Abstract
Importance
Adult survivors of childhood cancer are known to be at risk for treatment-related adverse health outcomes. A large population of survivors has not been evaluated using a comprehensive systematic clinical assessment to determine the prevalence of chronic health conditions.
Objective
Following systematic exposure-based medical assessments of a large cohort of adult survivors of childhood cancer, determine the prevalence of adverse health outcomes and the proportion associated with treatment-related exposures.
Design, Setting, and Participants
Presence of health outcomes was ascertained among 1713 adult (median age 32 years, range 18-60) survivors of childhood cancer (median time from diagnosis 25 years, range 10-47) enrolled in the St. Jude Lifetime Cohort Study since 10/1/2007 and followed through 10/31/2012.
Main Outcome Measures
Age-specific cumulative prevalence of adverse outcomes by organ system and sex-adjusted attributable fraction percentages with 95% confidence intervals were calculated.
Results
Using clinical criteria, the crude prevalence of adverse health outcomes was highest for pulmonary [65.2%(95% CI, 60.4-69.8%)], auditory [62.1%(95% CI, 55.8-68.2%)], endocrine-reproductive [62.0%(95% CI, 59.5-64.6%)], cardiac [56.4(95% CI, 53.5-59.2%)] and neurocognitive [48.0%(95%CI, 44.9-51.0%)] function, whereas abnormalities impacting hepatic [13.0%(95% CI, 10.8-15.3%)], skeletal [9.6%(95% CI, 8.0-11.5%)], renal [5.0%(95% CI, 4.0-6.3%)] and hematopoietic [3.0%(95% CI: 2.1-3.9%)] function were less common. Attributable fractions were highest for endocrine-reproductive disorders [88.4%(95% CI, 80.1-93.3%)] to 100%, but considerably lower for conditions highly prevalent in the general population such as hypertension [9.3%(95%CI, −16.3-29.2%)], dyslipidemia [15.5%(95% CI, 10.2-20.5%)], and obesity [42.1%(95% CI, 34.4-48.9%)]. Among survivors at risk for adverse outcomes following specific cancer treatment modalities, the estimated cumulative prevalence at 50 years of age was 21.6%(95% CI, 19.3-23.9%) for cardiomyopathy, 83.5%(95% CI, 80.2-86.8%) for heart valve disorder, 76.8%(95% CI, 73.6-80.0%) for pituitary dysfunction, 81.3%(95% CI, 77.6-85.0%) for pulmonary dysfunction, 86.5%(95% CI, 82.3-90.7%) for hearing loss, 40.9%(95% CI, 32.0-49.8%) for breast cancer, 31.1%(95% CI, 27.3-34.9%) for Leydig cell failure, and 31.9%(95% CI, 28.0-35.8%) for primary ovarian failure. At age 45 years, the estimated cumulative prevalence of any chronic health condition is 95.2% (95% CI 94.8-98.6%) and 80% (95% CI 73.0-86.6%) for a serious, life-threatening or disabling chronic condition.
Conclusion and Relevance
Systematic risk-based medical assessments of adults treated for childhood cancer identified a substantial number of previously undiagnosed problems that are typically prevalent in an older population underscoring the need for ongoing health monitoring and intervention of this population.
Keywords: Childhood cancer, late effects, long-term follow-up, health screening
Introduction
Curative therapy for pediatric malignancies has produced a growing population of adults formerly treated for childhood cancer who are at risk for health problems1-3 that appear to increase with aging.2-5 The prevalence of cancer-related toxicities that are systematically ascertained through formal clinical assessments has not been well studied. Ongoing clinical evaluation of well-characterized cohorts is critical to advance knowledge about the influence of aging on cancer-related morbidity and mortality, and to guide the development of health screening recommendations and health preserving interventions. The objective of this investigation was to determine, through systematic comprehensive medical assessment, the general health status of long-term survivors of childhood cancer and prevalence of treatment complications following predisposing cancer treatment-related exposures.
Methods
Participants
Following provision of written informed consent, eligible survivors were enrolled in the ongoing IRB-approved St. Jude Lifetime Cohort Study (SJLIFE) using recruitment strategies described previously.6,7 The objective of the SJLIFE study is to establish a lifetime cohort of survivors treated at St. Jude Children’s Research Hospital (SJCRH) to facilitate prospective periodic medical assessment of health outcomes among adults surviving pediatric malignancies. Eligibility for SJLIFE includes attained age of 18 years or older, treatment for cancer at SJCRH, and survival 10 or more years post diagnosis. The order of recruitment of eligible survivors was randomly determined by allocating subjects to blocks of size 50. This study included participants who were within the first 59 consecutive recruitment blocks (Supplemental Figure 1). Through the 59th recruitment block, 2888 survivors were potentially eligible. Of 2843 confirmed eligible, 1837 (64.6%) enrolled in the study. This analysis included 1713 participants (60.3% of eligible) diagnosed and treated between 1962 and 2001, enrolled on study since 10/01/2007, and followed until 10/31/2012, who had completed on-campus medical evaluations. Non-participants included 680 who actively or passively elected not to participate, 277 who expressed interest in participating but had not completed their campus visit, 124 who completed questionnaires but did not receive on-campus medical assessment, and 49 who were lost to follow-up.
Medical record abstraction documented the type and cumulative doses of treatment, information on surgical interventions, acute life-threatening organ toxicities, primary cancer recurrences, chronic health conditions, and subsequent neoplasms. Race and ethnicity were self-reported by participants and ascertained for non-participants by administrative record review of race/ethnicity reported by parents at diagnosis. Participants completed comprehensive health questionnaires prior to their clinical assessment. All participants underwent a core battery of evaluations comprised of history and physical examination with resting heart rate, blood pressure, 12-lead electrocardiography, and laboratory studies including complete blood count/differential, comprehensive metabolic panel, fasting lipid profile, insulin and hemoglobin A1C, assessments of thyroid and gonadal function, urinalysis, and a comprehensive physical performance assessment including measurement of body composition and neuromuscular system integrity. Participation also involved a clinical evaluation consistent with the risk-based screening and surveillance recommended by the Children’s Oncology Group (COG Guidelines).8 The risk-based portion of the assessment included additional laboratory tests and evaluations of organ function (e.g., echocardiography, pulmonary function testing, audiological testing, ophthalmology evaluation, neurocognitive testing, bone mineral density testing).
Screening for organ dysfunction
Medical assessments were completed according to the COG Guidelines considering history of transfusion, exposure to specific chemotherapeutic agents or radiation impacting target organs and tissues, hematopoietic cell transplantation, and graft versus host disease. Supplemental Table 1 summarizes the number of survivors at risk for various outcomes based on exposure to specific therapeutic modalities, the screening test(s) for specific exposures, and criteria for positive screening by organ system. Precise criteria for positive screening outcomes are provided in Supplemental Table 2.
Screening for subsequent adult neoplasms (SNs)
Survivors treated with radiation were considered at risk for solid SNs. With the exception of colonoscopy in survivors treated with abdominal and/or pelvic radiation and breast imaging in young women treated with chest radiation, risk-based screening for solid SNs involved history and physical examination. The complete blood count was used to assess for myelodysplasia and hematological SNs in survivors treated with alkylating agents, anthracyclines, and epipodophyllotoxins.
Validation of and classification of medical events
Medical records were routinely obtained to validate selected medical conditions diagnosed before the SJLIFE evaluation, including all SNs, all major cardiovascular events, and other severe/chronic organ dysfunction. Medical records were also obtained after SJLIFE participation to confirm diagnoses of conditions identified or suspected from the preliminary results of screening evaluations. Chronic health conditions were classified using Common Terminology Criteria for Adverse Events (CTCAE, version 4.0, National Cancer Institute) as mild (grade 1), moderate (grade 2), severe (grade 3), or life-threatening/disabling (grade 4).9
Statistical analysis
T-tests, chi-squared statistics and Fisher exact tests were used to compare participants to non-participants. Percentages of those with adverse organ system outcomes were calculated by exposure status and by whether the diagnosis occurred prior to, at or after the SJLIFE visit for specific risk (exposure) categories, for any treatment-related risk, for no cancer treatment-related risk, and overall. Age-and sex-attributable fractions (AF), reported as percentages with 95% confidence intervals (CI), were calculated for adverse outcomes included in the core assessment battery.10 These compare survivors who were exposed to those non-exposed within treatment categories, with treatment exposure preceding the health condition under consideration. A priori levels of significance were 2-tailed (p < .05). Kaplan Meier methodology was used to estimate the age-specific prevalence of adverse outcomes.11 SAS version 9.2 (Cary, N.C.) was used for all analysis.
