Abstract
Modular segmental replacement system (MSRS) is one of the options for Limb conservation surgery in bone tumors. The study analyses a single center experience of use of MSRS for limb conservation in cases of primary bone tumors. Retrospective analysis was done for a series of cases of limb salvage procedures done over a five year period. All Patients with bone tumors who underwent limb salvage procedures utilising MSRS prosthesis were included in the study. The patients’ record were perused for pre operative staging; neoadjuvant therapy used, if any; surgical procedure done; follow-up for prosthesis related complications and overall survival achieved. Total of 50 cases studied,included 28 males and 22 females . Median age at diagnosis of 28 (10–73) years. Tumor localized in lower limb in 38 patients, and upper limb in 12 patients. Tumors were malignant in 28 patients (56 %) and benign in 22 (44 %). The most common diagnosis was osteosarcoma (21 patients (42 %)) . The median resection length was 15 cm (range 6–25). High grade tumors (grade 2a and 2b) was found in 27 of 29 cases(93.1 %) . 14 patients had prosthesis related complications. The mean follow-up was 5 years (range: 3–7). 42 patients of 50 were alive with the endoprosthesis at the last follow-up. Survival rate of prosthesis is 84 %. The modular segmental-replacement system prosthesis favoured by us in limb sparing surgery for bone tumors results in satisfactory results in terms of tumor control and limb function.
Keywords: Bone tumor, Limb salvage, Modular prosthesis
Introduction
Amputation had been the standard method of treatment for most bone sarcomas, but the 1980s witnessed the development of limb-sparing surgery for most malignant bone tumors. Today, limb-sparing surgery is considered safe and routine for approximately 90 % of patients with extremity malignant bone tumors .[1] Advances in orthopaedics, bioengineering, radiographic imaging, radiotherapy, and chemotherapy have contributed to safer, more reliable surgical procedures. Currently, the three most popular options are using an endoprosthesis, allograft-prosthetic composite and biological reconstructions. Each of those methods has its short- and long-term advantages and disadvantages, and a surgeon should consider each patient individually.[2] We describe a single centre experience of using modular segmental-replacement system prosthesis for limb salvage.
Patient and Method
Study Design
A retrospective analysis of 50 patients treated by Limb Salvage procedures in bone tumors during a 5 year period (2004–2008) at a large tertiary care referral centre.
Inclusion Criteria
All Patients with bone tumors who underwent limb salvage procedures utilising modular segmental-replacement system prosthesis
Exclusion Criteria
Patients with bone tumors in whom limb salvage was not possible. The criteria used to determine if the limb was worthy of salvage were as follows:
The major neurovascular bundle is free of tumor.
Wide resection of the affected bone with a normal muscle cuff in all directions is possible.
All previous biopsy sites and all potentially contaminated tissues can be removed en bloc.
Bone can be resected 3 to 4 cm beyond abnormal uptake as determined by bone scan
The adjacent joint and joint capsule can be resected.
Adequate motor reconstruction can be accomplished by regional muscle transfers.
Adequate soft tissue coverage is possible to decrease the risk of skin flap necrosis and secondary infection.
Investigations
Standard Investigations done at our Center for all the patients include X ray of the part involved and of the chest, Computed Tomography of the part and of the chest, and magnetic resonance imaging of the part and, in some patients,magnetic resonance angiograph. All these findings were recorded. Following non invasive investigations, biopsy of the lesion is performed. At our institute we prefer core needle biopsies/J-Needle biopsies (image guided biopsies are preferred in deep seated tumors which are difficult to palpate). An open biopsy was performed in a few selected cases when core biopsies have failed to achieve a diagnosis. All biopsies were performed by a trained oncosurgeon and planned in such a way that the biopsy scar can be safely included in the incision while performing definitive surgery. At our institution the use of FNAC is generally restricted to the confirmation of suspected metastasis. Metastatic workup also includes Positron Emission Tomography/Computed Tomography (PET/CT) and Tc 99 M Bone Scan.
