Abstract
The introduction of C4d in daily clinical practice in the late nineties aroused an ever-increasing interest in the role of antibody-mediated mechanisms in allograft rejection. As a marker of classical complement activation, C4d made it possible to visualize the direct link between anti-donor antibodies and tissue injury at sites of antibody binding in a graft. With the expanding use of C4d worldwide several limitations of C4d were identified. For instance, in ABO-incompatible transplantations C4d is present in the majority of grafts but this seems to point at ‘graft accommodation’ rather than antibody-mediated rejection. C4d is now increasingly recognized as a potential biomarker in other fields where antibodies can cause tissue damage, such as systemic autoimmune diseases and pregnancy. In all these fields, C4d holds promise to detect patients at risk for the consequences of antibody-mediated disease. Moreover, the emergence of new therapeutics that block complement activation makes C4d a marker with potential to identify patients who may possibly benefit from these drugs. This review provides an overview of the past, present, and future perspectives of C4d as a biomarker, focusing on its use in solid organ transplantation and discussing its possible new roles in autoimmunity and pregnancy.
Keywords: acute allograft rejection, chronic rejection, complement, transplantation, transplant pathology
In the 1950s it was generally accepted that allograft rejection occurred due to T-cell–mediated cellular cytotoxicity. The introduction of C4d staining in daily clinical practice in the late nineties aroused an ever-increasing interest in the role of antibody-mediated mechanisms in allograft rejection. C4d as a marker made it possible to visualize, for the first time, the direct link between anti-donor antibodies and tissue injury at sites of antibody binding. It is illustrative that C4d as a biomarker has been called ‘a magic marker’, because of its stability, its strong association with antibody-mediated rejection (AMR), and finally, its major impact on graft survival and patient treatment.1
However, with the expanding use of C4d by transplant pathologists worldwide, several shortcomings of C4d were identified, and C4d appeared to be a less-sensitive marker than initially thought. For instance, in ABO-incompatible transplantations C4d is present in the majority of grafts, but this does not seem to be alarming, and certainly does not seem to indicate acute AMR or an inferior graft prognosis.2 In addition, molecular studies provided insight, suggestive of a complement-independent form of AMR or C4d-negative AMR, in which C4d is obviously not helpful as a diagnostic tool.3
C4d is now increasingly recognized as a potential biomarker in other fields where antibodies can cause tissue damage, such as systemic autoimmune diseases and pregnancy. In all these fields, C4d holds promise to detect patients at risk for the consequences of antibody-mediated disease. Moreover, the emergence of new therapeutics that inhibit complement activation makes C4d a marker with potential to identify patients who may possibly benefit from these drugs.
This review provides an overview of the past, present, and future perspectives of C4d as a biomarker, focusing on its use in transplantation and discussing its possible new roles in autoimmunity and pregnancy. For this purpose, a group of experts were interviewed about the role of C4d within their fields of expertise and challenged to think about the following issues:
Will we still be using C4d in 10 years time, and if not, what alternatives would you suggest?
What would you like to investigate if you would receive funding to be spent on research in the field of C4d?
What is your take home message for readers and listeners?
The interviews form the backbone of this review, together with a review of the recent literature on C4d. We would like to motivate readers to listen to the audio files that can be found online (Supplementary Material online), which include highlights, quotes and authors’ comments on both the state of the art and controversies in the field of C4d. A summary of this reviews most important points is given in Box 1.
Box 1. Summary and take home points.
C4d is a widely used marker for antibody-mediated rejection in the kidney, heart, pancreas, and possibly lung allografts.
In ABO-incompatible grafts, C4d is not a useful test, and may even indicate graft accommodation.
In pregnancy, C4d at the fetal–maternal interface indicates antibody-mediated rejection of ‘the fetal allograft’, as was demonstrated in antiphospholipid antibody-induced fetal loss.
C4d shows that the complement system is involved: If complement targeted therapies will be part of our future treatment options, a marker such as C4d will be needed to identify patients susceptible for those kind of (expensive) treatments.
Alternatives for C4d are emerging (genomics, molecular diagnostics, and endothelial transcripts) and if proven useful, effort will be made to transform these techniques or their progeny to practical tests.
BIOLOGY OF C4d
The human complement system
The complement system is an ancient component of the innate immune system. Complement activation is a non-specific, potent force. Once activated, it makes no distinction between self and non-self. Therefore, its activation is as tightly controlled as its natural regulation.2 The three pathways by which the complement system can become activated, namely, the classical, lectin, and alternative pathway, converge at the level of C3 and proceed into the formation of the membrane attack complex on complement-activating surfaces, causing direct tissue injury by perforation of the cell membrane. In addition, potent anaphylatoxins C3a and C5a are being formed in the process, which elicit the recruitment of other inflammatory cells to the site of activation (Figure 1a).
Figure 1. The complement system and C4d.
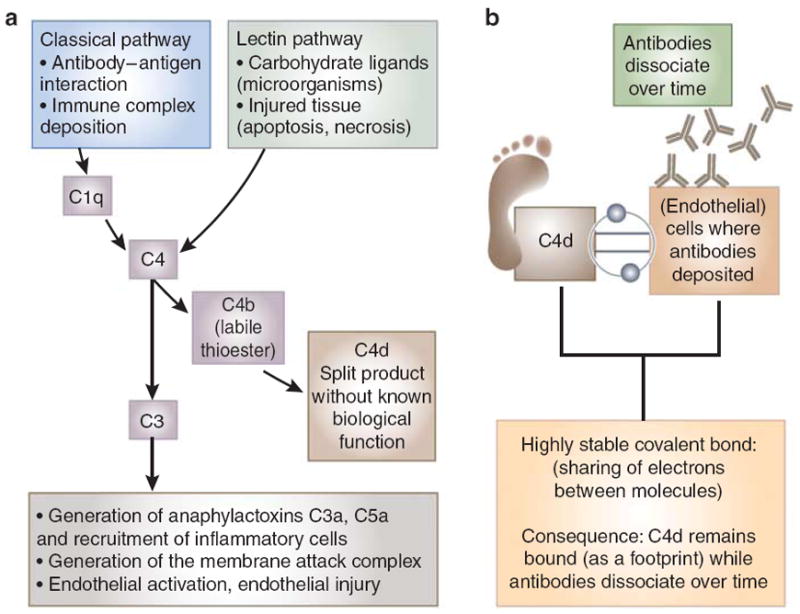
(a) The classical pathway of complement is initiated via binding of its recognition molecule C1q to immune complex deposits, antibody-antigen binding or charged molecules. When C1q becomes activated, it subsequently activates its natural substrate C4. C4d is a split product of C4 activation, without a biological function. Although C4d is mainly interpreted as a trace of classical pathway activation, it must be kept in mind that C4 can also be derived from the lectin pathway. Mannose-binding lectin (MBL) or ficolins binding to carbohydrate ligands on the surface of a wide variety of pathogens results in activation of the lectin pathway and cleavage of C4. Consequently, C4d may be generated without prior antibody binding. Classical complement activation converges with other pathways at the level of C3 and proceeds into the formation of the membrane attack complex on complement-activating surfaces, causing direct tissue injury by perforation of the cell membrane. In addition, potent anaphylatoxins C3a and C5a are being formed in the process, which elicit the recruitment of other inflammatory cells to the site of activation. (b) C4d as a footprint for antibody-mediated tissue injury. C4b, the larger molecule that C4d is derived from, has an internal thioester in the molecule, giving it the ability to form a covalent bond with target cells. When C4d is cleaved from C4b, the covalent bond between C4d and the tissue remains intact. Covalently bound C4d has a higher chance to remain at the site of complement activation than the antibodies themselves, which dissociate over time. C4d is anchored tightly to the tissue and therefore acts as a footprint of antibody-mediated tissue injury.
The classical pathway and generation of C4d
The classical pathway of complement is initiated via binding of its recognition molecule C1q to immune complex deposits, antibody-antigen binding, or charged molecules. When C1q becomes activated, it subsequently activates its natural substrate C4. C4d is a split product of C4 activation, without a biological function.1,4 Although C4d is mainly interpreted as a trace of classical pathway activation, it must be kept in mind that C4 can also be generated via the lectin pathway. Mannose-binding lectin (MBL) or ficolins binding to carbohydrate ligands on the surface of a wide variety of pathogens results in activation of the lectin pathway and cleavage of C4.5,6 Consequently, C4d may be generated without prior antibody binding (Figure 1a).
