Abstract
Paraganglioma is a rare neoplasm arising from carotid body usually benign and constitute 0.5 % of all total body tumors. They constitute 60–70 % of head and neck paraganglioma and resemble other paragangliomas of the body like glomus jugulare, glomus tympanicum, and pheochromocytoma. This is a retrospective analysis of the medical records of carotid body paraganglioma cases. Nine patients operated during the study period and the follow up traced were included in the study. Seven females and 2 males were analysed. Six had tumor on the left side and 3 had on the right side. All the cases surgical excision was done by a tranverse incision as 2 patients had Shamblin I, 5 patients had Shamblin II, and 1 patients Shamblin IIIa. All the Shamblin I had tumor away from the carotids and were easily dissected without vessel damage, a sub adventitial tumor excision was performed in all the 5 cases of Shamblin II, 1 case of Shamblin IIIa was dissected with difficulty without sacrificing or vessel reconstruction. Paraganglioma of the carotid body should be considered as a differential diagnosis for painless lateral neck masses. Larger tumors need a multidisciplinary team of head and neck with vascular surgeons for better results.
Keyword: Carotid body tumor, Paraganglioma, Modified Shamblin classification, Embolization
Introduction
Paraganglioma is a rare neoplasm arising from carotid body usually benign and constitute 0.5 % of all total body tumors [1–7]. They constitute 60–70 % of head and neck paraganglioma and resemble other paragangliomas of the body like glomus jugulare, glomus tympanicum, and pheochromocytoma [1].
Van Haller in 1743 first described the carotid body while the term paraganglia was first described by Kohn [8, 9]. Acronyms for the term paraganglia like carotid body tumor, glomus tumor, chemodectomas, and nonchromaffin tumor are less accurate and are obselete [10–12]. The tumor arises from paraganglionic cells of the carotid body which develops from both mesodermal elements of the third branchial arch and neural elements originating from the neural crest ectoderm and are more common in women [1–5]. Hereditary factors are reflected in 7–9 % of the cases and 10 % of the cases are bilateral [2, 4]. Most of the tumors are benign and malignant changes are seen in 6–12 % of cases with no clear histological features of malignant changes [3–5].
Methodology
This is a retrospective analysis of the medical records of carotid body paraganglioma cases operated in the department of head and neck oncosurgery during January 2005 to September 2012. All the preoperative, intraoperative and postoperative data were analyzed for each patient and the follow up by the medical records. Nine patients (mean age 31.77) operated during the study period and the follow up traced were included in the study. Seven females (mean age 29.42 years) and 2 males (mean age 40 years) with a mean presentation time of 5.44 months were analysed. Six had tumor on the left side and 3 had on the right side. (Table 1) All the patients had painless slow growing neck mass just anterior to the sternomastoid muscle at the level of the hyoid bone with none of the patients having signs of increased catecholamine secretion with all other baseline parameters being normal.
Table 1.
Clinical details management and outcome
| Sl no | Age | Sex | Smoking | History | Size(cm) | Shambling types | Embolization | Tumor(s) | Surgery (incision) | Complication(s) | Follow up moths | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | F | No | 4 | <4 | I | No | L | transverse | none | 7 | no |
| 2 | 23 | F | No | 6 | <4 | I | No | L | transverse | none | 24 | no |
| 3 | 41 | M | No | 6 | <4 | II | No | L | transverse | none | 14 | no |
| 4 | 27 | F | No | 8 | >4 | IIIa | No | R | transverse | none | 29 | no |
| 5 | 39 | M | Y | 6 | <4 | II | No | L | transverse | 10th,12th weakness | 46 | no |
| 6 | 28 | F | No | 4 | <4 | II | No | R | transverse | none | 48 | no |
| 7 | 31 | F | No | 5 | <4 | II | No | L | transverse | none | 56 | no |
| 8 | 33 | F | No | 4 | >4 | II | No | L | transverse | 10th,12th weakness | 54 | no |
| 9 | 29 | F | No | 6 | <4 | II | No | R | transverse | none | 38 | no |
| Mean age = 31.77 | Mean h/o 5.44 mon | Mean F/U 35.11 mon | ||||||||||
None of the cases had family history or hereditary factors of the condition or association with higher altitudes. The neck masses were examined clinically and suspected of the tumor due to the transmitted pulsation. No fine needle aspiration was done as clinically the tumor was suspected of as a vascular lesion with provisional diagnosis of carotid body tumor as the surface marking related it to the carotid pulsation and its bifurcation. Clinically the average size of the mass was less than 5 cm and bruit was auscultated in all the cases.
