Abstract
In 2009, the United Nations Estimated that 33.2 Million People worldwide were living with human immunodeficiency virus type 1 (HIV-1) infection and that 2.6 million people had been newly infected.1 The need for effective HIV-1 prevention has never been greater. In this review, we address recent critical advances in our understanding of HIV-1 transmission and acute HIV-1 infection. Fourth-generation HIV-1 testing, now available worldwide,2,3 will allow the diagnosis of infection in many patients and may lead to new treatments and opportunities for prevention.
THE HIV-1 TRANSMISSION EVENT
More than 80% of adults infected with HIV-1 became infected through the exposure of mucosal surfaces to the virus; most of the remaining 20% were infected by percutaneous or intravenous inoculations.1 The risk of infection associated with different exposure routes varies,4 but no matter what the transmission route, the timing of the appearance of viral and host markers of infection is generally uniform and follows an orderly pattern.5 Immediately after exposure and transmission, as HIV-1 is replicating in the mucosa, submucosa, and draining lymphoreticular tissues (Fig. 1),6,7 the virus cannot be detected in plasma; this so-called eclipse phase generally lasts 7 to 21 days.8,9 Once HIV-1 RNA reaches a concentration of 1 to 5 copies per milliliter in plasma, the virus can be detected with the use of sensitive qualitative methods of nucleic acid amplification10; at concentrations of 50 copies per milliliter, HIV-1 can be detected by means of quantitative clinical assays used to monitor viral load.11 The stages that define acute and early HIV-1 infection are characterized by the sequential appearance of viral markers and antibodies in the blood (Fig. 2).5 More sensitive, fourth-generation tests, which detect both antigens and antibodies, shrink the virus-positive–antibody-negative window by about 5 days.12 Testing for viral RNA in plasma closes this gap by an additional 7 days.
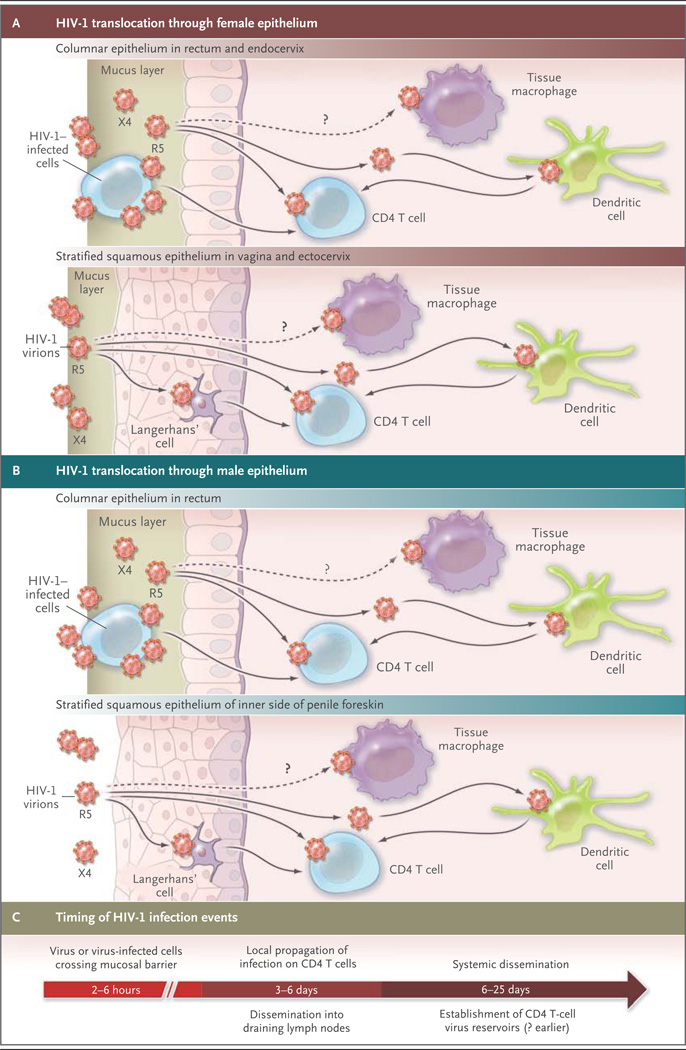
Figure 1. Progression from HIV-1 Transmission to Productive Clinical Infection.