Neoadjuvant Therapy
Before consideration of limb preservation, the patients were appropriately staged and assessed at a multidisciplinary tumor board meet. All patients with a proven histopathology of osteosarcoma and ewings sarcoma were given neoadjuvant chemotherapy. At our centre we use 3 cycles of IAP(Ifosfamide 1.5 gm/m2 Day 1–3, Adriamycin 25 mg/m2 Day 1–3, Cisplatin 90 mg/m2) as neoadjuvant for Osteosarcomas and 4 cycles of IE/VAC (alternating, Ifosfamide 2 gm/m2 Day 1–3, Etoposide 100 mg/m2 Day 1–3, Vincristine 1.4 mg/m2, Adriamycin 60 mg/m2, Cyclophosphamide 600 mg/m2) for Ewing’s sarcoma. Response to chemotherapy was assessed by imaging modalities and compared with previous imaging findings.
Surgical Approach
The surgery was performed according to the general principles of limb salvage surgery as outlined previously. We have used the modular segmental-replacement system prosthesis (with a bipolar component) for the proximal part of the humerus and the proximal part of the femur,and a simple-hinge component (Howmedica) for the knee joint, the proximal part of the tibia, and the distal part of the femur. All stems are cemented in place. We have used extra cortical fixation for femoral and tibial components but not humeral components.
Postoperative Follow-up
Isometric exercises and mobilization with crutches were started on 2nd postoperative day. The patients thereafter received adjuvant chemotherapy tailored to the degree of response seen to the neoadjuvant chemotherapy as evident on histopathology report.
Post Discharge Follow-up
The patients were followed up on quarterly basis for initial two years and thereafter on six monthly intervals. On follow-up visits, a thorough clinical examination was carried along with digital X-ray of the part. Chest imaging was done routinely by digital X ray and CT Scan of the chest done, if a suspect lesion was seen on the X ray chest. Bone scan was done as a routine on yearly basis while PET-CT was reserved for suspected metastatic lesions.
Result
Demography
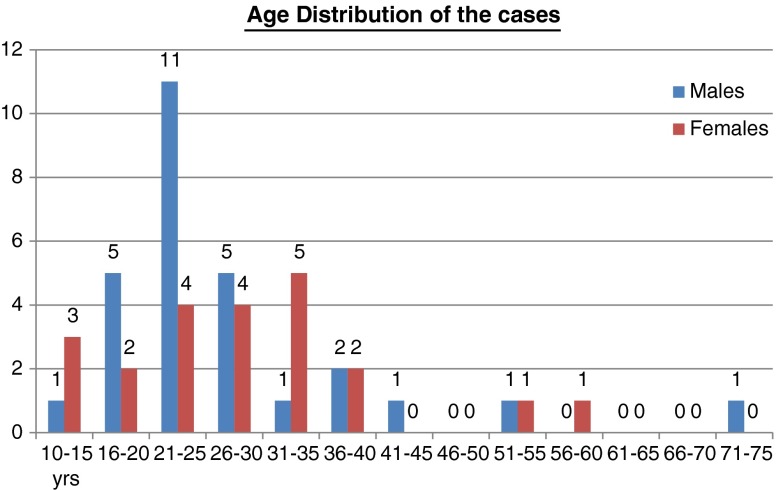
In the study period a total of 50 cases were studied which included 28 males and 22 females with a median age at diagnosis of 28 (10–73) years. The age distribution of the cases is as per Fig. 1.
Fig. 1.
Age distribution of the cases
Clinical Presentation
Upper limb involvement was present in 12 patients and lower limb in 38 patients. Tumor was localized in proximal femur in 2 patients, distal femur in 22 patients, proximal tibia in 11 patients, distal tibia in 3 patients, proximal humerus in 8 patients and distal humerus in 4 patients. The malignant bone tumors were present in 28 patients (56 %), benign tumors in 22 (44 %),. The most common diagnosis was osteosarcoma in 21 patients (42 %), Ewing sarcoma in 4 patients (8 %), Chondrosarcoma in 3(6 %) and giant cell tumor of bone in 22 patients (44 %). Figure 2 represents a composite distribution of tumor type and anatomical site.
Fig. 4.