C4d as a footprint of antibody-mediated cell injury
It is interesting that C4d is a biomarker even though it is an inactive split product of the complement cascade. C4d has been called ‘a footprint’ of antibody-mediated tissue injury. This nickname is based on the unusual phenomenon that C4b, the larger molecule that C4d is derived from, has an internal thioester in the molecule, giving it the ability to form a covalent bond with any free hydrogen group on target cells. When C4d is cleaved from C4b, the covalent bond between C4d and the tissue remains intact. Covalently bound C4d has a much longer half-life, and therefore remains at the site of complement activation, whereas antibodies bind to tissue by hydrostatic, van der Waals type of interactions. The ‘footprint effect’ of the internal thioester of C4d (Figure 1b) becomes strikingly apparent when the blood stream can clear all soluble/weakly bound molecules quickly, as happens with antibodies at endothelial surfaces. Covalently bound C4d will not be affected, because it is anchored tightly to the tissue and therefore serves as a footprint of antibody-mediated tissue injury.
THE DISCOVERY OF C4d AS A CLINICAL MARKER FOR AMR
From hyperacute rejection episodes it was known that donor-specific antibodies (DSAs), either anti-human lymphocyte antigen (HLA) or anti-ABO, had the capacity to destroy a graft.7-9 However, although there was speculation about a role for allo-antibodies in other forms of rejection apart from the hyperacute form, it was unclear what fraction of acute rejection episodes had a humoral component and how to recognize that an AMR was present. The publications of Feucht et al.10,11 in the early nineties marked a turning point in the history of solid organ transplantation. Feucht showed that patients with suspected antibody-mediated injury in the renal graft had a linear C4d staining pattern in peritubular capillaries and that the presence of C4d was associated with impaired graft function. Remarkably, these initial publications received relatively little attention in the transplant community.10,12,13 At the turn of the century the group of Collins et al.14 tested for presence of C4d along with other markers of endothelial activation or injury in renal transplant biopsies suspected of AMR. C4d was found in each of 10 renal biopsies of patients with circulating DSA and morphological signs suspicious of AMR, and in none of 14 controls with acute cellular rejection without detectable DSA.14 This work embodied the connection of dots between C4d, the presence of DSA and a selection of histomorphological features of AMR, which after four decades of an intensive search for a marker was nothing short of revolutionary. A few years later, the correlation with graft survival that Feucht et al. had already reported on in 1993 was confirmed by other groups.4,15 This led to general acceptance of the usefulness of C4d in the identification of acute AMR. In 2003 ‘C4d’ was incorporated in the Banff classification16 (Box 2).
Box 2. State of the art: Banff guidelines for the diagnosis of AMR.
-
The diagnosis of antibody-mediated rejection (AMR) in renal allografts is currently based on criteria established during the Banff conference on Allograft Pathology in 2007, which include the three following cardinal features:
Morphologic evidence of acute or chronic tissue injury
Immunopathological staining for C4d in peritubular capillaries
Presence of circulating antibodies to donor human lymphocyte antigen or other antigens expressed on donor endothelial cells.
It is recommended that every renal, cardiac, and pancreas allograft biopsy should be stained for C4d.
-
C4d staining is considered positive only when depositions are found in the following anatomical locations:
-
C4d is scored semiquantitatively in four categories:
No C4d staining (0% of (peritubular) capillaries)
Minimal C4d staining (0–10% of (peritubular) capillaries)
Focal C4d staining (10–50% of (peritubular) capillaries)
Diffuse C4d staining (>50% of (peritubular) capillaries)
Immunofluorescence is a more sensitive method to detect C4d than immunohistochemistry, by approximately one grade of the scoring system.
Although a diffuse C4d staining is defined as positive, the definition and clinical significance of ‘minimal’ and ‘focal’ C4d staining remain debated issues. Most experts consider a focal staining pattern as a red flag, especially when detected on paraffin-embedded tissue or in the presence of donor-specific antibody and/or suspicious histopathological features.
C4D IN CURRENT CLINICAL PRACTICE
For the kidney a consensus was reached that a diagnosis of AMR requires the simultaneous presence of DSA, distinguishable histopathological findings and deposition of C4d in peritubular capillaries (Figures 2 and 3a). Most centers involved in the management of transplant recipients have incorporated routine C4d staining in diagnostic pathology evaluation of all renal allograft biopsies.16 A solid base for regular C4d staining of biopsied allograft tissue is now established for heart transplantation and pancreas transplantation.17-19 For other transplanted organs such as the lung, the usage of C4d staining is still controversial.20,21 In liver and short bowel transplantation C4d seems to have no additional diagnostic value.22
Figure 2. Diagnosing acute antibody-mediated rejection.
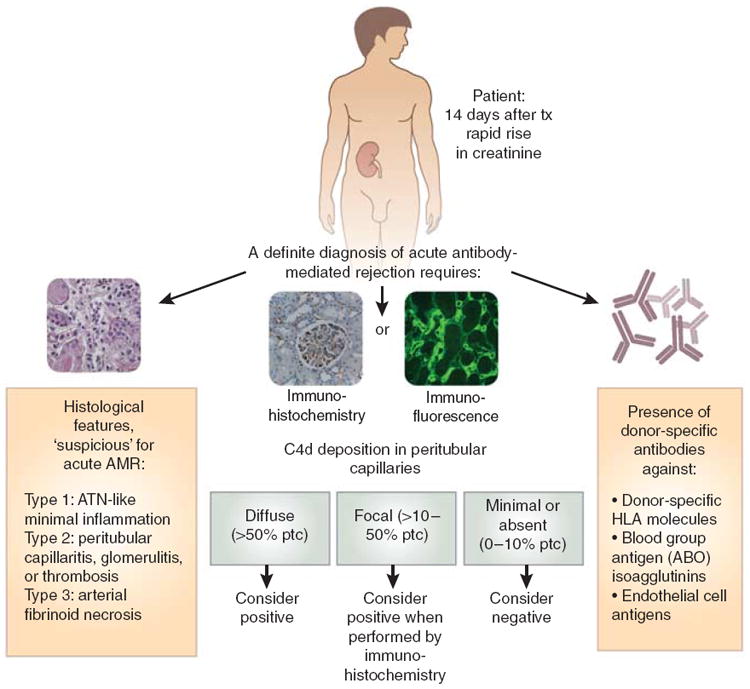
This flowchart shows that the diagnosis of acute antibody-mediated rejection requires the presence of histological features, a positive C4d stain, and the presence of donor-specific antibodies. AMR, antibody-mediated rejection; ATN, acute tubular necrosis; HLA, human lymphocyte antigen; tx, treatment.
Figure 3. C4d staining patterns in different clinical settings.
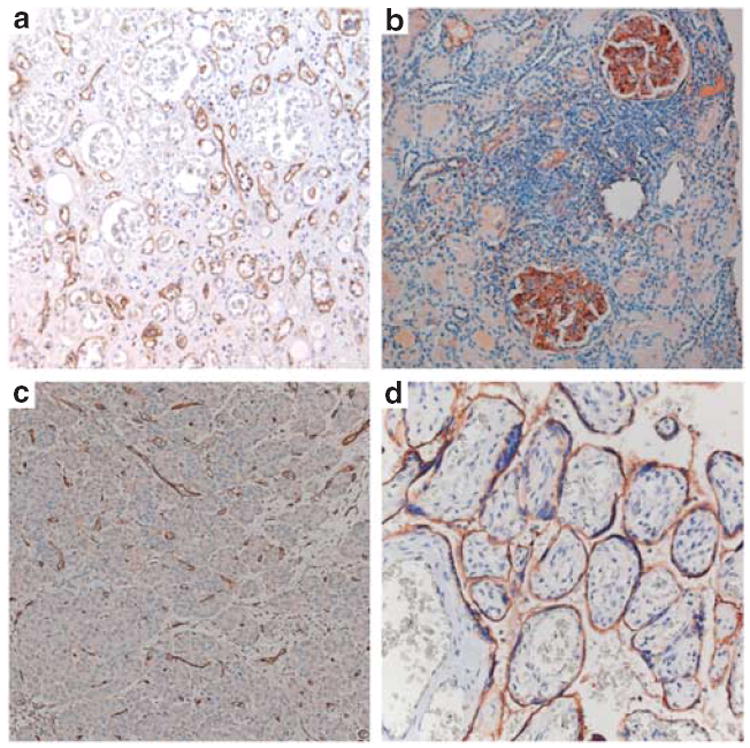
(a) Acute AMR of a kidney graft with typical peritubular capillary staining of C4d on paraffin-embedded tissue. (b) Glomerular C4d in a native kidney biopsy of a patient with lupus nephritis and thrombotic microangiopathy. (c) C4d in a pancreas graft with typical staining of C4d in interacinar capillaries, suggestive for AMR. (d) Placental C4d in a placenta from a patient with antiphospholipid syndrome and an intrauterine fetal death in this pregnancy. C4d is positive at the fetal–maternal interface on the maternal side of the syncytiotrophoblast, suggesting severe antibody-mediated injury leading to impaired placental development, impaired nutrient exchange, intrauterine fetal growth restriction, and finally, fetal death. AMR, antibody-mediated rejection.
