Abstract
Reverse-micelle forming amphiphilic homopolymers with carboxylic acid and quaternary amine substituents are used to selectively enrich biomarker peptides and protein fragments from human serum prior to matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) analysis. After depletion of human serum albumin (HSA) and immunoglobulin G (IgG), low abundance peptide biomarkers can be selectively enriched and detected by MALDI-MS at clinically relevant concentrations by using the appropriate homopolymer(s) and extraction pH value(s). Three breast cancer peptide biomarkers, bradykinin, C4a, and ITIH4, were chosen to test this new approach, and detection limits of 0.5 ng/mL, 0.08 ng/mL, and 0.2 ng/mL, respectively, were obtained. In addition, the amphiphilic homopolymers were used to detect prostate specific antigen (PSA) at concentrations as low as 0.5 ng/mL by targeting a surrogate peptide fragment of this protein biomarker. Selective enrichment and sensitive MS detection of low abundance peptide/protein biomarkers by these polymeric reverse micelles should be a sensitive and straightforward approach for biomarker screening in human serum.
INTRODUCTION
Biomarkers are biological compounds that can indicate normal or pathogenic biological processes or pharmacological responses to a therapeutic intervention.1, 2 Pathologically-relevant biomarkers are often low molecular weight peptides and proteins that are secreted into the bloodstream as a result of the disease process.3 Such peptides and proteins have had a tremendous impact on clinical management of human diseases, especially for cancer. Various analytical methods have been developed for the detection and discovery of biomarkers or biomarker patterns of major human diseases. Among these methods, enzyme-linked immunosorbent assays (ELISA) are the prominent tool for quantifying clinical biomarkers.4, 5 These immunoassays have extraordinary sensitivity and specificity for target analytes, but their development is costly ($100,000–$2 million per biomarker),6, 7 and they typically can only detect one analyte at a time. These limitations make immunoassays cumbersome for the detection of all biomarkers of interest. For the foreseeable future, though, ELISAs will likely remain the standard methods for detecting biomarkers in clinical settings, but research to develop more broadly applicable methods is necessary and on-going.
Mass spectrometry (MS) is recognized as a promising approach for detecting biomarkers, particularly multiple ones simultaneously.2, 8–10 Two general MS-based approaches are typically used for the analysis of proteins in biological fluids after appropriate enrichment and pre-fractionation steps – liquid chromatography with electrospray ionization mass spectrometry (LC/ESI-MS) and matrix-assisted laser desorption/ionization (MALDI) MS. LC/ESI-MS is a widely used tool for sensitive detection, identification, and quantification of peptide and protein biomarkers in biological fluids, especially because it can detect hundreds or even thousands of peptides/proteins in a given sample. MALDI-MS approaches do not usually detect as many different peptides and proteins as LC/ESI-MS methods, but they do provide nice advantages with regard to small sample sizes and high throughput when combined with specialized affinity enrichment/fractionation techniques such as surface enhanced laser desorption ionization (SELDI) chips.11–16 Indeed, MALDI-MS has become a powerful approach for screening novel disease-related biomarkers.17–22
While various MS-based approaches can and have successfully detected biomarkers in biological fluids, human serum still represents a challenging matrix for detecting peptides and proteins because of (i) its complexity, (ii) high levels of proteins (60–80 mg/mL), and (iii) compound concentration ranges spanning at least nine orders of magnitude. The complexity of serum, of course, also makes it a very informative source for finding biomarkers; however, 65–97% of serum proteins, by concentration, is HSA and IgG.23 The presence of these proteins makes detection of the less abundant peptides/proteins very difficult. Several protein depletion methods have been used to remove HSA and IgG effectively, allowing for further detection of low abundance peptide/protein biomarkers in serum samples.24, 25 Depleted serum samples can then be analyzed by MALDI-MS to detect peptides and proteins of potential interest in biological fluids.26–28 In most cases, though, peptides and protein biomarkers of low abundance still cannot be directly detectedwithout enrichment relative to other proteins in serum.
SELDI-MS has been applied extensively in biomarker research to help simplify peptide/protein analyses in serum by enriching peptides and proteins of interest via various affinity capture chemistries.11–14 Numerous other techniques have also been recently employed to effectively enrich and detect biomarkers of interest in biological fluids, such as a magnetic bead-based platforms,7 porous silicon-based arrays,27, 29 and solid-liquid phase change nanoparticles.30 These techniques have shown applicability for biomarker analyses, but they have not shown clinically relevant detection sensitivities, sufficient selectivity, or adequate simplicity, especially for the analysis of complex samples like serum.