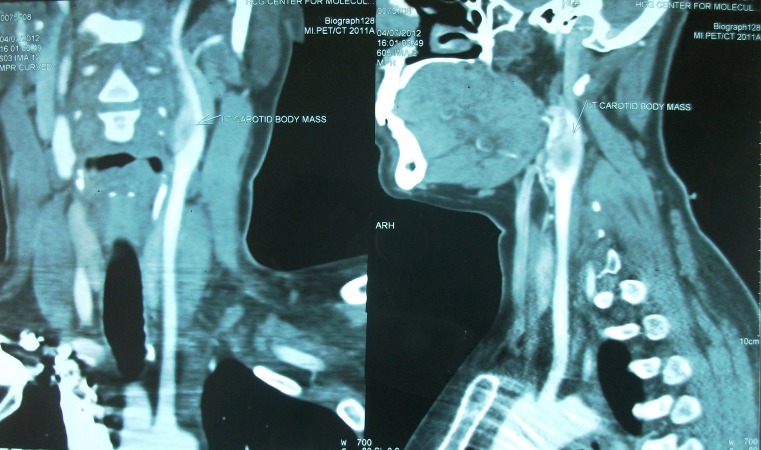
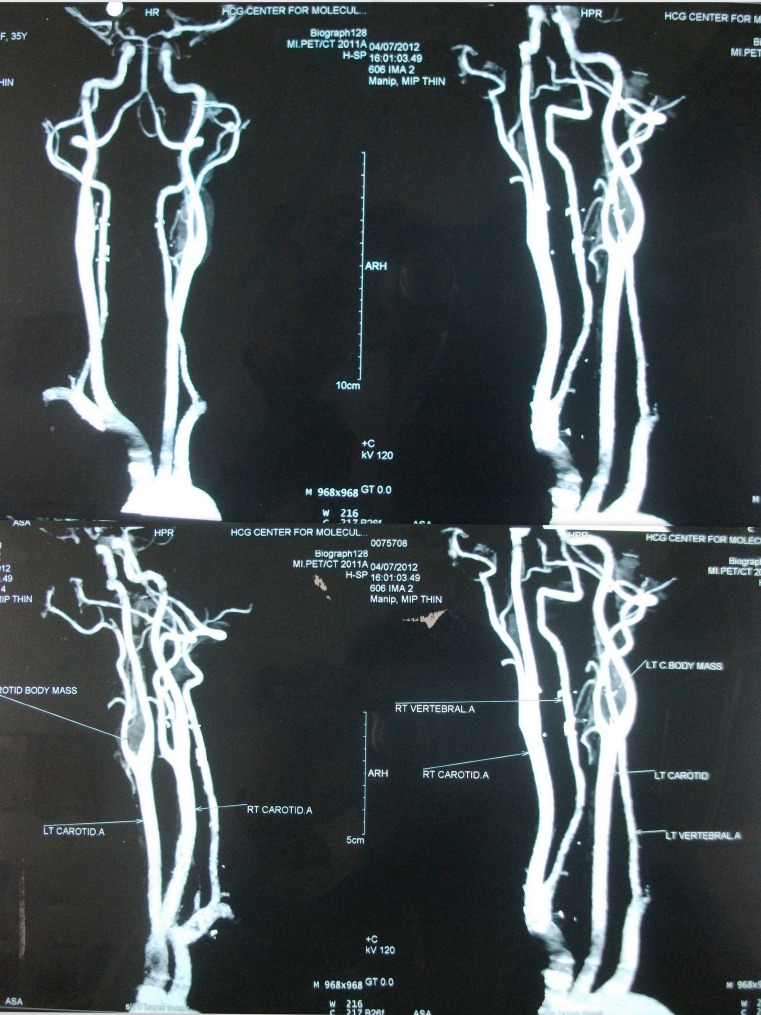
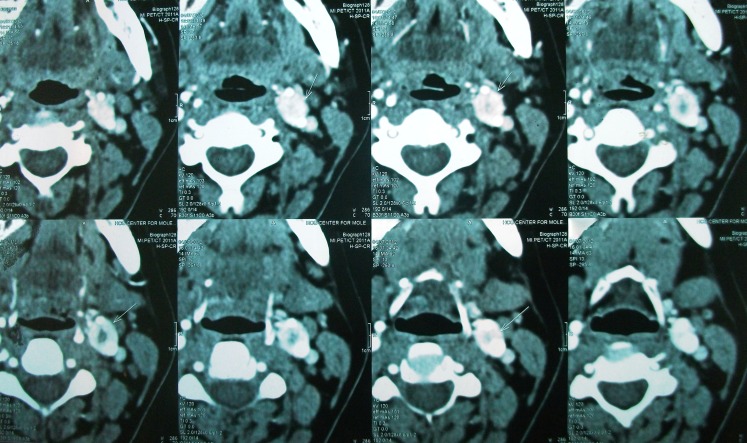
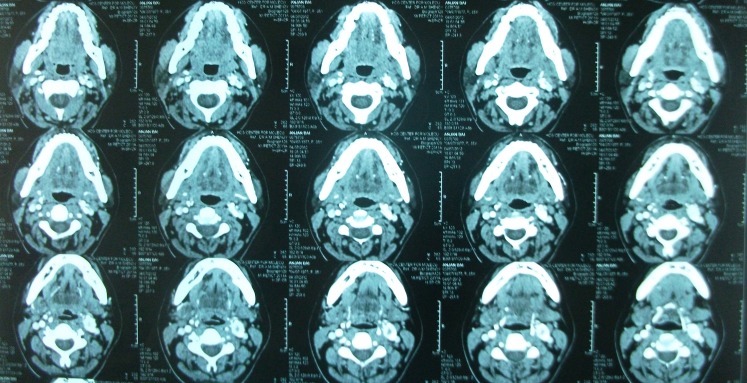
Contrast enhanced CT scan was done in all cases which demonstrated paraganglioma at the carotid bifurcation. Contrast angiography was done in three cases of Shamblin II to delineate the feeders which was not possible.