(a) Giant cell tumor proximal end femur (b) Hip prosthesis placed after resection of upper end femur
Fig. 2.
Composite distribution of tumor types with their anatomical localisation
Surgical Resection
The median resection length was 15 cm (range 6–25): the longest resections were performed in distal femur (median 16 cm, range 12–25), followed be proximal femur (median 15 cm, range 6–25) proximal tibia (median 12 cm, range 7–15) . No extra reconstructive interventions were needed for soft tissue coverage in any patients.
Histopathology
High grade tumors (grade 2a and 2b) were found in the majority of malignant bone tumors, 27 of 29 (93.1 %) . Detailed histological findings and tumor grades are shown in Table 1.
Table 1.
Histpathological grade distribution of the operated tumors
| Tumor type | ||||||
|---|---|---|---|---|---|---|
| Benign Bone tumors | ||||||
| Grade 1 | Grade 2 | Grade 3 | Total | |||
| Giant cell Tumor | 1 | 7 | 14 | 22 | ||
| Malignant Bone Tumors | ||||||
| Grade 1a | Grade 1b | Grade 2a | Grade 2b | Grade 3 | Total | |
| Osteosarcoma | 1 | 1 | 4 | 15 | 0 | 21 |
| Ewing sarcoma | 0 | 0 | 1 | 3 | 0 | 4 |
| Chondrosarcoma | 0 | 0 | 0 | 3 | 0 | 3 |
| Total | 1 | 1 | 5 | 21 | 0 | 28 |
Complications
14 patients had complications details of which, treatment methods, and final results are shown in Table 2.
Table 2.
Complications, their treatment and final outcome
| Complication | Diagnosis | Treatment | Final result |
|---|---|---|---|
| Infection | GCT (2) | Curettage | NED(2) |
| OS (3) | Curettage | NED(3) | |
| Local recurrance | OS (3) | Forequarter amputation (2) | DOD(3) |
| Re-resection (1) | |||
| Loosening | GCT (3) | Implant Reapplied (3) | NED(3) |
| OS (2) | Implant Reapplied (2) | NED (2) | |
| Periprosthetic fracture | OS (1) | Elongation of Endoprosthesis | NED (1) |
* Number in brackets represents number of patients
DOD died of disease; NED no evidence of disease
Follow Up
The mean follow-up was 5 years (range: 3–7). 17 of 50 patients had follow-up for more than 5 years. Five of our patients developed pulmonary metastasis during follow up after surgery. Four patients underwent pulmonary metastatectomy and are disease free at the last follow up. Patients who died due to tumor are as follows: 6 of 21 patients with osteosarcoma (30.0 %), 1 of 4 patients with Ewing sarcoma (25 %)1 of 3(33.3 %) patients with chondrosarcoma. In total, during the follow-up period, 8 patients died due to tumor. 42 patients of 50 were still living with the endoprosthesis at the last follow-up. Survival rate of prosthesis is 84 %. At the last control examination, 38 patients showed no evidence of the primary disease.
Discussion
Primary malignant bone tumors are relatively uncommon lesions. Before the 1970s, management routinely consisted of trans bone amputations or disarticulations, with dismal survival rates (10 % to 20 %). With the development of more effective chemotherapeutic agents and treatment protocols in the 1970s and 1980s, survival rates improved. This,along with better imaging modalities, allowed the focus of management to shift to limb preservation[1–3] Osteosarcoma is the commonest malignant bone tumor.[4] It accounted for 42 % of all bone tumors and 75 % of the malignant type in our series. Before the use of chemotherapy (which began in the 1970s), osteosarcoma was treated primarily with surgical resection (usually amputation). Despite such good local control, more than 80 % of patients subsequently developed recurrent disease that typically presented as pulmonary metastases.[4] The high recurrence rate indicates that most patients have micro metastatic disease at the time of diagnosis. The“neoadjuvant (pre-operative) chemotherapy” has the theoretical advantage of addressing these occult micro metastases. It has been found to facilitate subsequent surgical removal by causing tumor shrinkage and also by“sterilizing” the reactive zone around the tumor by destroying microscopic disease at the periphery of the primary lesion.[5, 6] Additionally, in some patients with a relative contraindication to limb salvage, such as a pathologic fracture in the upper extremity, the use of chemotherapy with a favourable response may allow limb salvage to be considered.[7] It also provides oncologists with an important risk parameter. Patients in whom there has been a good histopathologic response to neoadjuvant chemotherapy (>95 % tumor cell kill or necrosis) have a better prognosis than those whose tumors do not respond as favourably.[8] Hence, we use neoadjuvant chemotherapy as a routine for osteosarcoma and ewings sarcoma. In our series, during the follow-up, 8 out of 28 cases of malignant tumors died. The cumulative 5-year survivorship was 38 %. Other similar series report 5 year survivor ship ranging from 28 % to 76 %.[9] It must be borne in mind there were relatively larger percentage of high grade tumors in our series and also that it includes chondrosarcoma which do have a viable and effective chemotherapy regimen.