In Box 3, guidelines are given on how to interpret various test outcomes of C4d staining and DSA test results that clinicians who work with C4d commonly encounter in daily clinical practice. Furthermore, detailed information on how a C4d stain should be best performed is given in the Supplementary Material. Finally, Box 4 elaborates on current treatment options for AMR.23-26
Box 3. Issues for clinicians working with C4d in daily practice.
-
Focal staining
In paraffin-embedded tissue combined with a positive test for donor-specific antibody (DSA) and histopathological features: Most laboratories consider this as ‘positive for antibody-mediated rejection (AMR)’. It is advised to treat the patient.
In frozen tissue combined with a positive test for DSA and histopathological features: This is sometimes called a ‘probable AMR’. Most experts would consider treatment, however, prospective studies should investigate this group further.
-
C4d positivity without detectable DSA
-
Possible explanations:
Allo-antibodies are present, but they are not detected by standard anti-HLA assays (for example, anti-endothelial antibodies).
Allo-antibodies are absorbed by the graft, as is sometimes shown by reappearance of allo-antibodies in the blood after graft nephrectomy.
Allo-antibodies are not present and C4d deposition is caused by something else than DSA (for instance autoimmune disease, i.e., lupus nephritis or any form of lectin pathway activation).
-
How to proceed?
In case of diffuse C4d staining in peritubular capillaries and histological evidence for AMR: Most experts would advise to treat the patient for AMR.
In case of focal C4d staining and histological evidence for AMR: Check for other possible underlying diseases. If no other cause can be found: Treat the patient for AMR.
In case of focal or diffuse C4d staining and absence of histological changes the decision as to whether to treat for AMR is more uncertain. Treating or close follow-up are both suitable options.
-
-
C4d positivity in grafts without histological abnormalities
In ABO-incompatible grafts: In case of no histopathology and no graft dysfunction: It is suggested to interpret this as ‘graft accommodation’. No treatment is necessary, but close follow-up is strongly advised, as it is unknown what happens with these grafts during long-term follow-up.
In positive cross-matched patients (presensitized patient), this should not be seen as graft accommodation and is a rare and more worrying situation than the above: In case of normal histology and no graft dysfunction: Interpret as probable AMR and consider treating the patient. Prospective studies on the long-term follow-up of this group of patients are awaited.
Box 4. Therapy options for AMR.
Although much progress has been made in understanding the etiology of antibody-mediated rejection (AMR) and diagnosing the condition, it remains to be elucidated what treatment option is most beneficial. AMR is relatively unresponsive to therapies targeting T-lymphocytes used in acute cellular rejection such as steroids, cyclosporine, tacrolimus, and sirolimus. This issue has not been addressed in a randomized controlled fashion so far. Therapeutic strategies reported in the literature in case reports, case series, and cohort studies are the following:23
The suppression of the T-cell–dependent antibody response (steroids, cyclosporine, tacrolimus, and sirolimus)
The removal of donor reactive antibody (plasmapheresis)
The blockade of the residual allo-antibody (IvIg)
The depletion of naive and memory B cells (rituximab)
The blockade of complement component C5 by monoclonal antibodies (eculizumab)
There is large variability between transplant centers around the world in their specific therapeutic approach, but generally a combination of IvIg, plasmapheresis, and recently, rituximab is used. Trials (especially randomized controlled trials) investigating the effects of targeted complement blockade are awaited.
DEBATED ISSUES 1: C4D IN CHRONIC REJECTION EPISODES
Soon after the introduction of C4d in daily clinical practice, the phenomenon of a diffuse C4d staining pattern was frequently observed years after transplantation and was associated with chronic changes in the graft.27,28 This was in contrast with the idea that antibodies were only involved in hyperacute and acute rejection episodes. The presence of C4d in the late and chronic rejection episodes prompted clinicians and researchers to the hypothesis that an antibody component was present in forms of chronic rejection.
The concrete arguments that underline the role of antibodies in chronic rejection are as follows: First, in experimental models of non-human primates with transplanted kidneys (with no immunosuppressive drugs) progression to chronic graft injury and loss consistently goes through four stages: alloantibody production, deposition of C4d in peritubular capillaries and sometimes glomeruli, chronic histopathological changes, and finally, graft loss.29
Second, several large (prospective) studies showed that presence of circulating anti-HLA antibodies are associated with late graft failure.30-32 Third, histological changes associated with late graft loss, such as glomerular double contours, peritubular capillary basement membrane multilayering, interstitial fibrosis, and fibrous intimal thickening in arteries, are found in close association with C4d deposition in peritubular capillaries and presence of anti-HLA antibodies: In about 30–40% of biopsies with late graft dysfunction, C4d can be detected in peritubular capillaries.27,32-36
On the basis of the current understanding, criteria were proposed to diagnose chronic AMR in 2007. To establish this diagnosis, three elements should be present:
Histological evidence of chronic injury
Immunopathological evidence of antibody-mediated graft injury (C4d deposition)
Evidence of antibodies reactive against the donor
However, there are several controversies surrounding chronic AMR. Most importantly there is no clear-cut definition of what is meant by ‘chronic’. For some it simply means burned-out scar formation. Others use the word in a broader sense, thinking that there are chronic changes with some kind of activity so that chronicity still has an ongoing active component (and thus, a potential for treatment). Here the advantage of C4d is that it indicates recent (weeks) activity of an active immunological process.
It is unknown why DSAs cause acute rejection in one patient and chronic rejection in another, or even both sequentially in the same patient. Factors such as antibody titer, antibody avidity, and the extent of resistance (or accommodation) of the graft endothelium to complement activation could be responsible for this phenomenon. More research in this field is certainly needed, as the lack of insight into the natural history of chronic AMR now entails that the optimal therapy for chronic AMR remains undetermined.
DEBATED ISSUES 2: C4d-NEGATIVE AMR
Antibodies mainly damage a graft by targeting the endothelium of the graft’s microcirculation. This concept is the basis for the molecular studies performed by Sis et al.33 since 2007. These studies have elegantly uncovered a possible new form of AMR, namely, C4d-negative AMR (which has been described in a chronic, but not in acute settings). This important finding is currently the most interesting challenge for the concept of C4d as a biomarker, as the first studies using endothelial transcripts combined with DSA show excellent sensitivities for AMR (although less specificity than C4d), for chronic AMR.3 As a marker of antibody interaction with the tissue, it is not inconceivable that this or a simpler derivative method will partly or fully replace C4d in future. In 2007, a retrospective study of biopsies from 1320 transplanted patients showed that more than 40% of cases with transplant glomerulopathy—a histological lesion considered relatively characteristic for chronic AMR—were C4d negative, despite the fact that anti-HLA antibodies were detected in 73% of patients.33 This work was followed by studies looking at mRNA levels of genes involved in endothelial activation and injury. Interestingly, biopsies with high expression of these endothelial transcripts in combination with circulating DSA showed concurrent histopathological lesions of AMR (such as capillaritis, glomerulitis, transplant glomerulopathy, and fibrosis/atrophy) and had poor outcomes.3,37 Many of these active AMR cases would have been missed otherwise: Only 40% of kidneys with high endothelial gene expression and histopathological signs of chronic AMR were C4d positive.37
So far, two groups have confirmed the concept of C4d-negative AMR. In sensitized recipients, Loupy et al.38,39 showed that C4d or capillaritis in 3-month protocol biopsies were risk factors for later transplant glomerulopathy, and capillaritis was predictive even in the absence of C4d. The other evidence came from Haas and Mirocha,40 who investigated patients with DSAs who had a biopsy during the first 3 months after transplantation. Patients with a C4d-negative biopsy who were not treated for AMR had a higher rate of progression to transplant glomerulopathy than those who were treated for AMR post-biopsy.40
Because of these complexities a working group was established at the 2011 Banff Conference to refine criteria for diagnosis of chronic AMR in the kidney, and to investigate whether C4d-negative AMR should be incorporated in the Banff classification.
If there is indeed a C4d-negative form of AMR, it presumably is due to a pathophysiological mechanism that is complement independent. There are two types of experimental studies that recently provided some insight into this mechanism: Reed and group41 have set up an in vitro model of cultured endothelial cells, to which allo-antibodies can be added. The authors were able to show that allo-antibodies themselves can alter the state of the endothelium in the absence of complement or other inflammatory cells. In response to allo-antibodies, endothelial cells started expressing proinflammatory molecules, increased growth factor and adhesion molecules such as E-selectin, P-selectin, ICAM-1, VCAM-1, and CX3CL1.41 Subsequently, it was demonstrated that adding natural killer cells or macrophages together with antibodies to cultured endothelial cells could damage the endothelial cells even more severely, through Fc receptor interactions.42,43 Apparently, antibodies can induce injury through interaction with leukocytes such as natural killer cells, without complement as a mediator. In vivo, this has been recently demonstrated in mouse heart allografts44 and NK cells have been detected within glomerular and peritubular capillaries in human biopsies showing AMR.45 New diagnostic and therapeutic approaches are warranted to approach these cases in future.