Results
Participant characteristics
Table 1 provides demographic characteristics of study participants and compares characteristics of survivors who completed a campus visit to non-participants presumed to be eligible. Survivors who did not complete campus evaluations were more likely to be male and older, have a longer elapsed time from diagnosis, and were somewhat less likely to have received radiation and selected treatment exposures than those who completed the clinical evaluation. Supplemental Table 3 summarizes selected chemotherapy and radiation dose distributions of participants.
Table 1. Demographic, Treatment Exposures, and Diagnostic Characteristics of SJLIFE Campus Visit Participants (n=1713) and Non-Participants (n=1130).
| Characteristic | Total (n=2843) |
Participants (n=1713) |
Non- Participants (n=1130) |
P-value |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Sex | <.001 | |||
| Female | 1365 (48.0) | 880 (51.4) | 485 (42.9) | |
| Male | 1478 (52.0) | 833 (48.6) | 645 (57.1) | |
| Race | .27 | |||
| White | 2456 (86.4) | 1493 (87.2) | 963 (85.2) | |
| Black | 360 (12.7) | 203 (11.8) | 157 (13.9) | |
| Other | 27 (0.9) | 17 (1.0) | 10 (0.9) | |
| Hispanic Ethnicity | .80 | |||
| Yes | 31 (1.1) | 18 (1.1) | 13 (1.1) | |
| No | 2812 (98.9) | 1695 (99.0) | 1117 (98.9) | |
| Primary Diagnosis | ||||
| Leukemia | ||||
| Acute lymphoblastic leukemia | 1204 (42.3) | 765 (44.7) | 439 (38.9) | |
| Acute myeloid leukemia | 77 (2.7) | 38 (2.2) | 39 (3.5) | |
| Other leukemia | 9 (0.3) | 6 (0.4) | 3 (0.3) | |
| Lymphoma | ||||
| Hodgkin lymphoma | 328 (11.5) | 218 (12.7) | 110 (9.7) | |
| Non-Hodgkin lymphoma | 155 (5.5) | 78 (4.6) | 77 (6.8) | |
| CNS Tumors | ||||
| Astrocytoma/Glioma | 127 (4.5) | 67 (3.9) | 60 (5.3) | |
| Medulloblastoma and PNET | 54 (1.9) | 38 (2.2) | 16 (1.4) | |
| Ependymoma | 19 (0.7) | 15 (0.9) | 4 (0.4) | |
| Other | 41 (1.4) | 21 (1.2) | 20 (1.8) | |
| Sarcoma | ||||
| Ewing sarcoma family of tumors |
87 (3.1) | 58 (3.4) | 29 (2.6) | |
| Osteosarcoma | 119 (4.2) | 71 (4.1) | 48 (4.3) | |
| Rhabdomyosarcoma (RMS) | 84 (3.0) | 47 (2.7) | 37 (3.3) | |
| Non-RMS | 46 (1.6) | 17 (1.0) | 29 (2.6) | |
| Embryonal tumors | ||||
| Germ cell tumor | 44 (1.5) | 20 (1.2) | 24 (2.1) | |
| Neuroblastoma | 131 (4.6) | 64 (3.7) | 67 (5.9) | |
| Wilms tumor | 160 (5.6) | 94 (5.5) | 66 (5.8) | |
| Other | ||||
| Hepatoblastoma | 8 (0.3) | 4 (0.2) | 4 (0.4) | |
| Melanoma | 5 (0.2) | 4 (0.2) | 1 (0.1) | |
| Retinoblastoma | 109 (3.8) | 66 (3.9) | 43 (3.8) | |
| Carcinomas | 27 (0.9) | 16 (0.9) | 11 (1.0) | |
| Other neoplasms | 9 (0.3) | 6 (0.4) | 3 (0.3) | |
| Age at Diagnosis (Year) | .49 | |||
| Mean (SD) | 7.5 (5.5) | 7.5 (5.5) | 7.4 (5.4) | |
| Median | 6.0 | 6.0 | 6.0 | |
| Range | 0.0-28.0 | 0.0-24.0 | 0.0-28.0 | |
| <1 | 173 (6.1) | 95 (5.6) | 78 (6.9) | |
| 1-4 | 958 (33.7) | 591 (34.5) | 367 (32.5) | |
| 5-9 | 699 (24.6) | 411 (24.0) | 288 (25.5) | |
| 10-14 | 597 (21.0) | 359 (21.0) | 238 (21.1) | |
| 15-19 | 394 (13.9) | 245 (14.3) | 149 (13.2) | |
| 20-24 | 22 (0.8) | 12 (0.7) | 10 (0.9) | |
| Years from Diagnosis | <.001 | |||
| Mean (SD) | 26.3 (7.8) | 25.6 (7.6) | 27.4 (7.9) | |
| Median | 25.8 | 25.1 | 27.2 | |
| Range | 10.9-48.3 | 10.9-47.9 | 11.9-48.3 | |
| 10-19 | 665 (23.4) | 434 (25.3) | 231 (20.4) | |
| 20-29 | 1276 (44.9) | 789 (46.1) | 487 (43.1) | |
| 30-39 | 761 (26.8) | 433 (25.3) | 328 (29.0) | |
| 40-49 | 141 (5.0) | 57 (3.3) | 84 (7.4) | |
| Treatment Exposure | ||||
| Radiation | 1742 (61.3) | 1108 (64.7) | 634 (56.1) | <.001 |
| Anthracyclines | 1630 (57.3) | 1001 (58.4) | 629 (55.6) | .14 |
| Alkylating Agents | 1723 (60.6) | 1068 (62.4) | 655 (57.9) | .02 |
| Platinum | 260 (9.1) | 152 (8.9) | 108 (9.6) | .54 |
| Glucocorticoids | 1513 (53.2) | 964 (56.3) | 549 (48.6) | <.001 |
| Epipodophyllotoxins | 1110 (39.0) | 694 (40.5) | 416 (36.8) | .05 |
| Antimetabolites | 1609 (56.6) | 994 (58.0) | 615 (54.4) | .06 |
| Age at Recruitment | <.001 | |||
| Mean (SD) | 33.8 (8.2) | 33.1 (8.1) | 34.9 (8.4) | |
| Median | 33.3 | 32.0 | 34.0 | |
| Range | 18.0-66.0 | 18.0-60.0 | 22.0-66.0 | |
| 18-24 | 397 (14.0) | 279 (16.3) | 118 (10.4) | |
| 25-29 | 563 (19.8) | 348 (20.3) | 215 (19.0) | |
| 30-34 | 657 (23.1) | 390 (22.8) | 267 (23.6) | |
| 35-39 | 521 (18.3) | 314 (18.3) | 207 (18.3) | |
| 40-44 | 380 (13.4) | 221 (12.9) | 159 (14.1) | |
| 45-49 | 211 (7.4) | 108 (6.3) | 103 (9.1) | |
| 50-66 | 114 (4.0) | 53 (3.1) | 61 (5.4) | |
| Duration of Follow-up (Years) | ||||
| Before SJLIFE Visit | ||||
| Mean (SD) | 25.6 (7.6) | |||
| Median (IQR) | 25.1 (19.9-31.2) | |||
| After SJLIFE Visit | ||||
| Mean (SD) | 2.8 (0.9) | |||
| Median (IQR) | 2.8 (2.1-3.5) |
P-values from Chi-squared test comparing participants to non-participants SD – standard deviation; IQR – interquartile range
The distribution of cancer diagnoses among cancer survivors diagnosed before age 20 years in the US is estimated to be 18.3% leukemia, 18.7% lymphoma, 14.6% CNS tumors, 11.8% sarcoma, 16.6% embryonal tumors, and 8.2% other diagnoses [Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973-2009), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2012, based on the November 2011 submission].
Risk-based medical assessments
Table 2 summarizes the prevalence of selected treatment-related toxicities detected by risk-based screening associated with specific treatments. The overall prevalence of a given late effect represents the sum total of cases with the condition diagnosed before the SJLIFE evaluation, directly as a result of the SJLIFE evaluation, and after but unrelated to the SJLIFE evaluation.