HIV-1 must traverse several tissue layers in the female vagina or rectal mucosa to come into contact with appropriate receptive cells (Panel A). The CCR5 (R5) viral strain has selective transmission advantages that remain poorly explained, and R5 variants make up the majority of transmitted and founder viruses. CXCR4pt(X4) variants are transmitted only rarely. Founder viruses come into contact with Langerhans’ cells or CD4 T cells in squamous epithelium; CD4 T cells can also be infected by viruses bound to submucosal dendritic cells. It is not clear whether submucosal macrophages are an initial target, since most founder viruses poorly infect macrophages in vitro. The challenge for HIV-1 transmission in the male genital tract differs somewhat from that in the vagina because of differences in anatomy, but the penile foreskin and urethra harbor critical virus-receptive cells (Panel B). Virus–cell interactions in the male submucosa are likely to be similar to those in female submucosa, with viral targets including Langerhans’ cells, other submucosal dendritic cells, and CD4 cells. Removal of the foreskin through elective circumcision can prevent at least 60% of HIV-1 infections in men.6 Although the time required for HIV-1 virions or virus-infected cells to traverse epithelial barriers is short (hours), it probably takes as long as 3 to 6 days for HIV-1 infection and propagation to occur and for the virus to spread beyond submucosal CD4 T cells (Panel C). Dissemination into draining lymph nodes and the systemic circulation rapidly follows, with establishment of the CD4 T-cell viral reservoirs. Studies in nonhuman primates of the timing of response to postexposure prophylaxis with antiretroviral drugs suggests that the time to establishment of the CD4 T-cell reservoir may be as short as 24 hours.7
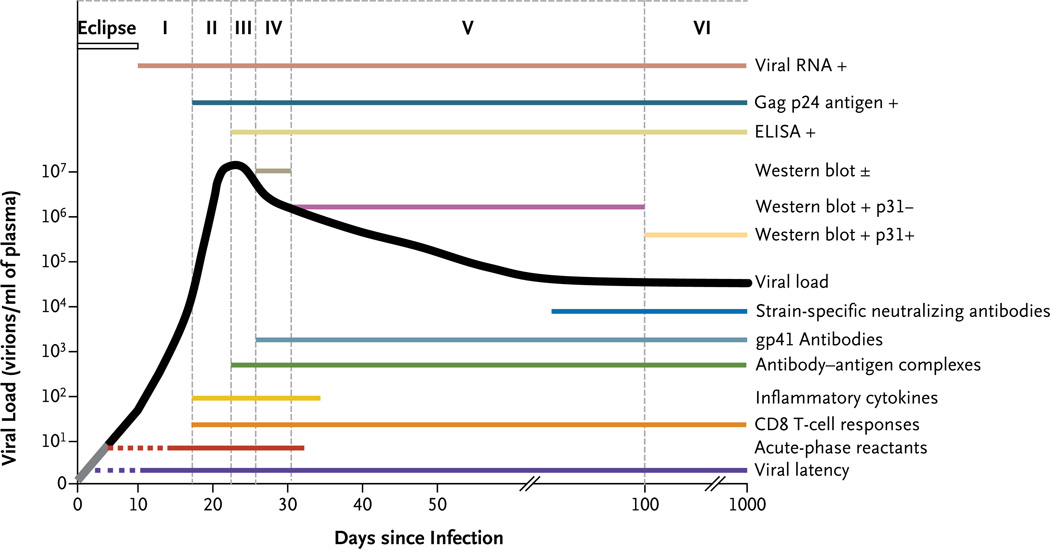
Figure 2. Natural History and Immunopathogenesis of HIV-1 Infection.
The progression of HIV-1 infection can be depicted as six discrete stages7 (indicated by Roman numerals). These stages are defined according to the results of standard clinical laboratory tests (listed above the curve for viral load). The stages are based on the sequential appearance in plasma of HIV-1 viral RNA; the gag p24 protein antigen; antibodies specific for recombinant HIV-1 proteins, detected with the use of an enzyme-linked immunosorbent assay (ELISA); and antibodies that bind to fixed viral proteins, including p31, detected on Western immunoblot. A plus sign indicates a positive test result, a minus sign a negative result, and a plus-minus sign a borderline-positive result. The lines below the viral-load curve show the timing of key events and immune responses that cannot be measured with standard clinical laboratory assays, beginning with the establishment of viral latency. Acute-phase reactants include elevated levels of serum amyloid protein A. CD8 T-cell responses lead to the appearance of escape mutants concurrently with inflammatory cytokines in plasma. Immune complexes of antibodies with viral proteins, such as the HIV-1 envelope glycoprotein (gp41), precede the first appearance of free antibodies to gp41. Strain-specific antibodies to gp41 that neutralize the virus do not appear until sometime close to day 80. The portion of the line for viral latency that is dotted reflects uncertainty as to exactly when latency is first established; the dotted line for acute-phase reactants indicates that not all patients have elevated levels of reactants at this early point in the process of infection; the gray segment of the black line for viral load reflects the inability to measure very low viral loads.