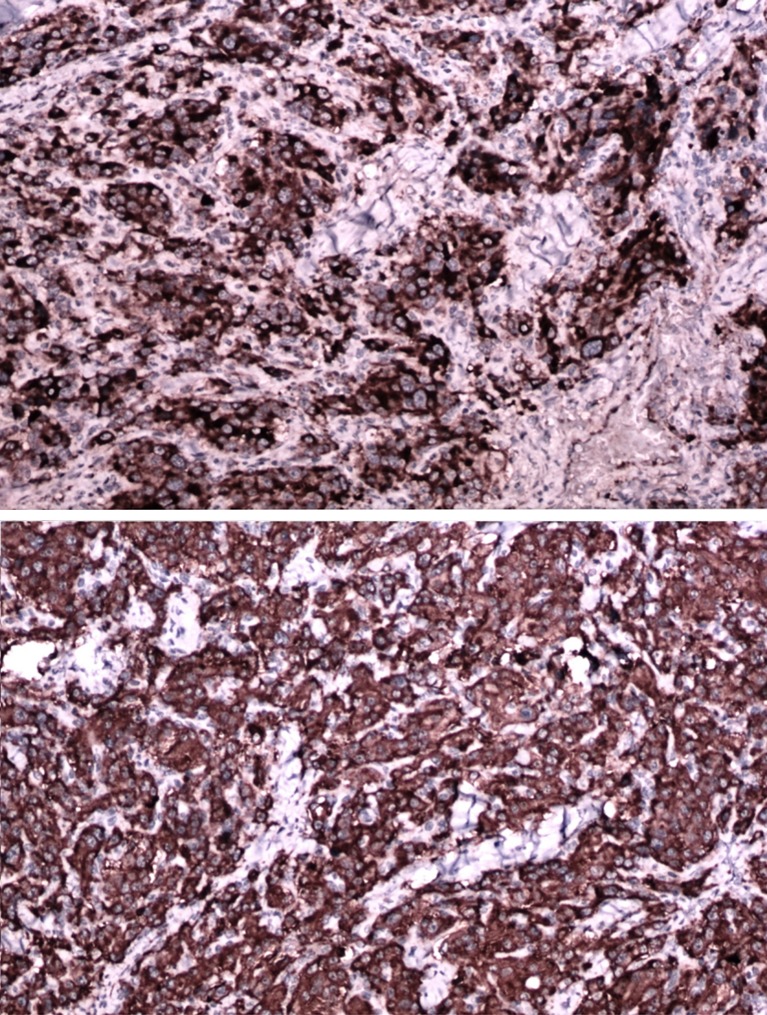
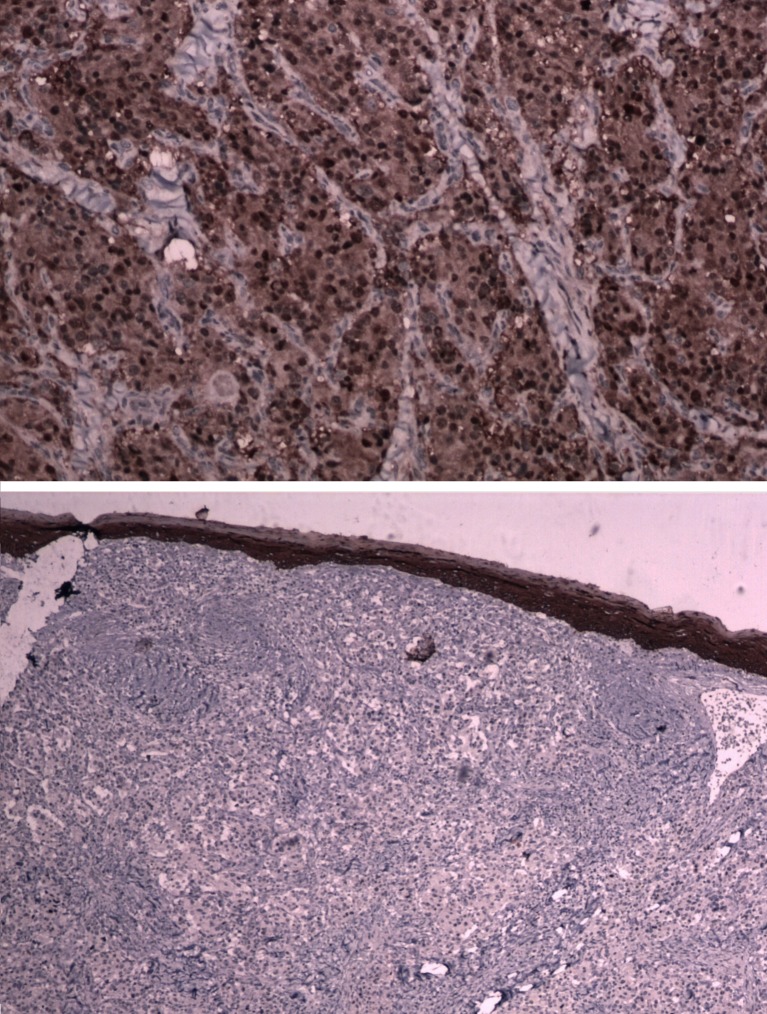
Digital substraction angiography was done in 1 case of Shamblin IIIa for embolization, but embolization was not done as extravasation was feared. Chest and neck x-rays were normal in all cases. All the patients were operated with a classical transverse incision as we had only smaller tumors, where dissection was expected to be effortless and no reconstruction was needed. No immediate or postoperative major complications were observed in our study. (Figures 1, 2, 3 and 4) None of the patients had cervical lymph node enlargement or evidence of distant metastasis clinically or intraoperatively. The tumor was confirmed on histopathology and immuno-histochemistry where all the tumor cells were positive for synaptophysin and chromogranin, negative for cytokeratin. (Figure 5) The sustentacular cells were positive S-100. (Figure 6)
Fig. 1.
