHR data facilitates projection of costs and benefits of intervention. This functionality will also be very useful for clinical quality assurance and program evaluation, if applied beyond a research program.
Table 2.
Sample UW-CTRI smoking cessation clinic X study recruitment report by department and encounter provider (office visits only)
| Number | Percent | No. of eligible patients | Ignored | Rate (%) | Yesa | Rate (%) | No | Rate (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Total clinic report (weeks 1–5) | |||||||||
| Total patients seen (distinct count) | 619 | ||||||||
| Identified smokers | 253 | ||||||||
| Clinic smoking rate | 41 | ||||||||
| Among 253 smokers | |||||||||
| Interested in study (rate) | 158 | 62.5 | |||||||
| Not interested in study | 44 | 17.4 | |||||||
| Prompt Ignored (not asked about study) | 51 | 20.2 | |||||||
| Clinic X (performance detail by week for individual medical assistants) | |||||||||
| HM | 79 | 17 | 21.5 | 47 | 59.5 | 15 | 19.0 | ||
| EL | 81 | 10 | 12.3 | 56 | 69.1 | 15 | 18.5 | ||
| SL | 70 | 4 | 5.7 | 52 | 74 | 14 | 20.0 |
aInterested in the study
THE PLANNING PROCESS
The integration of the EHR with a smoking cessation research project requires considerable time and planning effort, involving representatives with three types of expertise: (1) technical and information technology (IT), (2) research study operations, and (3) clinicians familiar with workflow within the healthcare system. Typically, health systems/clinics buy an EHR system from a vendor who then assists in implementation and provides ongoing support and updates, including new functionalities. Purchasers have options for tailoring the design of any base system that is purchased, so that features and functionality of systems developed by the same EHR vendor can substantially vary across health systems. Generally, health system IT staff members are trained by the vendor to maintain and operate the EHR, to add specific functionalities, and to design and oversee reporting functions. The scope of the “tailoring” that is possible within a system varies based on the system architecture. This ability of clinics or clinic systems to modify their EHRs for enhanced functions allows researchers to collaborate with clinics or health systems by modifying a particular EHR component for research purposes; if programming changes require vendor involvement, this can involve considerably more expense. Thus, researchers should consider modifying research methods or goals to ensure that EHR changes can be made by the healthcare system per se or clinics locally.
In the context of research, the activities described above are regulated by both HIPAA privacy and institutional review board (IRB) regulations. Privacy rules within each health system limit the level and mechanism of access to data by the research team to only data elements approved in the consent given by the participant (in this case a verbal consent to be referred was approved.) This paper primarily focuses on privacy and IRB rules in relation to those consenting to referral via the EHR; additional methods are needed to obtain other data the study may require, such as clinical or demographic information on those who do not consent. It is safe to say that, in general, research applications can leverage only a portion of the advantages and efficiencies that are available from EHR's in purely applied use. The constraints imposed by regulatory oversight require ongoing consideration during the planning process.
The research being described was developed in collaboration with three health systems in southern Wisconsin: Dean Clinic, Mercy Health System, and Aurora Healthcare. At the time of the initial development of the project, this necessitated working with two different EHR vendor platforms. Two models for the development were used. Since two of the health systems worked with the same EHR vendor, the researchers successfully reached out to this vendor to design most of the core functionality. In the second model, where one health system was using a different vendor platform, functional changes were developed, working with the health system IT staff.
The first model began with the study's principal investigator contacting the EHR vendor to discuss a collaboration opportunity. A team was formed, comprising key research staff leaders, vendor representatives (including operations support, IT/programming, reporting system staff, and client service staff), and representatives from the health system IT groups. The process started with the research team presenting a basic flowchart of desired EHR functional capacities, i.e., to identify smoking status, prompt the roomer to invite patients to participate in the study, provide the specific text for an invitation, notify study staff when a patient expresses interest, inform patient's physician of study participation and which medicine/s the patient would be receiving, and provide necessary study data. EHR vendor staff then discussed and presented various strategies to provide those capabilities. This resulted in a skeletal outline of a design to integrate the research requirements into the EHR functionality.
At this point, the development team worked to keep the clinic work process simple to learn and easy to fit into normal workflow. Throughout the process, draft solutions were reviewed jointly by the study research team and the vendor to ensure that desired functionality had been obtained. Once the team agreed on the model, the vendor team built detailed, step by step “build guides” for health system IT staff to use, to make needed modifications in their EHR, and to support the study. The build guides made it possible for health system IT staff to change their EHR within 4–6 weeks.
