Abstract
The etiology and pathogenesis of human autoimmune diseases remain unknown despite intensive investigations. Although remarkable progress has been accomplished through genome wide association studies in the identification of genetic factors that may predispose to their occurrence or modify their clinical presentation to date no specific gene abnormalities have been conclusively demonstrated to be responsible for these diseases. The completion of the human and chimpanzee genome sequencing has opened up novel opportunities to examine the possible contribution of human specific genes and other regulatory elements unique to the human genome, such as microRNAs and non-coding RNAs, towards the pathogenesis of a variety of human disorders. Thus, it is likely that these human specific genes and non-coding regulatory elements may be involved in the development or the pathogenesis of various disorders that do not occur in non-human primates including certain autoimmune diseases such as Systemic Sclerosis and Primary Sjögren's Syndrome. Here, we discuss recent evidence supporting the notion that human specific genes or human specific microRNA and other non-coding RNA regulatory elements unique to the human genome may participate in the development or in the pathogenesis of Systemic Sclerosis and Primary Sjögren's Syndrome.
Keywords: Human specific genes, microRNAs, Non-coding RNA, Autoimmune diseases, Systemic Sclerosis, Sjögren's Syndrome
1. Introduction
The etiology and pathogenesis of human autoimmune diseases remain unknown despite intensive investigations. The extensive genome-wide association studies conducted in the last decade have led to the identification of numerous genetic factors that may predispose to their occurrence or modify their clinical presentation. However, to date no specific gene abnormalities have been conclusively demonstrated to be responsible. One of the most remarkable consequences of the extensive DNA sequencing studies accomplished in the last two decades was the realization of the extremely high conservation of coding gene sequences among various species. Indeed, comparison of the human and chimpanzee genomes indicated that these two species differ by less than 2% in the complement of expressed genes [1] and it has been reported that there are less than 200 genes in humans that are not present in chimpanzees, our closest evolutionary relative [1,2]. The completion of the human and chimpanzee genome sequencing has opened up novel opportunities to examine the role of human-specific genes in human development as well as the possible contribution of human-specific genes and other regulatory elements unique to the human genome towards the pathogenesis of a variety of human disorders. Thus, in addition to being responsible for human specific traits, such as bipedalism, highly enlarged brain cortex, and human language, it is likely that human specific genes and other regulatory elements unique to the human genome may be involved in the development of various disorders that do not occur in non-human primates including certain autoimmune diseases such as Systemic Sclerosis (SSc) and Primary Sjögren's Syndrome (SS). This remarkable observation allows to raise the possibility that certain human diseases, such as SSc or SS, that do not have a counterpart in chimpanzees or in other non-human primates may occur or may develop owing to either structural alterations in human-specific genes. Alternatively, these disorders may be caused by functional derangement of other human-specific regulatory elements, such as microRNAs (miRNA) or non-coding RNAs (ncRNA), or may be the result of functional abnormalities caused by deletions of genes or regulatory elements from the chimpanzee genome that have been lost during recent evolutionary events and are absent from the human genome. Here, recent evidence supporting the notion that human specific genes or human specific microRNA and other human-specific regulatory elements may participate in the development or in the pathogenesis of SSc and SS will be discussed.
2. Human specific genes
The identification of human specific genes and other regulatory elements unique to the human genome has accelerated the study of recent evolutionary events in human development and has provided novel pathways to the study of the pathogenesis of a variety of human disorders. Numerous observations have already been described documenting the important role of human specific genes particularly in relation to brain and brain cortex development [3–8]. Notable examples include the identification of the FOXP2 gene which encodes an essential homeodomain protein required for human speech [5], the demonstration that the AHI1 gene regulates axon guidance [2], and the discovery that genes encoding ASPM and microcephalin are essential for normal cerebral cortical growth and may be responsible for the substantial increase in brain cortex size in humans [8]. However, as pointed out by Stahl and collaborators [9,10], human specific genes rather than creating new signaling pathways are more likely to be involved in modulation or evolutionary regulation of previously developed and highly conserved pathways. Thus, in addition to being responsible for human specific traits, such as bipedalism, highly enlarged brain cortex, and human language, it is likely that human specific genes and other regulatory elements unique to the human genome may be involved in important regulatory roles including the regulation of important immunological functional pathways.
3. Pathologic conditions associated with human specific genes
Despite the potential importance of the study of human specific genes to human biology and pathology, only a few of these genes have been extensively studied in human disorders. Among these are genes encoded by the neuroblastoma breakpoint family (NBPF) which are associated with the development of neuroblastoma in humans [11,12], the nuclear pore complex-interacting protein (NPIP) which has been reported to be overexpressed in retinal tissues of patients affected with macular degeneration [13], and TBC1D3, an oncogene overexpressed in breast and prostate cancers that encodes a protein with a specific domain capable of modulating growth factor receptor signaling [10]. There is, however, extensive recent interest in the possible role of human-specific genes and human-specific genomic regulatory elements in the development of complex human diseases including certain autoimmune diseases [14].
