Abstract
Allogeneic islet grafts are subject to rejection by both auto- and alloimmune responses when transplanted into diabetic individuals. T cells play a critical role in the initiation and perpetuation of both autoimmunity and allograft rejection. T cells up-regulate Fas and become sensitive to FasL-mediated killing following antigenic stimulation. Therefore, we tested if immunomodulation with an apoptotic form of FasL chimeric with streptavidin (SA-FasL) is effective in preventing the rejection of allogeneic C57BL/6 islet grafts in chemically diabetic NOD mice. C57BL/6 splenocytes and pancreatic islets were biotinylated and engineered to display the SA-FasL protein on their surface. Female NOD mice (6–7 weeks old) were treated with streptozotocin to induce diabetes and transplanted 5 days later with C57BL/6 islets engineered with SA-FasL in conjunction with transient treatment with rapamycin (3.0 mg/kg daily for days 0–19). Graft recipients were also systemically immunomodulated by intraperitoneal injection of 5 × 106 donor SA-FasL–engineered splenocytes on days 1, 3, and 5 after islet transplantation. This regimen resulted in the survival of all allogeneic islet grafts for the 250-day observation period, compared with a mean survival time (MST) of 14.2 ± 3.9 days for the control group. The survival effect was SA-FasL specific, with all NOD mice transplanted with control streptavidin protein–engineered islet grafts and treated with SA-engineered splenocytes under transient cover of rapamycin rejecting their grafts with an MST of 39.8 ± 8.5 days (P < .01). Taken together, these data demonstrate that immunomodulation with SA-FasL–engineered allogeneic islet grafts and splenocytes is effective in overcoming rejection in female NOD mice with preexisting autoimmunity with important clinical implications.
T cells play a critical role in initiating and perpetuating immune responses to allogeneic islets. Importantly, autoimmunity that causes the destruction of pancreatic beta cells is also initiated and perpetuated by T cells, because NOD mice transgenic for an insulin gene modified to lack a dominant CD4+ T-cell epitope do not develop spontaneous diabetes.1 There is considerable experimental and clinical evidence that allograft rejection in individuals with type 1 diabetes is subject to both auto- and alloreactive destructive immune responses.2 Therefore, T cells serve an important target for immunomodulation to prevent both auto- and alloreactive immune responses for long-term survival of allogeneic islet grafts and potential achievement of tolerance. Inasmuch as T cells up-regulate Fas receptor following antigenic stimulation and become sensitive to Fas-FasL-mediated killing,3 we assessed if immunomodulation with FasL serves an effective means of preventing allogeneic islet graft rejection in diabetes-prone female NOD mice. Given that FasL is apoptotic when expressed as a membranous protein and has no/minimal apoptotic activity and may serve as an antiapoptotic molecule in soluble form when cleaved from the cell surface by metalloproteinases,4 we recently generated a novel form of FasL protein chimeric with streptavidin (SA-FasL).5 We have shown that this molecule exists as tetramers and oligomers with potent apoptotic activity on Fas-expressing T cells.5 Importantly, this molecule can transiently be expressed on cells and tissues for immunomodulation.5–7 Indeed, we have recently demonstrated that the direct display of SA-FasL on allogeneic pancreatic islets was effective in preventing their rejection when transplanted under transient cover of rapamycin in a BALB/c-to-C57BL/6 mouse model that lacks preexisting autoimmunity to beta cells.6 In the present study, we investigated whether immunomodulation with SA-FasL protein has efficacy in preventing the rejection of allogeneic islets in female NOD recipients with preexisting autoimmunity.
METHODS
Mice and Recombinant Proteins
C57BL/6 mice served as donors for allogeneic splenocytes and pancreatic islets, and 6–7-week-old female NOD mice were used as recipients. Female NOD mice were purchased from Jackson Laboratory (Bar Harbor, ME), and C57BL/6 mice were bred and housed under specific pathogen-free conditions at our institution in accordance with the Institutional Guide for the Care and Use of Laboratory Animals. Recombinant SA-FasL and streptavidin (SA) proteins were produced in the laboratory using the Drosophila Expression System (DES) expression system (Life Technologies).8
Engineering Pancreatic Islets and Splenocytes with SA-FasL Protein
Pancreatic islets were harvested from 8–12-week-old C57BL/6 females under anesthesia with the use of a standard protocol as previously described.6 Islets were engineered by incubating in 5 μmol/L EZ-Link Sulfo-NHS-LC-Biotin solution (Thermo Scientific) in phosphate-buffered saline solution (PBS) at room temperature for 30 minutes followed by extensive washing to remove free biotin. Biotinylated islets were then incubated in PBS containing SA-FasL proteins (~200 ng protein/450-550 islets) or an equimolar amount of SA as a control at room temperature for 30 minutes. Engineered islets were washed 3 times with PBS and loaded into tubing for transplantation. Spleens were harvested from 8–12-week-old C57BL/6 females or males, processed into single-cell suspensions, and engineered with SA-FasL as previously described.8 Splenocytes engineered with an equimolar amount of SA protein served as control. The levels of SA-FasL and SA on the cell surface were assessed using fluorescein isothiocyanate–labeled antistreptavidin antibodies in flow cytometry.
