SUMMARY
The M56 gene encodes M56, one of three proteins comprising the murine cytomegalovirus (MCMV) terminase, an enzyme that packages DNA into capsids and cleaves the DNA into progeny genomes. Deletion of M56 from a bacterial artificial chromosome (BAC) clone of the MCMV genome was lethal, as the mutant BAC failed to reconstitute infectious virus. Reintroduction of M56 at an ectopic locus complemented the deletion, allowing reconstitution of a virus that replicated with wild type efficiency. Reintroduction of M56 sequences encoding an N-terminal epitope fusion was lethal, as was a mutation targeting a region in M56 implicated as an ATPase active site. In contrast, a frame shift mutation in M56a, an open reading frame that overlaps M56, had no effect on viral replication. We conclude that the protein product of M56a is dispensable while M56 residues comprising the proposed ATPase active site are critical for terminase function and viral replication.
Herpesviruses replicate their genomes in a manner similar to the large double-stranded DNA bacteriophage. Linear genomic DNA is replicated to form concatemers that are then packaged into preformed capsids and cleaved to produce capsids containing unit length progeny genomes (Brown et al., 2002). This process is carried out by an enzyme called terminase. Phage terminases have two subunits (Black, 1989), while herpesvirus terminases appear to have three. The terminase subunits of human cytomegalovirus (HCMV) are UL51, UL56, and UL89. DNA translocation is believed to be ATP-dependant, and indeed, UL89 contains Walker A and B motifs (Walker et al., 1982) indicative of an ATP binding pocket, but UL89 has not been shown to have ATPase activity in vitro. In contrast, a C-terminal portion of UL56 expressed in E. coli has been shown to have ATPase activity (Hwang & Bogner, 2002) and mutations within a putative ATP binding site decreased this activity (Scholz et al., 2003). However, the importance of these residues for viral replication has not been confirmed. Recently, peptides encoded by UL56a, an open reading frame (ORF) that overlaps much of UL56, have been detected in HCMV virions (Varnum et al., 2004) but the relevance of the UL56a gene product to terminase function or viral replication is not known.
To facilitate mutagenesis of the murine cytomegalovirus (MCMV) gene M56 that encodes M56, the MCMV ortholog of the HCMV terminase subunit UL56, we developed a cis complementation system (Hahn et al., 2003) in which M56 was deleted from a bacterial artificial chromosome (BAC) clone of the MCMV genome, then complemented in cis by reintroduction of wild type or mutant sequences at an ectopic location by site-specific Tn7-mediated transposition. BACs thus complemented were assessed for their ability to reconstitute viruses and the growth properties of such viruses were characterized.
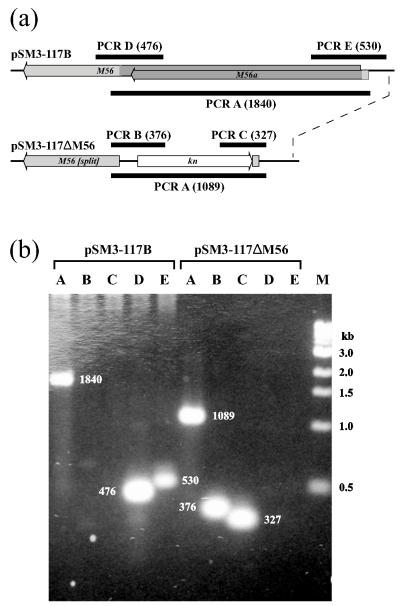