Table 2a. Prevalence of Cardiovascular and Pulmonary Late Effects in At-Risk Populations Following Exposure-Based Screening.
| Diagnosis before SJLIFE |
Diagnosis related to SJLIFE |
Diagnosis after SJLIFE |
Overall Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential Late Effect |
Screening test | Exposure Status | Number at riska |
N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI |
| Cardiovascular | |||||||||||||||
| Cardiomyopathy | Echocardiogram | Anthracyclines Anthraquinones Radiation to heart |
1214 | 32 | (2.6) | [1.8-3.7] | 38 | (3.1) | [2.2-4.3] | 6 | (0.5) | [0.2-1.1] | 76b | (6.2) | [5.0-7.8] |
| Heart valve disorder |
Echocardiogram | Radiation to heart | 501 | 31 | (6.2) | [4.2-8.7] | 235 | (46.9) | [42.5-51.4] | 18 | (3.6) | [2.1-5.6] | 284c | (56.7) | [52.2-61.1] |
| Conduction disorder |
Electrocardiogram | Anthracyclines Anthraquinones Radiation to heart |
1214 | 13 | (1.1) | [0.6-1.8] | 154 | (12.7) | [10.9-14.7] | 2 | (0.2) | [0.0-0.6] | 169d | (14.0) | [12.0 – 16.0] |
| Any cardiac condition |
As indicated above | Any cancer treatment-related risk |
1214 | 64 | (5.3) | [4.1-6.7] | 564 | (46.5) | [43.6-49.3] | 56 | (4.6) | [3.5-5.9] | 684 | (56.4) | [53.5-59.2] |
| Cardiovascular Risk Factors | |||||||||||||||
| Hypertension | Blood pressure | Ifosfamide Cisplatin/Carboplatin Methotrexate Radiation to kidney Nephrectomy Radiation to hypothalamus- Pituitary axis |
1508 | 232 | (15.4) | [13.6-17.3] | 94 | (6.2) | [5.1-7.6] | 16 | (1.1) | [0.6-1.7] | 342e | (22.7) | [20.6-24.9] |
| Dyslipidemia | Fasting lipid panel | Cisplatin/Carbopla tin Radiation to hypothalamus- pituitary |
807 | 186 | (23.0) | [20.2-26.1] | 256 | (31.7) | [28.5-35.1] | 49 | (6.1) | [4.5-7.9] | 491f | (60.8) | [57.4-64.2] |
| Obesity | Body mass index (BMI) |
Radiation to hypothalamus- pituitary |
714 | 158 | (22.1) | [19.1-25.4] | 187 | (26.2) | [23.0-29.6] | 0 | (0.0) | 345g | (48.3) | [44.6-52.1] | |
| Pulmonary | |||||||||||||||
| Abnormal pulmonary function |
Pulmonary function tests |
Busulfan Carmustine/Lomu stine Bleomycin Radiation to lungs Thoracotomy |
417 | 121 | (29.0) | [24.7-33.6] | 149 | (35.7) | [31.1-40.5] | 2 | (0.5) | [0.1-1.7] | 272h | (65.2) | [60.4-69.8] |
At risk by treatment exposure as defined in the COG Guidelines, see supplemental Table 1 for detailed exposures and potential late effects evaluated by risk-based screening.
60.5% were CTCAE v.4 Grade 3-4
9.9% were CTCAE v.4 Grade 3-4
2.4% were CTCAE v.4 Grade 3-4
0.6% were CTCAE v.4 Grade 3-4
None were CTCAE v.4 Grade 3-4
100% were CTCAE v.4 Grade 3-4
21.0% were CTCAE v.4 Grade 3-4
CTCAE v.4 percentages includes only those who fulfill criteria for “at risk” as defined by COG Guidelines.
Prevalence of and severity of organ dysfunction
Impaired pulmonary, cardiac, endocrine and nervous system function were most prevalent (detected in 20% or more of those at risk). Among survivors exposed to pulmonary toxic cancer treatments, 65.2% (95% CI, 60.4-69.8%) had abnormal pulmonary function, with 35.7% (95% CI, 31.1-40.5%) identified during the SJLIFE evaluation. The highest prevalence occurred among those treated with lung radiation (74.4%, 95% CI, 69.1-79.2%) Supplemental Table 1), followed by those treated with bleomycin (73.3%, 95% CI, 61.9-82.9) and thoracotomy (53.2%, 95% CI, 44.1-62.0%). Among survivors exposed to cardiotoxic therapies, 56.4% (95% CI, 53.5-59.2%) had cardiac abnormalities, with 46.5% (95% CI, 43.6-49.3%) newly discovered as a result of the SJLIFE evaluation. Heart valve abnormalities, most frequently mild to moderate tricuspid and/or mitral valve regurgitation, were diagnosed in 56.7% (95% CI, 52.2-61.1%) of survivors exposed to cardiac-directed radiation. The prevalence of systolic dysfunction among survivors exposed to anthracyclines and/or cardiac-directed radiation therapy was 6.2% (95% CI, 5.0-7.8%). Sixty-two percent (62.0%, 95% CI, 59.5-64.5%) of survivors developed endocrine disorders. Hypothalamic-pituitary axis (HPA) or thyroid dysfunction was established before SJLIFE participation in more than 90%. The prevalence of disorders affecting the HPA, thyroid, and male gonadal function and female gonadal function was 61.0% (95% CI, 57.3-64.7), 13.8% (95% CI, 11.6-16.1%), 66.4% (95% CI, 61.1-71.6%) and 11.8% (95% CI, 9.2-14.7%), respectively, for those exposed to radiation impacting these organs and/or alkylating agents. Nervous system abnormalities included a spectrum of neurosensory, neurocognitive, and neurologic deficits. The most common adverse neurosensory outcome was hearing loss, prevalent among 62.1% (95% CI, 55.8-68.2%) of survivors exposed to platinum agents or ear irradiation. Cataracts were detected in 20.6% (95% CI, 18.3-23.1%) of the population exposed to eye radiation, glucocorticoids and/or busulfan; 28.5% (95% CI, 23.1-33.9%) of persons with cataracts and glucocorticoid exposure had not received eye irradiation. The prevalence of any neurocognitive impairment among survivors exposed to central nervous system treatment was 48.0% (95% CI, 44.9-51.0%). The most frequent deficits were in mathematics (29.2%, 95% CI, 25.6-32.8%), memory (25.4%, 95% CI, 21.9-28.9%) and processing speed (24.4%, 95% CI, 21.0-27.8%). Peripheral neuropathy was identified in 21.9% (95% CI, 19.8-24.2%) of survivors treated with vinca alkaloid or platinum chemotherapy.
In contrast, the prevalence of hematopoietic, hepatic, skeletal, and urinary tract dysfunction below < 20% (Table 2 and Supplemental Table 1). The prevalence of a positive hepatopathy screen was 13.0% (95% CI, 10.8-15.3%) among at-risk survivors treated with antimetabolite chemotherapy or liver irradiation. Hepatitis C was the most common transfusion-acquired infection, affecting 6.8% (95% CI, 5.5-8.2%) of those at risk. Risk-based screening identified 1.0% (95% CI, 0.5-1.6%) of hepatitis C cases not previously diagnosed. Assessment of skeletal toxicity was limited to bone mineral density testing; osteoporosis was identified in only 9.6% (95% CI, 8.0-11.5%) of those treated with radiation to the hypothalamic-pituitary axis, glucocorticoids and/or methotrexate. The overall prevalence of kidney dysfunction was 5.0% (95% CI, 4.0-6.3%), divided equally between those with a previously established diagnosis of chronic kidney disease and those presenting with occult kidney dysfunction identified by the SJLIFE laboratory evaluation. Abnormalities of blood counts were detected in only 3.0% (95% CI, 2.1-3.9%) of survivors at risk for myelodysplasia/secondary leukemia following treatment with alkylating agents, anthracycline or epipodophyllotoxin chemotherapy.
Based upon this clinically-evaluated cohort, 98.2% (95% CI, 97.5-98.8%) of participants had a chronic health condition. Distributions of chronic health conditions by CTCAE v.4 grades are provided in Supplemental Table 4. A serious, life-threatening, or disabling chronic health condition (CTCAE v.4 Grade 3-4) occurred in 67.6% (95% CI, 65.3-69.8%) of survivors. The overall cumulative prevalence of a chronic condition is estimated to be 95.5% (95% CI, 94.8-98.6%) by age 45 years and 93.5% (95% CI, 86.7-97.3) 35 years after cancer diagnosis. The cumulative prevalence of a Grade 3-4 chronic condition is estimated to be 80.5% (95% CI, 73.0-86.6%) and 75.1% (95% CI, 68.0-80.9%) at 45 years of age and 35 years after cancer, respectively.