Our group has been investigating the merits of amphiphilic homopolymers as peptide extraction materials in conjunction with detection by MALDI-MS. These materials provide an inexpensive, simple, and flexible means of fractionating peptides of interest for detection by MS. Reverse micelles formed by negatively charged polymer (I) or positively charged polymer (II) can be used to enrich peptides according to their pI values, and the pI cutoff can be readily tuned by adjusting the aqueous solution pH.31–34 In addition, the presence of the homopolymers during the MALDI analysis results in significant signal enhancements for enriched peptides, enabling reproducible ion signals at concentrations as low as 10 fM.33
Here, we investigate the capability of these polymeric reverse micelles to selectively target and detect low abundance peptide and protein biomarkers in human serum. Our previous work with these polymeric reverse micelles showed that peptides could be selectively detected in the presence of other peptides from simple protein digests. In this work, we demonstrate that they also can target peptides present at very low concentrations in much more complex mixtures (e.g. serum) when the appropriate combination of reverse micelles are used for extraction. Three potential breast cancer biomarkers (i.e. bradykinin, C4a, and ITIH4)35 were studied to test the merits of this approach. In addition, prostate specific antigen (PSA) was also selected as a test protein biomarker. We find that our amphiphilic homopolymers are capable of enriching the biomarkers of interest when the appropriate extraction conditions are chosen, and MALDI-MS is able to provide detection limits for these biomarkers in serum that are clinically relevant (sub ng/mL). We expect that these polymeric materials along with MALDI-MS detection could be a simple, yet powerful, approach for rapidly screening serum samples for disease biomarkers. The approaches described here are the first step toward coupling the polymeric extraction materials with LC/MS detection to provide a sensitive and quantitative analysis of biomarkers.
EXPERIMENTAL SECTION
Reagents
Pooled normal human serum was acquired from Innovative Research (Novi, MI). ProteoSeek™ HSA/IgG removal kit was obtained from Pierce Biotechnology (Rockford, IL). Bradykinin (MW 1060.2 Da, pI 12.5, RPPGFSPFR), C4a (MW 1626.8 Da, pI 11.5, NGFKSHALQLNNRQ), and ITIH4 (MW 2358.6 Da, pI 6.0, SSRQLGLPGPPDVPDHAAYHPF) were purchased from American Peptide Company (Sunnyvale, CA). Prostate specific antigen (PSA: MW 34 kDa, pI 7.6), dithiothreitol (DTT), trifluoroacetic acid (TFA), α-cyano-hydroxycinnamic acid (α-CHCA), acetonitrile (ACN), toluene, tris(hydroxymethyl)aminomethane (Tris), and tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl) were obtained from Sigma-Aldrich (St. Louis, MO). Immobilized trypsin was acquired from Promega (San Luis Obispo, CA). Tetrahydrofuran (THF) was purchased from Fisher Scientific (Pittsburgh, PA) and then distilled over Na/Ph2CO before use. All other chemicals were used as provided. The water used in preparation of all solutions was obtained from a Milli-Q water purification system (Millipore, Bedford, MA).
Reverse Micelle Formation
Negatively Charged Reverse Micelles Formed by Polymer I
The procedure for synthesizing the amphiphilic homopolymer I has been reported elsewhere.36, 37 The number average molecular weight (Mn) of the polymer, which was determined using size exclusion chromatography was found to be 23 kDa with a polydispersity index (PDI) of 1.30. Reverse micelle solutions of polymer I were prepared by dissolving 10 mg of homopolymer I in toluene to obtain a concentration of 1.0 x 10−4 M, and two equivalents of water per equivalent of carboxylic acid were added into toluene to create the water pool in the reverse micelle interiors. This solution was sonicated until an optically clear solution was obtained and then used for the liquid-liquid extraction.

Positively Charged Reverse Micelles Formed by Polymer II
Amphiphilic homopolymer II was synthesized according to our previously reported procedure.34 Reverse micelle solutions of polymer II were prepared by dissolving 10 mg of polymer II (Mn ~ 28 kDa, PDI = 1.22) in toluene for a final concentration of 1.0 x 10−4 M, and two equivalents of water per equivalent of quaternary ammonium group were added. The solution was sonicated until a visibly clear solution was obtained. This solution was then used for the liquid-liquid extraction.

Depletion of HSA and IgG from Human Serum
The ProteoSeek™ HSA/IgG removal kit was used to remove HSA and the major subclasses of IgG from unprocessed human serum. In this experiment, the immobilized Anti-HSA/Anti-IgG gel was swirled to obtain a homogeneous suspension, and then 660 μL of the gel slurry was added into a spin column using a cut pipette tip. Next 30 μL of unprocessed human serum containing spiked peptide/protein biomarkers was added to the gel slurry and the spin column was capped and vortexed briefly to form a homogeneous suspension. This mixture solution was incubated for 30 minutes at 37°C and vortexed every 5 minutes to keep the mixture suspended. The bottom of the spin column was removed and the column was placed into a Centricon tube and centrifuged at 1,000 x g for 2 minutes. The filtrate containing the serum sample with HSA and IgG removed was used for further analysis.