The characteristic appearance in the blood of viral markers of acute HIV-1 infection belies an extremely complicated and still poorly understood series of virus–host cell interactions in the tissues (Fig. 1).4,13 Given the varied routes of viral transmission — cervicovaginal, penile, rectal, oral, percutaneous, intravenous, in utero — and the distinctly different histologic features of these tissues, it is not surprising that several cell types are candidates for early infection. More is known about vaginal transmission than about other routes, and the study of human tissue explants14,15 and the Indian rhesus macaque model of vaginal transmission of the simian immunodeficiency virus (SIV)13,16–18 have been informative (Fig. 1). The preponderance of evidence implicates CD4 T cells and Langerhans’ cells as the first targets of the virus,14,15 but other dendritic cells may play an important accessory role.19 However, recent observations of mucosally transmitted strains of HIV-1 reveal that monocyte-derived macrophages are generally poor targets for infection as compared with CD4 T cells.20,21
Regardless of the route of viral transmission and the first cells infected, within a few days, viral replication converges on the lymphoreticular system of the gastrointestinal tract (i.e., gut-associated lymphoid tissue).22–25 In this tissue, in both humans and macaques, the phenotype of most productively infected cells appears to be the resting CD4 T cell lacking activation markers and expressing low levels of the chemokine receptor CCR5.16–18 Many of these cells express α4β7 integrin receptors and type 17 helper T (Th17)–cell surface markers.26,27 (Since these receptors are also detected on T cells harvested from the genital mucosa, they may play an important role in HIV acquisition.28) The rapid expansion of HIV-1, first in gut-associated lymphoid tissue and then systemically,25,29 along with a sharp rise in plasma levels of viral RNA, is clinically important because of the coincident irreversible destruction of reservoirs of helper T cells and the establishment of viral latency (defined as the silent integration of HIV-1 DNA into the genomes of resting T cells, an effect that has stymied curative treatment efforts30,31).
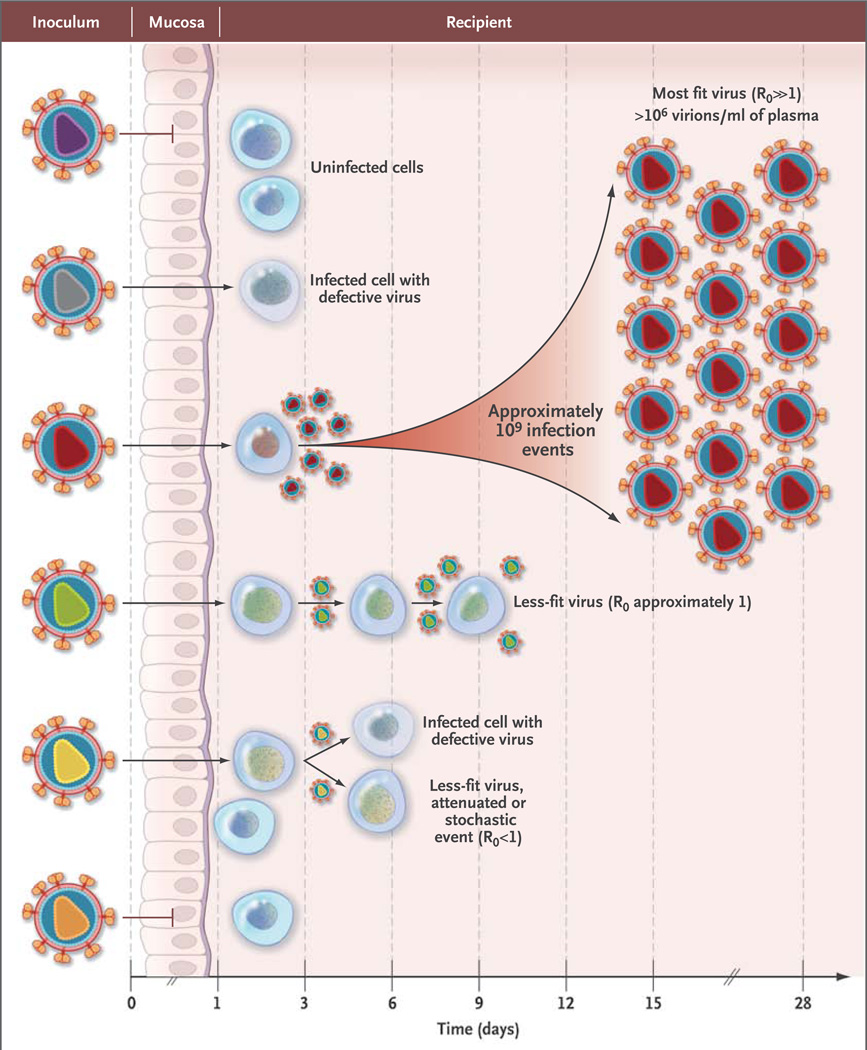
Rather than being genetically homogeneous, RNA viruses, including HIV-1, consist of complex mixtures of mutant and recombinant genomes called quasi-species. Genetic studies of the HIV-1 quasi-species in patients with chronic infection as compared with patients with acute infection have brought some clarity to the qualitative and quantitative aspects of HIV-1 transmission.8 Figure 3 depicts the HIV-1 transmission event,8,9,32,33 in which the inoculum (e.g., semen, cervicovaginal secretions, or blood) contains a complex genetic quasi-species of viruses, of which only a very small number are likely to broach mucosal barriers and establish infection. Lee and colleagues9 developed a model that allows transmitted viral genomes to be inferred from a phylogenetic analysis of the viral quasi-species that replicate in the weeks after infection. Empirical analyses based on single-genome amplification of HIV-1 RNA in plasma or HIV-1 DNA in blood lymphocytes have provided robust evidence to support this model.8,20,21,33–38 A single virion is responsible for HIV-1 transmission in approximately 80% of heterosexuals but in only about 60% of men who have sex with men and about 40% of injection-drug users.8,20,21,33–35 In injection-drug users, as many as 16 transmitted virions have been found to be responsible for productive infection,36 which would be consistent with the absence of a mucosal barrier to transmission. The phenotypes of cloned proviruses corresponding to transmitted (or founder) viruses are nearly always CD4 and CCR5 T-cell tropic variants and exhibit neutralization-sensitivity patterns that are typical of primary viral strains. These phenotypic properties are present at the moment of transmission, when the virus encounters the first target cell; they are not the consequence of viral adaptation to the new host.8,20,21
Figure 3. Model of HIV-1 Transmission.