BAC pSM3-117B, an infectious clone of the MCMV genome, contains a lacZα-mini-attTn7 site that permits insertion of sequences into the attTn7 locus via Tn7-mediated transposition (Luckow et al., 1993). It was made from pSM3-117K by Flip recombinase-mediated removal of a kanamycin-resistance (kn) marker (Hahn et al., 2003). BAC pSM3-117ΔM56 was constructed by replacing most of M56 (nucleotides 86,400 to 88,120; 21716071) with kn (Fig. 1a). Briefly, kn from pACYC177 was PCR-amplified (Wang et al., 2008) using primers M56-pACYC177-F (GAACATGGGACCGTTGATGAAACGGTAGATCTGCTGCCCGAGCTGGGGCAGCAGCTCGGACGATTTATTCAACGAAGCC) and M56-pACYC177-R (GTCTGTGCGCGGTATGTATAAGCGGAGGGGTAGGGGAGTCGACCTGCTCGCGCGCAACGTGCCAGTGTTACAACCAATT). The product was DpnI-restricted then electroporated into pSM3-117B-containing E. coli strain DY380 cells that had been induced at 42 °C for 15 min to express λ recombinases (Yu et al., 2000). Colonies containing recombinant BACs were selected on plates containing 50 μg/ml kanamycin, 50 μg/ml chloramphenicol, and 50 μg/ml tetracycline. One clone was designated pSM3-117ΔM56 after confirmation of correct structure using the five PCR reactions illustrated in Fig. 1a (for primer sequences see Table 1, Supplementary Data). Reaction A was predicted to amplify a 1840-bp product from pSM3-117B or a 1089-bp product from pSM3-117ΔM56. Reactions B and C were predicted to produce 376- and 327-bp products from pSM3-117ΔM56, respectively, but no products from pSM3-117B. Reactions D and E were predicted to produce 476- and 530-bp products from pSM3-117B, respectively, but no products from pSM3-117ΔM56. That each reaction produced the predicted results (Fig. 1b) confirmed that pSM3-117ΔM56 has the predicted structure and lacks a functional M56 locus. Transfection of pSM3-117ΔM56 DNA into mouse NIH3T3 cells (Wang et al., 2008) failed to reconstitute an infectious virus, consistent with a critical role for M56 in viral replication.
Fig. 1.
Deletion of the M56/M56a locus. (a) The native M56/M56a locus in pSM3-117B is compared to the predicted structure of pSM3-117ΔM56. M56 and M56a ORFs are shown as gray shaded arrows with dark gray indicating the region that is deleted and replaced by kn (white arrow) in pSM3-117ΔM56. Black bars represent the products of diagnostic PCR reactions (A-E) with their predicted sizes (bp) shown in parentheses. (b) The products of PCR reactions using pSM3-117B or pSM3-117ΔM56 as templates were separated on 1% agarose, stained with ethidium bromide, and visualized with UV light. The predicted sizes (bp) of each product are indicated. Sizes (kb) of markers (lane M) are indicated to the right.
Sequence analysis of the M56 region revealed an ORF, designated M56a, that overlaps M56 (Fig. 1a) and has homology to UL56a. As the importance of M56a or its hypothetical protein product M56a were not known, and as the deletion in pSM3-117ΔM56 disrupts both M56 and M56a (Fig. 1a), sequences from the M56a start to the M56 stop were included in efforts to complement the deletion. The Tn7 transposition shuttle pFastBac1 (Invitrogen) was modified to include the HCMV major immediate early promoter (MIEP), a multiple cloning site for insertion of M56/M56a sequences, and a 3′ IRES-gfp reporter (Fig. 2a). This shuttle, designated pMA178B, was made by ligation of annealed oligonucleotides MOL126/MOL127 into BamHI/HindIII-restricted pFastBac1 (Invitrogen), then restriction of the resulting plasmid with NdeI/MfeI and insertion of a 2.1-kb AseI/MfeI fragment from pIRES2-EGFP (Clontech). Shuttle plasmids containing wild type or mutant M56/M56a sequences were subsequently constructed. The sequences of oligonucleotides used for plasmid construction are given in Table 1 (Supplementary Data). Sanger dideoxy sequencing was used to confirm the entire sequence of each M56/M56a insertion.
Fig. 2.
