Abstract
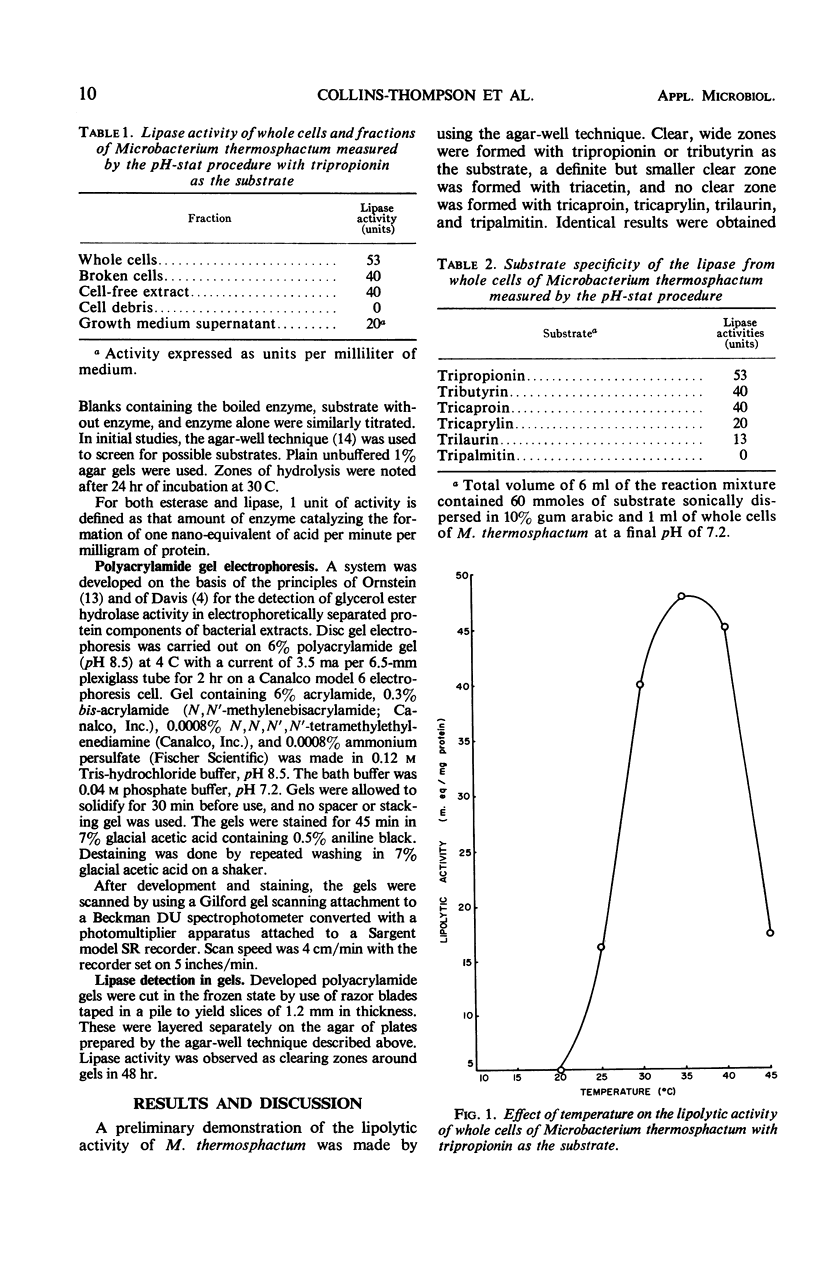
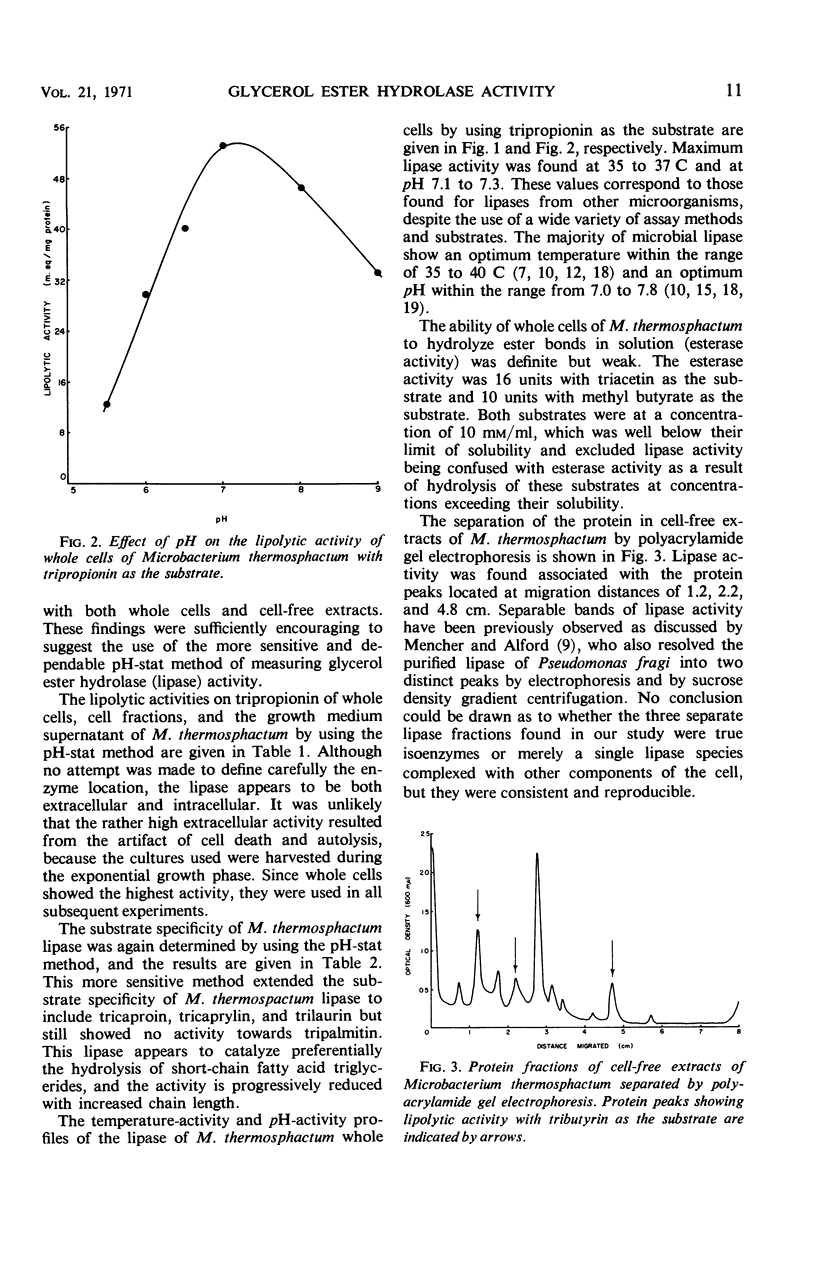
Microbacterium thermosphactum possesses a significant glycerol ester hydrolase (lipase, EC 3.1.1.3) activity and a weak but definite carboxylic ester hydrolase (esterase, EC 3.1.1.1) activity. Harvested whole cell preparations contained 53 units of lipase activity with tripropionin as the substrate. This activity decreased with an increasing chain length of fatty acid in the triglyceride to 13 units with trilaurin as the substrate and no activity with tripalmitin. Maximum lipase activity was found at a temperature of 35 to 37 C and at a pH of 7.1 to 7.3. Lipase activity was associated with three different protein peaks when the protein of cell-free extract was fractionated by polyacrylamide gel electrophoresis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DESNUELLE P., CONSTANTIN M. J., BALDY J. Technique potentiométrique pour la measure de l'activité de la lipase pancréatique. Bull Soc Chim Biol (Paris) 1955;37(2-3):285–290. [PubMed] [Google Scholar]
- Hodgkiss W., Ordal Z. J., Cann D. C. The comparative morphology of the spores of Clostridium botulinum type E and the spores of the "OS mutant". Can J Microbiol. 1966 Dec;12(6):1283–1284. doi: 10.1139/m66-170. [DOI] [PubMed] [Google Scholar]
