Abstract
An unknown primary tumor (UPT) is defined as a biopsy-proven malignancy whose anatomic origin remains unidentified after diagnostic evaluation. The estimated incidence of unknown primary tumors is 2 %–7 % of all malignancies. In 15–25 % of cases, the primary site cannot be identified even on postmortem examination. The management of these patients remains a clinical challenge. The aim of this study was to determine the role of 18FDG-PET CT in evaluation of primary tumor and its influence on therapeutic management. Fifty patients with histologically-proven metastases of UPT were included. For all patients, the conventional diagnostic work-up was unsuccessful in localizing the primary site. Whole-body PET/CT images were obtained approximately 60 min after intravenous injection of 350–425 MBq of (18) F-FDG. PET/(CT) depicted histologically verified primary tumors in 21of 46 patients (P > .05), achieving detection rates of approx. 61 % in patients presenting with cervical lymph node metastases from unknown primary tumors, and 40 % in those with extra cervical disease presentation. A positive predictive value of 72 % to 92 % was seen for all patients, depending on category of clinical presentation. In this study, PET/CT detection of additional metastases in 14.2 % (3 cases out of 21 true positives) influenced change in management plan. Considering recent studies and the results of this study, whole body FDG-PET/CT has to be considered a useful tool in evaluating metastases from an UPT, allowing an identification of primary tumors in 42 %, and modifying the stage of the disease and oncological treatment in about 50 % of cases. These results suggest the use of PET/CT with FDG in an early phase of the diagnostic evaluation to optimize the management of these patients.
Keywords: Unknown primary tumour, PET/CT
Introduction
Carcinoma of unknown primary tumour (UPT) is a diverse group of cancers that is defined by the presence of histological confirmed metastases in the absence of an identifiable primary malignancy despite a standardized diagnostic approach, comprising thorough clinical history, and physical examination, and laboratory investigations, and histological evaluation of routinely stained sections [1]. This may represent a very challenging problem both from the diagnostic and the therapeutic point of view [2, 3].
The criteria for UPT include a biopsy proven malignancy for which the anatomic origin is unknown after a medical history has been obtained, a detailed physical examination has been performed, and liver and kidney function tests, blood tests, chest radiography, abdomen and pelvis computed tomography (CT), and mammography or a prostate-specific antigen (PSA) test have been performed. UPT presents metastatic dissemination patterns that may be different from those observed in oncology with known primary tumours:
Short symptomatic pre-diagnostic intervals and clinically fast tumour growth have been observed.
UPT becomes symptomatic at the time of dissemination, whereas the primary site remains symptomatically silent.
The more routine use of electron microscopy and immunohistochemistry and the emerging field of molecular genetics are contributing to the more precise diagnosis of neoplasms.
The primary tumour is identified post mortem in 50 % to 75 % of cases. Post-mortem studies identify the lung or the pancreas as the site of the primary in about half of these cases [4]. The primary tumour is more rarely found in the liver, bile ducts, colon, rectum, or kidneys.
The typical patient with UPT presents with symptoms referable to a metastatic site. The initial work-up, including physical examination, laboratory and radiographic study performed to identify the primary site includes:
Medical history, physical examination, including testicular palpation for men and breast examination for women
-
Histology and cytology with immunohistology.
Light microscopic evaluation of biopsy material places the tumor into one of five histological categories, which then guides further evaluation:- Adenocarcinoma—approximately 70 %
- Poorly differentiated carcinoma—15 to 20 %; an additional 10 % represent poorly differentiated adenocarcinoma
- Poorly differentiated neoplasm—less than 5 %
- Squamous cell carcinoma—5 %
- Neuroendocrine carcinoma—less than 5 %
CT neck, chest, abdomen and pelvis
Women: gynaecological investigation
Routine laboratory, PSA (men >40 years), AFP, beta-hCG
Additional diagnostic procedures depending on working diagnosis, if this is of therapeutic consequence
AFP, alpha-fetoprotein; beta-hCG,
CT, computed tomography; PSA, prostate-specific antigen
The detection of primary tumor in patients with UPT may be important for several reasons, including finding a tumour and allowing therapy to be targeted as appropriately as possible.