Islet Transplantation and Systemic Treatment with Donor SA-FasL–Engineered Splenocytes
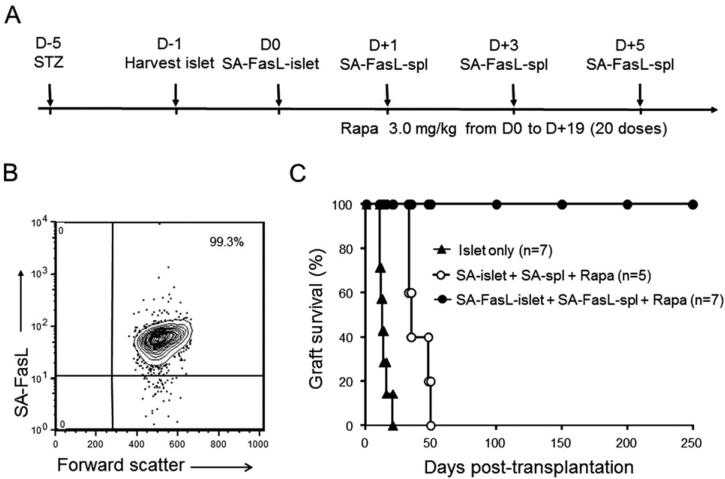
To expediate these studies, diabetes was induced in 6–7-week-old female NOD mice by intravenous injection of streptozotocin (220 mg/kg) and confirmed by 2 consecutive blood glucose readings >300 mg/dL. Diabetic mice were transplanted with SA-FasL– or SA-engineered C57BL/6 islets under the kidney capsule (450-550 islets/mouse). Recipients were injected intraperitoneally with 3.0 mg/kg rapamycin (LC Company) starting on the day of transplantation daily for 20 days. Graft recipients were also treated with 5 × 106 C57BL/6 SA-FasL–engineered splenocytes intraperitoneally on days 1, 3, and 5 after islet transplantation (Fig 1A). Animals were monitored for diabetes, and those with 2 consecutive daily measurements of >250 mg/dL blood glucose level were considered to be diabetic.
Fig 1.
SA-FasL–engineered allogeneic islet grafts survive indefinitely in chemically diabetic female NOD mice. (A) Schematic diagram of the immunomodulation protocol used in the present study. Female young (6–7 weeks old) NOD mice were made diabetic with the use of streptozotocin and then transplanted 5 days later with allogeneic female C57BL/6 SA-FasL–engineered islets (SA-FasL–islet) under the kidney capsule (~500 islets/mouse) in conjunction with transient use of rapamycin (Rapa; 3.0 mg/kg daily for 20 doses). Graft recipients were also given SA-FasL–engineered donor splenocytes (5 × 106 cells/injection) intraperitoneally on days 1, 3, and 5 after islet transplantation. Unmodified islets or streptavidin control protein–engineered islets (SA-islet) and splenocytes (SA-spl) were used as control samples. (B) Flow cytometric analysis of SA-FasL protein on the surface of engineered splenocytes. (C) Allogeneic islet graft survival.
RESULTS
Pancreatic islets and splenocytes were effectively engineered with SA-FasL protein with almost 100% efficacy, with SA-FasL protein detected on the surface of all pancreatic islets (not shown) and splenocytes (Fig 1B) as assessed by confocal microscopy and flow cytometry, respectively. All chemically diabetic female NOD mice transplanted with SA-FasL–engineered allogeneic C57BL/6 islets and treated with SA-FasL–engineered donor splenocytes under transient cover of rapamycin (3.0 mg/kg daily for 20 doses) achieved euglycemia during a 250-day observation period (Fig 1C). In marked contrast, recipients transplanted with control SA-engineered islet grafts and treated with SA-engineered donor splenocytes under the transient cover of rapamycin rejected their grafts with a mean survival time (MST) of 39.8 ± 8.5 days. However, this rejection rate was delayed compared with that of unmodified control grafts without donor splenocytes and rapamycin treatment (MST 14.2 ± 3.9 days; P < 0.01), possibly because of the rapamycin and/or SA-engineered splenocytes.