4. Human diseases not described in chimpanzees
Although many human diseases have homologues in various animal species including quite distant evolutionary species, such as the mouse and other rodents, there are certain human disorders that have not been described in the closest human evolutionary relatives such as apes and chimpanzees. A very extensive search was performed recently by our group to document the presence of either SSc or SS in chimpanzees. This included a detailed review of the available published medical and veterinary literature, as well as, inquiries to the Office of Comparative Pathology at the National Institutes of Health (S. Jimenez; unpublished). From these extensive search and inquiries, it became apparent that these two diseases have not been described or encountered in chimpanzees.
Although the etiology of SSc and SS is unknown and the complex pathogenesis of both disorders has not been fully elucidated, it is generally recognized that immunological abnormalities play a crucial role [15–20]. Indeed, both SSc and SS are characterized by T and B cell hyperactivity [15–20] as well as by the participation of monocytes and tissue macrophages, particularly during the initial or early stages of SSc [21,22]. These prominent immunological abnormalities include alterations in innate immunity with expression of the type I interferon signature, infiltration of affected tissues with oligoclonally expanded T cell populations, production of specific autoantibodies, and numerous alterations in T and B cell subpopulations [23–30]. It is also of importance that both SSc and SS are characterized by B cell hyperactivity and development of tertiary lymphoid organs and/or B cell infiltration of affected tissues, decreased switched and unswitched peripheral memory cells in SS and increased CD19 expression indicative of B cell activation in SSc.
5. Relative over-reactivity of human lymphocytes compared to lymphocytes from chimpanzees
Despite the remarkable sequence conservation between the genomes of humans and chimpanzees [1,2], it has become well recognized that some immune responses capable of mediating strong immunological events in humans occur at a much less intense level in chimpanzees. Thus, it appears that immunologic reactivity to certain immune stimuli is muted or absent in chimpanzees compared to humans. For example, it has been described that HIV infection is much less severe in chimpanzees and there is no progression to full blown AIDS with a maintenance of substantially higher levels of CD4 T cells in the chimpanzees in comparison to humans [31,32]. There is also evidence to suggest that there are remarkable differences in the pathological responses between humans and chimpanzees to infection with hepatitis C virus (HCV). For example, the development of antibodies to HCV viral envelope proteins is less frequent and substantially muted in chimpanzees compared to humans, and the severe complications of HCV infections, including fibrosis of the liver, cirrhosis, and terminal liver failure are much less common in chimpanzees than in humans [33,34]. Other evidence to indicate that the immunological responses of chimpanzees are muted compared to those of humans are the development of milder reactions to varicella infections in chimpanzees [35] and their very low, if any, susceptibility to infections with major human pathogens such as Epstein-Barr virus and mycoplasma pneumoniae [36].
A remarkable and catastrophic event further pointed out the substantial differences in the immune responses between humans and non-human primates. This event was a Phase 1 clinical trial of TGN1412, a humanized monoclonal anti-CD28 receptor antibody [37]. This biological agent had been developed and tested extensively in Cynomolgus macaques who tolerated its administration without side effects. A clinical trial in 2006 administered an extremely low dose of this antibody, equivalent to 1/500th of the highest dose that had been tested in Cynomolgus macaques to six human volunteers with highly deleterious consequences, including the development of a severe cytokine storm which led to massive multiple organ failure and nearly death in the recipients [37]. Although the mechanisms responsible for this catastrophic event have not been fully elucidated as discussed recently [38,41], numerous studies have demonstrated remarkable differences in the immune response between lymphocytes from chimpanzees and humans, and have uncovered a striking over-reactivity of human lymphocytes to a variety of T cell stimuli [42–44]. These studies demonstrated that human lymphocytes manifest a generalized over-reactivity in comparison to lymphocytes from chimpanzees and that this over-reactivity was probably responsible for the catastrophic events of the anti-CD28 antibody clinical trial.