In 1994, studies that evaluated the usefulness of PET with 18 F-FDG in detecting the primary tumour in patients with UPT began to be published. The reason was the technique’s capacity to detect different tumour types in all of the body non-invasively, and in a single examination.
Material and Methods
Patients
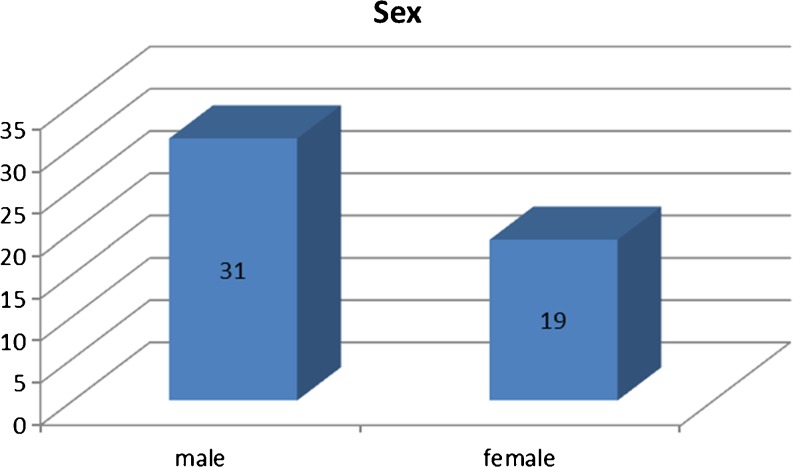
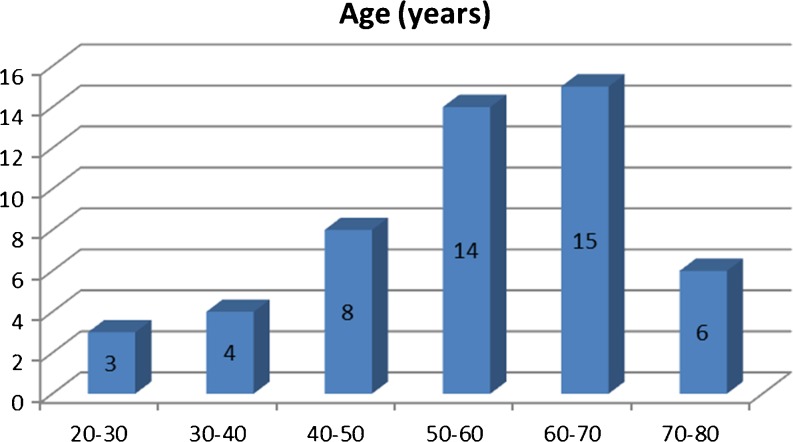
From May 2008 to May 2010, 50 consecutive patients with cancer of an unknown primary tumor were enrolled in the study, which was conducted in accordance with guidelines approved by this institution. In all these cases, the primary tumour could not be detected by conventional diagnostic modalities including CT/MRI scans, mammography, endoscopies, and tumour marker assays. There were 31 men and 19 women, in an age range of 29–76 years (Figs. 1 and 2).
Fig. 1.
Sex distribution of cases
Fig. 2.
Age distribution of cases
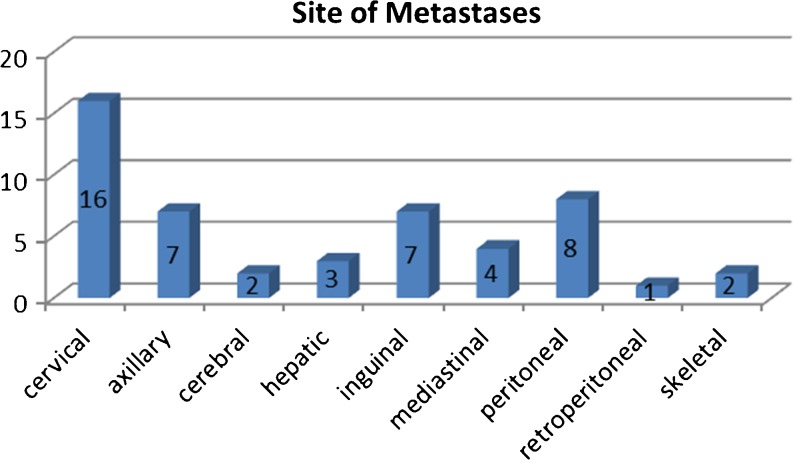
There were 35 lymph node metastases; 16 of which cervical, 7 axillary, 7 inguinal, 4 mediastinal, and 1 retroperitoneal. There were 8 peritoneal metastases, 3 liver metastases, 2 skeletal metastases, and 2 cerebral metastases (Fig. 3).