Percentage of adverse outcomes associated with treatment exposure
For conditions detected by comprehensive screening with the core battery of evaluations, Table 3 summarizes the prevalence of chronic health conditions by exposure to specific high-risk treatment as defined by the COG Guidelines, and the fraction attributable (AF) to the exposure. Cancer treatment was associated with a high proportion (88.4% to 100%) of cases of endocrinopathy, although the AF associated with diabetes mellitus was lower [41.7% (95% CI, 12.2-61.3%)]. Risk factors for cardiovascular disease (e.g., hypertension, dyslipidemia, obesity) were highly prevalent among both exposed and unexposed survivor groups and, as such, had a smaller proportion of cases associated with cancer treatment. Other conditions with a high percentage of cases associated with cancer treatment included kidney dysfunction [AF 65.7% (CI, 21.7-85.0%)] and cardiac ischemia [AF 57.1% (CI, 36.4-71.0%)]. In contrast, the prevalence of arrhythmia or conduction disorders was not associated with cardiotoxic treatment exposures in survivors.
Table 2b. Prevalence of Endocrine/Reproductive Late Effects in At-Risk Populations Following Exposure-Based Screening.
| Diagnosis before SJLIFE |
Diagnosis related to SJLIFE |
Diagnosis after SJLIFE |
Overall Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential Late Effect |
Screening test | Exposure Status | Number at riska |
N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI |
| Endocrine/ Reproductive | |||||||||||||||
| Hypothalamic- pituitary axis (HPA) disorders (one or more) |
Screening for HPA deficiencies: Growth & pubertal progress, Menstrual history, Insulin growth factor-1 (IGF-1), 8 am serum cortisol, Luteinizing hormone (LH), Follicle stimulating hormone (FSH), Estradiol or AM testosterone, Thyroid stimulating hormone (TSH), Free T4 |
Radiation to hypothalamus- pituitary (dose >=18 Gy) |
685 | 355 | (51.8) | [48.0-55.6] | 57 | (8.3) | [6.4-10.6] | 6 | (0.9) | [0.3-1.9] | 418 | (61.0) | [57.3-64.7] |
| Diabetes mellitus |
Fasting serum glucose |
Radiation to hypothalamus- pituitary |
714 | 35 | (4.9) | [3.4-6.8] | 13 | (1.8) | [1.0-3.1] | 8 | (1.1) | [0.5-2.2] | 56f | (7.8) | [6.0-10.1] |
| Primary hypothyroidismb |
TSH | Radiation to neck | 910 | 117 | (12.9) | [10.8-15.2] | 7 | (0.8) | [0.3-1.6] | 1 | (0.1) | [0.0-0.6] | 125g | (13.8) | [11.6-16.1] |
| Primary ovarian failure |
Menstrual history, FSH, Estradiol |
Alkylating agents Radiation to female reproductive system |
553 | 44 | (8.0) | [5.8-10.5] | 20 | (3.6) | [2.2-5.5] | 1 | (0.2) | [0.0-1.0] | 65h | (11.8) | [9.2-14.7] |
| Male germ cell dysfunctiond,e |
Semen sample analysis |
Alkylating agents Radiation to male reproductive system |
328 | 9 | (2.7) | [1.3-5.1] | 209 | (63.7) | [58.3-68.9] | 0 | (0.0) | 218i | (66.4) | [61.1-71.6] | |
| Leydig cell failured |
Morning testosterone, LH |
Alkylating agents Radiation to male reproductive system |
574 | 25 | (4.4) | [2.8-6.4] | 37 | (6.4) | [4.6-8.8] | 4 | (0.7) | [0.2-1.8] | 66j | (11.5) | [9.0-14.4] |
| Any endocrine condition |
As indicated above |
As indicated above | 1423 | 531 | (37.3) | [34.8-39.9] | 332 | (23.3) | [21.2-25.6] | 20 | (1.4) | [0.9-2.2] | 883 | (62.0) | [59.5-64.6] |
At risk by treatment exposure as defined in the COG Guidelines, see supplemental Table 1 for detailed exposures and potential late effects evaluated by risk-based screening.
Results presented for evaluation of central and primary hypothyroidism and thyroid nodules exclude 39 patients with prior thyroidectomy.
Results presented for evaluation of hypogonadotropic hypogonadism and primary ovarian failure exclude 50 women with bilateral oophorectomy.
Results presented for evaluation of Leydig cell failure exclude 1 man with bilateral orchiectomy.
Results presented for evaluation of germ cell dysfunction exclude 246 at risk patients who declined semen analysis due to history of established fertility (81), infertility (43), inability to provide a sample (18) or personal reasons (107).
32.0% were CTCAE v.4 Grade 3-4
None were CTCAE v.4 Grade 3-4
None were CTCAE v.4 Grade 3-4
97.7% were CTCAE v.4 Grade 3-4
None were CTCAE v.4 Grade 3-4
CTCAE v.4 percentages includes only those who fulfill criteria for “at risk” as defined by COG Guidelines.
Cumulative prevalence of chronic health conditions
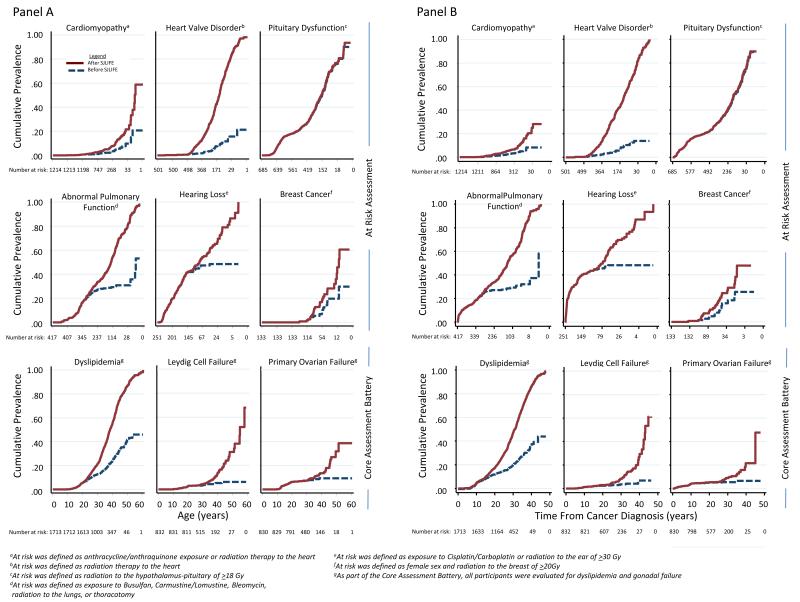
Figure 1 shows the age-specific and time from cancer prevalence of chronic health conditions for certain organ specific outcomes. The estimated prevalence of specific conditions was substantially higher following risk-based screening, highlighting the subclinical nature of many outcomes. For example, the estimated prevalence of a heart valve disorder among those age 40 years treated with chest radiation increased from 5.7% (95% CI, 3.5-7.9%) to 37.2% (95% CI, 33.0-41.4% after echocardiography screening. In contrast, risk-based screening had little influence on the estimated prevalence for pituitary disorders; diagnoses of most of these conditions were established before SJLIFE participation.
Figure 1.
Cumulative prevalence of chronic health conditions for representative group of organ-specific outcomes according to age (Panel A) and time from cancer diagnosis (Panel B). Dashed blue line reflects cumulative prevalence based on proportion diagnosed with condition before participation in SJLIFE. Solid red line reflects cumulative prevalence based on proportion diagnosed with condition following the SJLIFE medical assessment and followed until 10/31/2012.
Prevalence of subsequent neoplasms
A total of 272 survivors developed one or more SNs including 335 solid and 13 hematological SN (Table 4). For SNs identified directly as a result of the SJLIFE evaluation, abnormalities on physical examination (n=17), laboratory testing (n=2) and imaging (n=13) facilitated detection of 32 of 44 cases. Suspicious skin lesions were the most common physical finding leading to diagnosis of SN, followed by palpable masses, and abnormal mental status. Detection of hematuria on urinalysis among survivors treated with nephrotoxic chemotherapy resulted in diagnosis of 2 cases of renal cell carcinoma. Breast cancers diagnosed in 13 women resulted from follow-up of imaging abnormalities; none of the lesions was palpable on exam. In addition, 12 survivors had SNs identified as incidental findings on risk-based screening (e.g., renal cell mass detected on bone density testing) or imaging performed in the context of other research studies (e.g., meningiomas detected on brain MRI).