6. Regulation of gene expression by non-coding genomic elements
The last decade has witnessed a remarkable revolution in the understanding of the fine regulatory mechanisms controlling the patterns of gene expression of the complement of genes encoding translated proteins [45,46]. Indeed, numerous RNA regulatory elements that are not translated into proteins and that are capable of exerting a remarkable modulation of gene expression, collectively referred to as non-coding RNAs (ncRNAs), have been identified in the human genome [47–49]. These elements include miRNAs, long-intergenic non-coding RNAs (lincRNAs), and several other regulatory RNA species [50–58], including the recently identified circular RNAs [59–61]. It should be emphasized that the function of these novel RNA species is just beginning to be characterized. The most extensively studied ncRNA regulatory species are miRNAs [53–58]. MicroRNAs comprise a large family of small (21–23 nt) RNAs that exhibit a high degree of structural and functional conservation throughout species and their biogenesis is quite complex [62–64]. Binding of miRNA to the 3' untranslated regions (3'UTRs) of target genes is highly-specific and as a result of such sequence-specific binding miRNAs are capable of repressing the translation of target genes and/or inducing the degradation of target gene translated mRNA [65–68]. Binding to target mRNAs with perfect or near perfect complementarity induces mRNA degradation, whereas imperfect complementarity often induces translational repression. A 7–8 nt sequence in the 5' end of miRNAs, referred to as the “seed sequence”, is critical for efficient targeting. To date approximately 1,000 miRNAs encoded in the human genome have been identified and it has been shown that they are expressed in a tissue-specific and cell-type-specific manner. Individual miRNAs can target numerous mRNAs and individual mRNAs can be targeted by multiple miRNAs, providing a remarkably vast regulatory potential [59–64]. Although substantial progress has already been accomplished in the understanding of miRNA biogenesis and in the functional characterization of numerous miRNAs, the role of lincRNAs and other non-coding RNA species remains for the most part uncharacterized, except for notable progress with some of them such as HOTAIR [69–71]. Another level of complexity has recently been introduced regarding the regulatory role of non-coding RNA species with the identification of a new species of RNA, circular RNA. This novel species of RNA may play a crucial role in the regulation of miRNA function as they may act as reservoirs of large amounts of miRNAs and have numerous binding sites for specific miRNAs, a property that allows them to act as a miRNA sponge, effectively preventing the binding of miRNA to their specific targets [59–61].
Although the role of these regulatory RNA species is just beginning to become clarified, it has already become apparent that they may play crucial roles during normal development, as well as, in the pathogenesis of various genetically determined and, more importantly, acquired disorders. Furthermore, the existence of an organism-specific miRNA regulation for important protein-coding genes is very likely. Indeed, differential expression of certain miRNA has been described in the brains of human, chimpanzee, and macaques employing microarrays [72,73]. Moveover, of direct relevance to the topic discussed here, was the identification of miR-941 a human-specific miRNA which emerged de novo in the human lineage [74]. This human-specific miRNA is highly expressed in pluripotent cells and preferentially targets genes in the hedgehog and insulin-receptor pathways, pathways that have received recent attention for their possible involvement in the pathogenesis of SSc [75,76].
7. Other regulatory human-specific genomic changes
On the other hand, it is also likely that some important regulatory elements present in the chimpanzee genome may have been deleted and lost during evolution. Indeed, recent studies identified molecular events most likely to cause significant regulatory alterations in humans [3,77,78]. These alterations involved complete deletions of sequences highly conserved between chimpanzees and other mammals. These deletions occurred almost exclusively in non-coding regions of the human genome and, interestingly, were enriched in proximity to genes involved in steroid hormone signaling such as the androgen receptor and in various neural functions including elements involved in expression of specific brain regions in humans.
8. Role of ncRNA in the pathogenesis of autoimmune diseases
Numerous recent studies have examined the role of miRNAs in the pathogenesis of various autoimmune [79–89] and fibrotic disorders including SSc [90–100]. Furthermore, the involvement of human-specific miRNAs in the regulation of the phenotypic conversion of epithelial and endothelial cells into activated myofibroblasts has also been recently described [101–105]. Thus, the role of RNA regulatory species in the pathogenesis of these autoimmune diseases deserves attention and should be intrusively investigated as they may provide novel targets for therapeutic interventions.
9. Conclusion
The studies reviewed here provide strong support to the notion that human specific genes, the newly emerging classes of organism-specific ncRNAs and the existence of organism-specific aspects of mRNA-driven gene regulation, including miRNAs, long ncRNAs and other ncRNA species (Table I), may play a crucial role in the mechanisms that are responsible for the phenotypic differences between humans and chimpanzees with regard to the development of human specific autoimmune diseases. These potentially important mechanisms are illustrated diagrammatically in Figure 1. Thus, we posit that certain human autoimmune disorders including SSc and SS, which have not been described in chimpanzees, may occur exclusively in humans owing to increased or uncontrolled immunological reactivity in humans determined by either expression of human specific genes, or by alterations in regulatory networks. These alterations may be the result of the effects of human-specific genes or of differences in regulatory RNA species, such as human specific miRNAs and other short, circular, or long ncRNAs. We further posit that experimental investigations to explore this hypothesis may provide valuable information regarding the pathogenesis of these common and currently incurable systemic autoimmune disorders and may provide novel therapeutic targets for these disorders.
Table 1.