Fig. 3.
Distribution of sites of metastases
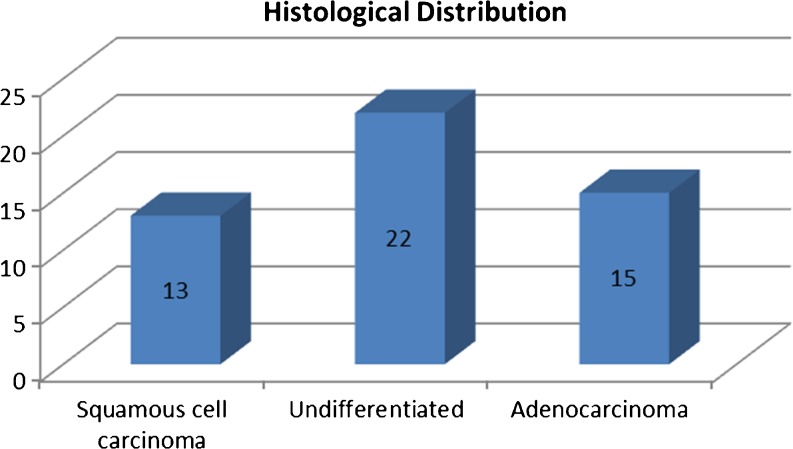
Histologic findings of at least one metastatic site were available in all patients and revealed adenocarcinoma in 22 patients and squamous cell carcinoma in 13 patients. In 15 patients, histopathology findings revealed undifferentiated carcinoma (Fig. 4).
Fig. 4.
Histologic distribution of the metastases
Patients were assigned the following two groups:
patients with extra-cervical metastases,
patients with cervical metastases.
A complete medical history and thorough physical examination had been performed in all patients. Furthermore, all patients underwent conventional diagnostic studies that included comprehensive laboratory analysis, imaging studies, tumour marker assays, immunohistochemistry, and endoscopic procedures where indicated. In all patients, the primary tumor had remained elusive.
PET/CT Imaging
Dual-modality PET/CT was performed by using a Biograph scanner. Patients had been instructed to fast for a minimum of 6 h prior to starting the examination. Blood samples collected before the injection of the radioactive tracer ensured blood glucose levels in the normal range.
The 18 F-FDG- PET/CT emission data of the whole body distribution of the tracer was acquired in 3D mode. Images reconstruction was then performed, and image analysis of the whole body 18 F- FDG- PET/CT scan was evaluated by nuclear medicine physician, generating a clinical report after reviewing previous imaging results and clinical information.
Statistical Analysis
Data analysis was performed for all patients, for patients with extra-cervical lymph node involvement (Group 1), and patients with cervical lymph node metastases (Group 2). Sensitivity and positive predictive values regarding the detection of the primary tumours were calculated.
A confidence level of less than 0.05 was considered to be statistically significant. Demographic data of all patients is summarized in Table 1.
Table 1.