A genetically and phenotypically diverse quasi-species of virus is present in the semen, cervicovaginal secretions, or blood of persons with chronic HIV-1 infection, but most often, only a single virion or virally infected cell is transmitted and leads to productive clinical infection. Other viruses may breach the mucosal or cutaneous surfaces, but they generally do not result in productive infection or contribute to it, presumably because such viruses are defective or less fit or simply fail to come into contact with susceptible target cells. R0 represents the basic reproductive ratio, which corresponds to the number of secondary infections caused by one infected cell. If this number falls below 1, infection is extinguished. In acute infection, the number of productively infected cells and the concentration of free virus in the plasma increase exponentially, with an estimated R0 of 8.32
INITIAL INNATE IMMUNE RESPONSES TO HIV-1
The first signal of an immune response to HIV-1 infection is the appearance of acute-phase reactants, including alpha 1-antitrypsin and serum amyloid A, in plasma 3 to 5 days after transmission39 (Fig. 2). The steep rise in the HIV-1 viral load (ramp-up viremia) coincides with a large burst of inflammatory cytokines led by interferon-α and interleukin-1540 and a shower of plasma microparticles with surface phosphatidylserine, derived from infected and activated CD4 T cells undergoing apoptosis; these particles have immunosuppressive properties.41
The earliest cytokines are produced by dendritic cells, but later in the infective process, multiple cell types (e.g., monocytes, macrophages, natural killer [NK] cells, and T cells) also produce these mediators.42 Although cytokines enhance protective antiviral immune responses in acute HIV-1 infection, the cytokine storm probably also contributes to harmful immune activation and loss of CD4 T cells.
NK cells are activated in acute HIV-1 infection and, in vitro, kill cells infected with the virus.43 NK cells have a range of receptors that either enhance or inhibit their function. NK-cell immunoglobulin-like receptors interact with HLA molecules with some specificity for the peptides they bind.44 This activity might explain the genetic associations between certain NK-cell immunoglobulin-like receptors and HLA types with more favorable outcomes of infection.45
ADAPTIVE IMMUNE RESPONSES IN ACUTE HIV-1 INFECTION
The initial antibody response to the viral envelope is non-neutralizing and does not select for viral escape46 (Fig. 2). Antibodies that neutralize the transmitted founder virus are not detected until 3 months or more after infection.47 Although many of the targets of neutralizing antibodies are on the glycoprotein-120 component of the HIV-1 envelope, the initial antibody response to HIV-1 is focused on non-neutralizing sites of the glycoprotein 41 envelope stalk.46 It is not known why the initial HIV-1 antibody response is directed (or misdirected) to ineffective envelope sites, but the response may be related in part to the relative abundance of non-native HIV-1 envelope molecules when glycoprotein 41 is exposed, whereas the exposure of functional native envelope trimers is rare.48 Similarly, other potentially protective antibodies directed against envelope proteins, such as antibodies that neutralize the founder viral strain or those that mediate antibody-dependent cellular cytotoxicity, do not arise until weeks after transmission.47,49,50 By the time a potentially effective antibody response has developed, it is much too late to influence the course of the infection (Fig. 2).