Well circumscribed mass seen at left carotid bifurcation
Fig. 2.
Mild splaying of the external and internal carotids seen
Fig. 3.
Central non-enhancing 4 × 3 cm lesion seen
Fig. 4.
Lesion close to greater horn of hyoid and longus colli
Fig. 5.
Tumor cells positive for synaptophysin and chromogranin
Fig. 6.
Tumor cells negative for cytokeratin and sustentacular cells positive for S-100
Results
All the cases surgical excision was done by a tranverse incision and none of the tumors were seen compressing and involving both the vessels with no reconstruction done. Two patients had Shamblin I, 5 patients had Shamblin II, and 1 patients Shamblin IIIa. All the Shamblin I had tumor away from the carotids and were easily dissected without vessel damage, a sub adventitial tumor excision was performed in all the 5 cases of Shamblin II, 1 case of Shamblin IIIa was dissected with difficulty without sacrificing or reconstruction of either the external or internal carotid arteries.
Complete excision of the tumors was seen in all cases while no postoperative major complications were reported. The hypoglossal and vagus nerve had weakness in 2 cases but complete recovery was seen over 3 months. Follow up period ranged from 6 months to 5 years with a mean follow up of 2.5 years. Follow up was done 3 monthly for the first year and 6 monthly later on. All the patients were clinically followed up with no finding suggestive of recurrence in all the 9 cases.
Discussion
Carotid body is a round, reddish-brown organ of the size of 2–6 mm situated in the semi adventitial tissue of the common carotid artery bifurcation [9, 13]. It is highly specialized in function supplied by a feeding vessels runs from the ascending pharyngeal artery a branch of external carotid artery and innervated through the glossopharyngeal and vagus nerves [9, 13]. Carotid body functions as an autonomic control of the respiratory and cardiovascular systems, as well as blood temperature [4, 14–16]. It is chemoreceptor organ that is stimulated by hypoxia, hypercapnea, and acidosis which controls the autonomic control drive by increasing the sympathetic flow [8, 9, 13].
The tumor usually present as a slow growing, non-tender, rubbery masses located just anterior to the sternocleidomastoid muscle at the level of the hyoid [4–6]. The tumor mass may transmit the carotid pulse or demonstrate a bruit or thrill and mobile in the lateral plane with limited mobility vertically [13, 17]. Symptoms like dysphagia, odynophagia, hoarseness of voice are seen if the tumor enlarges around the carotid vessels and X–XII cranial nerves [4–6]. Horner’s syndrome due to invasion or compression of cervical sympathetic chain, and syncope may be seen due to carotid sinus or internal carotid artery compression as the size of the tumor also matters in management [17]. Cranial nerve palsies are seen in 30 % of the cases with vagus being the commonest [18]. Catecholamine production may manifest as fluctuating hypertension, blushing, obstructive sleep apnea and palpitations [4–6].
Incorrect clinical diagnosis is rated upto 30 % which lead to unindicated attempts at biopsy or explorative surgery [19]. Fine needle aspiration with wide bore needle or biopsies of these tumors are dangerous as carotid artery aneurysms and elongation mimic the tumor in this area [20]. The high risk of FNA related bleeding can be minimised by proper technique, minimal needle calibre and minimal possible pricks [21, 22]. Color Doppler or CT scan guided FNA would be better to reduce complications if clinical diagnosis is inconclusive [23, 24].
Chief cells(type 1) are APUD (amine precursors uptake decarboxylation) type cells with copious cytoplasm and large round or oval nuclei [1]. Dense core granules that store and release catecholamines are seen in the cytoplasm and hyperplasia of these cells leads to paraganglia enlargement in chronic hypoxia [1]. Sustentacular cells (type 2) are elongated cells that closely resemble Schwann cells [1]. Kaspar et al reported 52 % of the patients to be males and the average age group to be 45 years with rare cases seen in children [25, 26]. These tumors are more seen in females and histologically resemble the normal architecture of the carotid body [27]. The tumors are highly vascular and clusters of cells called Zellballen are seen in between capillaries [27].
Malignant transformation is seen in 10 %, familial in 10 %, and bilateral in 10 % of sporadic cases where bilaterality may reach 30 % in the familial cases [18, 28].