Tryptic Digestion
For the protein biomarker detection (i.e. PSA), tryptic digestion of human serum depleted HSA/IgG containing the PSA protein was performed prior to liquid-liquid extraction. The digestion protocol that was used was based on one previously reported.38 In this experiment, 300 μL of depleted human serum was prepared in a 400 μL solution with 50 mM Tris and 1 mM of CaCl2 at pH of 7.4. To this solution, 100 μL of milli Q water and 10 μL of DTT (1 M) were added. This solution was left at 37 °C for 45 min and 40 μL of ACN was added to the solution. Then, the mixture solution was heated at 60 °C for 20 min to denature proteins that were present in the sample before 40 μL of immobilized trypsin was added. The resulting solution was vortexed every 1 h to keep the mixture suspended during incubation at 37 °C for 12 h. The digestion was stopped by filtering the solution through a 10K molecular weight cutoff Centricon filter and centrifuged at 12000 rpm for 20 min. The filtrates were used for further analysis.
Liquid- Liquid Extraction Procedure
A two-phase liquid-liquid extraction protocol similar to one previously described by our group was used.31, 39 Initially, each targeted peptide or protein biomarker was spiked into unprocessed human serum and incubated in the human serum for 24 h before HSA/IgG depletion in order to better mimic the effect of endogenous proteases on analyte stability. This 24 h incubation time was very important for bradykinin because this peptide is known to partially degrade in serum or plasma.35 For peptide biomarker detection, HSA/IgG-depleted human serum was diluted in a solution of 50 mM Tris/Tris-HCl and adjusted to the desired pH using 0.5 M HCl or 0.5 M NaOH. Solutions that had their pHs adjusted outside the optimal buffering range of Tris (i.e. pH 7 – 9) were tested for pH stability after mixing the solution with the toluene phase that was used for the extraction. In all cases, the solution pH was found to be stable within ± 0.2 pH units. The liquid-liquid extraction was performed by first mixing 400 μL of toluene solution of the polymeric reverse micelles (1.0 x 10−4 M) with 800 μL of aqueous sample solution and then vortexing this mixture. Centrifugation at 12000 rpm for 30 min was employed to break the resulting emulsion, and the two phases were separated. The aqueous phase was removed and the organic phase was dried to obtain a solid residue. This dried residue was then redissolved in 15 μL of distilled THF and mixed with 30 μL of an α-CHCA matrix solution (0.16 M in 60:40:0.3% THF/H2O/TFA) for a final volume of 45 μL. 0.8 μL of this solution was directly spotted on an Anchorchip MALDI target for MALDI-MS analysis. The general extraction protocol for peptide and protein biomarkers is illustrated in Scheme 1. For some biomarkers (i.e. ITIH4, PSA), two-step extraction protocols were also used as described in the Results and Discussion section.
Scheme 1.
The general liquid-liquid extraction protocol for peptide and protein biomarker detection.
Instrumentation
A Bruker Autoflex III MALDI time-of-flight mass spectrometer was used to perform MALDI-MS analysis. All mass spectra were obtained in reflectron mode and represent an average of 500 shots acquired at 35% laser power. The accelerating voltage was set at 20 kV. To prepare the sample for analysis, 0.8 μL of the sample/matrix solution was spotted on hydrophilic area of an Anchorchip MALDI target using the dried droplet method. A Bruker Daltonics’ Anchorchip MALDI target containing very small hydrophilic spots surrounded by a larger hydrophobic area has been previously shown to provide very sensitive analysis of peptides in the presence of reverse-micelle forming polymers.40 Analyte-enriched zones or “hot spots” on the small hydrophilic area of an Anchorchip target, which can be observed by microscope on the MALDI instrument, were irradiated by the laser to acquire peptide ion signals.