- MCLEAN R. A., SULZBACHER W. L. Microbacterium thermosphactum, spec: nov.; a nonheat resistant bacterium from fresh pork sausage. J Bacteriol. 1953 Apr;65(4):428–433. doi: 10.1128/jb.65.4.428-433.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencher J. R., Alford J. A. Purification and characterization of the lipase of Pseudomonas fragi. J Gen Microbiol. 1967 Sep;48(3):317–328. doi: 10.1099/00221287-48-3-317. [DOI] [PubMed] [Google Scholar]
- NASHIF S. A., NELSON F. E. Some studies on microbacteria from Iowa dairy products. Appl Microbiol. 1953 Jan;1(1):47–52. doi: 10.1128/am.1.1.47-52.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'LEARY W. M., WELD J. T. LIPOLYTIC ACTIVITIES OF STAPHYLOCOCCUS AUREUS. I. NATURE OF THE ENZYME PRODUCING FREE FATTY ACIDS FROM PLASMA LIPIDS. J Bacteriol. 1964 Nov;88:1356–1363. doi: 10.1128/jb.88.5.1356-1363.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Oterholm A., Ordal Z. J., Witter L. D. Purification and properties of a glycerol ester hydrolase (lipase) from Propionibacterium shermanii. Appl Microbiol. 1970 Jul;20(1):16–22. doi: 10.1128/am.20.1.16-22.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTTEM S., RAZIN S. LIPASE ACTIVITY OF MYCOPLASMA. J Gen Microbiol. 1964 Oct;37:123–134. doi: 10.1099/00221287-37-1-123. [DOI] [PubMed] [Google Scholar]
- SHAH D. B., WILSON J. B. EGG YOLK FACTOR OF STAPHYLOCOCCUS AUREUS. II. CHARACTERIZATION OF THE LIPASE ACTIVITY. J Bacteriol. 1965 Apr;89:949–953. doi: 10.1128/jb.89.4.949-953.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]