Metastasis confining to the neck is seen in 5 % of cases with multicentricity in 25 % of cases [26]. Albsoul et al reported 58 % of the incidences on the right side of the neck [1]. Smoking is reported as an etiological factor causing prolonged episodes of hypoxia and hypercapnia of the carotid body but most of the females are non smokers [1]. Baysal et al identified germline mutations in the succinate dehydrogenase, subunit D gene (SDHD) giving an genetic basis to head and neck paragangliomas [29]. Other subunits of SDH and related proteins were subsequently shown to be involved in HNPGL and sPGL [29–31]. These genes encode subunits or associated proteins of the succinate dehydrogenase complex, which plays a central role in energy metabolism as both an enzyme of tricarboxylic acid cycle (TCA) and as complex II of the mitochondrial respiratory chain, involved in oxidative phosphorylation [29].
Molecular and genetic studies have identified nine different susceptibility genes associated with head and neck paragangliomas [32]. The succinate dehydrogenase subunit D (SDHD) gene was first recognized as the susceptibility gene of paraganglioma syndrome type 1 (PGL 1) [32]. Subsequently, mutations of the B and C subunit genes (SDHB and SDHC) were described in patients with paraganglioma syndromes that were later designated as PGL type 3 and type 4 [29]. The SDHAF2 gene, also called SDH5, affects flavination of SDHA [33]. Later SDHA gene was identified as a tumor suppressor gene associated with paraganglial tumors [33]. Also a gene called TMEM127 was found to be mutated in pheochromocytomas, i.e. exclusively adrenal paraganglial tumors [33]. Meanwhile, it became evident that the spectrum of TMEM127 mutations extends to HNPs [29].
Mutations of subunits of SDH accumulates succinate where HIF-1 a key factor in hypoxia response is stabilized due to inhibition of HIF prolyl hydroxylases (PHDs) [34–36]. In hypoxia the HIF-1 protein is not degraded which enter the nucleus to stimulate gene transcription [34–36]. The concurrent SDH dysfunction releases reactive oxygen species (ROS) via the inhibition of SDH in its role as complex II of the respiratory chain which directly stabilizes the HIF1 [34–36]. So possible etiologic relations between high-altitude paraganglioma and the hypoxic drive have been reported [34–36]. Paraganglioma in head and neck has an higher prevalence (1:10) compared to its lower prevalence (1: 500,000) at sea levels [34–36]. An altitude of over 2,000 m has been referred to as marking the border of an increased incidence of non-chromaffin paragangliomas [34–36]. SDH genes mutations are least identified in high altitude paraganglioma cases and SDHB mutations in particular show a striking reduced penetrance [34–36]. The correlation between high altitude and hereditary paraganglioma syndromes influencing the tumor phenotype has been reported [37].
Albert in 1889 successfully excised the carotid paraganglioma after mortal failures by Reigner in 1880 and Maydl in1886 [38]. Scudder in 1903 demonstrated first successful removal of carotid body paraganglioma later in 1940 Gordon-Taylor described a safe subadvential plane of tumor dissection [39]. Mortality was 50–60 % and 15–30 % of patients suffered stroke before the advent of current vascular surgical techniques as larger tumors invariably needed common carotid artery ligation [18]. Surgical resection is the only treatment of choice for CBP, though preoperative chemoembolization and radiation therapy have been used in some centers [40]. Shamblin in 1971 classified the tumor based on its size, group I which could be easily dissected away from the vessel wall, group II which were intimately associated and compressed carotid vessels, but could be separated with careful subadventitial dissection and group III were large and typically encased the carotid artery requiring partial or complete vessel resection and replacement [41].
Shamblin’s classification classifies the risk with larger group III gives higher risks with cranial nerve and vascular injuries [1]. Luna-Ortiz et al modified the Shamblin’s grouping as: group I less than 4 cm in size with no surrounding or infiltrating the carotid and excision done without difficulty, group II more than 4 cm in size with partial surrounding or infiltrating the carotid and excision done with difficulty [42]. Group III includes IIIa, IIIb = I, II or III infiltration to any carotid vessel more than 4 cm in size and intimately infiltrating or surrounding the carotid vessels with difficulty requiring vascular repair, sacrifice or vessel replacement [42]. The Shamblin classification predicts only vascular morbidity without remarking on the neurological morbidity and reflects only the surgeon’s experience in dissecting the tumor [42].
Incidence of nerve injury is proportional to difficult surgical situation, larger tumors and also on vascular reconstructions [38]. Meticulous subadventitial dissection is advocated for Shamblin I and II while resection of the external and/or internal carotid artery for Shamblin III tumors involving the vessels [43]. Joint Vascular Research group (JVRG) in their meta-analysis study recommend that Duplex ultrasound scanning is the primary diagnostic investigation [1]. It depicts growth at carotid bifurcation and helps to define hypervascularity of growth [38]. Also used for preoperative assessment are angiography, digital subtraction angiogram (DSA), computed tomography (CT), CT Angiogram, magnetic resonance (MR) and MR Angiogram [44].