DEBATED ISSUES 3: C4D POSITIVITY AS A SIGN OF GRAFT ACCOMMODATION
Despite the experience that preformed antibodies against HLA- or blood group antigens due to pregnancy, blood transfusion, or prior transplants are a major cause of hyperacute rejection of renal allografts, the ever expanding deceased-donor waiting list led to the development of protocols enabling transplantation across these immunologic barriers. Japan and North America were the first to successfully transplant ABO-incompatible grafts in patients who had been pretreated with improved immunosuppressive regimens and plasmapheresis to remove preexisting antibodies.46,47 The follow-up of these cases revealed some unexpected phenomena, which served as the basis of a new and exiting concept: Stable graft accommodation.
As it soon became apparent that recurrence of low levels of anti-blood group antibodies occurred frequently in patients transplanted with an ABO-incompatible graft, there was concern about the development of AMR in these grafts. Not quite unexpectedly, diffuse C4d staining of peritubular capillaries in these biopsies was commonly found, even in protocol biopsies. However, the fact that more than 70–80% of ABO-incompatible grafts showed diffuse C4d positivity was a surprising finding, especially when compared with the marginal 30–40% diffuse positives observed in the group of patients with a positive cross-match for anti-HLA antibodies.48-50
Strikingly, in contrast to conventional transplants where a diffuse C4d stain is strongly associated with histological abnormalities such as capillaritis and transplant glomerulopathy, the ABO-incompatible kidneys as a rule show diffuse C4d positivity without histological tissue injury.51,52 A recent retrospective case–control study by Haas et al.53 indicated that persistent C4d-positive ABO-incompatible grafts without histological abnormalities are not subject to increased graft scarring, transplant glomerulopathy, or reduced renal function within the first year after transplantation.53 Moreover, ABO-incompatible grafts with persistent diffuse C4d positivity had significantly less chronic damage after 1 year. These puzzling observations can possibly be understood in the light of accommodation, in which a graft acquires resistance to humoral injury and continues to function well despite the constant presence of low levels of antibodies against the ABO antigens on the endothelium.
An underlying mechanism that might explain in part the development of graft accommodation, as suggested by Park et al.,54 could be the upregulation of complement regulatory proteins in endothelial cells, by which the initial activation of complement due to antibody binding is blocked at a point later in the cascade. This could explain the persistent presence of C4d without signs of microvascular injury,55 although why this happens frequently in the setting of ABO incompatibility, but at most rarely, in the setting of HLA-mismatched patients is poorly understood at the moment. Still, just as antibody-mediated graft injury cannot be completely accounted for by complement activation,37 complement inhibition alone does not appear to prevent chronic antibody-mediated graft injury.56
In conclusion, the common finding of C4d positivity in ABO-incompatible grafts without histological abnormalities currently forces pathologists to look more closely into the histology when trying to diagnose AMR in an ABO-incompatible graft, as a C4d stain in this group appears to signify something different than ‘rejection’ and cannot be reliably used as a diagnostic tool.
NEW FIELDS 1: C4D AND AMR IN OTHER TRANSPLANTED SOLID ORGANS
After the recognition of C4d as a tool to detect AMR in the transplanted kidney, this concept was soon translated to virtually all other transplanted solid organs. The transplanted organs in which the significance of C4d deposition has been most studied are the heart, lung, liver, and pancreas.
C4d in cardiac transplantation
Many groups have shown that linear C4d deposition along myocardial capillaries is a reliable-specific marker for antibody-mediated cardiac allograft rejection.17,57,58 Moreover, in line with earlier studies in the kidney, it was also shown that C4d positivity in the heart is an independent predictor of cardiac dysfunction and of cardiac mortality.19,59 The International Society for Heart and Lung Transplantation recommends that a diagnosis of AMR in a cardiac allograft can be justified when there is clinical evidence of graft dysfunction, histological evidence of acute capillary injury, and immunopathological evidence for C4d capillary positivity on endomyocardial biopsies. According to the International Society for Heart and Lung Transplantation, positive histological features indicative of AMR are necessary to warrant C4d staining.60 However, a recent publication by Fedrigo et al.19 casted doubt upon this approach, and suggested that C4d should instead be performed routinely on endomyocardial graft biopsies: The authors investigated 985 endomyocardial biopsies from 107 heart transplant recipients by staining them immunohistochemically for C4d. Intragraft C4d capillary deposition was present in 34%, but only 7% had AMR based on the International Society for Heart and Lung Transplantation criteria. Interestingly, C4d positivity, even without the presence of DSA, impaired graft function, or histological features of AMR, was independently associated to a higher mortality risk (unadjusted hazard ratio in patients with positive C4d staining, without DSA or loss of graft function).19 This study supports the concept of routine C4d staining, as no correlation between histology alone and clinical status could be elicited. In response to the emerging data, the Banff group reached consensus recommending specific time points to monitor DSA as well as C4d staining on every cardiac allograft biopsy, interpreting C4d staining only in myocardial capillaries and scoring as diffuse (>50% of capillaries), focal (<50%), or negative, but accepting only diffuse staining as positive.61
C4d in lung transplantation
Hyperacute and acute AMR episodes are well documented in lung transplantation: In such cases, diffuse alveolar damage, neutrophilic infiltrates, and post-transplant pulmonary capillary injury are typical histological findings that are distinct from cellular rejection and less responsive to corticosteroid treatment. C4d deposition in such cases was detected in several studies, mainly in septal capillaries, and was associated with parenchymal injury, clinical status, and the presence of DSA or anti-endothelial antibodies.21,62,63
However, a consistent anatomical deposition pattern of C4d in the lung was more problematic to identify than in the kidney (peritubular capillaries), the heart (myocardial capillaries), or even the pancreas (interacinar capillaries). A study by Wallace et al.64 could not describe any specific staining pattern in 68 lung allograft biopsy specimens using currently available techniques. Focal nonspecific staining occurred just as often in cases with suspected AMR compared with more chronic forms of rejection.64 Probably, the anatomy of the lung complicates pattern recognition, as the frequent occurrence of alveolar hemorrhage and septal damage give rise to nonspecific staining patterns, which makes it hard to score pulmonary allograft biopsies.
Compared with kidney transplants, the role of AMR and C4d in chronic pulmonary allograft rejection (bronchiolitis obliterans syndrome) is heavily debated. Magro et al.65 reported on evident C4d deposition in a series of 13 single-lung transplant patients with bronchiolitis obliterans syndrome, who had circulating anti-endothelial antibodies. Westall et al.66 investigated septal capillary C4d staining early after lung transplantation. Complement staining was not associated with acute cellular or chronic rejection, or with morphological features of AMR, but in a subgroup analysis the authors identified nine cases who developed early bronchiolitis obliterans syndrome. Interestingly, these cases showed significant lung allograft C3d/C4d deposition along with light-microscopic features suggestive of AMR, suggesting that C4d staining could potentially have a role in the identification of patients at risk of developing a chronic humoral form of pulmonary allograft rejection.66 Although these results point into the direction of antibody-mediated processes in chronic pulmonary graft rejection, these results should be replicated in larger cohorts to make any definitive statement.
In conclusion, the presence in one patient of both anti-HLA antibodies or anti-endothelial antibodies and pathological findings suspicious for AMR (including C4d staining) should be seen as a strong evidence for AMR of the pulmonary allograft, but there is not enough evidence for C4d as a marker for AMR in the lung graft to perform routine C4d staining on all pulmonary allograft biopsies.
C4d in pancreas transplantation
Few studies are available describing the histological and immunohistochemical features of rejection episodes of the pancreas, compared with other transplanted solid organs. The first large cohort appeared in 2009 including 27 pancreas biopsies, showing that C4d deposition in interacinar capillaries (Figure 3c) is associated with de novo DSA and impaired graft outcome, suggestive of AMR. These results were followed by a study that reported on a correlation between interacinar C4d staining with several serum and urine pancreas rejection markers. A third study discussing the role of AMR in simultaneous pancreas–kidney transplantation was performed in 2010, confirming that presence of C4d was associated with impaired pancreas survival.18
In all studies, only C4d staining in interacinar capillaries of the pancreas was demonstrated to correlate with circulating DSA. Coinciding histological parameters included capillaritis, edema, active septal inflammation, acinar inflammation, and acinar cell injury/necrosis. These findings led to the inclusion of C4d staining in the Banff classification for pancreas transplant pathology.61 However, to date no prospective studies have been performed evaluating the effect of treatment targeted at antibody-mediated injury, or reporting on long-term follow up of C4d-positive vs. C4d-negative pancreas grafts. These will be future challenges. Meanwhile, it is advised to stain all pancreas biopsies for C4d, with diffuse positive staining as indicative of AMR and focal positivity as suspected for AMR.