Currently, 80 % to 85 % of patients with primary malignant bone tumors involving the extremities (osteosarcoma, Ewing’s sarcoma, and chondrosarcoma) can be treated safely with wide resection and limb preservation with or without reconstruction.[9] Simon et al. [10] published the first evidence-based study supporting the benefits of limb-salvage procedures for the treatment of bone tumors. Their multicenter study, which included 227 patients with osteosarcoma of the distal end of the femur, reported the rates of local recurrence, metastasis, and survival. Three groups of patients where studied: patients in group 1 had a limb-sparing procedure, patients in group 2 had an above knee amputation, and in group 3, a hip disarticulation was the procedure of choice. The Kaplan-Meier curves of the patients who survived and the percentage of patients without recurrent disease showed no statistical difference among the three surgical groups after a mean length of follow-up of 5.5 years (Mantel-Cox test: P = .8). Limb-salvage surgery was as safe as an amputation in the management of patients with high-grade osteosarcoma. Thereafter, there have been multiple recent studies which indicate that limb preservation is the norm is bone tumors.[11–14]
Limb-salvage procedures can be divided into two groups: arthrodesis or arthroplasty. An arthrodesis is usually obtained using bone allografts [14], vascularised autografts[15] or both. An arthrodesis provides a stable, durable reconstruction which requires limited postoperative follow-up. But the inherent disadvantages include the loss of joint function, increased energy expenditure, and the additional abnormal mechanical stress to other joints.[16]
An arthroplasty preserves the joint. This can be accomplished with an allograft [17, 18] or a metallic prosthesis.[19] Early metal designs were custom made, resulting in obvious manufacturing delays between diagnosis and reconstruction; consequently, intraoperative flexibility was limited .However, malignant bone tumors are dynamic tumors that change with time and treatment. Hence, currently endoprosthetic reconstruction is performed with the use of modular prosthesis (Figs. 3 and 4). Modularity of prosthetic design allows intraoperative flexibility based on the final amount of tissue resected. A rigorous rehabilitation program can be initiated immediately after implantation, allowing early joint range of motion and weight bearing. Prosthetic reconstruction carries a lower risk of deep infection than do allografts, and non union is not a concern because there are no osteosynthesis sites. Endoprosthetic use also avoids the risk of disease transmission and immune responses that exists with allograft reconstruction. Longevity, complications, and functional outcome vary by anatomic site, type of prosthesis, and fixation technique.[17, 20] In our series, we have used the modular segmental-replacement system prosthesis as the preferred modality for limb preservation. The advantages of the modular segmental-replacement system prosthesis include simplicity, adaptability, and reduced operating time. We had an overall complication rate of 28 % and a prosthetic survivorship of 84 % at 5 year. Many studies have been performed to investigate endoprosthetic survival rates after tumor resection, but the results cannot be summarized and systematic review cannot be performed, mostly because of a small number of patients, as well as different models and principles of endoprosthesis. Tumor endoprosthetic survival rates are mostly about 60 % to 80 % at 5 years, and 40–70 % at 10 years.[21–23] For the current rotating-hinge knee design, reported follow-up is limited to approximately 10 years. Malawer et al. [24] in 1995 showed an 83 % survival of prostheses at 5 years and 67 % at 10 years. They had a revision rate of 15 %, infection rate of 13 %, amputation rate of 11 %, and local recurrence rate of 6 %. Overall, 44 % of patients had at least one complication. In an attempt to determine prosthesis and extremity survivorship, Horowitz et al. [25] reviewed their experience with 93 prosthetic reconstructions over 8 years: 16 proximal femur, 61 distal femur, and 16 proximal tibia. Minimum follow-up was 24 months (mean, 80 months). Prosthesis survival at 5 years was 88 %, 59 %, and 54 % for proximal femur, distal femur, and proximal tibia reconstructions, respectively. The overall event-free prosthesis survival was 63 % at 5 years and 36 % at 10 years. Limb survival for the entire group was 87 % at 5 years and 81 % at 10 years.