MR imaging and contrast CT are more effective noninvasive modalities comparing with duplex ultrasonography, especially for small tumors [45]. Digital substraction angiographic evaluation of blood supply to the tumor is essential to discern individual feeding vessels [1]. Embolization should be done to the vessels which are supra-selectively catheterized and determined not to allow free reflux of contrast medium into the internal carotid artery [1]. The carotid angiography demonstrates tumor vascularization and widening of the carotid bifurcation by a well-defined tumor blush (“lyre sign”), which is classic pathognomonic angiographic finding [6, 11]. Surgical excision of these vascular tumors lead to significant intraoperative bleeding which advocate the role of preoperative embolization [46, 47]. Supraselective feeder vessel embolization from the internal carotid or external carotid arteries are difficult because of the very short feeders [46, 47]. Complete obliteration of all tumor vascularity is not essential in reducing size and vascularity and surgical excision should be performed in 48 h to minimize revascularization edema or a local inflammatory response [28]. Albsoul reported lesser nerve damage in embolized cases compared to non embolised [20]. The preoperative embolization can be performed by ethanol or polyvinyl alcohol and not necessary in management of small tumors <4 cm [1]. Injection of Onyx through a combined arterial and venous route has also been reported to offer complete embolization of the tumor [48].
Percutaneous injection of cyanoacrylate glue has proven safe and effective which obliterates the tumor bed also [49, 50]. The risks of delayed glue migration and presence of collaterals and retrograde filling of small vessels arising from the ICA and vertebral artery which could prove fatal should be in remembered [49, 50]. Its effective in paragangliomas of the carotid body as the process is not limited by the number of arterial feeders, their origins, arterial tortuosities, atherosclerotic disease, or induced vasospasm [49, 50]. Schick et al initially described the use of pre-operative arterial embolisation which decreases blood loss and subsequent transfusion rates [51]. They reported 25 % shrinking of the tumour size if done within 48-h of surgery in medium to large CBTs with well-defined feeding vessels [51]. Some have reported no effect on blood loss, transfusion requirements or duration of surgery following embolisation [51]. The size of the tumor dictates the type of incision and the recent advances have reduced the cerebrovascular complication to minimal [1]. Smaller and earlier detected tumors have lesser complications with nerve damages reaching 14 % with tumors over 4 cm [1]. The modern surgical techniques have reduced the risk of postoperative stroke in carotid body paraganglioma resection to less than 5 % [45].
Immediate repair of the internal carotid artery is warranted if the vessel is encased by the tumor and other complication of massive bleeding is taken care of preoperative embolization [45]. In non embolized cases clamping the carotid arteries is useful with internal carotid shunts like the Pruitt-Inahara double balloon occlusive internal carotid shunt [45]. The placement of this shunt through incision on the common carotid artery contributes to the adequate bleeding control from the common and internal carotid arteries, as well as brain protection giving a clean and dry operative field during tumor removal [45].
Radiotherapy is recommended for giant and recurrent tumors, and with malignant transformation with metastatic to the regional lymph nodes [52]. Injuries to the hypoglossal and vagus are seen upto 20–40 % with larger tumor of Shamblin III variety with damage to be permanent in 20 % [52]. Lazar at al found no recurrence after complete resection while literature reports upto 6 % of cases [52]. Periodic duplex monographic scanning to identify graft stenosis should be done in vessel reconstructed individuals [52].
Conclusion
Paraganglioma of the carotid body should be considered as a differential diagnosis for painless lateral neck masses. Classification of the tumors based on modified Shamblin grading using vascular contrast studies help reduce intraoperative morbidity. Larger tumors need a multidisciplinary team of head and neck with vascular surgeons for better results.
References
- 1.Albsoul NM, Alsmady MM, Al-Aardah MI. Carotid body paraganglioma management and outcome. Eur J Sci Res. 2009;37(4):567–574. [Google Scholar]
- 2.Sykes JM, Ossoff RH. Paragangliomas of the head and neck. Otolaryngol Clin N Am. 1986;19:755–767. [PubMed] [Google Scholar]
- 3.Sobol SM, Dailey JC. Familial multiple cervical paragangliomas: report of a kindred and review of the literature. Otolaryngol Head Neck Surg. 1990;102:382–390. doi: 10.1177/019459989010200413. [DOI] [PubMed] [Google Scholar]