C4d in liver transplantation
In the liver there are several excellent studies available, but results are variable as well as the C4d staining pattern: In different studies, emphasis is being put on sinusoidal staining, portal vein staining, central vein staining, and even stromal staining in the portal tract. There seems to be no agreement.22 And even beyond that, studies have reported significant C4d staining in cases that are not directly related to rejection, such as autoimmune hepatitis, or viral hepatitis. There might be a different role for complement in rejection of the liver, as many complement components are produced in this organ. The endothelium of the liver could thus be more resistant to complement-induced damage. In fact, this may partly explain the relatively low frequency of liver rejection in general, as well as the possibility of ABO-incompatible transplantation. Overall, in liver transplantation C4d is not a useful diagnostic marker to detect AMR.
NEW FIELDS 2: C4d IN NATIVE RENAL DISEASE
The detection of capillary C4d in kidney transplants was the logical consequence of previous studies of the classical complement cascade in normal and diseased native kidneys,67 including also other mammalian kidneys.68 After the discovery of C4d as a biomarker in transplantation, many studies have sought evidence for C4d deposition in native kidneys, mainly in the setting of autoimmunity.
In native kidney disease, peritubular capillary C4d staining was investigated in many forms of glomerulonephritis,67,69-73 where peritubular capillary C4d staining was virtually never observed. The only exception was lupus nephritis, in which granular peritubular capillary staining has been rarely described, which should be kept in mind when a diagnosis of AMR in a transplanted systemic lupus erythematosus (SLE) patient is considered. Recurrence of the original disease should then be ruled out.
Glomerular C4d deposition on the other hand is a relatively common finding in native-diseased kidneys. Xing et al. recently investigated which complement pathways were involved in anti-neutrophil autoantibody-associated vasculitis. Interestingly, they detected glomerular C4d only in a small subgroup of patients with anti-neutrophil autoanti-body-negative pauci-immune glomerulonephritis, whereas in the anti-neutrophil autoantibody-positive patients, it was absent.73 The authors could not identify glomerular deposition of C1q and most C4d-positive cases were also MBL positive. This is an example of C4d positivity that does not seem to be linked primarily to classic pathway activation. MBL positivity may instead be associated with exposure of carbohydrate (sugar) moieties in damaged glomeruli or glomerular basement membrane, an infectious pathogenesis, or just a consequence of tissue damage or remodeling. Although interesting from an etiological point of view, it is not likely that C4d will be used as a diagnostic marker in anti-neutrophil autoantibody-associated vasculitis in the near future.
In lupus nephritis, glomerular C4d deposition can be detected in the majority of cases with a full-house immuno-fluorescence pattern, as a result of immune complex deposition (Figure 3b).67,74 In one study, biopsies of patients with lupus nephritis with prominent diffuse glomerular C4d staining had detectable glomerular microthrombi significantly more often than biopsies of patients with focal or mild C4d staining.71 This relation between thrombotic micro-angiopathy and glomerular C4d has been confirmed in renal biopsies of patients with antiphospholipid syndrome, a similar antibody-mediated autoimmune disease leading to endothelial damage and thrombosis in all vascular beds.75 Apparently, uncontrolled or abundant complement activation can cause severe damage to the glomerular endothelium to such an extent that a thrombotic microangiopathy can develop. This is in line with the occasionally observed thrombotic microangiopathy in cases of C4d-positive acute AMR.76 Furthermore, this mechanism also has a role in atypical Hemolytic Uremic Syndrome, where a genetic defect in complement regulation causes widespread microthrombosis.77 In the setting of thrombotic microangiopathies independent of the underlying disease, performing a C4d stain might help clinicians understand the mechanisms of renal microvascular thrombosis. A positive C4d stain could indicate that complement is involved, and could even guide future treatment, for instance, with complement inhibitors. However, this needs further basic study, and its clinical utility must await trials of complement inhibitory therapies.
NEW FIELD 3. C4d IN PREGNANCY: ANTIBODY-MEDIATED PREGNANCY LOSS?
The analogy between pregnancy and transplantation was made as early as 1953, when Peter Medawar introduced the concept of ‘the fetal allograft’.78 Failure of placentation, which may be triggered by immune mechanisms, underlies a spectrum of common pregnancy disorders.79 Defective placentation is known to occur in a substantial proportion of cases of early pregnancy loss, with reduced trophoblast invasion into both the decidua and spiral arteries.80 Similar to the primary defect in preeclampsia, fetal growth restriction and still birth are the resultant reduction in uteroplacental blood flow. However, in settings of recurrent miscarriage (>3 consecutive miscarriages) this might be different: The more miscarriages a women experiences, the higher the chance of an underlying maternal condition.81
In certain autoimmune diseases, such as SLE and anti-phospholipid syndrome, recurrent early and late miscarriage occur up to 20 times more often than in the normal population, and placental insufficiency leading to preeclampsia and fetal growth restriction are also of increased prevalence.82,83 In antiphospholipid syndrome, it has been established that pathogenic antibodies bind to trophoblast.84 The question is: Can these pregnancy losses and other complications be interpreted as ‘antibody-mediated’? A recent study by Cohen et al demonstrated that complicated pregnancies of patients with SLE and antiphospholipid syndrome share several pathophysiological aspects with AMR85 (Figure 4). Interestingly, placental C4d was detectable in the majority of SLE and antiphospholipid syndrome cases (>60%) in a diffuse staining pattern at the fetal-maternal interface (Figure 3d), whereas in normal pregnancies C4d was always negative. Excessive placental C4d was related to impaired fetal outcome due to fetal loss or due to prematurity in the setting of preeclampsia. These studies extend previous work showing increased C4d in placentas from patients with antiphospholipid syndrome,86 and they argue that C4d is associated with clinical outcomes. Both antiphospholipid antibodies and DSA seem to bind at the interface where cells from the one individual (mother or host) meet the other (fetus or graft), and C4d functions as a footprint for antibody-mediated tissue injury. These results point at a role for complement in disease pathogenesis and possible role for C4d as a biomarker to verify that this pathway is activated in pregnancy complication. Identification of patients with C4d and antibody-mediated pregnancy morbidity, for instance after a late pregnancy loss or following multiple miscarriages, might direct their therapy.
Figure 4. Analogy between AMR and antibody-mediated pregnancy loss.
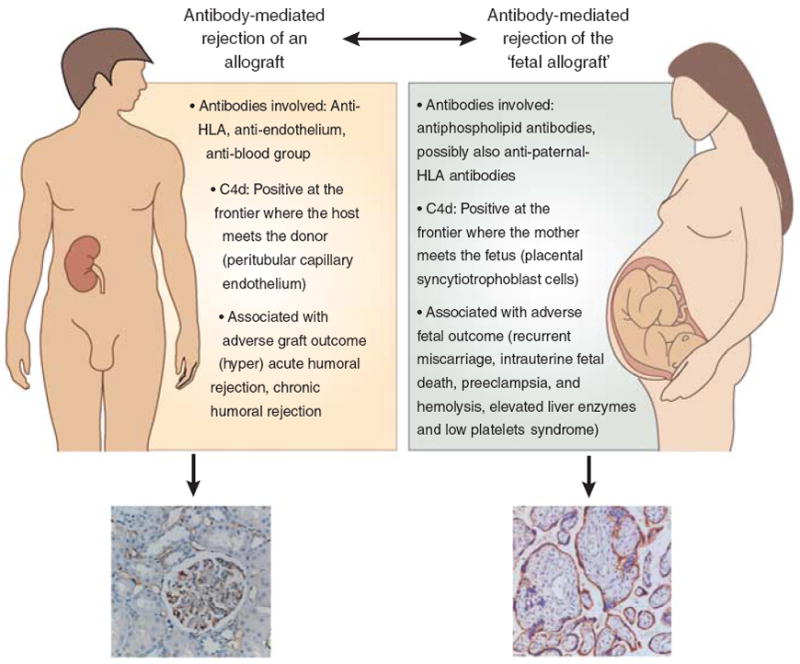
In this scheme the analogy between antibody-mediated rejection of a transplanted graft and ‘antibody-mediated pregnancy loss’ is schematically shown. HLA, human lymphocyte antigen.