RESULTS AND DISCUSSION
I. Effect of extraction pH
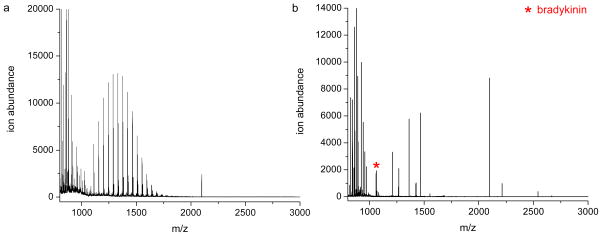
Initially, we investigated if our extraction protocol could even extract and detect peptides of interest from human serum. Serum was spiked with bradykinin at a final concentration of 5 x 10−8 M. Highly abundant serum proteins (e.g., HSA and IgG) are one of the major obstacles in proteomic analysis of human serum samples. Consequently, we used antibody columns to deplete HSA and IgG prior to extraction. After depletion of HSA and IgG, the serum was extracted by negatively charged reverse micelles at a pH of 7.7. In this case, only peptides and proteins with pI values above 7.7, including bradykinin (pI = 12.5), are expected to be extracted by the reverse micelles. MALDI spectra before and after extraction clearly indicate that bradykinin can only be detected after the serum sample is extracted (Figure 1). Before extraction, the MALDI spectrum (Figure 1a) is dominated by a distribution of polyethyleneglycol (PEG) ions with mass differences of 44 Da; PEG is used as a stabilizer in the antibody depletion buffer. These interferences mask most of the signal from peptides in serum. After extraction with the reverse micelles, however, PEG remains in the aqueous phase due to its hydrophilic nature, and peptides with pI values above 7.7 can be enriched and successfully detected in the organic phase (Figure 1b). This result demonstrates that our procedure can extract and detect bradykinin from complex human serum, but other serum peptides, including those with higher ion abundances than bradykinin, can also be extracted by reverse micelles at this pH. To confirm that the ion measured at m/z 1060 was indeed bradykinin, we performed control experiments in which the same procedure was used to extract human serum that had not been spiked with bradykinin. Figure S1 in the supporting information shows one example of the results from such experiments. It is clear that no ions are detected at m/z 1060, indicating that there are no interfering compounds in serum that are extracted at this pH and detected at m/z 1060. Similar control experiments were done for all the other extractions described below.
Figure 1.
MALDI mass spectra of human serum with bradykinin (5.0 x 10−8M) before (a) and after (b) extraction at pH 7.7 with the negatively charged polymeric reverse micelles. The distribution of peaks centered around m/z 1300 in (a) are polyethylene glycol ions from the HSA/IgG depletion column.
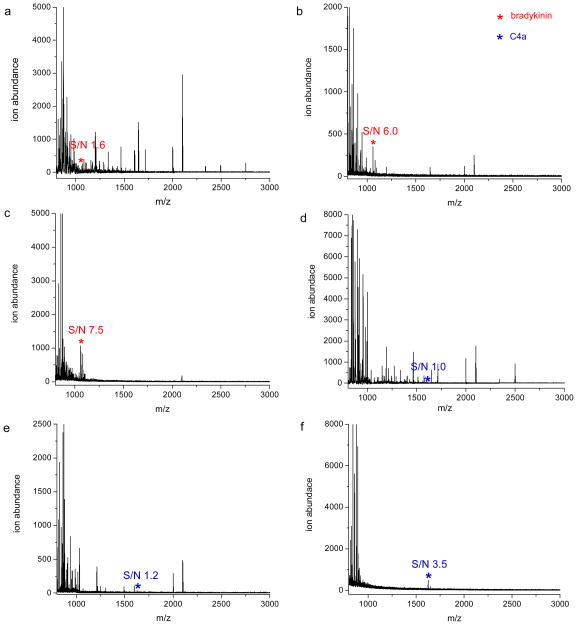
To lower the detection limit for bradykinin, we predicted that we would have to find an optimal extraction pH to minimize interferences from other compounds in serum and the likely signal suppression that is associated with the other ions observed in Figure 1b. Because electrostatic interactions are the main driving force for selective extraction of peptides by the polymeric reverse micelles31, 32, 34, 39, the effect of aqueous solution pH on the extraction selectivity of two peptide biomarkers (i.e. bradykinin and C4a) was investigated. Each peptide biomarker was spiked into human serum at various concentrations, and after HSA/IgG depletion, the sample was extracted at different aqueous phase pH values. Bradykinin and C4a are best detected at a high extraction pH value (10.1). This is probably due to the fact that these peptides have very high pI values and there are few peptides in serum with pI values above 10.1. Given this result, we selected this extraction pH to determine the approximate limit of detection (LOD) for these peptides in human serum. The estimated LOD values for bradykinin and C4a extracted at pH 10.1 with the negatively charged polymer are 5.0 x 10−10 M (0.5 ng/mL) and 5.0 x 10−11 M (0.08 ng/mL), respectively as shown in Figure 2. For comparison, MALDI spectra of spiked serum samples with 5.0 x 10−10 M bradykinin and 5.0 x 10−11 M C4a obtained at two other extraction pH values (6.8 and 8.7) are also demonstrated in Figure 2. Overall, these results indicate a more sensitive analysis of these peptide biomarkers is possible when only a subset of the serum peptides are extracted, and this can be accomplished by extracting the sample at a relatively high pH (10.1). The identities of bradykinin and C4a were confirmed by control experiments similar to that shown in Figure S1.