- 4.Myers EN, Johnson JT. In: Neoplasms. Otolaryngology-head and neck surgery. Cummings CW, Fredrickson JM, Harker LA, Krause CJ, Schuller DE, editors. St Louis: Mosby Year Book; 1993. pp. 1590–1597. [Google Scholar]
- 5.Kyriakos M. Pathology of selected soft tissue tumors of the head and neck. In: Thawley SE, Panje WR, editors. Comprehensive management of head and neck tumors. Philadelphia: W.B. Saunders; 1987. pp. 1261–1264. [Google Scholar]
- 6.Maves MD. Vascular tumors of the head and neck. In: Bailey BJ, Johnson JT, Kohut RI, Pillsbury HC, Tardy ME, editors. Head and neck surgery-otolaryngology. Philadelphia: JB Lippincott; 1993. pp. 1397–1409. [Google Scholar]
- 7.Rush BF., Jr Current concepts in the treatment of carotid body tumors. Surgery. 1962;52:679. [PubMed] [Google Scholar]
- 8.Attia AA, Abdalla H, Mamoun S, El-Sebai H. Carotid body ganglioma. J Egypt Natl Cancer Inst. 2001;13(2):79–84. [Google Scholar]
- 9.Milewski C. Morphology and clinical aspects of paragangliomas in the area of head-neck. HNO. 1993;41:526–531. [PubMed] [Google Scholar]
- 10.Balatsouras DG, Eliopoulos PN, Economou CN. Multiple glomus tumors. J Laryngol Otol. 1992;106:538–543. doi: 10.1017/S0022215100120080. [DOI] [PubMed] [Google Scholar]
- 11.Mayer R, Fruhwirth J, Beham A, Groell R, Poschauko J, Hackl A. Radiotherapy as adjunct to surgery for malignant carotid body paragangliomas presenting with lymph node metastases. Strahlenther Onkol. 2000;176:356–360. doi: 10.1007/PL00002343. [DOI] [PubMed] [Google Scholar]
- 12.Pacheco-Ojeda L. Malignant carotid body tumors: report of 3 cases. Ann Otol Rhinol Laryngol. 2001;110:36–40. doi: 10.1177/000348940111000107. [DOI] [PubMed] [Google Scholar]
- 13.Davidovic LB, Djukic VB, Vasic DM, Sindjelic RP, Duvnjak SN. Diagnosis and treatment of carotid body paraganglioma: 21 years of experience at a clinical center of Serbia. World J Surg Oncol. 2005;3:10. doi: 10.1186/1477-7819-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kairemo KJA, Hopsu EVM. Radioimmunodetection of chemodectoma by In-111 labeled anti-CEA antibody. Clin Nucl Med. 1990;15:900–903. doi: 10.1097/00003072-199012000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Saldana MJ, Salem LE, Travezan R. High altitude hypoxia and chemodectomas. Hum Pathol. 1973;4:251–263. doi: 10.1016/S0046-8177(73)80012-7. [DOI] [PubMed] [Google Scholar]
- 16.Heath D. The human carotid body in health and disease. J Pathol. 1991;164:1–8. doi: 10.1002/path.1711640102. [DOI] [PubMed] [Google Scholar]
- 17.Patetsios P, Gable DR, Garrett WV, Lamont JP, Kuhn JA, Shutze WP. Management of carotid body paragangliomas and reviews of 30 year experience. Ann Vasc Surg. 2002;16(3):331–338. doi: 10.1007/s10016-001-0106-8. [DOI] [PubMed] [Google Scholar]
- 18.Dimakakos P, Kotsis T. Carotid body paraganglioma: review and surgical management. Eur J Plast Surg. 2001;24:58–65. doi: 10.1007/s002380100231. [DOI] [Google Scholar]
- 19.Masilamani S, Duvuru P, Sundaram S. Fine needle aspiration cytology diagnosis of a case of carotid body tumour. Singap Med J. 2012;53(2):e36. [PubMed] [Google Scholar]
- 20.Por YC, Lim DT, Teoh MK, Soo KC. Surgical management and outcome of carotid body tumours. Ann Acad Med Singap. 2002;31:141–144. [PubMed] [Google Scholar]
- 21.Rosa M, Sahoo S. Bilateral carotid body tumour: the role of fine-needle aspiration biopsy in the preoperative diagnosis. Diagn Cytopathol. 2008;36:178–180. doi: 10.1002/dc.20775. [DOI] [PubMed] [Google Scholar]
- 22.Jayaram G, Kaliaperumal S, Kumar G. Bilateral carotid body tumour diagnosed on cytology. Acta Cytol. 2005;49:690–692. [PubMed] [Google Scholar]
- 23.Das DK, Gupta AK, Chowdhury V, et al. Fine-needle aspiration diagnosis of carotid body tumour: report of a case and review of experience with cytologic features in four cases. Diagn Cytopathol. 1997;17:143–147. doi: 10.1002/(SICI)1097-0339(199708)17:2<143::AID-DC11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Muhm M, Polterauer P, Gstöttner W, et al. Diagnostic and therapeutic approaches to carotid body tumours. Review of 24 patients. Arch Surg. 1997;132:279–284. doi: 10.1001/archsurg.1997.01430270065013. [DOI] [PubMed] [Google Scholar]
- 25.Westerband A, Hunter GC, Citora I, et al. Current trends in the detection and management of carotid body tumors. J Vasc Surg. 1998;28(1):84–92. doi: 10.1016/S0741-5214(98)70203-4. [DOI] [PubMed] [Google Scholar]
- 26.Kotelis D, Rizos T, Geisbüsch P, Attigah N, Ringleb P, Hacke W, Allenberg JR, Böckler D. Late outcome after surgical management of carotid body tumors from a 20-year single-center experience. Langenbeck’s Arch Surg. 2008 Jul 17 [DOI] [PubMed]
- 27.Kawai A, Healey J, Wilson S, Huvos A, Yeh S. Carotid body paraganglioma metastatic to the bone: report of two cases. Skeletal Radiol. 1998;27:103–107. doi: 10.1007/s002560050346. [DOI] [PubMed] [Google Scholar]