The second model, in which the research team worked directly with the health system IT staff to modify the EHR for the remaining health system, followed essentially the same steps. In this process, however, the local IT staff had less freedom to change the basic architecture of the data system, resulting in some reduced functionality, discussed later under LESSONS LEARNED.
RECRUITMENT RESULTS
The success of recruitment is influenced by many factors beyond the ease of the referral system, e.g., the nature of the trial, degree of risk, availability of alternative treatments, and degree of involvement of the clinic itself in the trial. Patient acceptance of a referral may depend on such factors and upon others as well, e.g., the time required and the perceived likelihood of treatment success. Also, patients are more likely to volunteer for a clinical trial addressing an existing critical health problem than one targeting preventive care (such as smoking cessation) [39–42]. These differences complicate any comparison of recruitment results across different clinical and research domains. The EHR has been used to recruit patients for various types of epidemiologic research [43] and to increase industrial trial participation within medical groups [24]. In one comparison study, the use of an EHR was shown to decrease the number of interviews (and time) required for recruitment, resulting in almost a 10-fold increase in the number of qualifying individuals [44].
Within this study, recruitment results are measured along two dimensions: the rate of adherent use of the referral system developed through the EHR and the net rate of study referral of smokers at each clinic. The first set of results (rate of adherent use of the EHR) is arrayed in Table 3. These two measures are important because they parallel two key tobacco intervention adherence measures from the PHS Clinical Practice Guideline on Treating Tobacco Use and Dependence [45]: ask rate (percent of people asked about their smoking) and assist rate (percent of those asked who are provided with treatment assistance). As indicated in Lesson 7 below, this rate is significantly affected by variation between the EHR platforms. Even with these limitations, the assist rates compare favorably with those in other studies of EHR-prompted provider interventions [32].
Table 3.
Adherent use of the EHR
| Adherence indicator | Health system A | Health system B |
|---|---|---|
| (N = 21,707)a | (N = 42,508)b | |
| Patients asked about smoking at visit (% (N)) | 100 (21,707) | 68.4 (28,784) |
| Smokers identified (N) | 4,269 | 8,009 |
| Smokers assisted (% (N)) | 62.6 (2,673) | 49.7 (3,983) |
aAll data expressed as people, with multiple visits collapsed
bEncounter (visit) data; data will be pulled at end of study to allow analysis to be done in terms of patients asked and assisted. The net ask and assist rates will increase in this final analysis within this health system, since individuals are often not asked about smoking on repeat visits, and each individual assisted is only listed as assisted at one of their visits
Data from the first half of the recruitment in the present study (N = 1,071) show a final study initiation rate of all smokers in health system A of 12.1 % of all smokers identified by the clinic during the study period (Table 4). The true smoker referral rate at health system B cannot be calculated at this time due to the real-time reporting limitations of that EHR, which allows only counts of number of patient encounters and does not identify particular patients at visits (see notes in Table 3). At the end of the study, a data extract will allow for identification of unique patients in this health care system, permitting meaningful comparison across the two systems. However, because encounter referrals do not translate directly into referrals of individual patients, the data available at this time reflect functionality within each health care system, but do not permit ready comparison across systems. The rates show that, in health care system A, slightly over half of smokers are provided treatment assistance, and about half of patient encounters result in such assistance in health care system B. These rates represent responses to an unexpected smoking study invitation to patients making ordinary primary care visits.
Table 4.
Study recruitment results by health system from July 1, 2010 to November 30, 2011
| Patient data (mandatory prompts) | Visit data (no mandatory prompts) | Percentages | |
|---|---|---|---|
| Number of people | Number of visitsa | ||
| Health system A | |||
| Patients seen | 21,707 | NA | |
| Smokers identified | 4,269 | 19.7 of patients | |
| Smokers referred to study | 1,498 | 35.1 of smokers | |
| Total consented from health system A | 515b | 34.4 of smokers referred | |
| Health system B | |||
| Clinic visits | 28,784 | NA | |
| Visits where smoking was documented | 8,009 | 27.8 of visits | |
| Visits resulting in a study referral | 1,360c | 16.9 of visits | |
| Total consented from health system B | 556b | 40.9 of smokers referred | |
The third health system had not started recruitment at the time of this paper. They utilize the health system A platform
aSmoking status was not assessed at all visits in this health system. Total patient visits during this period for health system B was 42,508
bSix percent of those referred have either been scheduled for the consent visit or have not yet been reached. The final net referral rate will be slightly higher than the figure given
cStudy visits resulting in referral equal the number of smokers referred to the study
LESSONS LEARNED
By engaging in this research using the EHR, the study team has learned important lessons that may prove useful to other researchers.