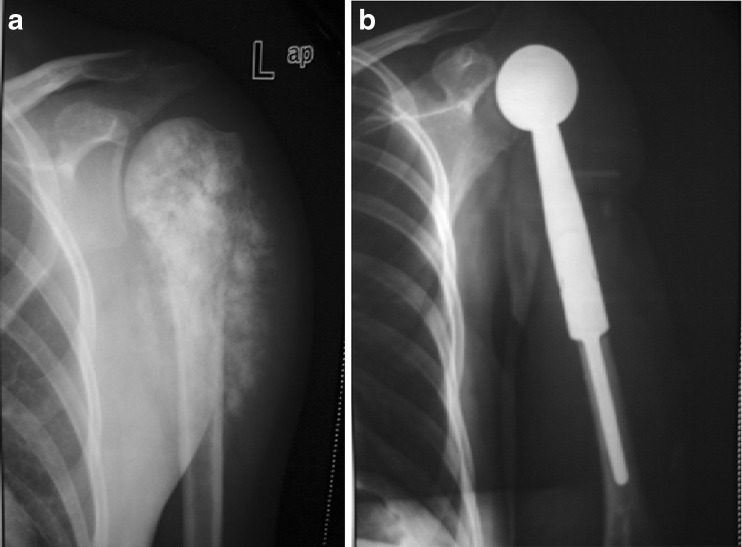
Fig. 3.
(a) Osteosarcoma of upper end humerus (b) Resection of tumor followed by shoulder prosthesis placed
Loosening, dislocation is the most common complication after primary or secondary femur endoprosthetic reconstruction, regardless the indication. In our series we noted 5 patients (10 %) with loosening of endoprosthesis, who were then managed successfully with re application of endoprosthesis. Periprosthetic fracture occurred in 1(2 %) patient during sport activity and was managed with endoprosthetic elongation. their series, Malawer et al. noted aseptic loosening as cause for failure in approximately 20 % at 5 years and 30 % at 10 years. Similarly, a review of other series too note an incidence of this complication ranging from 5–30 % . Another common complication is infection. The incidence of infection was 10 % in our study. In the previous literature, the rate of deep infection has ranged from 4 to more than 30 %.[26] Curettage, debridement and irrigation were performed in these patients, we had satisfactory final results.
The location of bone tumors in the growing areas of bone commonly mandates the removal of the affected growth plate. Subsequent continued growth in the contra lateral extremity results in limb-length inequality which result in orthopaedic dysfunction.[17] Expandable prosthesis were developed to address this issue. Custom expandable prostheses system consists of a fixed stem with a screw or a multiple plate extension mechanism. The obvious disadvantage in these systems is that a surgical procedure is required for the subsequent expansions.[27] The Stanmore expandable prosthesis (Stanmore Implants, Stanmore Middlesex, United Kingdom) allows non-invasive expansion. When the implanted prosthesis is placed at the center of a rotating electromagnetic field, the poles of a magnet within the implant are captured, causing it to rotate in synchrony. The external field rotates at a fixed speed, causing the implant to expand at a rate of 0.23 mm per minute (1 mm every 4 min).[28] We do not have experience with these systems.