The first CD8 T-cell responses appear days before the peak of viremia and focus on between one and three distinct epitopes (short antigenic peptides bound to HLA molecules that are derived from HIV-1 proteins) most commonly found in HIV-1 proteins nef and gag.51 These first T-cell responses select escape mutants (which cannot be recognized by killer CD8 T cells), with complete replacement of the original viral amino acid sequence by the new sequence in 10 to 21 days.52 These initial T-cell responses are followed by new T-cell responses to other epitopes, which often escape as well. A combination of strong T-cell responses, producing chemokine (C-C motif) ligand 4 (CCL4), and a focus on epitopes with high levels of variability (entropy) favors rapid escape.53 These CD8 T cells also express perforin — a protein closely associated with cell-mediated cytotoxicity — which suggests that they can kill infected cells.54
Other CD8 T-cell responses do not appear to select escape mutants — or must do so very slowly. Some of these T cells may be functionally deficient, but most appear to be effective, focusing on regions of the virus that can mutate, but at the cost of making the virus less efficient in replication.55 These latter T cells are likely to contribute to the control of HIV-1. As this T-cell response evolves, the plasma viral load falls (Fig. 2). The rate of loss of virus containing the epitopes recognized by the early T-cell responses that drive escape provides a measure of the rate of killing (or removal) of virus-infected cells in vivo.52 Other factors, such as loss of susceptible cells (given the extreme depletion of activated CD4 T cells in gut-associated lymphoid tissue), probably also play a part in lowering the initial peak viral load.23,56
During acute HIV-1 infection, irrevocable depletion of CD4 T lymphocytes from the gastrointestinal tract23 and some other lymphoid tissues has been observed in humans and rhesus macaques.25 In humans, adjunctive damage to the mucosal barriers may allow leakage of gut bacterial products into otherwise sterile tissues and to the bloodstream, leading to further immune activation that can promote HIV replication and have other adverse consequences.57 The rapid, early, and massive loss of CD4 T cells in lymphoid organs (which is poorly reflected in CD4 T-cell counts in blood) probably accounts for the weak CD4 T-cell responses in cases of acute HIV-1 infection.
The importance of CD8 T cells in controlling acute HIV-1 infection is consistent with studies in the rhesus macaque SIV model showing that in vivo depletion of CD8 T cells abrogated viral control in both acute infection and chronic infection.58 In addition, many studies in macaques have shown that vaccines that stimulate SIV-specific CD8 T-cell responses can attenuate subsequent SIV infection.59 These data are also consistent with extensive work showing that in patients with certain HLA types, particularly HLA-B57 (and the very closely related HLA-B58) and HLA-B27, viral control is often better than average, with a lower virus set point and longer survival in the absence of antiretroviral therapy.60 The B27, B57, and B58 molecules present highly conserved parts of the virus to T cells, so that the virus can escape immune control only at the cost of replicative fitness.55
DETECTION OF ACUTE HIV-1 INFECTION
In the absence of a high degree of clinical suspicion, the symptoms associated with acute HIV-1 infection are often too vague or nonspecific to lead to a diagnosis.61 In the absence of antibody seroconversion, confirmation of acute infection requires detection of HIV-1 RNA or p24 antigen, but tests designed for this purpose have heretofore not been routinely available. In public health settings, a cross-sectional screening strategy that involves searching for HIV RNA in pooled, antibody-negative samples has been used to increase detection.61 This approach has been used to detect acute HIV-1 infection, with a prevalence of 0.5 cases detected per 1000 persons tested, in North Carolina, to 4.0 cases per 1000, in San Francisco; acute infection accounted for 5 to 10% of all cases of HIV in both places.
As an alternative and more practical strategy, an enzyme-linked immunosorbent assay that can concomitantly detect viral p24 antigen and antiviral antibodies has been developed and approved for clinical use.2,3,61 This test can increase the number of patients with acute HIV-1 infection whose condition is diagnosed at a time when they are most infectious to others.3 It is anticipated that a rapid point-of-care test will also be developed for the purpose of detecting acute HIV-1 infection. The implementation of these tests across the United States in public health and commercial laboratories can be expected to dramatically increase the number of patients with acute HIV-1 infection who will require care.
PUBLIC HEALTH CONSEQUENCES OF ACUTE HIV-1 INFECTION
The per-person probability of transmitting HIV-1 is most closely correlated with the viral burden in blood; each time the viral burden in an HIV-1–infected person increases by a factor of 10, the risk of transmission is expected to increase by a factor of 2.5.62 The risk of contagion from patients with acute, early infection appears to be much higher than that from patients with established infection,63 at least in part because of the high viral load and the homogeneity of viral variants clearly capable of causing infection. In the rhesus macaque SIV model, plasma from animals with acute infection is up to 750 times as infectious, on a per-virion basis, as plasma from animals with chronic infection.64 The reduced risk of contagion from patients with chronic infection probably results from the presence of neutralizing antibodies, which are not evident in acute infection.