Lesson 1: flexible design process
The study team must be prepared to change study procedures in light of EHR capabilities. With the limited financial resources for programming costs that is typical of most research studies, the design process may require finding the “best fit” between the ideal research model, clinic workflow, and core functional capabilities of the EHR. For example, there are limited options for determining how and where the study invitation becomes visible and whether or not some response is mandatory (i.e., is it a required field or not?). In one of the EHR systems used for this study, it was not possible to automate the appearance of the pop-up study invitation, reducing staff compliance. Optimally, efficient research operations require coordinated adjustments among EHR design, research methods, and clinic practices, with the latter being least congenial (and forgiving) to change.
Lesson 2: awareness of the health system IT work process
Modification of the EHR requires IT staff time, which is costly and limited in availability. Can the study afford to pay for IT staff time? Will the health system administration make this research project a priority for the IT department? Obviously, programming changes need to be kept as simple as possible to achieve the desired research purpose. One lesson we derived from the current research is the importance of estimating IT staff work requirements separately for each participating health care system. In fact, one strategy for controlling IT expenses is to conduct the research in only a single health care setting. This, of course, would limit the generalizability of the findings. The fact that the research goal (smoking cessation) was tied to an important quality measure for the health systems helped the study obtain assistance for free or at greatly reduced costs.
Lesson 3: language and communication
Researchers speak a different language than EHR programmers. Often they are not fully aware of the meaning of each other's basic terminology (e.g., best practice alerts and build guides vs. IRB requirements, CONSORT data requirements, and eligibility criteria). The make-up of the planning team is critical for an efficient development process. All three elements, clinic operations, EHR/IT, and research, need to be represented during the design phase. The research team needs to contain members able to bridge across clinic and EHR/IT team members. Such bridging staff may be developed prior to research implementation through a series of meetings among team members and by researchers, actually spending time observing clinic workflow and/or learning IT functionality and terminology.
Lesson 4: adapt to EHR differences
Studies that involve more than one EHR need to accommodate differences in work process that reflect differences in EHRs. There are differences between EHR vendor platforms as well as variation within the same EHR platform deployed across two health systems. These dissimilarities can have an effect on study recruitment rates and real-time data availability. What may appear to be a superficial difference in work processes between systems can result in significant variation in performance and require altered study procedures for the different clinics. The likely effect of the lack of automated reminders on the “ask” and “assist” rates in this study in health care system B has already been mentioned. This difference, which arose out of a difference between EHR structures, could only be (partially) addressed by a human intervention—research staff stationed in this system's clinics had to be more vigilant in training and follow-up with clinic staff members to remind them to convey study invitations. Despite training, MAs, with the harder-to-use system, sometimes had an initial period of improper use of the procedure (e.g., inviting people into the study and having them accept, but then not initiating the study referral). This increased the burden for both research and clinic staff and resulted in some loss of referrals compared to the more automated EHR system.
Lesson 5: limit design changes
Making EHR changes after the study goes into the field can be difficult and costly in terms of time and financial resources and relations with the health care system and staff and EHR vendor. A lesson learned in this instance is to minimize postlaunch modifications by thoroughly walking through all EHR prompts and functionalities with clinical staff prior to programming.
Lesson 6: privacy
EHRs, which make health information so easy to retrieve, also trigger understandable concerns about privacy. In the team with the vendor-developed build guides (system A), the build guide for limited study access to the EHR ultimately could not be used because of privacy concerns of the health system privacy officer. While a less “elegant” alternative solution (secure email) was quickly developed and the study proceeded without delay, earlier attention should be paid to health system's privacy approach related to access by an outside research team.
Lesson 7: understanding the clinical context of EHR use and data
The ability to extract meaning from EHR data requires a clear understanding of how the clinic staff use the EHR, how the EHR entry fields can be accessed by the staff, and the leadership provided by the clinic. For instance, in the current study, we have found that EHR use and effects on the study were significantly affected by small differences in how clinic staff was able to access recruitment scripts in the EHR and by the commitment of clinic leadership to smoking cessation. In other words, obtained data reflect not just the EHR itself, but the clinical context into which it is introduced. The lesson learned in this case is that we learned to do careful on-site investigation of each clinic prior to its incorporation into the study in order to train and support the introduction of the EHR modifications as well as to give feedback on staff performance.