It is pertinent to mention that limb salvage surgery requires high-level infrastructure which includes an experienced multidisciplinary team of orthopaedic surgeons and oncologists, high-quality prostheses, a good tissue bank for allografts, adequate blood bank facility and state-of-the-art intensive care facilities and rehabilitation training modules. The prosthesis itself entails a major cost burden. Thus, limb salvage protocols can be implemented in limited, tertiary level centres. Arthrodesis, as mentioned earlier, remains a viable option for limb preservation where such detailed infrastructure does not exist. The Van Nes rotationplasty is another alternative for skeletally immature individuals .When an above knee amputation is indicated, a “more functional” limb that will act as a below knee amputation can be obtained with this procedure. In a study by Lindner et al. [29], out of 136 patients with a high grade osteosarcoma, 79 were treated by limb salvage, 21 by Van Nes rotationplasty,and 33 by amputation . The patients were then followed for a mean of 43 months. The authors demonstrated that the functional result of the Van Nes rotationplasty was superior to that of amputation or limb salvage. In our Center, we have treated a select few cases with arthrodesis and rotation arthroplasty too, but the number is too few to draw any meaningful comparison.
Conclusion
The surgical management of patients with malignant tumors of bone is challenging. The modular segmental-replacement system prosthesis favoured by us in limb sparing surgery for bone tumors results in satisfactory results in terms of tumor control and limb function. The case selection needs to be precise to obtain good long term results. It has inherent disadvantage of requirement of specialised infrastructure. Amputation remains as a valid procedure in cases where limb preservation is not possible.
References
- 1.Enneking WF. An abbreviated history of orthopaedic oncology in North America. Clin Orthop. 2000;374:115–24. doi: 10.1097/00003086-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Eckardt JJ, Yang RS, Ward WG, Kelly C, Eilber FR. Endoprosthetic reconstruction for malignant bone tumors and nonmalignant tumorous conditions of bone. In: Stauffer RN, Erlich MG, Fu FH, Kostuik JP, Manske PR, Sim FH, editors. Advances in operative orthopaedics. 3. St. Louis: Mosby; 1995. pp. 61–83. [Google Scholar]
- 3.Choong PF, Sim FH. Limb-sparing surgery for bone tumors: new developments. Semin Surg Oncol. 1997;13:64–9. doi: 10.1002/(SICI)1098-2388(199701/02)13:1<64::AID-SSU10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 4.Ries LAG, Smith MA, Gurney JG, et al (1999) Cancer incidence and survival among children and adolescents: United States SEER program 1975–1995. Bethesda, Md: National Cancer Institute; NIH Pub No 99–4649. Available at http://seer.cancer.gov/publications/childhood/
- 5.Link MP, Eilber F. Osteosarcoma. In: Pizzo PA, Poplack DG, editors. Principles and practice of pediatric oncology. 3. Philadelphia: Lippincott-Raven; 1997. pp. 889–920. [Google Scholar]
- 6.Weis LD. Common malignant bone tumors: Osteosarcoma. In: Simon MA, Springfield D, editors. Surgery for bone and soft-tissue tumors. Philadelphia: Lippincott-Raven; 1998. pp. 265–74. [Google Scholar]
- 7.Ebeid W, Amin S, Abdelmegid A. Limb salvage management of pathologic fractures of primary malignant bone tumors. Cancer Control. 2005;12:57–61. doi: 10.1177/107327480501200107. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Helman LJ. Strategies to explore new approaches in the investigation and treatment of osteosarcoma. Cancer Treat Res. 2009;152:517–28. doi: 10.1007/978-1-4419-0284-9_31. [DOI] [PubMed] [Google Scholar]
- 9.Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R. Local and systemiccontrol after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop. 1999;358:120–27. [PubMed] [Google Scholar]