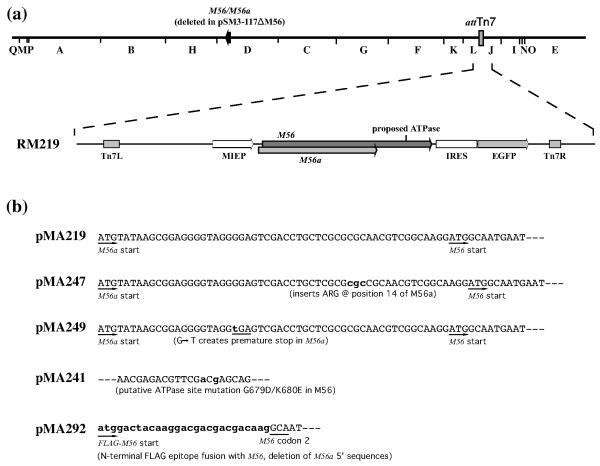
Complementation in cis of the M56/M56a deletion. (a) A HindIII map of the MCMV genome illustrating the positions of the M56/M56a locus and the attTn7 site. Expanded below is the transposed region (between Tn7L and Tn7R) of RM219 showing the MIEP (open arrow), M56 and M56a (gray arrows), and the IRES-gfp marker cassette. (b) Wild type (pMA219) and mutations in M56/M56a sequences cloned in the indicated shuttle plasmids are shown. Nonviral sequences or nucleotide changes are shown in lowercase and bold.
Wild type M56/M56a sequences were PCR-amplified using primers MOL129 and MOL153. The product was ligated into pGEM®-T Easy (Promega) then transferred as a 2.8-kb EcoRI fragment into EcoRI-restricted pMA178B to make plasmid pMA219. Sequences between Tn7R and Tn7L of pMA219 were transposed into pSM3-117ΔM56 to generate pSM3-117ΔM56-t219, as previously described (Hahn et al., 2003). Briefly, pMA219 was transformed into DH10B strain E. coli containing pSM3-117ΔM56 and helper plasmid pMON7124 (Luckow et al., 1993). Transformants were selected on plates containing 25 μg/ml chloramphenicol, 50 μg/ml kanamycin, 7 μg/ml gentamycin, 10 μg/ml tetracycline, 200 μg/ml X-gal, and 40 μg/ml isopropylthio-β-D-galactoside and incubated for 48 h at 37°C. White colonies in which lacZα was disrupted by transposition into the lacZα-mini-attTn7 site of pSM3-117ΔM56 were further screened for correct transposition using three PCR reactions as previously described (Wang et al., 2008).
BAC pSM3-117ΔM56-t219 was transfected into mouse NIH3T3 cells. Reconstitution of a virus designated RM219 was evidenced by the appearance of GFP+/CPE+ foci 5 - 10 days post-transfection. The predicted genome structure of RM219 is shown in Fig. 2a. The sequence of the ectopic M56/M56a insertion in RM219 was confirmed by Sanger dideoxy sequencing of DNA isolated from virions as previously described (McVoy et al., 1998).
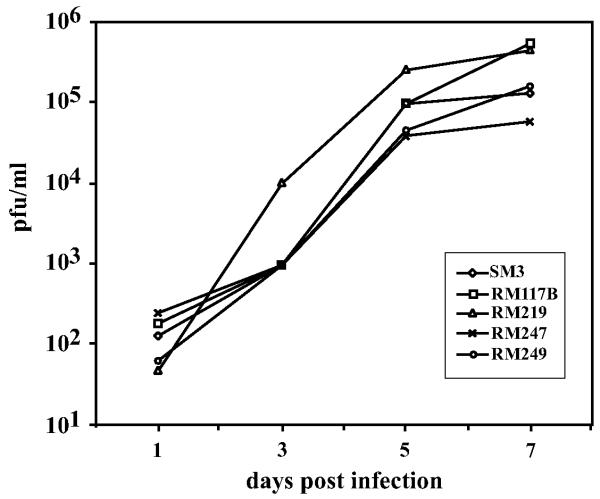
To compare the growth properties of RM219 to wild type virus, NIH3T3 cells were infected at an m.o.i. of 0.1. Cells were washed 3 h post-infection and culture supernatants were collected daily and titrated by limiting-dilution in 96-well plate cultures as described (Cui et al., 2008). The replication kinetics and efficiency of virus RM219 were similar to those of parental of viruses SM3 (derived from pSM3) and RM117B (derived from pSM3-117B) (Fig. 3). Therefore, the ectopic M56/M56a sequences were able to complement in cis the deletion of native M56/M56a sequences without significant loss of viral replication efficiency.