Lesson 8: clinic workflow is critical
Any change in the EHR made for research that adds time to clinic processes affects workflow and patient care, which is not acceptable to the health system unless the perceived benefit to the patient is sufficiently great. The EHR changes for this study were implemented only because the interruption in workflow was modest, and the perceived gain, great. On average, the interaction and documentation process required less than 2 min for each smoker or 6–10 min over the course of a full clinic day for each medical assistant, assuming a smoking rate in the 15–25 % range.
The EHR is a powerful tool that can greatly facilitate treatment research implementation via a translational, team science approach. Using this tool can streamline recruitment, facilitate communication with collaborating clinicians, and create a more easily translatable “product” from the research. Ultimately, the test of an EHR design for research is that it supplies requisite research data and facilitates translation, but it also should satisfy the same needs as pertain to EHR use generally: is its use feasible in terms of clinic workflow and operations, does it store and provide useful information, and does it enhance patient care. This research study modified the EHR so that clinicians could enhance the health of their patients by increasing access to evidence-based smoking cessation treatment. Simultaneously, it enables study researchers to gauge both treatment effectiveness and reach in the overall population of smokers seen in the primary care clinic. Thus, in this and future research, the EHR has the potential to enhance research efficiency, improve the yield and accuracy of data, and facilitate the effective translation of research interventions into clinical practice.
Acknowledgments
This research was supported by the National Cancer Institute (5P50CA143188-13 and 5K05CA139871-04) and the Wisconsin Partnership Program. The clinical trials are registered as NCT01122238, NCT01116986, and NCT01120704.
Footnotes
Implications
Practice: Patients identified as tobacco users can be seamlessly linked to research and treatment resources by the primary care team using prompting from the electronic health record (EHR) with minimal physician or staff time. The patient need not be motivated to quit immediately in order to begin intervention.
Policy: EHR's are being rapidly implemented in clinic settings, but their optimal research use requires extensive research/clinic team collaboration. Such use may facilitate the rapid translation of research findings into clinical practice by creating a replicable model that integrates specialized treatment into primary care. In this way, the clinical translation cycle can be shortened, an important NIH policy goal.
Research: Incorporating EHR applications into research can significantly reduce patient recruitment time and costs. The use of EHR for recruitment, team delivery of research interventions, and quality monitoring provides a rare opportunity for the efficient and replicable implementation of research and treatment procedures across health care settings. The goal is more rapid translation of research into clinical practice.
References
- 1.Davis D, Evans M, Jada A, et al. The case for knowledge translation: shortening the journey from evidence to effect. BMJ. 2003;327:33–35. doi: 10.1136/bmj.327.7405.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapka J, Goins KV, Pbert L, Ockene JK. Translating efficacy research to effectiveness studies in practice: lessons from research to promote smoking cessation in community health centers. Health Promot Pract. 2004;5:245–255. doi: 10.1177/1524839904263713. [DOI] [PubMed] [Google Scholar]
- 3.Burnam MA. Measuring outcomes of care for substance use and mental disorders. New Dir Ment Health Serv. 1996;71:3–17. doi: 10.1002/yd.23319960303. [DOI] [PubMed] [Google Scholar]
- 4.Goldblatt EM, Lee WH. From bench to bedside: the growing use of translational research in cancer medicine. Am J Transl Res. 2010;2:1–18. [PMC free article] [PubMed] [Google Scholar]