Table 2c. Prevalence of Neurocognitive and Neurosensory Late Effects in At-Risk Populations Following Exposure-Based: Screening.
| Diagnosis before SJLIFE |
Diagnosis related to SJLIFE |
Diagnosis after SJLIFE |
Overall Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential Late Effect |
Screening test | Exposure Status | Numb er at riska |
N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI |
| Neurocognitive | |||||||||||||||
| Neurocognitive impairment |
Neuropsychologi cal testing |
Antimetabolite therapy Cranial irradiation Neurosurgery |
1062 | 90 | (8.5) | [6.9-10.3] | 415 | (39.1) | [36.1-42.1] | 4 | (0.4) | [0.1-1] | 509b | (48.0 | [44.9-51.0] |
| Neurosensory | |||||||||||||||
| Ocular toxicity | Ophthalmology consultation |
Busulfan Corticosteroids Radiation to eye |
1127 | 120 | (10.6) | [8.9-12.6] | 183 | (16.2) | [14.1-18.5] | 9 | (0.8) | [0.4-1.5] | 312c | (27.6) | [25.1-30.4] |
| Hearing loss | Otoscopy, tympanometry and conventional pure-tone audiometry |
Cisplatin/Carboplatin Radiation to ear (dose >30Gy) |
251 | 116 | (46.2) | [39.9-52.6] | 38 | (15.1) | [10.9-20.2] | 2 | (0.8) | [0.1-2.8] | 156d | (62.1) | [55.8-68.2] |
| Neuropathy | Modified total neuropathy scale (mTNS) |
Cisplatin/Carboplatin Vinblastine/ Vincristine |
1422 | 55 | (3.9) | [2.9-5.0] | 241 | (16.9) | [15.0-19.0] | 16 | (1.1) | [0.6-1.8] | 312e | (21.9) | [19.8-24.2] |
At risk by treatment exposure as defined in the COG Guidelines, see supplemental Table 1 for detailed exposures and potential late effects evaluated by risk-based screening.
58.4% were CTCAE v.4 Grade 3-4
17.0% were CTCAE v.4 Grade 3-4
53.8% were CTCAE v.4 Grade 3-4
1.9% were CTCAE v.4 Grade 3-4
CTCAE v.4 percentages includes only those who fulfill criteria for “at risk” as defined by COG Guidelines.
Comment
This report delineates the type and prevalence of specific health conditions systematically ascertained across multiple organ systems among a large, histologically heterogeneous population of adults formerly treated for childhood cancer. In contrast to published studies, the SJLIFE study prospectively applied consistent risk-based screening to quantify the burden of chronic disease among long-term childhood cancer survivors. These results provide precise estimates of the prevalence of treatment-related morbidities among long-term childhood cancer survivors and an enumeration of the chronic health conditions known to be associated with early mortality in the general population. Unique from previous publications, the present study also quantifies the substantial proportion of previously undiagnosed disease among cohort members, underscoring the need for ongoing follow-up and assessment.
Prior studies investigating long-term outcomes of adults treated for cancer during childhood have largely relied on survivor self-report of outcomes or registry data.2-5 U.S. research programs reporting outcomes based on medical assessments have featured relatively small cohorts, including those with pediatric-aged survivors.12-14 A previous study retrospectively evaluated the prevalence of adverse outcomes that were identified through late effects clinic evaluations undertaken from 1996 to 2004 among 1362 five-year survivors of childhood cancer (median age 24.4 years) in the Netherlands.1 Medical assessments were performed according to standardized follow-up protocols; however, specific screening methodologies and total numbers screened for each condition were not described. Their findings confirmed the burden of morbidity present in a young adult cohort (88% were younger than 35 years). At an average follow-up of 17 years, 75% of survivors experienced at least one adverse event; 40% had at least one severe, life-threatening or disabling event. Our results extend these findings in an older survivor population by documenting yield from risk-based screening according to standardized guidelines and by demonstrating the age-specific burden of particular chronic health conditions followed for a mean of 26.3 years from diagnosis. Moreover, the focus on exposure-driven, risk-based screening increases the relevance of our findings, considering the fact that despite the substantial evolution of therapeutic approach for various pediatric malignancies over the last 50 years, most of the specific treatment modalities prompting screening remain in use.15,16 Analyses evaluating outcomes related to the evolution of “packaging” these modalities over time and its influence on the prevalence of organ-specific outcomes for clinical diagnostic groups will be the subject of future investigations.
For some organ systems evaluated, the results of risk-based assessment revealed a substantial number of previously undiagnosed problems that are typically observed in older populations.17-21 This had a marked effect on the estimates of age-specific organ dysfunction. Comparing the prevalences of our outcomes to those reported in previously published studies is difficult as the latter often represent clinically manifest conditions,2-5 those derived from inconsistent screening practices administered over a long period of time,1 or those applied to convenience cohorts.13,14 Recent studies implementing systematic screening in younger survivor cohorts have similarly identified a high prevalence of abnormalities after selected systems were evaluated, e.g., pulmonary.13 In our cohort, the prevalence of newly discovered neurocognitive and neurosensory deficits, heart valve disorders and pulmonary dysfunction were particularly striking. Considering the median age of this cohort was only 32 years, these data are concerning and may indicate a pattern of accelerated or premature aging. Evaluation of the contribution of predisposing host and treatment factors to this phenomenon will be the focus of future research.
The primary aim of our study was to establish the prevalence of late health effects following systematic screening after predisposing cancer treatment-related exposures, with a particular emphasis on preclinical disease manifestations. For analytical purposes we dichotomized screening outcomes, which included a spectrum of conditions of varying severity, as present or absent. Ninety-eight percent of our cohort had one or more chronic health condition with 67.6% having a severe or life-threatening/disabling condition by CTCAEv4.0 (Grade 3-4). While some findings may not immediately influence on the health status of survivors, their presence may reflect early disease outcomes that may be remediated or at least monitored prospectively to assess the relationship to future decline in function. For example, adult survivors of childhood leukemia who received 24 Gy cranial irradiation demonstrated reduced cognitive status and memory on formal neuropsychological testing.22 The abnormalities detected did not affect functional status measures like employment, but are consistent with early onset mild cognitive dementia, underscoring the need for longitudinal evaluation as this group ages.
Exposure-specific, risk-based screening resulted in identification and referral for treatment of some conditions that are amenable to remediation. These included low stage occult breast cancers identified by breast imaging in women treated with chest radiation, and cardiomyopathy identified by echocardiography among those exposed to anthracyclines and chest radiation. In contrast, the yield from screening for other outcomes, e.g., myelodysplasia and kidney dysfunction, was negligible. Low yield from laboratory assessments of hematological and biochemical parameters has been reported in a younger survivor cohort followed just over 10 years.13 Confirmation of these findings in this older and larger cohort provides reassurance that these conditions do not increase in prevalence with aging. Collectively, the data from risk-based screening also provide clinically relevant information about the magnitude of risk and preclinical manifestations of common late effects to guide refinement of health screening recommendations.
Assessment of all survivors with a core laboratory battery permitted evaluation of associations of specific cancer treatment and chronic health conditions. As expected, endocrine and reproductive disorders were largely associated with previous treatment with radiation and alkylating agents. The association of cancer treatment with conditions highly prevalent in the general population, such as obesity and diabetes, was lower. For example, an increased risk of metabolic syndrome or its components has been observed among cancer survivors treated with HPA irradiation.23 However, within the SJLIFE cohort, the AF of obesity, diabetes mellitus, dyslipidemia and hypertension ranged from 9%-42% among survivors. The current report describes the occurrence of health outcomes within childhood cancer survivors following the initial cross-sectional clinical assessment. In depth analyses are underway to identify predictors of and risk profiles for specific outcomes, which take into consideration the inter-relationships between genetics, demographic and lifestyle factors, treatment exposures, and co-morbidities. The ongoing prospective follow-up of these patients will also provide additional insights into the longitudinal changes in health outcomes within an aging survivor population.