Demographic data of enrolled patients
| No | Age | Sex | Metastases | Histology | Primary | Positivity | Addl Mets (biopsy proven) |
|---|---|---|---|---|---|---|---|
| 1 | 54 | M | Axillary | Sq carcinoma | Lung | TP | – |
| 2 | 29 | F | Cervical | Adenocarcinoma | Lung | TP | – |
| 3 | 63 | F | Axillary | Adenocarcinoma | Uterus | TP | – |
| 4 | 57 | M | Cervical | Undiff carcinoma | Not identified | – | – |
| 5 | 64 | M | Cervical | Adenocarcinoma | Pancreas | TP | – |
| 6 | 76 | M | Mediastinal | Sq carcinoma | Lung | TP | Other lung |
| 7 | 44 | M | Peritoneal | Undiff carcinoma | Not identified | – | – |
| 8 | 43 | M | Cervical | Undiff carcinoma | Nasopharynx | TP | – |
| 9 | 61 | M | Cervical | Sq carcinoma | Oropharynx | TP | – |
| 10 | 70 | M | Axillary | Undiff carcinoma | Not identified | – | – |
| 11 | 49 | F | Inguinal | Undiff carcinoma | Not identified | – | – |
| 12 | 33 | M | Peritoneal | Adenocarcinoma | Not identified | – | – |
| 13 | 62 | F | Hepatic | Adenocarcinoma | Pancreas | – | – |
| 14 | 71 | M | Cervical | Adenocarcinoma | Colon | FP | – |
| 15 | 58 | F | Axillary | Undiff carcinoma | Not identified | – | – |
| 16 | 55 | F | Retroperitoneal | Adenocarcinoma | Not identified | – | – |
| 17 | 48 | M | Inguinal | Sq carcinoma | Not identified | – | – |
| 18 | 53 | M | Peritoneal | Undiff carcinoma | Not identified | – | – |
| 19 | 60 | F | Inguinal | Undiff carcinoma | Not identified | – | – |
| 20 | 58 | F | Cervical | Sq carcinoma | Oropharynx | TP | – |
| 21 | 47 | M | Mediastinal | Adenocarcinoma | Not identified | – | – |
| 22 | 33 | M | Axillary | Adenocarcinoma | Not identified | – | – |
| 23 | 65 | M | Peritoneal | Adenocarcinoma | Colon | TP | – |
| 24 | 28 | M | Inguinal | Sq carcinoma | Not identified | – | – |
| 25 | 26 | F | Mediastinal | Undiff carcinoma | Not identified | – | – |
| 26 | 70 | M | Cervical | Sq carcinoma | Oropharynx | TP | Pulmonary |
| 27 | 60 | M | Cervical | Adenocarcinoma | Oropharynx | FP | – |
| 28 | 61 | F | Inguinal | Sq carcinoma | Not identified | – | – |
| 29 | 54 | F | Peritoneal | Adenocarcinoma | Not identified | – | – |
| 30 | 56 | F | Inguinal | Sq carcinoma | Anal canal | TP | – |
| 31 | 71 | M | Axillary | Undiff carcinoma | Not identified | – | – |
| 32 | 68 | M | Hepatic | Adenocarcinoma | Colon | TP | – |
| 33 | 64 | M | Cervical | Sq carcinoma | Nasopharynx | TP | – |
| 34 | 62 | F | Cervical | Undiff carcinoma | Not identified | – | – |
| 35 | 63 | M | Inguinal | Undiff carcinoma | Not identified | – | – |
| 36 | 59 | M | Cervical | Sq carcinoma | Esophagus | TP | – |
| 37 | 73 | F | Peritoneal | Adenocarcinoma | Stomach | TP | – |
| 38 | 46 | M | Skeletal | Adenocarcinoma | Lung | TP | – |
| 39 | 62 | F | Hepatic | Adenocarcinoma | Not identified | – | – |
| 40 | 54 | M | Cerebral | Adenocarcinoma | Lung | TP | – |
| 41 | 34 | F | Cervical | Adenocarcinoma | Colon | FP | – |
| 42 | 56 | M | Cervical | Undiff carcinoma | Not identified | – | – |
| 43 | 54 | M | Cervical | Undiff carcinoma | Not identified | – | – |
| 44 | 63 | M | Cervical | Sq carcinoma | Not identified | – | – |
| 45 | 49 | M | Cerebral | Undiff carcinoma | Not identified | – | – |
| 46 | 54 | F | Peritoneal | Adenocarcinoma | Not identified | – | – |
| 47 | 39 | F | Axillary | Adenocarcinoma | Breast | TP | – |
| 48 | 52 | M | Skeletal | Adenocarcinoma | Lung | FP | – |
| 49 | 49 | F | Peritoneal | Adenocarcinoma | Colon | TP | – |
| 50 | 62 | M | Mediastinal | Sq carcinoma | Lung | TP | Skeletal |
Observations
Primary Tumor Diagnosis
PET scan data sets helped identify more primary tumours than any of the other imaging modalities, although the difference did not prove to be statistically significant.