Figure 2.
MALDI mass spectra of human serum with 5.0 x 10−10 M (0.5 ng/mL) bradykinin and extracted at pH values of 6.8 (a), 8.7 (b) and 10.1 (c). MALDI mass spectra of human serum with 5.0 x 10−11 M (0.08 ng/mL) C4a and extracted at pH values of 6.8 (d), 8.7 (e) and 10.1 (f).
II. Extraction and detection of a peptide biomarker with a mid-range pI
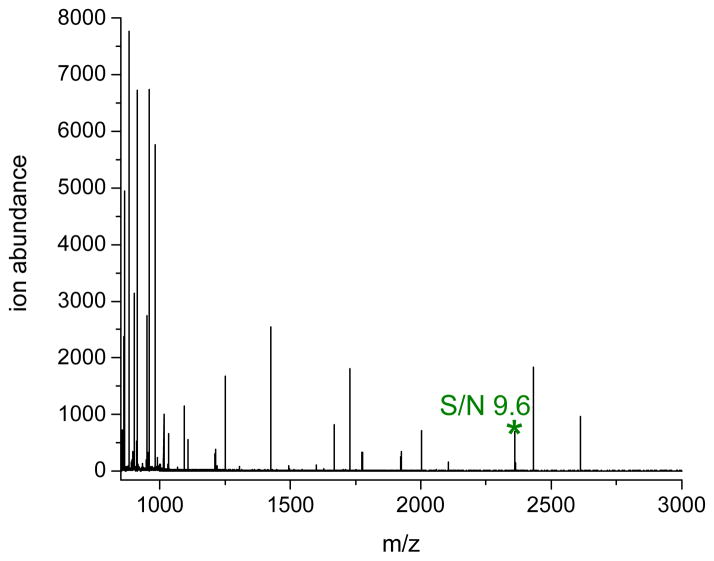
While use of a high extraction pH minimizes interferences from serum peptides, not all biomarkers are amenable to this approach because many peptides have lower pI values. Thus, we investigated the ability of our extraction protocol to enrich a peptide biomarker (i.e. ITIH4: pI 6.0) with a lower pI. According to the results shown in Figure 2a, 2b, 2d and 2e, many unknown peptides in human serum have pI values between 6.8 and 8.7. Therefore, extraction of ITIH4 peptide at a single aqueous solution pH might not result in the successful removal of these unknown peptides. To address this problem, we investigated a new two-step extraction procedure. After HSA/IgG depletion, the negatively charged polymer is first used to extract and remove the peptides and proteins with pI values above 7.4. Then, the remaining aqueous phase, still containing ITIH4, is extracted with the positively charged polymer (II) at the same pH (7.4). At this second step, only serum peptides or proteins with pI values below 7.4, which includes ITIH4, are expected to be enriched and detected by MALDI-MS. Using this two-step approach, we obtained an estimated LOD of 1.0 x 10−10 M (0.2 ng/mL) for ITIH4 (Figure 3). If only a single step extraction is used with the positively charged polymer at a pH of 7.4, our estimated LOD is higher (5.0 x 10−9 M; 12 ng/mL).
Figure 3.
MALDI mass spectrum of human serum with 1.0 x 10−10 M (0.2 ng/mL) ITIH4 and extracted by the positively charged polymer at pH 7.4.
A valid question to ask is why the second extraction with the positively charged polymers is necessary if the first extraction removes many of the interfering peptides. Why not simply analyze the remaining aqueous phase that contains ITIH4? There are three reasons for the second extraction. First, direct analysis of the resulting aqueous phase after extraction with the negatively charged polymer would give interfering signals from the PEG ions that are used as a stabilizer in the antibody depletion buffer. Second, and more importantly, the second extraction by the positively charged polymer achieves a concentration factor of almost 20 fold (800 μL / 45 μL) as the peptides are pulled into the organic phase that is eventually dried and reconstituted to a volume of 45 μL for analysis. And third, previous work has shown that MALDI analysis in the presence of the amphiphilic polymer results in a signal enhancement of almost 10 fold.33
It should be noted that most of the peptides found in Figure 3 have different m/z ratios than the peptides found after extracting with the negatively charged polymer at pH 6.8 (Figures 2a and 2d). These results (see Table S1 in the Supporting Information) follow our expectation that only peptides with relatively low pIs (< 7.4) should be extracted by the positively charged polymer at pH 7.4 (Figure 3); whereas, only peptides with relatively high pIs (> 6.8) should be extracted by the negatively charged polymer at pH 6.8 (Figures 2a and 2d). A few peptides show up in both cases (m/z 1248.9, m/z 1719.0 and m/z 2000.2), and it is possible that these peptides have pI values between 6.8 and 7.4.