- 10.Simon MA, Aschliman MA, Thomas N, Mankin HJ. Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am. 2005;87:2822. doi: 10.2106/JBJS.8712.cl. [DOI] [PubMed] [Google Scholar]
- 11.Biermann JS, Adkins D, Benjamin R, et al. Bone cancer. J Natl Compr Canc Netw. 2007;5:420–437. doi: 10.6004/jnccn.2007.0037. [DOI] [PubMed] [Google Scholar]
- 12.Mankin HJ, Hornicek FJ, Rosenberg AE, Harmon DC, Gebhardt MC. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;429:286–91. doi: 10.1097/01.blo.0000145991.65770.e6. [DOI] [PubMed] [Google Scholar]
- 13.Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb: amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br. 2002;84:88–92. doi: 10.1302/0301-620X.84B1.12211. [DOI] [PubMed] [Google Scholar]
- 14.Enneking WF, Shirley PD. Resection-arthrodesis for malignant and potentially malignant lesions about the knee using an intramedullary rod and local bone grafts. J Bone Joint Surg Am. 1997;59:223–36. [PubMed] [Google Scholar]
- 15.Weinberg H, Kenan S, Lewis MM, et al. The role of microvascular surgery in limb-sparing procedures for malignant tumors of the knee. Plast Reconstr Surg. 1993;92:692–8. doi: 10.1097/00006534-199309001-00019. [DOI] [PubMed] [Google Scholar]
- 16.Marulanda GA, Henderson ER, Johnson DA, Letson GD, Cheong D. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15:13–20. doi: 10.1177/107327480801500103. [DOI] [PubMed] [Google Scholar]
- 17.Gebhardt MC, Flugstad DI, Springfield DS, et al. The use of bone allografts for limb salvage in high-grade extremity osteosarcoma. Clin Orthop Relat Res. 1991;270:181–96. [PubMed] [Google Scholar]
- 18.Sim FH, Frassica FJ. Use of allografts following resection of tumors of the musculoskeletal system. Instr Course Lect. 1993;42:405–13. [PubMed] [Google Scholar]
- 19.Bradish CF, Kemp HB, Scales JT, Wilson JN. Distal femoral replacement by custom-made prostheses. Clinical follow-up and survivorship analysis. J Bone Joint Surg Br. 1987;69:276–84. doi: 10.1302/0301-620X.69B2.3818760. [DOI] [PubMed] [Google Scholar]
- 20.Neel MD, Letson GD. Modular endoprostheses for children with malignant bone tumors. Cancer Control. 2001;8:344–8. doi: 10.1177/107327480100800406. [DOI] [PubMed] [Google Scholar]
- 21.Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop. 1996;322:207–23. [PubMed] [Google Scholar]
- 22.Kabukcuoglu Y, Grimer RJ, Tillman RM, Carter SR. Endoprosthetic replacement for primary malignant tumors of the proximal femur. Clin Orthop. 1999;358:8–14. [PubMed] [Google Scholar]
- 23.Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res. 2006;442:223–9. doi: 10.1097/01.blo.0000181491.39048.fe. [DOI] [PubMed] [Google Scholar]
- 24.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcosarcomas. J Bone Joint Surg Am. 1995;77:1154–65. doi: 10.2106/00004623-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz SM, Glasser DB, Lane JM, Healey JH. Prosthetic and extremity survivorship after limb salvage for sarcoma: how long do the reconstructions last? Clin Orthop. 1993;293:280–6. [PubMed] [Google Scholar]
- 26.Simon MA. Limb salvage for osteosarcoma in the 1980s. Clin Orthop. 1991;270:264–70. [PubMed] [Google Scholar]
- 27.Wilkins RM, Soubeiran A. The Phenix expandable prosthesis: early American experience. Clin Orthop Relat Res. 2001;382:51–8. doi: 10.1097/00003086-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Gupta A, Meswania J, Pollock R, et al. Non-invasive distal femoral expandable endoprosthesis for limb-salvage surgery in paediatric tumours. J Bone Joint Surg Br. 2006;88:649–54. doi: 10.1302/0301-620X.88B5.17098. [DOI] [PubMed] [Google Scholar]
- 29.Lindner NJ, Ramm O, Hillmann A, et al. The University of Muenster experience. Clin Orthop Relat Res. 1999;358:83–9. doi: 10.1097/00003086-199901000-00011. [DOI] [PubMed] [Google Scholar]