- 5.Lenfant C. Shattuck lecture—clinical research to clinical practice—lost in translation? The NEMJ. 2003;349:868–874. doi: 10.1056/NEJMsa035507. [DOI] [PubMed] [Google Scholar]
- 6.Thorndike AN, Rigotti NA, Stafford RS, Singer DE. National patterns in the treatment of smokers by physicians. JAMA. 1998;279:604–608. doi: 10.1001/jama.279.8.604. [DOI] [PubMed] [Google Scholar]
- 7.Whitlock EP, Orleans CT, Pender N, Allan J. Evaluating primary care behavioral counseling interventions: an evidence-based approach. Am J Prev Med. 2002;22:267–284. doi: 10.1016/S0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 8.Aveyard P, Brown K, Saunders C, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax. 2007;62:898–903. doi: 10.1136/thx.2006.071837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yano EM, Rubenstein LV, Farmer MM, et al. Targeting primary care referrals to smoking cessation clinics does not improve quit rates: implementing evidence-based interventions into practice. Health Serv Res. 2008;43:1637–1661. doi: 10.1111/j.1475-6773.2008.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumenthal DS. Barriers to the provision of smoking cessation services reported by clinicians in underserved communities. J Am Board Fam Med. 2007;20:272–279. doi: 10.3122/jabfm.2007.03.060115. [DOI] [PubMed] [Google Scholar]
- 12.Helgason AR, Lund KE. General practitioners' perceived barriers to smoking cessation—results from four Nordic countries. Scan J Public Health. 2002;30:141–147. doi: 10.1080/14034940210133799. [DOI] [PubMed] [Google Scholar]
- 13.Zwar NA, Richmond RL. Role of the general practitioner in smoking cessation. Drug Alcohol Rev. 2006;25:21–26. doi: 10.1080/09595230500459487. [DOI] [PubMed] [Google Scholar]
- 14.Bodenheimer T, Young DM, MacGregor K, Holtrop JS. Practice-based research in primary care: facilitator of, or barrier to, practice improvement? Ann Fam Med. 2005;3(Suppl 2):S28–32. doi: 10.1370/afm.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoves J, Connolly J, Cheung CK, et al. Electronic consultation as an alternative to hospital referral for patients with chronic kidney disease: a novel application for networked electronic health records to improve the accessibility and efficiency of healthcare. Qual Saf Health Care. 2010;19:e54. doi: 10.1136/qshc.2009.038984. [DOI] [PubMed] [Google Scholar]
- 16.Garrido T, Jamieson L, Zhou Y, Wiesenthal A, Liang L. Effect of electronic health records in ambulatory care: retrospective, serial, cross sectional study. BMJ. 2005;330:581. doi: 10.1136/bmj.330.7491.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemeth LS, Wessell AM. Improving medication safety in primary care using electronic health records. J Patient Saf. 2010;6:238–243. doi: 10.1097/PTS.0b013e3181fe401f. [DOI] [PubMed] [Google Scholar]
- 18.Harris SB, Glazier RH, Tompkins JW, et al. Investigating concordance in diabetes diagnosis between primary care charts (electronic medical records) and health administrative data: a retrospective cohort study. BMC Health Serv Res. 2010;10:347. doi: 10.1186/1472-6963-10-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linder JA, Kaleba EO, Kmetik KS. Using electronic health records to measure physician performance for acute conditions in primary care: empirical evaluation of the community-acquired pneumonia clinical quality measure set. Med Care. 2009;47:208–216. doi: 10.1097/MLR.0b013e318189375f. [DOI] [PubMed] [Google Scholar]
- 20.Lindholm C, Adsit R, Bain P, et al. A demonstration project for using the electronic health record to identify and treat tobacco users. Wis Med J. 2010;109:335–340. [PMC free article] [PubMed] [Google Scholar]
- 21.Boyle R, Solberg L, Fiore M. Use of electronic health records to support smoking cessation. Cochrane Database Syst Rev. 2011. [DOI] [PubMed]
- 22.Hsiao C-J, Hing E, Socey TC, Cai B. Electronic medical record/electronic health record systems of office-based physicians: United States, 2009 and preliminary 2010 estimates. http://www.cdc.gov/nchs/data/hestat/emr_ehr_09/emr_ehr_09.htm. Accessed 15 March 2012.