To take this concept further, a similar mechanism could have a role in other pregnancy-related disorders with a possible immunological background and a clinical course of miscarriages, fetal death, or early delivery. Indeed, reports are slowly emerging investigating the role of complement and C4d in preeclampsia and hemolysis, elevated liver enzymes and low platelets—syndrome87 and spontaneous early delivery.88 In a cohort of women with severe preeclampsia with and without underlying SLE/antiphospholipid syndrome (the PROMISSE study), Salmon et al.87 recently showed that not only excessive activation of complement, but also inadequate regulation could contribute to preeclampsia. The authors identified several mutations in complement regulatory genes in a subgroup of preeclamptic women, among which a new mutation involved in the regulation of C4 activation. These striking observations support the concept that C4 activation at the fetal–maternal interface is an essential mediator of both fetal loss and preeclampsia and that insufficient regulation of C4 leads to a more severe phenotype.
Although the diagnostic value of placental C4d has not been tested in a prospective manner, these studies would be helpful to investigate whether a positive placental C4d stain is a risk factor of a future complicated pregnancy.
CONCLUSION: PROS AND CONS FOR C4D AS A BIOMARKER IN TRANSPLANTATION, AUTOIMMUNITY, AND PREGNANCY
The introduction of C4d as a biomarker into the standard work-up of renal transplant biopsies has provoked an enormous amount of insight into the role of antibodies in different forms of allograft rejection. C4d is now one of the core diagnostic tools to identify AMR, and is being used in virtually all transplant centers around the world. The vast amount of research into the deposition patterns of C4d in different clinical settings, such as in the kidney, heart, pancreas, and lung transplantation, has taught us that antibodies contribute largely to both acute and chronic rejection episodes.
In analogy with solid organ transplantation, C4d has recently been demonstrated in pregnancy, in particular in the setting of ‘antiphospholipid antibody-mediated fetal loss’ and preeclampsia. In autoimmune settings C4d might have a role in identifying patients at risk of developing thrombotic complications. More research will nevertheless be needed to discover the full extend of C4d as a biomarker in these new fields.
This review has shown that there are certain drawbacks of using C4d. The difficulties of interpreting focal staining patterns, the relatively low sensitivity of C4d as a marker for AMR in late renal allograft biopsies, and its lack of utility as a marker for antibody-mediated injury in biopsies of ABO-incompatible allografts suggests that C4d has lost some of its magic during the past decade. However, most experts agree that if complement targeted therapies will be part of our future treatment options, a marker such as C4d will be needed to identify patients susceptible for those kind of expensive treatments. Taken together, with its unique ability to act as a footprint for antibody-mediated injury, C4d will likely remain to have a prominent role in transplantation, and possibly in pregnancy and autoimmunity (Box 5).
Box 5. Pros and cons for C4d as a biomarker.
-
Pro C4d
C4d staining is relatively inexpensive
C4d staining is easy to perform in basic laboratories
A diffuse staining pattern is relatively easy to interpret
C4d gives very few false positives (it is relatively specific)
C4d shows that the complement system is involved: If complement targeted therapies (eculizumab) will be part of our future treatment options, a marker such as C4d will be needed to identify patients susceptible for those kind of (expensive) treatments.
There are currently no reliable (prospectively tested) alternatives available
-
Contra C4d
C4d scoring is subjective and the issue of focal staining and C4d/donor-specific antibody discrepancies will not be solved
C4d is not sensitive for chronic (or chronic/active) antibody-mediated rejection
C4d is not helpful in ABO-incompatible grafts
Alternatives for C4d are emerging (genomics, molecular diagnostics, and endothelial transcripts) and if proven useful, great effort will be made to transform these techniques or their progeny into practical tests
Finally, we would like to encourage readers to listen to the audio files, in which a group of experts in the field of C4d give their answers to the questions raised in the introduction (Box 6 and Supplementary Material online).
Box 6. What would you like to investigate if you would receive funding to be spent on research in the field of C4d?
Robert Colvin: ‘Understand the mechanism of accommodation. There must be a mechanism that we can intervene with. And then I would like to try to find out if it is possible to induce the same endothelial state in the presence of anti-HLA antibodies’.
Mohamed Daha: ‘I want to understand the effect of modulated and injured tissue to complement activation. In addition, I would like to know what local production of factors that control the complement system do, and see if we can influence them to control disease’.
Cynthia Drachenberg: ‘One of the things that I find extremely puzzling is the contrast between acute AMR and chronic AMR. How do endothelial cells cope with this in the chronic setting and why do they behave so differently than in the acute form of AMR?’
Mark Haas: ‘What would be nice to do is to look at the genomics of ABO-incompatible grafts with no histologic signs of rejection, and see how this differs from AMR meeting current Banff criteria, from C4d positivity without histologic findings of rejection in non ABO-incompatible settings, and also what genomic changes have occurred compared to ABO-incompatible base-line biopsies.’
Volker Nickeleit: ‘I want to do a large prospective clinicopathological study with thousands of patients, protocol biopsies and regular monitoring of anti-HLA antibodies to find out answers to many unanswered questions we struggle with in daily clinical practice’.
Banu Sis: ‘I want to study the molecular phenotypes of early acute AMR in presensitized patients and C4d positive ABO-incompatible kidneys and compare molecular mechanisms of acute AMR to chronic AMR. Subtle molecular changes may help us to intervene before irreversible tissue injury takes place.’
Ming-hui Zhao: ‘Recent advances on complement research provide us a chance to rethink the role and mechanism of complement played in many native kidney diseases. C4d is not always a reflection of classical pathway of complement activation, but can be MBL derived too. I would like to investigate the different roles for classical and MBL pathway-activation further in the setting of autoimmune renal disease’.
Jane Salmon: ‘The most elegant approach would be to look at C4d in pregnancy in every possible direction in well-phenotyped patients with different pregnancy outcomes. Such a study would include next generation sequencing to identify variants of complement and complement regulatory proteins, measurement of C4d on erythrocytes and complement activation products in blood through pregnancy, and assessment of C4d deposition in placental tissues. Analyses would define pathways and predictors. ’
Supplementary Material
Acknowledgments
We kindly acknowledge Hanneke de Kort for providing constructive criticism to the manuscript as well as two images for Figure 3.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary Material. C4d expert interviews and compilations. Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
DISCLOSURE
All the authors declared no competing interests.