- 28.Kafie FE, Freischlag JA. Carotid body tumors: the role of preoperative embolization. Ann Vasc Surg. 2001;15:237–242. doi: 10.1007/s100160010058. [DOI] [PubMed] [Google Scholar]
- 29.Niemann S, Muller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26(3):268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 30.Astuti D, Latif F, Dallol A, et al. Gene mutations in the succinate dehydrogenase subunit SDHB cause susceptibility to familial pheochromocytoma and to familial paraganglioma. Am J Hum Genet. 2001;69:49–54. doi: 10.1086/321282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao HX, Khalimonchuk O, Schraders M, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schiavi F, Boedeker CC, Bausch B, Peczkowska M, Gomez CF, Strassburg T, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294(16):2057–2063. doi: 10.1001/jama.294.16.2057. [DOI] [PubMed] [Google Scholar]
- 33.Naji M, Zhao C, Welsh SJ, Meades R, Win Z, Ferrarese A, et al. (68)Ga- DOTA-TATE PET vs. (123)I-MIBG in identifying malignant neural crest tumours. Mol Imaging Biol. 2011;13(4):769–775. doi: 10.1007/s11307-010-0396-8. [DOI] [PubMed] [Google Scholar]
- 34.Pollard PJ, Briere JJ, Alam NA, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 35.Selak MA, Armour SM, MacKenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Semenza GL. HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J Bioenerg Biomembr. 2007;39:231–234. doi: 10.1007/s10863-007-9081-2. [DOI] [PubMed] [Google Scholar]
- 37.Bayley JP, Devilee P (2010) Warburg tumours and the mechanisms of mitochondrial tumour suppressor genes. Barking up the right tree? Curr Opin Genet Dev [DOI] [PubMed]
- 38.Sajid MS, Hamilton G, Baker DM, On Behalf Of Joint Vascular Research G A multicenter review of carotid body tumor management. Eur J Vasc Endovasc Surg. 2007;34:127–130. doi: 10.1016/j.ejvs.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Gordon-Taylor G. On carotid tumors. Br J Surg. 1940;28:163–172. doi: 10.1002/bjs.18002811003. [DOI] [Google Scholar]
- 40.Kollert M, Minovi A, Mangold R, Hendus J, Draf W, Bockmühl U. Cervical paragangliomas—tumor control and long-term functional results after surgery. Skull Base. 2006;16:185–192. doi: 10.1055/s-2006-950386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V, Herrera-Gomez A. Does Shamblin’s classification predict postoperative morbidity in carotid body tumors? a proposal to modify Shamblin’s classification. Eur Arch Otorhinolaryngol. 2006;263:171–175. doi: 10.1007/s00405-005-0968-4. [DOI] [PubMed] [Google Scholar]
- 42.Luna-Ortiz K, Rascon-Ortiz M, Villavicencio-Valencia V. Carotid body tumors: review of a 20-year experience. Oral Oncol. 2005;41:56–61. doi: 10.1016/j.oraloncology.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Pandey M, Chandramohan K, Sebastian P, Ramachandran K. An unusual bilateral cervical paraganglioma: a case report. Int J Oral Maxillofac Surg. 2002;31:335–337. doi: 10.1054/ijom.2001.0196. [DOI] [PubMed] [Google Scholar]
- 44.Christie A, Teasdale E. A comparative review of multidetector CT angiography and MRI in the diagnosis of jugular foramen lesions. Clin Radiol. 2010;65(3):213–217. doi: 10.1016/j.crad.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Defraigne JO, Sakalihassan N, Antoine P, Thiry A, Limet R. Carotid chemodectomas. Experience with nine cases with reference to preoperative embolization and malignancy. Acta Chir Belg. 1997;97:220–228. [PubMed] [Google Scholar]
- 46.Abud DG, Mounayer C, Benndorf G, Piotin M, Spelle L, Moret J. Intratumoral injection of cyanoacrylate glue in head and neck paragangliomas. AJNR Am J Neuroradiol. 2004;25:1457e62. [PMC free article] [PubMed] [Google Scholar]
- 47.Valavanis A. Preoperative embolization of the head and neck: indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986;7:943e52. [PMC free article] [PubMed] [Google Scholar]
- 48.Rimbot A, Mounayer C, Loureiro C, et al. Preoperative mixed embolization of a paraganglioma using Onyx. J Neuroradiol. 2007;34:334–339. doi: 10.1016/j.neurad.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Casasco A, Herbreteau D, Houdart E, et al. Devascularization of craniofacial tumors by percutaneous tumor puncture. AJNR Am J Neuroradiol. 1994;15:1233–1239. [PMC free article] [PubMed] [Google Scholar]
- 50.George B, Casasco A, Deffrennes D, et al. Intratumoral embolization of intracranial and extracranial tumors: technical note. Neurosurgery. 1994;35:771–773. doi: 10.1227/00006123-199410000-00031. [DOI] [PubMed] [Google Scholar]
- 51.Wax MK, Briant TD. Carotid body tumors: a review. J Otolaryngol. 1992;21(4):277–285. [PubMed] [Google Scholar]
- 52.Zabel A, Milker-Zabel S, Huber P, Schulz-Ertner D, Schlegel W, Wannenmacher M, Debus J. Fractionated stereotactic conformal radiotherapy in the management of large chemodectomas of the skull base. Int J Radiat Oncol Biol Phys. 2004;58:1445–1450. doi: 10.1016/j.ijrobp.2003.09.070. [DOI] [PubMed] [Google Scholar]