III. Detection of protein biomarker
Because proteins are also important biomarkers, we next investigated if our protocol could sensitively detect proteins in human serum. Instead of detecting the protein directly, however, we chose to target tryptic peptide fragment(s) of the protein of interest (i.e. prostate specific antigen (PSA)). PSA is a diagnostic marker of prostate cancer.41 It has been reported that the clinically relevant LOQ for PSA is around 4 ng/mL, and if PSA concentrations exceed this level, a prostate biopsy is recommended.42–44 To find a peptide to act as a surrogate for PSA, we first digested PSA with immobilized trypsin and identified HSQPWQVLVASR (m/z 1408, pI = 11.1) as a suitable peptide fragment to use for detecting the presence of PSA (see Figure S2 in the Supporting Information).
Both one-step and two-step extractions were investigated to maximize the detection sensitivity for PSA. In both experiments, the protein was spiked into human serum and then HSA and IgG were removed. In the one-step extraction approach, the depleted serum was digested by immobilized trypsin and then extracted at pH 10.1 with the negatively charged reverse micelles. Using this approach, the LOD of PSA was found to be approximately 5.0 x 10−10 M (17 ng/mL) as shown in Figure 4a. Because several unidentified peptides are apparent in the spectrum, we suspected that high pI peptides from high pI proteins acted to suppress the signal for the targeted PSA peptide fragment, thereby preventing a lower LOD from being obtained. A possible solution to this problem is to use a two-step extraction approach in which the high pI proteins are first removed before enzymatically digesting the sample. To do this, we extracted and removed all peptide and protein components with pI values above 9.1 using the negatively charged polymer at an aqueous pH value of 9.1. During this pre-digestion extraction step, PSA, which has a pI of 7.6, is expected to remain in the aqueous phase because it has a pI below 9.1. Then, the pH of the remaining aqueous phase was adjusted to pH 7.4 and digested by immobilized trypsin. Before the second extraction step, the aqueous pH was increased to 10.1 and then extracted using the negatively charged reverse micelles. During this second extraction, all peptide fragments with pI values above 10.1, including our target PSA peptide (m/z 1408, pI = 11.1), are expected to be enriched and detected by MALDI-MS. The LOD of this two-step extraction approach was found to be approximately 1.5 x 10−11 M (0.5 ng/mL) as shown in Figure 4b. These results show that two-step extractions can significantly decrease the LOD value of our method more than one order of magnitude.
Figure 4.
a.) MALDI mass spectrum of extracted serum that was spiked with 5.0 x 10−10 M (17 ng/mL) of PSA and digested with immobilized trypsin. The negatively charged polymer was used for the extraction, and the aqueous phase pH was 10.1 b.) MALDI mass spectrum of extracted serum that was spiked with 1.5 x 10−11 M(0.5 ng/mL) of PSA, extracted to remove high pI peptides and proteins, and then digested with immobilized trypsin. The negatively charged polymer was used for both the pre- and post-digestion extractions. The pre-digest extraction was done at a pH of 9.1, and the post-digestion extraction was performed at a pH of 10.1.
Given the high selectivity and relative simplicity of our approach, we believe that our extraction protocol along with MALDI-MS analysis might be an alternative for early stage screening of PSA. Additional method optimization by using polymers with different functionalities might be able to further improve the sensitivity of our protocol and increase its application to other peptide and protein biomarkers.
CONCLUSION
We have shown that after HSA/IgG depletion, polymeric reverse micelles along with MALDI-MS detection can be used to sensitively detect peptide and protein biomarkers in human serum. By simply adjusting the extraction solution pH, target peptide biomarkers can be selectively enriched and detected by MALDI-MS at clinically relevant concentrations (i.e. bradykinin: 0.5 ng/mL, C4a: 0.08 ng/mL, ITIH4: 0.2 ng/mL). Our protocol is also able to detect the PSA protein at clinically relevant concentrations (0.5 ng/mL) by targeting a surrogate peptide fragment of this protein. Overall, in its current form our extraction protocol along with MALDI-MS detection could be useful for early stage screening of peptide and protein biomarkers. Ultimately, however, quantitative analyses of biomarkers are necessary. In this regard, we have begun to investigate the ability to couple our reverse micelle extraction approaches with LC/ESI-MS detection because of the superior quantitative ability of this method. Future work will describe these efforts.