Fig. 3.
Growth of wild type and cis-complemented viruses. NIH3T3 cells were infected with the indicated viruses at an m.o.i. of 0.1. Viral titers in the culture supernatants were determined on the days post infection indicated.
To determine if the putative M56a protein is important for viral replication, two mutations were engineered into M56a 5′ of the M56 start codon. In plasmid pMA247 a 3-bp insertion that added an arginine to M56a was created by digestion of pMA219 with BssHII, blunt-ending with Klenow DNA polymerase, and religation. In plasmid pMA249 a single base insertion created a premature stop in addition to a frame shift in M56a (Fig. 2b). This was achieved by PCR amplification of pMA219 with primers MOL210b and MOL211, ligation of the product into pGEM®-T Easy, and transfer of a 0.45-kb BssHII/NdeI fragment to BsHII/NdeI-restricted pMA219.
The sequences in pMA247 and pMA249 were transposed into pSM3-117ΔM56 to produce pSM3-117ΔM56-t247 and pSM3-117ΔM56-t249 respectively. Both BACs reconstituted viruses (RM247 and RM249, respectively) that replicated with wild type kinetics and efficiencies (Fig. 3). Sequencing of virion DNA confirmed that RM247 and RM249 retained their respective mutations within their ectopic M56/M56a sequences. Moreover, while PCR reaction A amplified 1089- and 1840-bp products from RM219, RM247, and RM249 DNA, reaction E failed to amplify these DNAs yet produced an abundant 530-bp product from RM117B DNA (data not shown). Thus, the 1840-bp products of reaction A were presumably derived from the ectopic M56/M56a insertions while the native M56/M56a region remained disrupted in all three viruses. That the frame shift in M56a was maintained in virus RM249 yet caused no impairment in its replication demonstrates that M56a is fully dispensable for viral replication.
Hwang et al. reported an ATPase activity for a C-terminal portion of HCMV UL56 expressed in E. coli (Hwang & Bogner, 2002). Residues 709-716 were proposed as a putative ATP binding pocket and substitutions of residues within this region impaired the in vitro ATPase activity of the E. coli-expressed protein (Scholz et al., 2003). UL56 residues 709-716 are identical to M56 residues 674-681. To determine if this region is important for MCMV replication, the complementing M56 gene was modified to encode two amino acid changes, G679D and K680E (Fig. 2b). Plasmid pMA241 was constructed by PCR overlap extension. DNA from pMA219 was amplified using MOL189 and MOL190 or MOL191 and MOL192 to generate overlapping products containing two nucleotide changes. The two products were then mixed and amplified again using primers MOL189 and MOL191. The resulting product was double-digested with BbvCI/BamHI and ligated into BbvCI/BamHI-restricted pMA219 to produce plasmid pMA241. Sequences from pMA241 were transposed into pSM3-117ΔM56 to generate pSM3-117ΔM56-t241. Repeated attempts to reconstitute virus from pSM3-117ΔM56-t241 failed, suggesting that residues within the proposed ATP binding site are critical for the function of M56 during viral replication.