In all these cases, the primary tumour had remained unidentified by conventional diagnostic modalities including CT/MRI scans, mammography, endoscopies, and tumour marker assays.
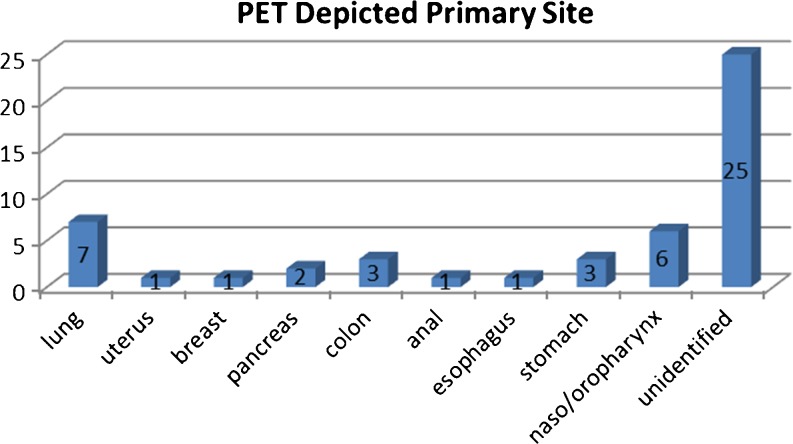
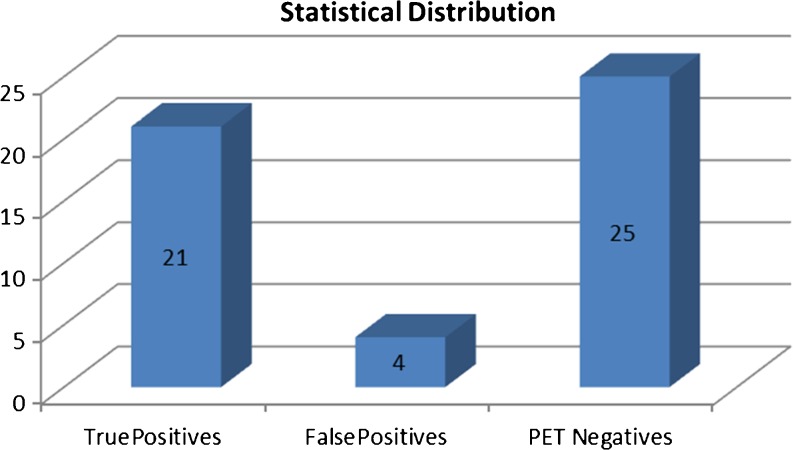
PET/(CT) depicted primary tumours in 25 (50 %) of 50 patients (P > .05, Chi square Test).
Histologic findings subsequently confirmed the diagnosis in 21 patients (Fig. 5).
Fig. 5.
Sites of Primary Tumour depicted by PET/CT
There were 4 (8 %) false-positive interpretations. In these cases, pathologic evaluation revealed two cases of colitis, one case each of pharyngitis, and pulmonary inflammation, without signs of malignancy. In 25 (50 %) patients, the primary site was not identified on PET/CT data (P > .05, Chi square Test) (Fig. 6).
Fig. 6.
Statistical classification of findings
Whole body FDG-PET/CT scan findings in the 50 cases are summarized in Table 2.
Table 2.