References
- 1.Nickeleit V, Mihatsch MJ. Kidney transplants, antibodies and rejection: is C4d a magic marker? Nephrol Dial Transplant. 2003;18:2232–2239. doi: 10.1093/ndt/gfg304. [DOI] [PubMed] [Google Scholar]
- 2.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sis B, Halloran PF. Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant. 2010;15:42–48. doi: 10.1097/MOT.0b013e3283352a50. [DOI] [PubMed] [Google Scholar]
- 4.Regele H, Bohmig GA, Habicht A, et al. Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: a contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol. 2002;13:2371–2380. doi: 10.1097/01.asn.0000025780.03790.0f. [DOI] [PubMed] [Google Scholar]
- 5.Berger SP, Roos A, Daha MR. Complement and the kidney: what the nephrologist needs to know in 2006? Nephrol Dial Transplant. 2005;20:2613–2619. doi: 10.1093/ndt/gfi166. [DOI] [PubMed] [Google Scholar]
- 6.Daha MR, van Kooten C, Roos A. Compliments from complement: a fourth pathway of complement activation? Nephrol Dial Transplant. 2006;21:3374–3376. doi: 10.1093/ndt/gfl515. [DOI] [PubMed] [Google Scholar]
- 7.Williams GM, Hume DM, Hudson RP, et al. ‘Hyperacute’ renal-homograft rejection in man. N Engl J Med. 1968;279:611–618. doi: 10.1056/NEJM196809192791201. [DOI] [PubMed] [Google Scholar]
- 8.Kissmeyer-Nielsen F, Olsen S, Petersen VP, et al. Hyperacute rejection of kidney allografts, associated with pre-existing humoral antibodies against donor cells. Lancet. 1966;2:662–665. doi: 10.1016/s0140-6736(66)92829-7. [DOI] [PubMed] [Google Scholar]
- 9.Porter KA. Morphological aspects of renal homograft rejection. Br Med Bull. 1965;21:171–175. doi: 10.1093/oxfordjournals.bmb.a070388. [DOI] [PubMed] [Google Scholar]
- 10.Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43:1333–1338. doi: 10.1038/ki.1993.187. [DOI] [PubMed] [Google Scholar]
- 11.Feucht HE, Felber E, Gokel MJ, et al. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464–470. doi: 10.1111/j.1365-2249.1991.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feucht HE, Lederer SR, Kluth B. Humoral alloreactivity in recipients of renal allografts as a risk factor for the development of delayed graft function. Transplantation. 1998;65:757–758. doi: 10.1097/00007890-199803150-00029. [DOI] [PubMed] [Google Scholar]
- 13.Kluth-Pepper B, Schneeberger H, Lederer SR, et al. Impact of humoral alloreactivity on the survival of renal allografts. Transplant Proc. 1998;30:1772. doi: 10.1016/s0041-1345(98)00424-2. [DOI] [PubMed] [Google Scholar]
- 14.Collins AB, Schneeberger EE, Pascual MA, et al. Complement activation in acute humoral renal allograft rejection: diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol. 1999;10:2208–2214. doi: 10.1681/ASN.V10102208. [DOI] [PubMed] [Google Scholar]
- 15.Herzenberg AM, Gill JS, Djurdjev O, et al. C4d deposition in acute rejection: an independent long-term prognostic factor. J Am Soc Nephrol. 2002;13:234–241. doi: 10.1681/ASN.V131234. [DOI] [PubMed] [Google Scholar]
- 16.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant. 2003;3:708–714. doi: 10.1034/j.1600-6143.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 17.Crespo-Leiro MG, Veiga-Barreiro A, Domenech N, et al. Humoral heart rejection (severe allograft dysfunction with no signs of cellular rejection or ischemia): incidence, management, and the value of C4d for diagnosis. Am J Transplant. 2005;5:2560–2564. doi: 10.1111/j.1600-6143.2005.01039.x. [DOI] [PubMed] [Google Scholar]
- 18.de Kort H, Munivenkatappa RB, Berger SP, et al. Pancreas allograft biopsies with positive c4d staining and anti-donor antibodies related to worse outcome for patients. Am J Transplant. 2010;10:1660–1667. doi: 10.1111/j.1600-6143.2010.03079.x. [DOI] [PubMed] [Google Scholar]
- 19.Fedrigo M, Gambino A, Tona F, et al. Can C4d immunostaining on endomyocardial biopsies be considered a prognostic biomarker in heart transplant recipients? Transplantation. 2010;90:791–798. doi: 10.1097/TP.0b013e3181efd059. [DOI] [PubMed] [Google Scholar]
- 20.Glanville AR. Antibody-mediated rejection in lung transplantation: myth or reality? J Heart Lung Transplant. 2010;29:395–400. doi: 10.1016/j.healun.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Magro CM, Pope HA, Klinger D, et al. Use of C4d as a diagnostic adjunct in lung allograft biopsies. Am J Transplant. 2003;3:1143–1154. doi: 10.1034/j.1600-6143.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 22.Neil DA, Hubscher SG. Current views on rejection pathology in liver transplantation. Transpl Int. 2010;23:971–983. doi: 10.1111/j.1432-2277.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 23.Lucas JG, Co JP, Nwaogwugwu UT, et al. Antibody-mediated rejection in kidney transplantation: an update. Expert Opin Pharmacother. 2011;12:579–592. doi: 10.1517/14656566.2011.525219. [DOI] [PubMed] [Google Scholar]
- 24.Sberro-Soussan R, Zuber J, Suberbielle-Boissel C, et al. Bortezomib as the sole post-renal transplantation desensitization agent does not decrease donor-specific anti-HLA antibodies. Am J Transplant. 2010;10:681–686. doi: 10.1111/j.1600-6143.2009.02968.x. [DOI] [PubMed] [Google Scholar]
- 25.Wahrmann M, Haidinger M, Kormoczi GF, et al. Effect of the proteasome inhibitor bortezomib on humoral immunity in two presensitized renal transplant candidates. Transplantation. 2010;89:1385–1390. doi: 10.1097/TP.0b013e3181d9e1c0. [DOI] [PubMed] [Google Scholar]
- 26.Walsh RC, Everly JJ, Brailey P, et al. Proteasome inhibitor-based primary therapy for antibody-mediated renal allograft rejection. Transplantation. 2010;89:277–284. doi: 10.1097/TP.0b013e3181c6ff8d. [DOI] [PubMed] [Google Scholar]
- 27.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574–582. doi: 10.1681/ASN.V123574. [DOI] [PubMed] [Google Scholar]
- 28.Bohmig GA, Exner M, Habicht A, et al. Capillary C4d deposition in kidney allografts: a specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol. 2002;13:1091–1099. doi: 10.1681/ASN.V1341091. [DOI] [PubMed] [Google Scholar]
- 29.Smith RN, Kawai T, Boskovic S, et al. Four stages and lack of stable accommodation in chronic alloantibody-mediated renal allograft rejection in Cynomolgus monkeys. Am J Transplant. 2008;8:1662–1672. doi: 10.1111/j.1600-6143.2008.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hourmant M, Cesbron-Gautier A, Terasaki PI, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16:2804–2812. doi: 10.1681/ASN.2004121130. [DOI] [PubMed] [Google Scholar]
- 31.Terasaki PI, Ozawa M. Predictive value of HLA antibodies and serum creatinine in chronic rejection: results of a 2-year prospective trial. Transplantation. 2005;80:1194–1197. doi: 10.1097/01.tp.0000174338.97313.5a. [DOI] [PubMed] [Google Scholar]
- 32.Worthington JE, Martin S, Al-Husseini DM, et al. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75:1034–1040. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 33.Sis B, Campbell PM, Mueller T, et al. Transplant glomerulopathy, late antibody-mediated rejection and the ABCD tetrad in kidney allograft biopsies for cause. Am J Transplant. 2007;7:1743–1752. doi: 10.1111/j.1600-6143.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 34.Regele H, Exner M, Watschinger B, et al. Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant. 2001;16:2058–2066. doi: 10.1093/ndt/16.10.2058. [DOI] [PubMed] [Google Scholar]
- 35.Sijpkens YW, Joosten SA, Wong MC, et al. Immunologic risk factors and glomerular C4d deposits in chronic transplant glomerulopathy. Kidney Int. 2004;65:2409–2418. doi: 10.1111/j.1523-1755.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- 36.Vongwiwatana A, Gourishankar S, Campbell PM, et al. Peritubular capillary changes and C4d deposits are associated with transplant glomerulopathy but not IgA nephropathy. Am J Transplant. 2004;4:124–129. doi: 10.1046/j.1600-6143.2003.00294.x. [DOI] [PubMed] [Google Scholar]
- 37.Sis B, Jhangri GS, Bunnag S, et al. Endothelial gene expression in kidney transplants with alloantibody indicates antibody-mediated damage despite lack of C4d staining. Am J Transplant. 2009;9:2312–2323. doi: 10.1111/j.1600-6143.2009.02761.x. [DOI] [PubMed] [Google Scholar]
- 38.Loupy A, Suberbielle-Boissel C, Hill GS, et al. Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant. 2009;9:2561–2570. doi: 10.1111/j.1600-6143.2009.02813.x. [DOI] [PubMed] [Google Scholar]
- 39.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA) Am J Transplant. 2011;11:56–65. doi: 10.1111/j.1600-6143.2010.03364.x. [DOI] [PubMed] [Google Scholar]
- 40.Haas M, Mirocha J. Early ultrastructural changes in renal allografts: correlation with antibody-mediated rejection and transplant glomerulopathy. Am J Transplant. 2011 doi: 10.1111/j.1600-6143.2011.03647.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Reed EF. Effect of antibodies on endothelium. Am J Transplant. 2009;9:2459–2465. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee CY, Lotfi-Emran S, Erdinc M, et al. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324–1334. doi: 10.1097/01.tp.0000287457.54761.53. [DOI] [PubMed] [Google Scholar]
- 43.Uehara S, Chase CM, Kitchens WH, et al. NK cells can trigger allograft vasculopathy: the role of hybrid resistance in solid organ allografts. J Immunol. 2005;175:3424–3430. doi: 10.4049/jimmunol.175.5.3424. [DOI] [PubMed] [Google Scholar]