These findings should be considered in the context of study limitations. Results could be influenced by selection bias considering the 60% participation rate for onsite comprehensive evaluations. However, the lack of substantial differences between the studied and the source population of SJLIFE in the relative frequencies of demographic, disease, or neighborhood characteristics reduces concerns about selective non-participation.7 It is possible that differences in attained age and time from diagnosis between participants and non-participants could bias results if the older non-participants who had a greater elapsed time from treatment had more chronic health conditions. Because of enrollment priorities based on treatment exposures in this dynamic cohort, the study population does not precisely reflect the distribution of histologies that would be expected in a long-term childhood cancer survivorship cohort. For example, the proportion of those with leukemia is somewhat higher, and those with brain cancer lower, than would be anticipated in a large random sample of survivors. Those relative proportions will tend to balance as recruitment and enrollment in this ongoing study continue over time. In addition, the yield of screening is likely underestimated in the SJLIFE cohort as many had been previously screened as participants in the pediatric long-term follow-up clinic at St. Jude. Moreover, the absence of controls in our study precluded assessment of the actual clinical effect of screening. Failure to undertake uniform evaluations among all cohort participants also precluded the discovery of novel treatment-related outcomes. Finally, when interpreting the cumulative prevalence within our population it is important to keep in mind that the rates are based upon the experience of patients who were alive at the time of recruitment for clinical evaluation. Thus, these prevalence rates underestimate actual incidence if one assumes that the population of patients who met eligibility criteria, but died prior to recruitment to the SJLIFE cohort, experienced a high rate of morbidity prior to death. This assumption seems reasonable because reports of late mortality among childhood cancer survivors have indicated that death from second cancers, cardiac events, and pulmonary events are the most frequent causes.24
In summary, this study provides global and age-specific estimates of clinically ascertained morbidity in multiple organ systems in a large systematically evaluated cohort of long-term childhood cancer survivors. The percentage of survivors with one or more chronic health conditions prevalent in a young adult population was extraordinarily high. These data underscore the need for clinically-focused monitoring, both for conditions that have significant morbidity if not detected and treated early, such as second malignancies and heart disease, and also for those that if remediated can improve quality of life, such as hearing loss and vision deficits.
Supplementary Material
Table 2d. Prevalence of Other Metabolic Late Effects in At-Risk Populations Following Exposure-Based Screening.
| Diagnosis before SJLIFE |
Diagnosis related to SJLIFE |
Diagnosis after SJLIFE |
Overall Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential Late Effect |
Screening test | Exposure Status | Number at riska |
N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI |
| Hematology | |||||||||||||||
| Abnormal blood counts |
Complete blood count with differential |
Alkylating agents Anthracyclines Epipodophyllotoxins |
1375 | 16 | (1.2) | [0.7-1.9] | 20 | (1.5) | [0.9-2.2] | 4 | (0.3) | [0.1-0.7] | 40b | (3.0) | [2.1-3.9] |
| Hepatic | |||||||||||||||
| Hepatopathy | Alanine aminotransferas e (ALT), aspartate aminotransferas e (AST), bilirubin |
Mercaptopurine/Thio guanine Radiation to liver (dose >= 30 Gy) |
920 | 34 | (3.7) | [2.6-5.1] | 65 | (7.1) | [5.5-8.9] | 20 | (2.2) | [1.3-3.3] | 119c | (13.0) | [10.8-15.3] |
| Skeletal | |||||||||||||||
| Osteoporosis | Dual X-ray Absorptiometry |
Methotrexate Corticosteroids Radiation to hypothalamic- pituitary |
1142 | 23 | (2.0) | [1.3-3.0] | 87 | (7.6) | [6.1-9.3] | 0 | (0.0) | 110d | (9.6) | [8.0-11.5] | |
| Urinary tract | |||||||||||||||
| Kidney dysfunction |
Urinalysis BUN, Creatinine, Na, K, Cl, CO2, Ca, Mg, PO4 |
Ifosfamide Cisplatin/Carboplatin Methotrexate Radiation to kidney Nephrectomy |
1410 | 35 | (2.5) | [1.7-3.4] | 33 | (2.3) | [1.6-3.3] | 3 | (0.2) | [0.0-0.6] | 71e | (5.0) | [4.0-6.3] |
At risk by treatment exposure as defined in the COG Guidelines, see supplemental Table 1 for detailed exposures and potential late effects evaluated by risk-based screening.
None of the cases were CTCAE v.4 Grade 3-4
20.0% were CTCAE v.4 Grade 3-4
All cases were CTCAE v.4 Grade 3-4
15.2% were CTCAE v.4 Grade 3-4
CTCAE v.4 percentages includes only those who fulfill criteria for “at risk” as defined by COG Guidelines.
Table 2e. Prevalence of Transfusion-Associated Infectious Late Effects and Cancer Screening in At-Risk Populations Following Exposure-Based Screening.
| Diagnosis before SJLIFE |
Diagnosis related to SJLIFE |
Diagnosis after SJLIFE |
Overall Prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potential Late Effect |
Screening test | Exposure Status | Number at riska |
N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI | N | (%) | 95% CI |
| Infection, Transfusion Acquired | |||||||||||||||
| Hepatitis B | Hepatitis B surface antigen and core antibody |
Diagnosis before 1972 |
113 | 2 | (1.8) | [0.2-6.2] | 1 | (0.9) | [0.0-4.8] | 1 | (0.9) | [0.0-4.8] | 4b | (3.6) | [1.0-8.8] |
| Hepatitis C | Hepatitis C antibody |
Diagnosis before 1993 |
1437 | 75 | (5.2) | [4.1-6.5] | 14 | (1.0) | [0.5-1.6] | 8 | (0.6) | [0.2-1.1] | 97c | (6.8) | [5.5-8.2] |
| HIV | HIV serology (HIV 1 & 2 antibodies) |
Diagnosis between 1977-1985 |
640 | 2 | (0.3) | [0.0-1.1] | 1 | (0.2) | [0.0-0.9] | 0 | (0.0) | 3d | (0.5) | [0.1-1.4] | |
|
Cancer
Screening |
|||||||||||||||
| Subsequent neoplasmf |
Targeted screening based on specific subsequent neoplasm riske |
Any cancer treatment-related risk |
1536 | 202 | (13.2) | [11.5-14.9] | 43 | (2.8) | [2.0-3.8] | 30 | (2.0) | [1.3-2.8] | 275 | (18.0) | [16.0-19.9] |
At risk by treatment exposure as defined in the COG Guidelines, see supplemental Table 1 for detailed exposures and potential late effects evaluated by risk-based screening.
One case was CTCAE v.4 Grade 3-4 c 43.3% were CTCAE v.4 Grade 3-4 d All cases were CTCAE v.4 Grade 3-4
43.3% were CTCAE v.4 Grade 3-4
All cases were CTCAE v.4 Grade 3-4
CBC for myelodysplasia/acute myeloid leukemia, mammogram/breast MRI for breast cancer, colonoscopy for colorectal cancer, physical exam for other skin/solid neoplasms.
The total prevalence percentages for subsequent neoplasm count each person only once. Two participants had a second neoplasm (SN) diagnosed both before and at SJLIFE visit and another participant had an SN diagnosed before and after SJLIFE visit.
CTCAE v.4 percentages includes only those who fulfill criteria for “at risk” as defined by COG Guidelines.