Whole-body FDG- PET/CT findings in the 50 CUP syndrome cases enrolled
| Biopsy proven Mets | N | Histology | TP | Primary site | FP | PET CT indicated primary site | NI |
|---|---|---|---|---|---|---|---|
| Cervical LN | 16 | Adenoca (5) | 8 | Nasopharynx (5) | 3 | Colon (2) | 5 |
| Sq ca (6) | Lung (1) | Oropharynx (1) | |||||
| Undiff (5) | Esophagus (1) | ||||||
| Pancreas (1) | |||||||
| Extra cervical LN | 19 | Adenoca (5) | 6 | Lung (3) | – | 13 | |
| Sq ca (7) | Breast (1) | ||||||
| Undiff (7) | Uterus (1) | ||||||
| Anal Canal (1) | |||||||
| Cerebral | 2 | Adenoca (1) | 1 | Lung (1) | – | 1 | |
| Undiff (1) | |||||||
| Hepatic | 3 | Adenoca (3) | 2 | Stomach (1) | – | 1 | |
| Colon (1) | |||||||
| Peritoneal | 8 | Adenoca (6) | 3 | Colon (2) | – | 5 | |
| Undiff (2) | Stomach (1) | ||||||
| Skeletal | 2 | Adenoca (2) | 1 | Lung (1) | 1 | Lung (1) | – |
| Total | 50 | 21 | 4 | 25 |
TP True Positive, FP False Positive, NI Not identified
Statistical findings are shown in Table 3.
Table 3.
Statistical findings
| HPE positive | HPE negative | ||
| PET positive | 21 | 4 | |
| (13) Extracervical | (1) Extracervical | ||
| (8) Cervical | (3) Cervical | ||
| PET negative | 25 | *** | |
| (20) Extracervical | |||
| (5) Cervical | |||
| Diseased | Not Diseased | Total | |
| Positive test | True positives (a) | False-positives (b) | Test positives (a + b) |
| Negative test | False-negatives (c) | True negatives (d) | Test negatives (c + d) |
| Total | Total with disease(a + c) | Total without disease(b + d) | Total screened (a + b + c + d) |
*** Due to inherent study design True negatives cannot be obtained
Sensitivity = a/(a + c), Specificity = d/(b + d), Positive predictive value = a/(a + b), Negative predictive value = d/(c + d)
Analysis
Group 1: Extracervical Metastases
When only patients in group 1 were considered, PET/CT depicted 14 of 34 (41 %) primary tumours (P > .05, Chi square Test), with one false-positive finding.
Sensitivity (39.3 %) and positive predictive value (92.8 %) for assessment of patients in group 1 are summarized in Table 3 below.
Group 2: Cervical Metastases
When only patients in Group 2 were considered, PET/(CT) helped identify the primary tumour in 11of 16 (68.75 %) patients, with three false-positive findings (P > .05, Chi square Test).
Sensitivity (61.5 %) and positive predictive value (72.7 %) are shown in Table 4.
Table 4.
Statistical analysis (based on above shown formulas)
| Sensitivity | Positive predictive value | |
|---|---|---|
| Detection of the primary lesion in patients with cancer of unknown primary tumor | 45.6 % (21/46) | 84 % (21/25) |
| Detection of the primary tumor in patients with extra cervical metastases (group 1) | 39.3 % (13/33) | 92 % (13/14) |
| Detection of the primary tumor in patients with cervical metastases (group 2) | 61.5 % (8/13) | 72.2 % (8/11) |
FDG- PET/CT identified further unexpected parenchymal/bone metastases in 3 out of the 25 patients. Finally, in this patient population, the following oncological treatment was influenced by whole body FDG- PET/CT scan in a total of 25 patients:
21 cases in which the primary site was identified, specific oncological treatment was started;
In 3 cases in which additional metastases was detected on FDG-PET/CT, the disease stage was modified.
Discussion
Prognosis in patients with cancer of an unknown primary tumor is generally poor, with a median survival time ranging between 4 and 11 months; however, patient care and survival rates are dependent on the knowledge of the primary tumor and localized or disseminated disease [5]
The identification of the primary tumor may offer the possibility of a more specific and effective treatment with an improvement of survival time [6]. The key role of whole body FDG-PET scan in this field has been showed by previous studies, and these data led the German Consensus Conference to recognize, in 2001, the whole body FDG-PET study as a standard diagnostic technique, i.e. scientifically proved benefit and established clinical use in patient with Carcinoma of unknown primary syndrome (CUPS) [7]. At this time, to the best of our knowledge, there are only a few published papers on CUP syndrome and whole body FDG-PET/CT scan.
In the present study involving 50 cases of metastases from unknown primary tumour, detection rates of 50 % (25/50) were achieved. At the same time, the positive predictive value of 72 % to 92 % was seen for all patients, depending on the category (cervical/extra-cervical) of clinical presentation.