As antibodies have not been raised to the M56 protein, we sought to construct viruses encoding M56 fused with an N-terminal FLAG epitope tag. Plasmid pMA219 was PCR-amplified with MOL237 and MOL242 and the product ligated into pCR8/GW/TOPO (Invitrogen). A 2.4-kb EcoRI fragment was then excised and ligated into EcoRI-restricted pMA178B to produce plasmid pMA292. Transposition into pSM3-117ΔM56 produced pSM3-117ΔM56-t292 in which the native M56 AUG and upstream M56a sequences were replaced by sequences encoding an N-terminal FLAG epitope (Fig. 2b). Infectious virus cold not be reconstituted from pSM3-117ΔM56-t292, suggesting that the M56 protein was likely rendered nonfunctional by the N-terminal epitope fusion.
DNA maturation is an attractive target for the development of novel antivirals, and indeed, several compounds are known to block this process (Hwang et al., 2007, Krosky et al., 2000, Reefschlaeger et al., 2001, Underwood et al., 2004, Underwood et al., 1998, van Zeijl et al., 2000). Future drug discovery efforts would benefit from a better understanding of the protein composition, structure, and biochemical functions of terminase. Progress has been made in expressing terminase subunits and dissecting their biochemical activities in vitro. To confirm the importance of such activities for viral replication we developed a cis complementation system that facilitates mutagenic evaluation of the M56 terminase subunit. Deletion of the native M56/M56a locus was lethal but could be efficiently complemented by transposition of an ectopic copy of M56/M56a sequences. As native M56 is most probably expressed with late kinetics while ectopic expression from the MIEP likely occurs with early or immediate early kinetics, this result suggests that the kinetics of M56 expression are not critical.
Bacteriophage terminases use the energy from ATP hydrolysis to translocate DNA into capsids (Catalano, 2000, Feiss & Catalano, 2005, Rao & Black, 2005). The large subunits contain Walker A and B box motifs that form ATP binding pockets (Walker et al., 1982) and many have been shown to possess ATPase activities in vitro (Mitchell et al., 2002). Walker box motifs are highly conserved among the herpesvirus terminase subunits that include HCMV UL89 and its ortholog in herpes simplex virus type 1, UL15. Within these motifs homology even extends to phage terminase large subunits (Davison, 1992, Mitchell et al., 2002, Przech et al., 2003). Although a mutation in the Walker A box of UL15 confirmed its importance for viral replication (Yu & Weller, 1998), ATPase activity has not been demonstrated for UL15, UL89, or their orthologs in other herpesviruses. In contrast, UL56 lacks canonical Walker box motifs or sequence homology with bacteriophage terminase subunits, but a C-terminal region of UL56 has been shown to have ATPase activity when expressed in E. coli (Hwang & Bogner, 2002) and mutations within a putative ATP binding pocket affected a decrease in this activity (Scholz et al., 2003). That BAC pSM3-117ΔM56-t241 containing similar mutations in M56 was unable to reconstitute an infectious virus confirms that residues within the proposed ATP binding pocket are necessary for viral replication and supports the hypothesis that an ATPase-activity associated with this region (Scholz et al., 2003) is important for terminase function.
In 2004 Varnum et al. detected peptides within HCMV virions having amino acid sequences encoded by an unannotated ORF that was designated UL56a because it overlaps the UL56 gene (Varnum et al., 2004). We found that similar ORFs are conserved in MCMV, rat CMV, rhesus CMV, chimpanzee CMV, and tupaia herpesvirus, and that they encode hypothetical proteins that are highly conserved at the amino acid level (Fig. 4, Supplementary Data). While this suggests that these ORFs serve an important purpose, other herpesviruses, including guinea pig CMV, a close relative of MCMV and rat CMV (McGeoch et al., 2006), appear to lack UL56a homologs. Moreover, the strong nucleotide sequence conservation among the terminase genes that they overlap could account for the amino acid conservation observed in Fig. 4. That virus RM249 replicates with wild type efficiency in vitro clearly demonstrates that expression of the putative M56a protein is not important for replication of MCMV in cell culture. Why these overlapping ORFs have evolved in some viral genomes and what role the encoded proteins play in vivo remains to be determined.