- 23.Embi PJ, Jain A, Clark J, Bizjack S, Hornung R, Harris CM. Effect of a clinical trial alert system on physician participation in trial recruitment. Arch Intern Med. 2005;165:2272–2277. doi: 10.1001/archinte.165.19.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JL, The EHR. solution to clinical trial recruitment in physician groups. Health Manag Technol. 2006;27:22–25. [PubMed] [Google Scholar]
- 25.Wilke RA, Berg RL, Peissig P, et al. Use of an electronic medical record for the identification of research subjects with diabetes mellitus. Clin Med Res. 2007;5:1–7. doi: 10.3121/cmr.2007.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilcox A, Natarajan K, Weng C. Using personal health records for automated clinical trials recruitment: the ePaIRing Model. Summit on Translational Bioinformatics 2009, 136-140. http://www.ncbi.nlm.nih.gov/pubmed/21347187. Accessed 15 March 2012. [PMC free article] [PubMed]
- 27.Peterson KA, Fontaine P, Speedie S. The Electronic Primary Care Research Network (ePCRN): a new era in practice-based research. J Am Board Fam Med. 2006;19:93–97. doi: 10.3122/jabfm.19.1.93. [DOI] [PubMed] [Google Scholar]
- 28.Collins LM, Baker TB, Mermelstein RJ, Piper ME, Jorenby DE, Smith SS, et al. The Multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41(2):208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiore MC. The new vital sign. Assessing and documenting smoking status. JAMA. 1991;266:3183–3184. doi: 10.1001/jama.1991.03470220099036. [DOI] [PubMed] [Google Scholar]
- 30.McCullough A, Fisher M, Goldstein AO, Kramer KD, Ripley-Moffitt C. Smoking as a vital sign: prompts to ask and assess increase cessation counseling. J Am Board Fam Med. 2009;22:625–632. doi: 10.3122/jabfm.2009.06.080211. [DOI] [PubMed] [Google Scholar]
- 31.Luck MB, McIntire M, Blades N, Robinson W. Characteristics of women with gynecologic cancer who enter clinical trials. J Reprod Med. 2005;50:481–485. [PubMed] [Google Scholar]
- 32.Bentz CJ, Bailey BK, Bonin KE, et al. Provider feedback to improve 5A's tobacco cessation in primary care: a cluster randomized clinical trial. Nicotine Tob Res. 2007;9:341–349. doi: 10.1080/14622200701188828. [DOI] [PubMed] [Google Scholar]
- 33.Zhou YY, Unitan R, Wang JJ, et al. Improving population care with an integrated electronic panel support tool. Popu Health Manag. 2011;14:3–9. doi: 10.1089/pop.2010.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goss CH, Rubenfeld GD, Ramsey BW, Aitken ML. Clinical trial participants compared with nonparticipants in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:98–104. doi: 10.1164/rccm.200502-273OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone VE, Mauch MY, Steger K, Janas SF, Craven DE. Race, gender, drug use, and participation in AIDS clinical trials. Lessons from a municipal hospital cohort. J Gen Intern Med. 1997;123:150–157. doi: 10.1007/s11606-006-5022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe N. Clinical trials and the real world: selection bias and generalisability of trial results. Cardiovasc Drugs Ther. 2002;16:75–77. doi: 10.1023/A:1015327801114. [DOI] [PubMed] [Google Scholar]
- 38.Berger VW, Exner DV. Detecting selection bias in randomized clinical trials. Control Clin Trials. 1999;20:319–327. doi: 10.1016/S0197-2456(99)00014-8. [DOI] [PubMed] [Google Scholar]
- 39.Verheggen FW, Nieman F, Jonkers R. Determinants of patient participation in clinical studies requiring informed consent: why patients enter a clinical trial. Patient Educ Couns. 1998;35:111–125. doi: 10.1016/S0738-3991(98)00060-3. [DOI] [PubMed] [Google Scholar]
- 40.Moore S. A need to try everything: patient participation in phase I trials. J Adv Nurs. 2001;33:738–747. doi: 10.1046/j.1365-2648.2001.01715.x. [DOI] [PubMed] [Google Scholar]
- 41.Buckley B, Murphy AW, Byrne M, Glynn L. Selection bias resulting from the requirement for prior consent in observational research: a community cohort of people with ischaemic heart disease. Heart. 2007;93:1116–1120. doi: 10.1136/hrt.2006.111591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ives DG, Traven ND, Kuller LH, Schulz R. Selection bias and nonresponse to health promotion in older adults. Epidemiology. 1994;5:456–461. doi: 10.1097/00001648-199407000-00013. [DOI] [PubMed] [Google Scholar]
- 43.Sinha G. Electronic health records help recruit trial participants and track drug safety. J Natl Cancer Inst. 2008;100:384–385. doi: 10.1093/jnci/djn070. [DOI] [PubMed] [Google Scholar]
- 44.Rollman BL, Fischer GS, Zhu F, Belnap BH. Comparison of electronic physician prompts versus waitroom case-finding on clinical trial enrollment. J Gen Intern Med. 2008;23:447–450. doi: 10.1007/s11606-007-0449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update, U.S. Department of Health and Human Services, U.S. Public Health Service, Rockville, MD. 2008.