POTENTIAL MECHANISMS INVOLVED IN THE DEVELOPMENT OF HUMAN-SPECIFIC AUTOIMMUNE DISEASES
| • Human-specific post-translational protein modifications |
| • Acquisition of human-specific genes: |
| ◯ Gene duplication |
| ◯ Gene splicing |
| • Loss of functional non-human primate specific genes: |
| ◯ Deletions |
| ◯ Mutations |
| • Human-specific alterations in regulatory non-coding RNA: |
| ◯ piwi-interacting RNAs |
| ◯ microRNA |
| ◯ Long ncRNA |
| ◯ Long intergenic ncRNA |
| ◯ Circular RNA |
| • Human-specific mutations in long ncRNA protein-binding partners |
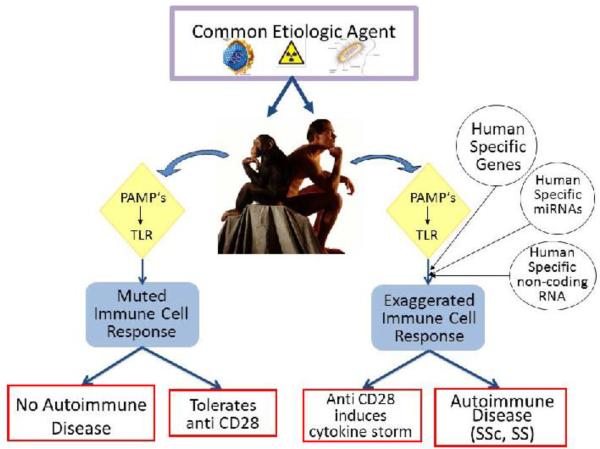
Figure 1. SCHEMATIC DIAGRAM ILLUSTRATING THE POSTULATED ROLE OF HUMAN SPECIFIC GENES AND OTHER HUMAN SPECIFIC GENOMIC REGULATORY ELEMENTS IN THE DEVELOPMENT OF AUTOIMMUNE DISEASES SUCH AS SSC AND SS.
A common etiologic agent is recognized by innate immunity cells through PAMP's (pathogen-associated molecular patterns) receptors which then activate specific toll like receptor (TLR) pathways to initiate adaptive immune responses. Some of these immune responses are muted in chimpanzees as evidenced for example by tolerance to anti CD28 antibodies, and, therefore, the chimpanzees do not develop SSc or SS. In contrast, in humans the immune response is exaggerated owing to the effects of either human-specific genes or to regulatory abnormalities mediated by human-specific miRNAs or human-specific short and long ncRNAs and as a result they develop SSc or SS.
Take Home Points
Human-specific genes and regulatory elements may play a role in autoimmune diseases not encountered in non-human primates such as Systemic Sclerosis (SSc) and Primary Sjögren's Syndrome (SS).
These unique genetic elements may include human-specific genes, microRNAs, or other non-coding RNAs species capable of modulating gene expression.
Although the etiology of SSc and SS is unknown, their exclusive occurrence in humans may result from uncontrolled immunological reactivity mediated by human-specific genes or non-coding RNA regulatory elements.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AM19606. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The expert assistance of Kenneth Brown is duly acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chimpanzee Sequencing and Analysis Consortium Initial sequence of the chimpanzee genome and comparison with the human genome. Nature. 2005;437:69–87. doi: 10.1038/nature04072. [DOI] [PubMed] [Google Scholar]
- 2.Clamp M, Fry B, Kamal M, et al. Distinguishing protein-coding and noncoding genes in the human genome. Proc Natl Acad Sci U S A. 2007;4(104):19428–33. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amadio JP, Walsh CA. Brain evolution and uniqueness in the human genome. Cell. 2006;126:1033–5. doi: 10.1016/j.cell.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Lambert N, Lambot MA, Bilhau A, et al. Genes expressed in specific areas of the human fetal cerebral cortex display distinct patterns of evolution. PLoS ONE. 2011;6:e17753. doi: 10.1371/journal.pone.0017753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konopka G, Bomar JN, Winden K, et al. Human-specific transcriptional regulation of CNS development genes by FOXP2. Nature. 2009;462:213–7. doi: 10.1038/nature08549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bian S, Sun T. Functions of noncoding RNAs in neural development and neurological diseases. Mol Neurobiol. 2011;44:359–73. doi: 10.1007/s12035-011-8211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salta E, DeStrooper B. Non-coding RNAs with essential roles in neurodegenerative disorders. Lancet Neurol. 2012;11:189–200. doi: 10.1016/S1474-4422(11)70286-1. [DOI] [PubMed] [Google Scholar]
- 8.Kouprina N, Pavlicek A, Mochida GH, et al. Accelerated evolution of the ASPM gene controlling brain size begins prior to human brain expansion. PLoS Biol. 2004;2:E126. doi: 10.1371/journal.pbio.0020126. Epub 2004 Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stahl PD, Wainszelbaum MJ. Human-specific genes may offer a unique window into human cell signaling. Sci Signal. 2009;2:59. doi: 10.1126/scisignal.289pe59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wainszelbaum MJ, Charron AJ, Kong C, et al. The hominoid-specific oncogene TBC1D3 activates Ras and modulates epidermal growth factor receptor signaling and trafficking. J Biol Chem. 2008;283:13233–42. doi: 10.1074/jbc.M800234200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandepoele K, Van Roy N, Staes K, et al. A novel gene family NBPF: intricate structure generated by gene duplications during primate evolution. Mol Biol Evol. 2005;22:2265–74. doi: 10.1093/molbev/msi222. [DOI] [PubMed] [Google Scholar]