In summary, amino acids proposed to function as an ATP binding pocket and to mediate an ATPase activity of M56 were confirmed to be of critical importance for viral replication, and an overlapping ORF of unknown function (M56a) was shown to be dispensable. In the future this system can provide a rapid genetic approach to complement progress made in defining the in vitro biochemical properties of terminase.
Supplementary Material
ACKNOWLEDGMENTS
We thank Martin Messerle for pSM3, Don Court for E. coli strain DY380, and the National Institutes of Health for their support.
REFERENCES
- Black LW. DNA packaging in dsDNA bacteriophages. Annu Rev Microbiol. 1989;43:267–92. doi: 10.1146/annurev.mi.43.100189.001411. [DOI] [PubMed] [Google Scholar]
- Brown JC, McVoy MA, Homa FL. In: Packaging DNA into Herpesvirus Capsids. In Structure-Function Relationships of Human Pathogenic Viruses. Bogner E, Holzenburg A, editors. Kluwer Academic/Plenum Publishers; London: 2002. [Google Scholar]
- Catalano CE. The terminase enzyme from bacteriophage lambda: a DNA-packaging machine. Cell Mol Life Sci. 2000;57:128–48. doi: 10.1007/s000180050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, McGregor A, Schleiss MR, McVoy MA. Cloning the complete guinea pig cytomegalovirus genome as an infectious bacterial artificial chromosome with excisable origin of replication. J Virol Methods. 2008;149:231–9. doi: 10.1016/j.jviromet.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ. Channel catfish virus: a new type of herpesvirus. Virology. 1992;186:9–14. doi: 10.1016/0042-6822(92)90056-u. [DOI] [PubMed] [Google Scholar]
- Feiss M, Catalano CE. Bacteriophage lambda terminase and the mechanisms of viral DNA packaging. In: Catalano CE, editor. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. Landes Biosciences; Georgetown, TX: 2005. pp. 5–39. [Google Scholar]
- Hahn G, Jarosch M, Wang JB, Berbes C, McVoy MA. Tn7-mediated introduction of DNA sequences into bacmid-cloned cytomegalovirus genomes for rapid recombinant virus construction. J Virol Methods. 2003;107:185–94. doi: 10.1016/s0166-0934(02)00232-x. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Bogner E. ATPase activity of the terminase subunit pUL56 of human cytomegalovirus. J Biol Chem. 2002;277:6943–8. doi: 10.1074/jbc.M108984200. [DOI] [PubMed] [Google Scholar]
- Hwang JS, Kregler O, Schilf R, Bannert N, Drach JC, Townsend LB, Bogner E. Identification of acetylated, tetrahalogenated benzimidazole D-ribonucleosides with enhanced activity against human cytomegalovirus. J Virol. 2007;81:11604–11. doi: 10.1128/JVI.01130-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosky PM, Ptak RG, Underwood MR, Biron KK, Townsend LB, Drach JC. Differences in DNA packaging genes and sensitivity to benzimidazole ribonucleosides between human cytomegalovirus strains AD169 and Towne. Antivir Chem Chemother. 2000;11:349–52. doi: 10.1177/095632020001100506. [DOI] [PubMed] [Google Scholar]
- Luckow VA, Lee SC, Barry GF, Olins PO. Efficient generation of infectious recombinant baculoviruses by site- specific transposon-mediated insertion of foreign genes into a baculovirus genome propagated in Escherichia coli. J Virol. 1993;67:4566–79. doi: 10.1128/jvi.67.8.4566-4579.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch DJ, Rixon FJ, Davison AJ. Topics in herpesvirus genomics and evolution. Virus Res. 2006;117:90–104. doi: 10.1016/j.virusres.2006.01.002. [DOI] [PubMed] [Google Scholar]