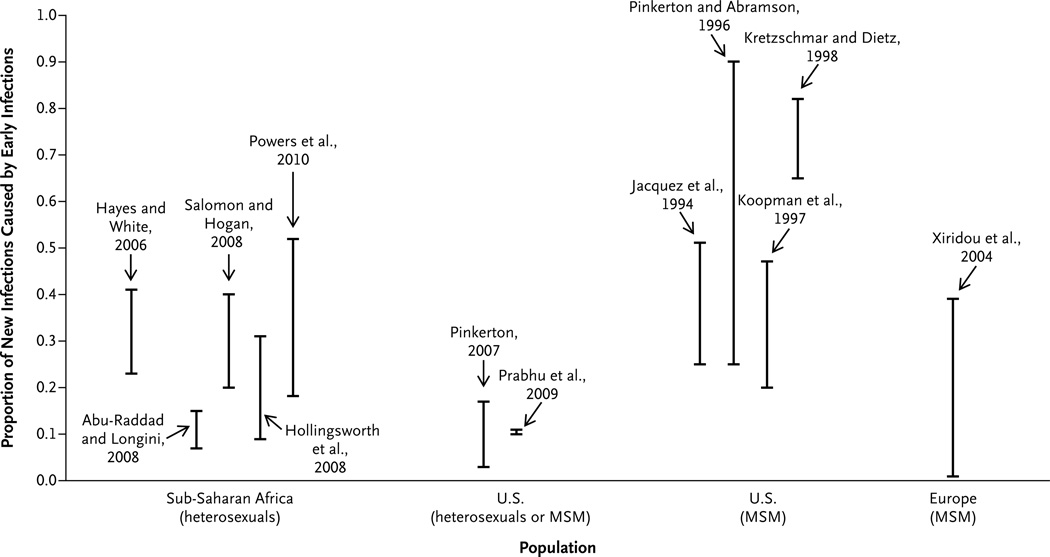
Mathematical models used to estimate the role of patients with acute infection in the spread of HIV-1 have produced strikingly different results, depending on the population studied and the assumptions used (Fig. 4).65–77 The epidemic phase used for modeling has been a critical determinant.78 In communities subject to a new epidemic, early infections are held to be responsible for a considerable share of HIV-1 transmission, since a larger proportion of infected persons have acute or early-stage disease rather than late-stage disease.79 Sexual behavior plays an important role in rates of infection, with high rates of partner change increasing the chances of contact with a person who has acute HIV-1 infection. In a recent comprehensive study conducted in Lilongwe, Malawi, in which both behavioral and biologic data were used, 38% of cases of HIV-1 were ascribed to sexual exposure to patients in the first 5 months of infection, even though there is a long-established epidemic in Malawi.75 The results of the Malawi study may be most relevant to the HIV-1 pandemic in sub-Saharan Africa.
Figure 4. Role of Acute and Early HIV-1 Infection in the Spread of HIV-1, According to Population Studies in Sub-Saharan Africa, the United States, and Europe.
Acute and early HIV-1 infection is responsible for secondary transmission of HIV-1, which is critical to the epidemic spread of the virus. A variety of models65–77 have generated widely varying estimates of the potential importance of acute and early HIV-1 infection, depending on the patient populations studied and the assumptions of the models. These models generally include people in whom the virus was detected before and during seroconversion (acute HIV-1 infection) and for several weeks thereafter (early infection) (see Hayes and White73 and Salomon and Hogan76). The estimates reflect the proportion of all transmissions during an individual patient’s entire infectious period. The extent to which this proportion corresponds to the proportion of all transmissions that occur during acute and early HIV-1 infection at the population level depends on the epidemic phase and the distribution of patterns of sexual contact in the population (see Pinkerton and Abramson77, Kretzschmar and Dietz,70 and Koopman et al.69). Transmission probabilities were drawn from the population category shown, but the reported estimates result from a range of hypothetical sexual-behavior variables that do not necessarily reflect a specific population (see Kretzschmar and Dietz70 and Abu-Raddad and Longini74). The range of estimates shown was extracted from the endemic-phase portion of graphs showing the proportion of new infections resulting from early HIV-1 infection over calendar time. I bars represent an estimate of the percentage of new HIV cases caused by people with acute or early HIV infection. MSM denotes men who have sex with men.
The importance of acute HIV-1 infection can also be seen in studies of phylogenetically related cases, such as the study reported by Brenner and colleagues.79 They state that more than half the patients with newly diagnosed early HIV-1 infection in Montreal are infected with viral variants that can be linked through phylogenetic studies, which suggests the presence of clusters of transmission, perhaps from patients with acute and early infection.
PREVENTING HIV-1 INFECTION
Effective HIV preventive strategies must be in place before or immediately after the transmission event. This is a tall order for antiviral prophylaxis, administered before or after exposure, or a vaccine. Indeed, the antibody responses directed against the HIV-1 envelope after the administration of vaccine regimens are not of long duration.80,81 Nevertheless, there are several points in the transmission event at which the founder virus may be vulnerable to inhibition by antibodies, ranging from the entry of virus or virus-infected cells into mucus in the genital tract to cell-to-cell transmission in genital tract submucosa (Fig. 1).82 Weakly neutralizing antibodies that mediate antibody-dependent, cell-mediated cytotoxicity or antibody-dependent cellular viral inhibition may have a protective effect by stimulating immune cells to produce anti–HIV-1 chemokines, such as CCL3, CCL4, and CCL5.81 In a recent vaccine efficacy trial conducted in Thailand, the partial prevention of HIV-1 acquisition observed may have been due to a transient antibody response83; the results could also be explained by the actions of one or more innate antiviral immune mechanisms.