Table 3. Chronic Health Conditions and Percent Associated with Cancer-Related Therapy.
| Potential Late Effect |
Screening test | Criteria for positive screeninga |
Exposure Groups |
Number | n | Prevalence | Attributable fractionb,c | ||
|---|---|---|---|---|---|---|---|---|---|
| (%) | 95% CI | (%) | 95% CI | ||||||
| Cardiovascular Risk Factors | |||||||||
| Hypertension | Blood pressure | BP > 140/90 | Total | 1713 | 387 | (22.6) | [20.6-24.7] | (9.3) | [−16.3-29.2] |
| Exposed | 1508 | 342 | (22.7) | [20.6-24.9] | |||||
| Unexposed | 205 | 45 | (22.0) | [16.5-28.3] | |||||
| Dyslipidemia | Fasting lipid panel | Total cholesterol ≥ 200 | Total | 1713 | 872 | (50.9) | [48.5-53.3] | (15.5) | [10.2-20.5] |
| mg/dL, or triglycerides ≥ | Exposed | 807 | 491 | (60.8) | [57.4-64.2] | ||||
| 150 mg/dL, or LDL ≥ 130 mg/dL or HDL < 40 mg/dL |
Unexposed | 906 | 381 | (42.1) | [38.8-45.3] | ||||
| Obese | Body mass index | BMI> 30.0 kg/m2 | Total | 1713 | 624 | (36.5) | [34.1-38.8] | (42.1) | [34.4-48.9] |
| (BMI) | Exposed | 714 | 345 | (48.3) | [44.6-52.1] | ||||
| Unexposed | 999 | 279 | (27.9) | [25.2-30.8] | |||||
| Cardiac | |||||||||
| Arrhythmia | Electrocardiogram | Detection of rhythm | Total | 1713 | 126 | (7.4) | [6.2-8.7] | (−17.8) | [−68.4-17.7] |
| abnormality | Exposed | 1214 | 85 | (7.0) | [5.6-8.6] | ||||
| Unexposed | 499 | 41 | (8.2) | [6.0-11.0] | |||||
| Conduction | Electrocardiogram | Detection of conduction | Total | 1713 | 243 | (14.2) | [12.6-15.9] | (−4.3) | [−33.9-18.8] |
| disorder | abnormality | Exposed | 1214 | 169 | (14.0) | [12.0-16.0] | |||
| Unexposed | 499 | 74 | (14.8) | [11.8-18.3] | |||||
| History of cardiac | Electrocardiogram | ECG abnormality | Total | 1713 | 387 | (5.7) | [20.6-24.7] | (57.1) | [36.4-71.0] |
| ischemia | indicating history of | Exposed | 501 | 48 | (9.6) | [7.2-12.5] | |||
| ischemia | Unexposed | 1212 | 49 | (4.1) | [3.0-5.3] | ||||
| Endocrine/Reproductive | |||||||||
| Hypogonadotropic | Menstrual history, | Amenorrhea before 40 | Total | 830 | 34 | (4.1) | [2.9-5.7] | (90.7) | [83.2-94.9] |
| hypogonadism | Follicle stimulating | years and estradiol | Exposed | 65 | 20 | (30.8) | [19.9-43.5] | ||
| (females)d | hormone (FSH), | below normal range and | Unexposed | 765 | 14 | (1.8) | [1.0-3.1] | ||
| Estradiol | FSH within or below normal range |
||||||||
| Hypogonadotropic | Luteinizing | Testosterone below | Total | 832 | 55 | (6.5) | [5.1-8.5] | (88.4) | [80.1-93.3] |
| hypogonadism | hormone (LH), | normal range and LH | Exposed | 88 | 23 | (26.2) | [17.3-36.6] | ||
| (males)e | Morning testosterone |
within or below normal range |
Unexposed | 744 | 32 | (4.3) | [3.0-6.0] | ||
| Central | Thyroid stimulating | Free T4 below normal | Total | 1674 | 78 | (4.7) | [3.7-5.8] | [89.2-98.9] | |
| hypothyroidismf | hormone (TSH), | range and TSH within or | (96.6) | ||||||
| Free T4 | below normal range | Exposed | 152 | 38 | (25.0) | [18.3-32.7] | |||
| Unexposed | 1522 | 40 | (2.7) | [1.9-3.6] | |||||
| Diabetes mellitus | Fasting serum | Fasting glucose ≥ 126 | Total | 1713 | 101 | (5.9) | [4.8-7.1] | (41.7) | [12.2-61.3] |
| glucose | mg/dL or hemoglobin ≥ | Exposed | 714 | 56 | (7.8) | [6.0-10.1] | |||
| hemoglobin A1C | A1C 6.4% | Unexposed | 999 | 45 | (4.5) | [3.3-6.0] | |||
| Primary | TSH, Free T4 | Free T4 below normal | Total | 1674 | 128 | (7.7) | [6.4-9.0] | (97.0) | [90.6-99.0] |
| hypothyroidismf | range and TSH above | Exposed | 910 | 125 | (13.8) | [11.6-16.2] | |||
| normal range | Unexposed | 764 | 3 | (0.4) | [0.1-1.1] | ||||
| Primary ovarian failure |
Menstrual history, | Amenorrhea before age | Total | 830 | 65 | (7.8) | [6.1-9.9] | (100.0) | |
| Follicle stimulating | < 40 years and FSH | Exposed | 553 | 65 | (11.8) | [9.2-14.7] | |||
| hormone (FSH),Estradiol |
above normal range | Unexposed | 277 | 0 | (0.0) | ||||
| Leydig cell failured | Morning | Testosterone below | Total | 832 | 71 | (8.5) | [6.7-10.6] | (96.5) | [91.2-98.6] |
| testosterone, LH | normal range and LH | Exposed | 574 | 66 | (11.5) | [9.0-14.4] | |||
| above normal range | Unexposed | 258 | 5 | (2.0) | [0.6-4.5] | ||||
| Hematology | |||||||||
| Abnormal blood | Complete blood | Abnormal blood counts | Total | 1713 | 49 | (2.9) | [2.1-3.8] | (5.6) | [−96.8-54.8] |
| counts | count with | consistent with | Exposed | 1375 | 40 | (3.0) | [2.1-3.9] | ||
| differential | cytopenia, myelodysplasia, myeloproliferative disorder |
Unexposed | 338 | 9 | (2.7) | [1.2-5.0] | |||
| Hepatic | |||||||||
| Hepatopathy | Alanine | ALT, AST, bilirubin | Total | 1713 | 205 | (12.0) | [10.5-13.6] | (14.5) | [−10.7-33.9] |
| aminotransferase | above reference range | Exposed | 920 | 119 | (13.0) | [10.8-15.3] | |||
| (ALT), aspartate aminotransferase (AST), bilirubin |
Unexposed | 793 | 86 | (10.9) | [8.8-13.2] | ||||
| Neurosensory | |||||||||
| Neuropathy | Modified total | Score on mTNS 4+ | Total | 1713 | 348 | (20.4) | [18.4-22.3] | (42.3) | [20.6-58.1] |
| neuropathy scale | Exposed | 1422 | 312 | (21.9) | [19.8-34.2] | ||||
| (mTNS) | Unexposed | 291 | 36 | (12.4) | [8.8-16.7] | ||||
| Urinary tract | |||||||||
| Kidney dysfunction | Urinalysis | Serum creatinine > 1.5 | Total | 1713 | 76 | (4.5) | [3.5-5.5] | (65.7) | [21.7-85.0] |
| BUN, Creatinine | mg/dL | Exposed | 1410 | 71 | (5.0) | [4.0-6.3] | |||
| Na, K, Cl, CO2, Ca, | eGFR < 90 | Unexposed | 303 | 5 | (1.6) | [0.5-3.8] | |||
| Mg, PO4 | mL/min/1.73m2 ± Abnormal urinalysis (e.g., proteinuria) ± Electrolyte alterations |
||||||||
| Hemorrhagic | Urinalysis | Hematuria | Total | 1713 | 15 | (0.9) | [0.5-1.4] | (−128.6) | [−534.0-17.6] |
| cystitis | Exposed | 1130 | 7 | (0.7) | [0.3-1.3] | ||||
| (microscopic hematuria) |
Unexposed | 583 | 8 | (1.4) | [0.6-2.7] | ||||
See supplemental Table 3 “Criteria for Defining Late Effects” for detailed information about the definitions for positive screening for specific late effects.
Attributable fraction indicates the percentage of cases in the cohort that are related to the specific treatment exposure, where Ae% = ×100%.
Ae%=Attributable fraction, Re=absolute risk in exposed, Ro==absolute risk in unexposed.
Negative values indicate that the risk for that chronic condition was less in the group that received the treatment exposure than in the group that did not receive the treatment exposure.
Results presented for evaluation of hypogonadotropic hypogonadism and primary ovarian failure exclude 50 women with bilateral oophorectomy.
Results presented for evaluation of Leydig cell failure exclude 1 man with bilateral orchiectomy.
Results presented for evaluation of central and primary hypothyroidism exclude 39 patients with prior thyroidectomy.
Table summarizes prevalence of chronic health conditions detected by comprehensive screening with the core battery of evaluations administered to all study participants.