- 12.Vandepoele K, Andries V, Van Roy N, et al. A constitutional translocation t(1;17) (p36.2;q11.2) in a neuroblastoma patient disrupts the human NBPF1 and ACCN1 genes. PLoS One. 2008;3:e2207. doi: 10.1371/journal.pone.0002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornan DM, Peirson SN, Hardcastle AJ, et al. Novel retinal and cone photoreceptor transcripts revealed by human macular expression profiling. Invest Ophthalmol Vis Sci. 2007;48:5388–96. doi: 10.1167/iovs.07-0355. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DN, Kehrer-Sawatzki H. Exploring the potential relevance of human-specific genes to complex disease. Hum Genomics. 2011;5:99–107. doi: 10.1186/1479-7364-5-2-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu YS, Kong J, Cheema GS, et al. The immunobiology of systemic sclerosis. Semin Arthritis Rheum. 2008;38:132–60. doi: 10.1016/j.semarthrit.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Chizzolini C. T cells, B cells, and polarized immune response in the pathogenesis of fibrosis and systemic sclerosis. Curr Opin Rheumatol. 2008;20:707–12. doi: 10.1097/BOR.0b013e32830c45ae. [DOI] [PubMed] [Google Scholar]
- 17.Sakkas LI, Chikanza IC, Platsoucas CD. Mechanisms of Disease: the role of immune cells in the pathogenesis of systemic sclerosis. Nat Clin Pract Rheumatol. 2006;2:679–85. doi: 10.1038/ncprheum0346. [DOI] [PubMed] [Google Scholar]
- 18.Del Galdo F, Artlett CM. T cells and B cells in the pathogenesis of systemic sclerosis: recent insights and therapeutic opportunities. Curr Rheumatol Rep. 2006;8:123–30. doi: 10.1007/s11926-006-0052-0. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson R, Vogelsang P, Volchenkov R, et al. The complexity of Sjögren's syndrome: novel aspects on pathogenesis. Immunol Lett. 2011;141:1–9. doi: 10.1016/j.imlet.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Peri Y, Agmon-Levin N, Theodor E, et al. Sjögren's syndrome, the old and the new. Best Pract Res Clin Rheumatol. 2012;1:105–17. doi: 10.1016/j.berh.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa O, Ishikawa H. Macrophage infiltration in the skin of patients with systemic sclerosis. J. Rheumatol. 1992 Aug;19(8):1202–6. [PubMed] [Google Scholar]
- 22.Kräling BM, Maul GG, Jimenez SA. Mononuclear cellular infiltrates in clinically involved skin from patients with systemic sclerosis of recent onset predominantly consist of monocytes/macrophages. Pathobiology. 1995;63(1):48–56. doi: 10.1159/000163933. [DOI] [PubMed] [Google Scholar]
- 23.Lafyatis R, York M. Innate immunity and inflammation in systemic sclerosis. Curr Opin Rheumatol. 2009;21:617–22. doi: 10.1097/BOR.0b013e32832fd69e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.York MR. Novel insights on the role of the innate immune system in systemic sclerosis. Expert Rev Clin Immunol. 2011;7:481–9. doi: 10.1586/eci.11.40. [DOI] [PubMed] [Google Scholar]
- 25.Bosello S, De Luca G, Tolusso B, et al. B cells in systemic sclerosis: a possible target for therapy. Autoimmun Rev. 2011;10:624–30. doi: 10.1016/j.autrev.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Sakkas LI, Xu B, Artlett CM, et al. Oligoclonal T cell expansion in the skin of patients with systemic sclerosis. J Immunol. 2002;168:3649–59. doi: 10.4049/jimmunol.168.7.3649. [DOI] [PubMed] [Google Scholar]
- 27.Mehra S, Walker J, Patterson K, et al. Autoantibodies in systemic sclerosis. Autoimmun Rev. 2013;12:340–54. doi: 10.1016/j.autrev.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Harris ML, Rosen A. Autoimmunity in scleroderma: the origin, pathogenetic role, and clinical significance of autoantibodies. Curr Opin Rheumatol. 2003;15:778–84. doi: 10.1097/00002281-200311000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Routsias JG, Tzioufas AG. Sjögren's syndrome-study of autoantigens and autoantibodies. Clin Rev Allergy Immunol. 2007;32:238–51. doi: 10.1007/s12016-007-8003-8. [DOI] [PubMed] [Google Scholar]
- 30.Tzioufas AG, Tatouli IP, Moutsopoulos HM. Autoantibodies in Sjögren's syndrome: clinical presentation and regulatory mechanisms. Presse Med. 2012;41:e451–60. doi: 10.1016/j.lpm.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 31.Heeney JL, Dalgleish AG, Weiss RA. Origins of HIV and the evolution of resistance to AIDS. Science. 2006;313:462–6. doi: 10.1126/science.1123016. [DOI] [PubMed] [Google Scholar]