If FDG- PET/CT (/CT) findings are positive, confirmatory biopsy is necessary because of the risk of false-positive results [8]. Literature on the exact causes of false positive FDG- PET/CT/CT results is scarce, although benign inflammatory lesions and pulmonary infarction have been reported aetiologies [9]. The oropharynx and the lung are the two most common locations of false-positive FDG- PET/CT/CT results [10].
In this study too, there was one false positive each in lung and oropharynx out of four false positive cases.
A further scope of FDG- PET/CT/CT is its ability to identify additional metastatic sites, which may have important implications for patient management. Despite intensive literature search, there appears to be no study that has investigated the role of FDG PET CT in modification of treatment plan. Therefore, the additional value of FDG- PET/CT/CT to patients with UPT and its cost-effectiveness should be further investigated.
Nevertheless, the failure rate of 50 % for non-detectable primary cancers points to how much room there is for improvement in optimizing diagnostic strategies in patients with cancer of an unknown primary tumor. In most cases, the primary tumor remains unidentified during the patients’ lifetime
These results support the idea that, in metastases from UPT, whole body PET/CT scan with FDG can have a clinical impact and should be performed early in the workup of the patients.
Conclusions
Considering recent studies and the results of this study, whole body FDG-PET/CT has to be considered a useful tool in evaluating metastases from an UPT, allowing an identification of primary tumours in 1 out of 2 cases, and modifying the stage of the disease and oncological treatment in about 50 % of cases. These results suggest that the use of PET/CT with FDG is complimentary in the diagnostic evaluation to optimize the management of these patients.
References
- 1.Abbruzzese JL, Lenzi R, Raber MN, et al. The biology of unknown primary tumors. Semin Oncol. 1993;20:238–243. [PubMed] [Google Scholar]
- 2.Dennis JL, Hvidsten TR, Wit EC, et al. Markers of adenocarcinoma characteristic of the site of origin: development of a diagnostic algorithm. Clin Cancer Res. 2005;11:3766–3772. doi: 10.1158/1078-0432.CCR-04-2236. [DOI] [PubMed] [Google Scholar]
- 3.Briasoulis E, Tolis C, Bergh J, Pavlidis N, ESMO Guidelines Task Force ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of cancers of unknown primary site (CUP) Ann Oncol. 2005;16(Suppl 1):i75–i76. doi: 10.1093/annonc/mdi804. [DOI] [PubMed] [Google Scholar]
- 4.Pavlidis N. Cancer of unknown primary: biological and clinical characteristics. Ann Oncol. 2003;14(Suppl 3):iii111–iii118. doi: 10.1093/annonc/mdg742. [DOI] [PubMed] [Google Scholar]
- 5.Culine S, Kramar A, Saghatchian M, et al. Development and validation of a prognostic model to predict the length of survival in patients with carcinomas of an unknown primary site. J Clin Oncol. 2002;20:4679–4683. doi: 10.1200/JCO.2002.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Chorost MI, Lee MC, Yeoh CB, Molina M, Ghosh BC. Unknown primary. J Surg Oncol. 2004;87:191–203. doi: 10.1002/jso.20099. [DOI] [PubMed] [Google Scholar]
- 7.Reske SN, Kotzerke J. FDG-PET for clinical use. Results of the 3rd German Interdisciplinary Consensus Conference, “Onko-PET III”, 21 Jul and 19 Sep 2000. Eur J Nucl Med. 2001;28:1707–1723. doi: 10.1007/s002590100626. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG- PET/CT in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 9.Nassenstein K, Veit-Haibach P, Stergar H, Gutzeit A, Freudenberg L, Kuehl H, et al. Cervical lymph node metastases of unknown origin: primary tumor detection with whole-body positron emission tomography/computed tomography. Acta Radiol. 2007;48:1101–1108. doi: 10.1080/02841850701581768. [DOI] [PubMed] [Google Scholar]
- 10.Kwee TC, Kwee RM. Combined FDG-PET/CT for the detection of unknown primary tumors: systematic review and meta-analysis. Eur Radiol. 2009;19:731–744. doi: 10.1007/s00330-008-1194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]