Table 4. Subsequent Neoplasms Among Cohort Participants.
| Total SN | Time from Primary Neoplasm to SN | SN Identified by Risk-Based Screening | Time from Primary Neoplasm to SN | |||||
|---|---|---|---|---|---|---|---|---|
| Subsequent Neoplasms | No. Cases | Median | 25th % | 75th % | No. Cases | Median | 25th % | 75th % |
| Total SN | 348 | 25.1 | 18.2 | 31.6 | 44 | 30.6 | 23.6 | 34.7 |
| Solid SN | ||||||||
| All Carcinomas | 233 | 25.4 | 18.5 | 30.8 | 28 | 27.2 | 21.9 | 33.8 |
| Breast | 39 | 24.9 | 17.7 | 30.4 | 13 | 22.6 | 17.2 | 30.4 |
| Cervix | 13 | 17.9 | 10.7 | 24.0 | 0 | |||
| Colon | 3 | 23.8 | 21.3 | 38.9 | 1 | 38.9 | 38.9 | 38.9 |
| Salivary Gland | 9 | 15.6 | 13.5 | 18.3 | 0 | |||
| Skin | 118 | 26.9 | 20.1 | 32.1 | 9 | 27.9 | 27.0 | 32.3 |
| Thyroid | 33 | 22.6 | 18.1 | 28.3 | 2 | 34.3 | 30.4 | 38.3 |
| Other Carcinomas | 18 | 34.5 | 23.9 | 36.5 | 3 | 23.9 | 23.2 | 34.6 |
| All CNS Tumors | 73 | 25.1 | 19.0 | 33.1 | 12 | 32.9 | 31.5 | 36.2 |
| Meningioma | 63 | 26.6 | 20.3 | 33.5 | 12 | 32.9 | 31.5 | 36.2 |
| Other CNS | 10 | 9.6 | 5.5 | 23.4 | 0 | |||
| Sarcoma | 19 | 23.2 | 17.8 | 31.9 | 3 | 32.2 | 29.7 | 41.8 |
| Other Solid SN | 10 | 20.8 | 14.4 | 26.2 | 1 | 13.5 | 13.5 | 13.5 |
| Hematological SN | ||||||||
| ALL | 1 | 2.5 | 2.5 | 2.5 | 0 | |||
| AML/MDS | 2 | 12.3 | 1.5 | 23.1 | 0 | |||
| Other Leukemia | 2 | 20.7 | 14.3 | 27.0 | 0 | |||
| Hodgkin Lymphoma | 3 | 2.1 | 1.0 | 17.0 | 0 | |||
| Non-Hodgkin Lymphoma | 3 | 38.0 | 3.5 | 38.9 | 0 | |||
| Other Hematological SN | 2 | 20.7 | 4.3 | 37.0 | 0 | |||
Includes 348 SNs diagnosed among 272 participants before SJLIFE evaluation, directly as a result of SJLIFE evaluation, and after/unrelated to SJLIFE evaluation. 44 out of 82 SNs discovered on or after the SJLIFE evaluation were identified directly as a result of risk-based screening.
Abbreviations: ALL – acute lymphoblastic leukemia; AML – acute myeloid leukemia; CNS – central nervous system; MDS – myelodysplastic syndrome; SN – subsequent neoplasm
Acknowledgements
Drs. Melissa M. Hudson and Kirsten K. Ness had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
We are grateful for the considerable efforts of the research, clinical and administrative staff who support the SJLIFE study and the survivors and their families from whom it is our privilege to learn.
Funding/ Support: This study was supported by the Cancer Center Support (CORE) grant CA 21765 (R. Gilbertson – PI) from the National Cancer Institute and by the ALSAC and the American Lebanese Syrian Associated Charities (ALSAC)
Role of the Sponsor: The NCI and ALSAC had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Research grant support: Supported by the Cancer Center Support (CORE) grant CA 21765 (R. Gilbertson – PI) from the National Cancer Institute and by the ALSAC.
Footnotes
Author Contributions
Study concept and design: Hudson, Gurney, Krull, Armstrong, Srivastava, Robison Acquisition of data: Hudson, Ness, Gurney, Chemaitilly, Krull, Armstrong, Nottage, Robison
Analysis and interpretation data: Hudson, Ness, Gurney, Mulrooney, Chemaitilly, Green, Krull, Armstrong, Nottage, Jones, Sklar, Srivastava, Robison
Drafting of the manuscript: Hudson, Ness, Mulrooney, Chemaitilly, Green, Armstrong, Nottage, Srivastava, Robison
Critical revision of manuscript for important intellectual content: Hudson, Ness, Gurney, Mulrooney, Chemaitilly, Krull, Armstrong, Nottage, Jones, Sklar, Srivastava, Robison
Statistical analysis: Ness, Nottage, Jones, Srivastava, Robison
Administrative, technical, or material support: Hudson, Ness, Gurney, Krull, Armstrong, Robison
Study supervision: Hudson, Ness, Mulrooney, Sklar, Srivastava, Robison
Conflict of Interest Disclosures: All authors have completed and submitted the ICJME Form for Disclosure of Potential Conflicts of Interest and none were reported.
Previous presentation of the information reported in the manuscript: An abstract from the preliminary analysis was presented at the 43rd Congress of the International Society of Paediatric Oncology, 25-30 October 2011, in Auckland, New Zealand.
References
- 1.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116(17):3129–3139. doi: 10.1182/blood-2009-06-229369. quiz 3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowling E, Yabroff KR, Mariotto A, McNeel T, Zeruto C, Buckman D. Burden of illness in adult survivors of childhood cancers: findings from a population-based national sample. Cancer. 2010;116(15):3712–3721. doi: 10.1002/cncr.25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rebholz CE, Reulen RC, Toogood AA, et al. Health care use of long-term survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2011;29(31):4181–4188. doi: 10.1200/JCO.2011.36.5619. [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude lifetime cohort study. Pediatr Blood Cancer. 2013;60(5):856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. [Accessed January 28, 2013];Children’s Oncology Group Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancer, Version 3.0. 2008 http://www.survivorshipguidelines.org.
- 9.Cancer Therapy Evaluation Program. at http://ctep.cancer.gov/reporting/ctc_v30.html.
- 10.Ruckinger S, von Kries R, Toschke AM. An illustration of and programs estimating attributable fractions in large scale surveys considering multiple risk factors. BMC Med Res Methodol. 2009;9:7. doi: 10.1186/1471-2288-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison PD. Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc; Cary, N.C.: 1995. [Google Scholar]
- 12.Staba Hogan MJ, Ma X, Kadan-Lottick NS. New health conditions identified at a regional childhood cancer survivor clinic visit. Pediatr Blood Cancer. 2013;60(4):682–687. doi: 10.1002/pbc.24360. [DOI] [PubMed] [Google Scholar]
- 13.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the Children’s Oncology Group Long-Term Follow-Up Guidelines. J Clin Oncol. 2012;30(35):4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wasilewski-Masker K, Mertens AC, Patterson B, Meacham LR. Severity of health conditions identified in a pediatric cancer survivor program. Pediatr Blood Cancer. 2010;54(7):976–982. doi: 10.1002/pbc.22431. [DOI] [PubMed] [Google Scholar]
- 15.Hudson MM, Neglia JP, Woods WG, et al. Lessons from the past: opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;58(3):334–343. doi: 10.1002/pbc.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green DM, Kun LE, Matthay KK, et al. Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. doi: 10.1002/pbc.24487. [Epub ahead of print Feb 15 2013] http://www.ncbi.nlm.nih.gov/pubmed/23418018. [DOI] [PMC free article] [PubMed]
- 17.Asbell PA, Dualan I, Mindel J, Brocks D, Ahmad M, Epstein S. Age-related cataract. Lancet. 2005;365(9459):599–609. doi: 10.1016/S0140-6736(05)17911-2. [DOI] [PubMed] [Google Scholar]
- 18.Frisina RD. Age-related hearing loss: ear and brain mechanisms. Ann N Y Acad Sci. 2009;1170:708–717. doi: 10.1111/j.1749-6632.2009.03931.x. [DOI] [PubMed] [Google Scholar]
- 19.Glisky EL. Changes in Cognitive Function and Human Aging. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- 20.Marin-Garcia J, Goldentha MJ, Moe GW. Aging and the Heart. Springer; U.S.: 2008. Cardiomyopathy and Heart Failure in Aging; pp. 209–238. [Google Scholar]
- 21.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20(10):1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armstrong GT, Reddick WE, Peterson R, et al. Dementia in adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst. 2013 doi: 10.1093/jnci/djt089. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Haas EC, Oosting SF, Lefrandt JD, Wolffenbuttel BH, Sleijfer DT, Gietema JA. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010;11(2):193–203. doi: 10.1016/S1470-2045(09)70287-6. [DOI] [PubMed] [Google Scholar]
- 24.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.