DISCUSSION
Allogeneic islet transplantation is an effective regimen for the treatment of type 1 diabetes.9 However, rejection reactions initiated and perpetuated by auto- and alloreactive T cells present a formidable barrier to widespread clinical application of allogeneic islet transplantation.9 The immunosuppressive agents used to control islet graft rejection in the clinic are not only ineffective, but may also contribute to the long-term graft failure owing to associated toxicity.9 Therefore, the development of novel immunomodulatory approaches that support indefinite islet allo-graft survival without chronic use of immunosuppression is sought. In the present study, we demonstrated that allogeneic islets engineered to display a novel form of SA-FasL protein with potent apoptotic activity on their surface when transplanted into diabetes-prone NOD recipients under the cover of a short course of rapamycin and systemic treatment with 3 doses of SA-FasL–engineered donor splenocytes have robust efficacy in supporting indefinite survival of allogeneic islet grafts. Inasmuch as insulitis is prevalent in 4-week-old female NOD mice,10 all 6–7-week-old female mice used in our study as graft recipients were expected to have a significant repertoire of autoreactive T cells. Complete lack of allogeneic islet rejection during the course of the 250-day observation period provides important evidence for the efficacy of our immunomodulatory regimen for the control of not only allo, but also autoreactive immunity. This prediction is consistent with our previous observations that SA-FasL protein preferentially induces apoptosis in autoreactive T-effector cells in NOD while sparing CD4+CD25+FoxP3+ T-regulatory (Treg) cells.11 Although we do not know the mechanistic basis of long-term allogeneic islet survival in the present study, it is tempting to speculate that active immune regulation, plausibly by Treg cells, may play a role. This notion is consistent with our recent studies demonstrating that SA-FasL–engineered allogeneic islets achieve localized tolerance by inducing Treg cells.6 Further studies are needed to assess if similar mechanisms play a role in the observed tolerance in spontaneous diabetes-prone NOD mice as used in this study.
Acknowledgments
Funding: American Diabetes Association ADA grant 1-12-BS-191, 5T32 HL076138-07 training fellowship (H.Z.), and Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
DISCLOSURE
The SA-FasL protein and ProtEx technology described in this article are licensed from the University of Louisville by ApoVax, Inc., Louisville, KY, for which H.S. serves as a Member of the Board and Chief Scientific Officer, and H.S. and E.S.Y. have significant equity interest in the company. The other author has no financial conflicts of interest.
REFERENCES
- 1.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Makhlouf L, Kishimoto K, Smith RN, et al. The role of autoimmunity in islet allograft destruction: major histocompatibility complex class II matching is necessary for autoimmune destruction of allogeneic islet transplants after T-cell costimulatory blockade. Diabetes. 2002;51:3202–3210. doi: 10.2337/diabetes.51.11.3202. [DOI] [PubMed] [Google Scholar]
- 3.Kabelitz D, Pohl T, Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly LA, Tai L, Lee L, et al. Membrane-bound Fas ligand only is essential for Fas-induced apoptosis. Nature. 2009;461:659–663. doi: 10.1038/nature08402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yolcu ES, Askenasy N, Singh NP, et al. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 6.Yolcu ES, Zhao H, Bandura-Morgan L, et al. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing T regulatory cells in mice. J Immunol. 2011;187:5901–5909. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askenasy N, Yolcu ES, Wang Z, et al. Display of Fas Ligand protein on cardiac vasculature as a novel means of regulating allograft rejection. Circulation. 2003;11:1525–1531. doi: 10.1161/01.cir.0000064893.96179.7e. [DOI] [PubMed] [Google Scholar]
- 8.Yolcu ES, Gu X, Lacelle C, et al. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol. 2008;181:931–939. doi: 10.4049/jimmunol.181.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorina P, Shapiro AM, Ricordi C, et al. The clinical impact of islet transplantation. Am J Transplant. 2008;8:1990–1997. doi: 10.1111/j.1600-6143.2008.02353.x. [DOI] [PubMed] [Google Scholar]
- 10.Signore A, Procaccini E, Toscano AM, et al. Histological study of pancreatic beta-cell loss in relation to the insulitis process in the non-obese diabetic mouse. Histochemistry. 1994;101:263–269. doi: 10.1007/BF00315913. [DOI] [PubMed] [Google Scholar]
- 11.Franke DD, Yolcu ES, Alard P, et al. A novel multimeric form of FasL modulates the ability of diabetogenic T cells to mediate type 1 diabetes in an adoptive transfer model. Mol Immunol. 2007;44:2884–2892. doi: 10.1016/j.molimm.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]