- McVoy MA, Nixon DE, Adler SP, Mocarski ES. Sequences within the herpesvirus-conserved pac1 and pac2 motifs are required for cleavage and packaging of the murine cytomegalovirus genome. J Virol. 1998;72:48–56. doi: 10.1128/jvi.72.1.48-56.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MS, Matsuzaki S, Imai S, Rao VB. Sequence analysis of bacteriophage T4 DNA packaging/terminase genes 16 and 17 reveals a common ATPase center in the large subunit of viral terminases. Nucleic Acids Res. 2002;30:4009–21. doi: 10.1093/nar/gkf524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przech AJ, Yu D, Weller SK. Point mutations in exon I of the herpes simplex virus putative terminase subunit, UL15, indicate that the most conserved residues are essential for cleavage and packaging. J Virol. 2003;77:9613–21. doi: 10.1128/JVI.77.17.9613-9621.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao VB, Black LW. DNA Packaing in Bacteriophage T4. In: Catalano CE, editor. Viral Genome Packaging Machines: Genetics, Structure, and Mechanism. Landes Biosciences; Gerogetown, TX: 2005. pp. 40–58. [Google Scholar]
- Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, Haerter M, Buerger I, Trappe J, Herrington JA, Haebich D, Ruebsamen-Waigmann H. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38-4766): in vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother. 2001;48:757–67. doi: 10.1093/jac/48.6.757. [DOI] [PubMed] [Google Scholar]
- Scholz B, Rechter S, Drach JC, Townsend LB, Bogner E. Identification of the ATP-binding site in the terminase subunit pUL56 of human cytomegalovirus. Nucleic Acids Res. 2003;31:1426–33. doi: 10.1093/nar/gkg229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MR, Ferris RG, Selleseth DW, Davis MG, Drach JC, Townsend LB, Biron KK, Boyd FL. Mechanism of action of the ribopyranoside benzimidazole GW275175X against human cytomegalovirus. Antimicrob Agents Chemother. 2004;48:1647–51. doi: 10.1128/AAC.48.5.1647-1651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood MR, Harvey RJ, Stanat SC, Hemphill ML, Miller T, Drach JC, Townsend LB, Biron KK. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72:717–25. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl M, Fairhurst J, Jones TR, Vernon SK, Morin J, LaRocque J, Feld B, O’Hara B, Bloom JD, Johann SV. Novel class of thiourea compounds that inhibit herpes simplex virus type 1 DNA cleavage and encapsidation: resistance maps to the UL6 gene. J Virol. 2000;74:9054–61. doi: 10.1128/jvi.74.19.9054-9061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnum SM, Streblow DN, Monroe ME, Smith P, Auberry KJ, Pasa-Tolic L, Wang D, Camp DG, 2nd, Rodland K, Wiley S, Britt W, Shenk T, Smith RD, Nelson JA. Identification of Proteins in Human Cytomegalovirus (HCMV) Particles: the HCMV Proteome. J Virol. 2004;78:10960–6. doi: 10.1128/JVI.78.20.10960-10966.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JE, Saraste M, Runswick MJ, Gay NJ. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. Embo J. 1982;1:945–51. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Nixon DE, McVoy MA. Definition of the minimal cis-acting sequences necessary for genome maturation of the herpesvirus murine cytomegalovirus. J Virol. 2008;82:2394–404. doi: 10.1128/JVI.00063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ellis HM, Lee EC, Jenkins NA, Copeland NG, Court DL. An efficient recombination system for chromosome engineering in Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:5978–83. doi: 10.1073/pnas.100127597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Weller SK. Genetic analysis of the UL 15 gene locus for the putative terminase of herpes simplex virus type 1. Virology. 1998;243:32–44. doi: 10.1006/viro.1998.9041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.