An alternative prevention strategy that is more readily available is that of offering antiretroviral agents to people at risk before or immediately after HIV exposure or as a means of secondary prevention.84,85 Use of the antiretroviral drug tenofovir as a topical prophylactic agent before viral exposure in women at high risk led to a 39% reduction in incident cases of HIV infection that was directly correlated with concentrations of the drug in mucosal tissue.86 Seven ongoing trials of oral preexposure prophylaxis have been under-taken.87 A multinational trial focused on men who have sex with men87 showed that a once-daily pill containing tenofovir plus emtricitabine provided an average of 44% protection over and above that conferred by the provision of comprehensive preventive services, including the provision of condoms and counseling.88 The level of protection varied widely, depending on how consistently participants used preexposure prophylaxis. The Centers for Disease Control and Prevention has issued preliminary recommendations for the use of preexposure prophylaxis by men who have sex with men.89 Work with rhesus macaques suggests that achieving high levels of antiviral agents in mucosal tissues shortly after exposure to SIV–HIV viral chimeras is critical for protection from infection.90 This work, along with the further exploration of new drug combinations, will probably play a role in the further development of preexposure and postexposure prophylaxis.
MANAGING ACUTE HIV-1 INFECTION
The health care provider has three responsibilities with respect to acute HIV-1 infection: detection; secondary prevention, which in some cases must include partner notification (and possibly postexposure prophylaxis with antiretroviral therapy); and initiation of antiretroviral therapy, if it is considered appropriate. Although the fourth-generation HIV tests have the capacity to detect acute HIV-1 infection, algorithms that will reduce the time to diagnosis and linkage to medical care must be put into place.3 Equally important, strategies must be developed to offer the best possible counseling in order to reduce further spread of HIV-1 and to break up the sexual networks that can form around patients with acute HIV-1 infection.91 However, partner notification in the United States has been limited92 and is only now being studied in resource-constrained countries.93 A recent study in Africa has highlighted the difficulty of explaining acute HIV-1 infection to study subjects in a manner that is likely to reduce further transmission.94
Alternatively, antiretroviral therapy could be used to suppress viral replication in order to reduce HIV-1 transmission. What constitutes the optimal use of antiretroviral therapy for patients with acute infection is unclear, in part because the extent of the personal health benefit derived from early use of antiretroviral therapy remains in question.95,96 There have been considerable differences in reported results of the clinical benefits of antiretroviral therapy for patients with primary HIV infection because in many cases antiretroviral therapy was initiated weeks or even months after HIV-1 acquisition — probably much too late to influence the course of disease. A few small studies have shown some benefit when therapy was provided before or during seroconversion, with some degree of immune preservation96 and with sustained reduction of blood viral load after antiretroviral therapy was discontinued. Moir et al. reported that patients treated soon after receiving the diagnosis had improved B-cell function.97 Very early administration of antiretroviral therapy may limit the size of the latent pool of HIV-1–infected CD4 T cells.98,99 Although encouraging, these results underscore the need for well-constructed clinical trials that will provide the basis for determining the overall cost–benefit ratio of antiretroviral therapy for acute HIV-1 infection and for balancing the public health benefits with the benefits for individual patients.
In the most recent treatment guidelines from the International Antiviral Society — USA, the authors argue that potential benefits to public and individual health may even now justify the treatment of patients with acute HIV infection, particularly those who are symptomatic.100 The authors of other guidelines have come to similar conclusions, setting the stage for regular treatment of persons with acute HIV infection. If anti-retroviral therapy is to be provided, the treatment regimen might include drugs that concentrate in the genital tract of men and women85 and an integrase inhibitor, the latter because of the rapidity with which this class of drugs lowers the viral load.101 Some pilot studies using multidrug regimens, such as those administered before HIV seroconversion, are in progress.102
CONCLUSIONS
The earliest events in acute HIV-1 infection determine the future health of the individual patient and the extent of transmission in the general population. Recent studies have unraveled many of the initial immune events of acute infection. With improved diagnostic tests, greater numbers of persons with acute HIV-1 infection will come to the attention of practicing physicians and public health officials. Although considerable progress has been made in understanding the HIV-1 transmission event, more studies are needed to develop optimal treatment and prevention strategies for people in the earliest stages of HIV-1 infection.
Acknowledgments
Supported by the National Institutes of Health and the Center for HIV–AIDS Vaccine Immunology.