- 32.Heeney J, Jonker R, Koomstra W, et al. The resistance of HIV-infected chimpanzees to progression to AIDS correlates with absence of HIV-related T-cell dysfunction. J Med Primatol. 1993;22:194–200. [PubMed] [Google Scholar]
- 33.Walker CM. Comparative features of hepatitis C virus infection in humans and chimpanzees. Springer Semin Immunopathol. 1997;19:85–98. doi: 10.1007/BF00945027. [DOI] [PubMed] [Google Scholar]
- 34.Bassett SE, Brasky KM, Lanford RE. Analysis of hepatitis C virus-inoculated chimpanzees reveals unexpected clinical profiles. J Virol. 1998;72:2589–2599. doi: 10.1128/jvi.72.4.2589-2599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen JI, Moskal T, Shapiro M, et al. Varicella in Chimpanzees. J Med Virol. 1996;50:289–92. doi: 10.1002/(SICI)1096-9071(199612)50:4<289::AID-JMV2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 36.Levine PH, Leiseca SA, Hewetson JF, et al. Infection of rhesus monkeys and chimpanzees with Epstein-Barr virus. Arch Virol. 1980;66:341–51. doi: 10.1007/BF01320630. [DOI] [PubMed] [Google Scholar]
- 37.Suntharalingam G, Perry MR, Ward S, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 38.St. Clair EW. The calm after the cytokine storm: lessons from the TGN1412 trial. J Clin Invest. 2008;118:1344–7. doi: 10.1172/JCI35382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hünig T. The storm has cleared: lessons from the CD28 superagonist TGN1412 trial. Nat Rev Immunol. 2012;12:317–8. doi: 10.1038/nri3192. [DOI] [PubMed] [Google Scholar]
- 40.Straud V, Kimberly R, Issacs JD. Bioligic therapies in rheumatology: lessons learned, future directions. Nat Rev Drug Discov. 2007;6:75–92. doi: 10.1038/nrd2196. [DOI] [PubMed] [Google Scholar]
- 41.Stebbings R, Findley L, Edwards C, et al. Cytokine storm in the phase 1 trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–31. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- 42.Eastwood D, Findlay L, Poole S, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol. 2010;161:512–26. doi: 10.1111/j.1476-5381.2010.00922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto PC, Stein LL, Hurtado-Ziola N, et al. Relative over-reactivity of human versus chimpanzee lymphocytes: implications for the human diseases associated with immune activation. J Immunol. 2010;184:4185–95. doi: 10.4049/jimmunol.0903420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Bleness M, Searles VB, Varki A, et al. Evolution of genetic and genomic features unique to the human lineage. Nat Rev Genet. 2012;13:853–66. doi: 10.1038/nrg3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gibcus JH, Dekker J. The context of gene expression regulation. F1000 Biol Rep. 2012;4:8. doi: 10.3410/B4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrett LW, Fletcher S, Wilton SD. Regulation of eukaryotic gene expression by the untranslated gene regions and other non-coding elements. Cell Mol Life Sci. 2012;69:3613–34. doi: 10.1007/s00018-012-0990-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khalil AM, Guttman M, Huarte M, et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A. 2009;106:11667–72. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guttman M, Amit I, Garber M, et al. Chromatin signature reveals over a thousand highly conserved large non-coding RNAs in mammals. Nature. 2009;458:223–7. doi: 10.1038/nature07672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mattick JS, Makunin IV. Non-coding RNA. Hum Mol Genet. 2006;15(Spec No 1):R17–29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- 50.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;16(43):904–14. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wapinski O, Chang HY. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–61. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 52.Moran VA, Perera RJ, Khalil AM. Emerging function and mechanistic paradigms of mammalian long non-coding RNAs. Nucleic Acids Research. 2012:1–10. doi: 10.1093/nar/gks296. doi:10.1093/nar/gks296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ambros V. The evolution of our thinking about microRNAs. Nat Med. 2008;14:1036–40. doi: 10.1038/nm1008-1036. [DOI] [PubMed] [Google Scholar]
- 54.Janga SC, Vallabhaneni S. MicroRNAs as post-transcriptional machines and their interplay with cellular networks. Adv Exp Med Biol. 2011;722:59–74. doi: 10.1007/978-1-4614-0332-6_4. [DOI] [PubMed] [Google Scholar]