Supplementary Material
Acknowledgments
This work was supported by the Office of Naval Research (N000140510501) and the National Science Foundation Center for Hierarchical Manufacturing (CMMI-0531171). This work was also partially supported by the NIGMS of the National Institutes of Health (GM-065255). We thank Dr. R. Thirumoorthy for the synthesis amphiphilic polymer precursor.
References
- 1.Atkinson AJ, Colburn WA, DeGruttola VG, Demets DL, Downing GJ. Clin Pharmacol Ther. 2001;69:89–95. [Google Scholar]
- 2.Neuhoff NV, Pich A. Drug Discovery Today: Technologies. 2005;2:361–367. doi: 10.1016/j.ddtec.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 3.Petricoin EE, Paweletz CP, Liotta LA. J Mammary Gland Biol Neoplasia. 2002;7:433–440. doi: 10.1023/a:1024042200521. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosi A, Airo F, Merkoci A. Anal Chem. 2010;82:1151–1156. doi: 10.1021/ac902492c. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Chen W, Qu L, Chen Y, He Q, Wang H, Wu J, Shou Z, Ju Z, Chen J. J Biomed Biotechnol. 2010;2010:121947–121951. doi: 10.1155/2010/121947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulovich AG, Whiteaker JR, Hoofnagle AN, Wang P. Proteomics Clin Appl. 2008;2:1386–1402. doi: 10.1002/prca.200780174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, Paulovich AG. Anal Biochem. 2007;362:44–54. doi: 10.1016/j.ab.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hawkridge AM, Muddiman DC. Annu Rev Anal Chem. 2009;2:265–277. doi: 10.1146/annurev.anchem.1.031207.112942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackermann BL, Hale JE, Duffin KL. Curr Drug Metab. 2006;7:525–539. doi: 10.2174/138920006777697918. [DOI] [PubMed] [Google Scholar]
- 10.Meng Z, Veenstra TD. J Proteomics. 2011 doi: 10.1016/j.jprot.2011.04.011. Article in Press. [DOI] [PubMed] [Google Scholar]
- 11.Issaq HJ, Conrads TP, Prieto DA, Tirumalai R, Veenstra TD. Anal Chem. 2003;75:148 A–155 A. [PubMed] [Google Scholar]
- 12.Bock MD, Seny DD, Meuwis MA, Chapelle JP, Louis E, Malaise M, Merville MP, Fillet M. J Biomed Biotechnol. 2010;2010:1–15. doi: 10.1155/2010/906082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu C. J Biomed Biotechnol. 2011;2011:1–6. doi: 10.1155/2011/245821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Léonard JF, Courcol M, Gautier JC. Methods Mol Biol. 2011:691. doi: 10.1007/978-1-60761-849-2_22. [DOI] [PubMed] [Google Scholar]
- 15.Tolson J, Bogumil R, Brunst E, Beck H, Elsner R, Humeny A, Kratzin H, Deeg M, Kuczyk M, Mueller GA, Mueller CA, Flad T. Lab Invest. 2004;84:845–856. doi: 10.1038/labinvest.3700097. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed FE. Current Proteomics. 2008;5:224–252. [Google Scholar]
- 17.Cazares LH, Diaz JI, Drake RR, Semmes OJ. Methods Mol Biol. 2008;428:125–140. doi: 10.1007/978-1-59745-117-8_7. [DOI] [PubMed] [Google Scholar]
- 18.Villanueva J, Philip J, Entenberg D, Chaparro CA, Tanwar MK, Holland EC, Tempst P. Anal Chem. 2004;76:1560–1570. doi: 10.1021/ac0352171. [DOI] [PubMed] [Google Scholar]
- 19.Tiss A, Smith C, Camuzeaux S, Kabir M, Gayther S, Menon U, Waterfield M, Timms J, Jacobs I, Cramer R. Proteomics. 2007;7:77–89. doi: 10.1002/pmic.200700746. [DOI] [PubMed] [Google Scholar]
- 20.Albrethsen J. Journal of Proteomics. 2011;74:765–773. doi: 10.1016/j.jprot.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Guo R, Pan C, Shen J, Liu C. J Cancer Res Clin Oncol. 2011;137:513–519. doi: 10.1007/s00432-010-0899-3. [DOI] [PubMed] [Google Scholar]
- 22.Batesona H, Saleema S, Loadmana PM, Sutton CW. J Pharmacol Toxicol Methods. 2011 Article in Press. [Google Scholar]