- 44.Hirohashi T, Chase CM, Della Pelle P, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. doi: 10.1111/j.1600-6143.2011.03836.x. e-pub ahead of print 9 November 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hidalgo LG, Sis B, Sellares J, et al. NK cell transcripts and NK cells in kidney biopsies from patients with donor-specific antibodies: evidence for NK cell involvement in antibody-mediated rejection. Am J Transplant. 2010;10:1812–1822. doi: 10.1111/j.1600-6143.2010.03201.x. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery RA, Zachary AA, Racusen LC, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation. 2000;70:887–895. doi: 10.1097/00007890-200009270-00006. [DOI] [PubMed] [Google Scholar]
- 47.Uchida K, Tominaga Y, Haba T, et al. ABO-incompatible renal transplantation—dissociation of ABO antibodies. Transplant Proc. 1998;30:2302–2303. doi: 10.1016/s0041-1345(98)00631-9. [DOI] [PubMed] [Google Scholar]
- 48.Fidler ME, Gloor JM, Lager DJ, et al. Histologic findings of antibody-mediated rejection in ABO blood-group-incompatible living-donor kidney transplantation. Am J Transplant. 2004;4:101–107. doi: 10.1046/j.1600-6135.2003.00278.x. [DOI] [PubMed] [Google Scholar]
- 49.Haas M, Ratner LE, Montgomery RA. C4d staining of perioperative renal transplant biopsies. Transplantation. 2002;74:711–717. doi: 10.1097/00007890-200209150-00021. [DOI] [PubMed] [Google Scholar]
- 50.Kanetsuna Y, Yamaguchi Y, Horita S, et al. C4d and/or immunoglobulins deposition in peritubular capillaries in perioperative graft biopsies in ABO-incompatible renal transplantation. Clin Transplant. 2004;18(Suppl 11):13–17. doi: 10.1111/j.1399-0012.2004.00241. [DOI] [PubMed] [Google Scholar]
- 51.Toki D, Ishida H, Setoguchi K, et al. Acute antibody-mediated rejection in living ABO-incompatible kidney transplantation: long-term impact and risk factors. Am J Transplant. 2009;9:567–577. doi: 10.1111/j.1600-6143.2008.02538.x. [DOI] [PubMed] [Google Scholar]
- 52.Haas M, Rahman MH, Racusen LC, et al. C4d and C3d staining in biopsies of ABO- and HLA-incompatible renal allografts: correlation with histologic findings. Am J Transplant. 2006;6:1829–1840. doi: 10.1111/j.1600-6143.2006.01356.x. [DOI] [PubMed] [Google Scholar]
- 53.Haas M, Segev DL, Racusen LC, et al. C4d deposition without rejection correlates with reduced early scarring in ABO-incompatible renal allografts. J Am Soc Nephrol. 2009;20:197–204. doi: 10.1681/ASN.2008030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park WD, Grande JP, Ninova D, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3:952–960. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 55.Haas M. The significance of C4d staining with minimal histologic abnormalities. Curr Opin Organ Transplant. 2010;15:21–27. doi: 10.1097/MOT.0b013e3283342ebd. [DOI] [PubMed] [Google Scholar]
- 56.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405–2413. doi: 10.1111/j.1600-6143.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 57.Hammond EH, Yowell RL, Nunoda S, et al. Vascular (humoral) rejection in heart transplantation: pathologic observations and clinical implications. J Heart Transplant. 1989;8:430–443. [PubMed] [Google Scholar]
- 58.Lones MA, Czer LS, Trento A, et al. Clinical-pathologic features of humoral rejection in cardiac allografts: a study in 81 consecutive patients. J Heart Lung Transplant. 1995;14:151–162. [PubMed] [Google Scholar]
- 59.Fedson SE, Daniel SS, Husain AN. Immunohistochemistry staining of C4d to diagnose antibody-mediated rejection in cardiac transplantation. J Heart Lung Transplant. 2008;27:372–379. doi: 10.1016/j.healun.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 61.Sis B, Mengel M, Haas M, et al. Banff ’09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]
- 62.Ionescu DN, Girnita AL, Zeevi A, et al. C4d deposition in lung allografts is associated with circulating anti-HLA alloantibody. Transpl Immunol. 2005;15:63–68. doi: 10.1016/j.trim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Magro CM, Deng A, Pope-Harman A, et al. Humorally mediated posttransplantation septal capillary injury syndrome as a common form of pulmonary allograft rejection: a hypothesis. Transplantation. 2002;74:1273–1280. doi: 10.1097/00007890-200211150-00013. [DOI] [PubMed] [Google Scholar]
- 64.Wallace WD, Reed EF, Ross D, et al. C4d staining of pulmonary allograft biopsies: an immunoperoxidase study. J Heart Lung Transplant. 2005;24:1565–1570. doi: 10.1016/j.healun.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 65.Magro CM, Ross P, Kelsey M, et al. Association of humoral immunity and bronchiolitis obliterans syndrome. Am J Transplant. 2003;3:1155–1166. doi: 10.1034/j.1600-6143.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 66.Westall GP, Snell GI, McLean C, et al. C3d and C4d deposition early after lung transplantation. J Heart Lung Transplant. 2008;27:722–728. doi: 10.1016/j.healun.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 67.Zwirner J, Felber E, Herzog V, et al. Classical pathway of complement activation in normal and diseased human glomeruli. Kidney Int. 1989;36:1069–1077. doi: 10.1038/ki.1989.302. [DOI] [PubMed] [Google Scholar]
- 68.Zwirner J, Felber E, Burger R, et al. Classical pathway of complement activation in mammalian kidneys. Immunology. 1993;80:162–167. [PMC free article] [PubMed] [Google Scholar]
- 69.Chen M, Xing GQ, Yu F, et al. Complement deposition in renal histopathology of patients with ANCA-associated pauci-immune glomerulonephritis. Nephrol Dial Transplant. 2009;24:1247–1252. doi: 10.1093/ndt/gfn586. [DOI] [PubMed] [Google Scholar]
- 70.Chen M, Daha MR, Kallenberg CG. The complement system in systemic autoimmune disease. J Autoimmun. 2010;34:J276–J286. doi: 10.1016/j.jaut.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Cohen D, Koopmans M, Kremer Hovinga I, et al. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460–2469. doi: 10.1002/art.23662. [DOI] [PubMed] [Google Scholar]
- 72.Kusunoki Y, Itami N, Tochimaru H, et al. Glomerular deposition of C4 cleavage fragment (C4d) and C4-binding protein in idiopathic membranous glomerulonephritis. Nephron. 1989;51:17–19. doi: 10.1159/000185234. [DOI] [PubMed] [Google Scholar]
- 73.Xing GQ, Chen M, Liu G, et al. Differential deposition of C4d and MBL in glomeruli of patients with ANCA-negative pauci-immune crescentic glomerulonephritis. J Clin Immunol. 2010;30:144–156. doi: 10.1007/s10875-009-9344-2. [DOI] [PubMed] [Google Scholar]
- 74.Kim SH, Jeong HJ. Glomerular C4d deposition indicates in situ classic complement pathway activation, but is not a marker for lupus nephritis activity. Yonsei Med J. 2003;44:75–80. doi: 10.3349/ymj.2003.44.1.75. [DOI] [PubMed] [Google Scholar]
- 75.Shen Y, Chen XW, Sun CY, et al. Association between anti-beta2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: a prospective study of 155 cases. Lupus. 2010;19:1195–1203. doi: 10.1177/0961203310368409. [DOI] [PubMed] [Google Scholar]
- 76.Noris M, Remuzzi G. Thrombotic microangiopathy after kidney transplantation. Am J Transplant. 2010;10:1517–1523. doi: 10.1111/j.1600-6143.2010.03156.x. [DOI] [PubMed] [Google Scholar]
- 77.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–1687. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 78.Medawar PB. Some immunological and endocrinological problems raised by the evolution of viviparity in vertebrates. Symp Soc Exp Biol. 1953;7:320–338. [Google Scholar]
- 79.Hiby SE, Apps R, Sharkey AM, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. J Soc Gynecol Investig. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Branch DW, Gibson M, Silver RM. Clinical practice. Recurrent miscarriage. N Engl J Med. 2010;363:1740–1747. doi: 10.1056/NEJMcp1005330. [DOI] [PubMed] [Google Scholar]
- 82.Ruiz-Irastorza G, Crowther M, Branch W, et al. Antiphospholipid syndrome. Lancet. 2010;376:1498–1509. doi: 10.1016/S0140-6736(10)60709-X. [DOI] [PubMed] [Google Scholar]
- 83.Cohen D, Berger SP, Steup-Beekman GM, et al. Diagnosis and management of the antiphospholipid syndrome. Br Med J. 2010;340:c2541. doi: 10.1136/bmj.c2541. [DOI] [PubMed] [Google Scholar]
- 84.Rote NS, Vogt E, DeVere G, et al. The role of placental trophoblast in the pathophysiology of the antiphospholipid antibody syndrome. Am J Reprod Immunol. 1998;39:125–136. doi: 10.1111/j.1600-0897.1998.tb00344.x. [DOI] [PubMed] [Google Scholar]
- 85.Cohen D, Buurma A, Goemaere NN, et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225:502–511. doi: 10.1002/path.2893. [DOI] [PubMed] [Google Scholar]
- 86.Shamonki JM, Salmon JE, Hyjek E, et al. Excessive complement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007;196:167–165. doi: 10.1016/j.ajog.2006.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salmon JE, Heuser C, Triebwasser M, et al. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee J, Romero R, Xu Y, et al. A signature of maternal anti-fetal rejection in spontaneous preterm birth: chronic chorioamnionitis, anti-human leukocyte antigen antibodies, and C4d. PLoS One. 2011;6:e16806. doi: 10.1371/journal.pone.0016806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