- 55.Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y, Shen XJ, Zou Q, et al. Biological functions of microRNAs: a review. J Physiol Biochem. 2011;67:129–39. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 57.Li LC, Okino ST, Zhao H, et al. Small dsRNAs induce transcriptional activation in human cells. Proc Natl Acad Sci U S A. 2006:10317337–42. doi: 10.1073/pnas.0607015103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;l9:219–30. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 59.Memczak S, Jens M, Elefsinioti A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013 Feb 27; doi: 10.1038/nature11928. doi; 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 60.Hansen TB, Jensen TI, Clausen BH, et al. Natural RNA circles function as efficent microRNA sponges. Nature. 2013 Feb 27; doi: 10.1038/nature11993. doi; 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 61.Kosik KS. Circles reshape the RNA world. Nature. 2013 Feb 27; doi: 10.1038/nature11956. doi; 10.1038/nature11956. [DOI] [PubMed] [Google Scholar]
- 62.Saj A, Lai EC. Control of microRNA biogenesis and transcription by cell signaling pathways. Curr Opin Genet Dev. 2011;21:504–10. doi: 10.1016/j.gde.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki HI, Miyazono K. Emerging complexity of microRNA generation cascades. J Biochem. 2011;149:15–25. doi: 10.1093/jb/mvq113. [DOI] [PubMed] [Google Scholar]
- 64.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 65.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cannell IG, Kong YW, Bushell M. How do microRNAs regulate gene expression? BioChem Soc Trans. 2008:1224–31. doi: 10.1042/BST0361224. [DOI] [PubMed] [Google Scholar]
- 67.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev BioChem. 2010;79:351–79. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 68.Djuranovic S, Nahvi A, Green R. A parsimonious model for gene regulation by miRNAs. Science. 2011;331:550–3. doi: 10.1126/science.1191138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Woo CJ, Kingston RE. HOTAIR lifts noncoding RNAs to new levels. Cell. 2007;29(129):1257–9. doi: 10.1016/j.cell.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–6. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu HY, Guo S, Xi J, et al. MicroRNA expression and regulation in human, chimpanzee, and macaque brains. PLoS Genet. 2011;7:e1002327. doi: 10.1371/journal.pgen.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Somel M, Liu X, Tang L, et al. MicroRNA-Driven Developmental Remodeling in the Brain Distinguishes Humans from Other Primates. PLoS Biol. 2011;9:e1001214. doi: 10.1371/journal.pbio.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu HY, He L, Fominykh K, et al. Evolution of the human-specific microRNA miR-941. Nat Commun. 2012;3:1145. doi: 10.1038/ncomms2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Beyer C, Distler JH. Morphogen pathways in systemic sclerosis. Curr Rheumatol Rep. 2013;15:299. doi: 10.1007/s11926-012-0299-6. [DOI] [PubMed] [Google Scholar]
- 76.Hsu E, Feghali-Bostwick CA. Insulin-like growth factor-II is increased in systemic sclerosis-associated pulmonary fibrosis and contributes to the fibrotic process via Jun N-terminal kinase- and phosphatidylinositol-3 kinase-dependent pathways. Am J Pathol. 2008;172:1580–90. doi: 10.2353/ajpath.2008.071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLean CY, Reno PL, Pollen AA, et al. Human-specific loss of regulatory DNA and the evolution of human-specific traits. Nature. 2011;10:216–9. doi: 10.1038/nature09774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fairbanks DJ, Fairbanks AD, Ogden TH, et al. NANOGP8: Evolution of a Human-Specific Retro-Oncogene. G3 (Bethesda) 2012;2:1447–57. doi: 10.1534/g3.112.004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pauley KM, Cha S, Chan EK. MicroRNA in autoimmunity and autoimmune diseases. J Autoimmun. 2009;32:189–94. doi: 10.1016/j.jaut.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leng RX, Pan HF, Qin WZ, et al. Role of microRNA-155 in autoimmunity. Cytokine Growth Factor Rev. 2011;22:141–7. doi: 10.1016/j.cytogfr.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 81.Alevizos I, Illei GG. MicroRNAs in Sjögren's syndrome as a prototypic autoimmune disease. Autoimmun Rev. 2010;9:618–21. doi: 10.1016/j.autrev.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kapsogeorgou EK, Gourzi VC, Manoussakis MN, et al. Cellular microRNAs (miRNAs) and Sjögren's syndrome: candidate regulators of autoimmune response and autoantigen expression. J Autoimmun. 2011;37:129–35. doi: 10.1016/j.jaut.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Ceribelli A, Nahid MA, Satoh M, et al. MicroRNAs in rheumatoid arthritis. FEBS Lett. 2011;1:3667–74. doi: 10.1016/j.febslet.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wittmann J, Jäck HM. microRNAs in rheumatoid arthritis: midget RNAs with a giant impact. Ann Rheum Dis. 2011;70(Suppl 1):i92–6. doi: 10.1136/ard.2010.140152. [DOI] [PubMed] [Google Scholar]