- 23.Adkins JN, Varnum SM, Auberry KJ, Moore RJ, Angell NH, Smith RD, Springer DL, Pounds JG. Mol Cell Proteomics. 2002;1:947–955. doi: 10.1074/mcp.m200066-mcp200. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed N, Barker G, Oliva K, Garfin D, Talmadge K, Georgiou H, Quinn M, Rice G. Proteomics. 2003;3:1980–1987. doi: 10.1002/pmic.200300465. [DOI] [PubMed] [Google Scholar]
- 25.Govorukhina NI, Gunnink AK, Van der Zee AGJ, De Jong S, De Bruijn HWA, Bischoff R. J Chromatogr A. 2003;1009:171–178. doi: 10.1016/s0021-9673(03)00921-x. [DOI] [PubMed] [Google Scholar]
- 26.Aresta A, Calvano CD, Palmisano F, Zambonin CG, Monaco A, Tommasi S, Pilato B, Paradiso A. J Pharm Biomed Anal. 2008;46:157–164. doi: 10.1016/j.jpba.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 27.Chen YQ, Bi F, Wang SQ, Xiao SJ, Liu JN. J Chromatogr B. 2008;875:502–508. doi: 10.1016/j.jchromb.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Hortin GL. Clin Chem. 2006;52:1223–1237. doi: 10.1373/clinchem.2006.069252. [DOI] [PubMed] [Google Scholar]
- 29.Yan H, Tajudin AA, Bengtsson M, Xiao SJ, Laurell T, Ekstrom S. Anal Chem. 2011 doi: 10.1021/ac200679t. [DOI] [PubMed] [Google Scholar]
- 30.Wang C, Sun Z, Ma L, Su M. Anal Chem. 2011;83:2215–2219. doi: 10.1021/ac103102h. [DOI] [PubMed] [Google Scholar]
- 31.Combariza MY, Savariar EN, Vutukuri DR, Thayumanavan S, Vachet RW. Anal Chem. 2007;79:7124–7130. doi: 10.1021/ac071001d. [DOI] [PubMed] [Google Scholar]
- 32.Rodthongkum N, Washington JD, Savariar EN, Thayumanavan S, Vachet RW. Anal Chem. 2009;81:5046–5053. doi: 10.1021/ac900661e. [DOI] [PubMed] [Google Scholar]
- 33.Rodthongkum N, Chen Y, Thayumanavan S, Vachet RW. Anal Chem. 2010;82:3686–3691. doi: 10.1021/ac1000256. [DOI] [PubMed] [Google Scholar]
- 34.Rodthongkum N, Chen Y, Thayumanavan S, Vachet RW. Anal Chem. 2010;82:8686–8691. doi: 10.1021/ac101922b. [DOI] [PubMed] [Google Scholar]
- 35.Broek IVD, Sparidans RW, Schellens JHM, Beijnen JH. J Chromatogr B. 2010;878:590–602. doi: 10.1016/j.jchromb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 36.Basu S, Vutukuri DR, Shyamroy S, Sandanaraj B, Thayumanavan S. J Am Chem Soc. 2004;126:9890–9891. doi: 10.1021/ja047816a. [DOI] [PubMed] [Google Scholar]
- 37.Basu S, Vutukuri DR, Thayumanavan S. J Am Chem Soc. 2005;127:16794–16795. doi: 10.1021/ja056042a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Govorukhina NI, Reijmers TH, Nyangoma SO, Van der Zee AG, Jansen RC, Bischoff R. J Chromatogr A. 2006;1120:142–150. doi: 10.1016/j.chroma.2006.02.088. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Escudero A, Azagarsamy M, Theddu N, Vachet RW, Thayumanavan S. J Am Chem Soc. 2008;130:11156–11163. doi: 10.1021/ja803082v. [DOI] [PubMed] [Google Scholar]
- 40.Rodthongkum N, Chen Y, Thayumanavan S, Vachet RW. Anal Chem. 2010;82:3686–3691. doi: 10.1021/ac1000256. [DOI] [PubMed] [Google Scholar]
- 41.Brawer MK. Seminars in Surgical Oncology. 2000;18:3–9. doi: 10.1002/(sici)1098-2388(200001/02)18:1<3::aid-ssu2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 42.Fortin T, Salvador A, Charrier JP, Lenz C, Lacoux X, Morla A, Choquet-Kastylevsky GV, Lemoine JM. Mol Cell Proteomics. 2009;8:1006–1015. doi: 10.1074/mcp.M800238-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ, Parnes HL, Coltman CA. Journal of the American Medical Association. 2005;294:66–70. doi: 10.1001/jama.294.1.66. [DOI] [PubMed] [Google Scholar]
- 44.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Mol Cell Proteomics. 2007;6:2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kulasingam V, Smith CR, Batruch I, Buckler A, Jeffery DA, Diamandis EP. J Proteome Res. 2008;7:640–647. doi: 10.1021/pr7005999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.