- 85.Chatzikyriakidou A, Voulgari PV, Georgiou I, et al. miRNAs and related polymorphisms in rheumatoid arthritis susceptibility. Autoimmun Rev. 2012;11:636–41. doi: 10.1016/j.autrev.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 86.Thamilarasan M, Koczan D, Hecker M, et al. MicroRNAs in multiple sclerosis and experimental autoimmune encephalomyelitis. Autoimmun Rev. 2012;11:174–9. doi: 10.1016/j.autrev.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 87.Junker A. Pathophysiology of translational regulation by microRNAs in multiple sclerosis. FEBS Lett. 2011;585:3738–46. doi: 10.1016/j.febslet.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 88.Junker A, Hohlfeld R, Meinl E. The emerging role of microRNAs in multiple sclerosis. Nat Rev Neurol. 2011;7:56–9. doi: 10.1038/nrneurol.2010.179. [DOI] [PubMed] [Google Scholar]
- 89.Iborra M, Bernuzzi F, Invernizzi P, et al. MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun Rev. 2012;11:305–14. doi: 10.1016/j.autrev.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 90.Jiang X, Tsitsiou E, Herrick SE, et al. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015–21. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vettori S, Gay S, Distler O. Role of microRNAs in Fibrosis. Open Rheumatol J. 2012;6:130–9. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chau BN, Xin C, Hartner J, et al. MicroRNA-21 Promotes Fibrosis of the Kidney by Silencing Metabolic Pathways. Sci Transl Med. 2012;4:121–18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang J, Huang W, Xu R, et al. MicroRNA-24 Regulates Cardiac Fibrosis after Myocardial Infarction. J Cell Mol Med. 2012 Jan 19; doi: 10.1111/j.1582-4934.2012.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lakner AM, Steuerwald NM, Walling TL, et al. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012 Jan 25; doi: 10.1002/hep.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang S, Banerjee S, de Freitas A, et al. Participation of miR-200 in Pulmonary Fibrosis. Am J Pathol. 2012;180:484–93. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pandit KV, Milosevic J, Kaminski N. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;157:191–9. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 97.Wei J, Battacharyya S, Tourtellotte QG, et al. Fibrosis in systemic sclerosis: emerging concepts and implications for targeted therapy. Autoimmun Rev. 2011;10:267–75. doi: 10.1016/j.autrev.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhattachharyya S, Wei J, Varga J. Understanding fibrosis in systemic sclerosis: shifting paradigms, emerging opportunities. Nat Rev Rheumatol. 2011;8:42–54. doi: 10.1038/nrrheum.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu H, Li Y, Qu S, et al. MicroRNA Expression Abnormalities in Limited Cutaneous Scleroderma and Diffuse Cutaneous Scleroderma. J Clin Immunol. 2012 Feb 4; doi: 10.1007/s10875-011-9647-y. [DOI] [PubMed] [Google Scholar]
- 100.Kawashita Y, Jinnin M, Makino T, et al. Circulating miR-29a levels in patients with scleroderma spectrum disorder. J Dermatol Sci. 2011;61:67–9. doi: 10.1016/j.jdermsci.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 101.Zavadil J, Narasimhan M, Blumenberg M, et al. Transforming growth factor-beta and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–61. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 102.Korpal M, Lee ES, Hu G, et al. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghosh AK, Nagpal V, Covington JW, et al. Molecular basis of cardiac endothelial-to-mesenchymal transition (EndMT): Differential expression of microRNAs during EndMT. Cell Signal. 2012;24:1031–6. doi: 10.1016/j.cellsig.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ikushima H, Miyazono K. TGF-ß signal transduction spreading to a wider field: a broad variety of mechanisms for context-dependent effects of TGF-ß. Cell Tissue Res. 2012;347:37–49. doi: 10.1007/s00441-011-1179-5. [DOI] [PubMed] [Google Scholar]
- 105.Kumarswamy R, Volkmann I, Jazbutyte V, et al. Transforming growth factor-β-induced endothelial-to-mesenchymal transition is partly mediated by microRNA-21. Arterioscler Thromb Vasc Biol. 2012;32:361–9. doi: 10.1161/ATVBAHA.111.234286. [DOI] [PubMed] [Google Scholar]