We thank Joseph Sodroski, Norman Letvin, David Goldstein, Kelly Soderberg, Kimberly Powers, Stuart Shapiro, and Margaret Johnston for helpful discussions and Ward Cates, David Burns, Joseph Eron, and William Miller for their careful review of an earlier version of the manuscript.
Footnotes
Dr. Cohen reports receiving consulting fees from GlaxoSmith-Kline and Merck; Dr. McMichael, receiving payment for the development of educational presentations from Henry Stewart Talks; and Dr. Haynes, receiving a research grant from Peregrine Pharmaceuticals. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Global report 2010. Geneva: UNAIDS; 2010. http://www.unaids.org/globalreport/documents/20101123_GlobalReport_full_en.pdf. [Google Scholar]
- 2.Branson BM. State of the art for diagnosis of HIV infection. Clin Infect Dis. 2007;45(Suppl 4):S221–S225. doi: 10.1086/522541. [DOI] [PubMed] [Google Scholar]
- 3.Eshleman SH, Khaki L, Laeyendecker O, et al. Detection of individuals with acute HIV-1 infection using the ARCHITECT HIV Ag/Ab Combo assay. J Acquir Immune Defic Syndr. 2009;52:121–124. doi: 10.1097/QAI.0b013e3181ab61e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiebig EW, Wright DJ, Rawal BD, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS. 2003;17:1871–1879. doi: 10.1097/00002030-200309050-00005. [DOI] [PubMed] [Google Scholar]
- 6.Weiss HA, Dickson KE, Agot K, Han-kins CA. Male circumcision for HIV prevention: current research and programmatic issues. AIDS. 2010;24(Suppl 4):S61–S69. doi: 10.1097/01.aids.0000390708.66136.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emau P, Jiang Y, Agy MB, Tian B, Bekele G, Tsai CC. Post-exposure prophylaxis for SIV revisited: animal model for HIV prevention. AIDS Res Ther. 2006;3:29. doi: 10.1186/1742-6405-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keele BF, Giorgi EE, Salazar-Gonza-lez JF, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee HY, Giorgi EE, Keele BF, et al. Modeling sequence evolution in acute HIV-1 infection. J Theor Biol. 2009;261:341–360. doi: 10.1016/j.jtbi.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palmer S, Wiegand AP, Maldarelli F, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–4536. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damond F, Avettand-Fenoel V, Collin G, et al. Evaluation of an upgraded version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test for HIV-1 load quantification. J Clin Microbiol. 2010;48:1413–1416. doi: 10.1128/JCM.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sickinger E, Jonas G, Yem AW, et al. Performance evaluation of the new fully automated human immunodeficiency virus antigen-antibody combination assay designed for blood screening. Transfusion. 2008;48:584–593. doi: 10.1111/j.1537-2995.2007.01583.x. [DOI] [PubMed] [Google Scholar]
- 13.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 14.Hladik F, Sakchalathorn P, Ballweber L, et al. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity. 2007;26:257–270. doi: 10.1016/j.immuni.2007.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boggiano C, Littman DR. HIV’s vagina travelogue. Immunity. 2007;26:145–147. doi: 10.1016/j.immuni.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Schuler T, Zupancic M, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [Erratum, Science 1999;286:2273.] [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZQ, Wietgrefe SW, Li Q, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proc Natl Acad Sci U S A. 2004;101:5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 19.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–476. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 20.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Bar KJ, Wang S, et al. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 2010;6(5):e1000890. doi: 10.1371/journal.ppat.1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veazey RS, DeMaria M, Chalifoux LV, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 23.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 26.Arthos J, Cicala C, Martinelli E, et al. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat Immunol. 2008;9:301–309. doi: 10.1038/ni1566. [DOI] [PubMed] [Google Scholar]
- 27.Kader M, Wang X, Piatak M, et al. Al-pha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2009;2:439–449. doi: 10.1038/mi.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicala C, Martinelli E, McNally JP, et al. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc Natl Acad Sci U S A. 2009;106:20877–20882. doi: 10.1073/pnas.0911796106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schacker T, Little S, Connick E, et al. Productive infection of T cells in lym-phoid tissues during primary and early human immunodeficiency virus infection. J Infect Dis. 2001;183:555–562. doi: 10.1086/318524. [DOI] [PubMed] [Google Scholar]
- 30.Chun TW, Engel D, Berrey MM, Shea T, Corey L, Fauci AS. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95:8869–8873. doi: 10.1073/pnas.95.15.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro RM, Qin L, Chavez LL, Li D, Self SG, Perelson AS. Estimation of the initial viral growth rate and basic reproductive number during acute HIV-1 infection. J Virol. 2010;84:6096–6102. doi: 10.1128/JVI.00127-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keele BF, Li H, Learn GH, et al. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med. 2009;206:1117–1134. doi: 10.1084/jem.20082831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abrahams MR, Anderson JA, Giorgi EE, et al. Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-Poisson distribution of transmitted variants. J Virol. 2009;83:3556–3567. doi: 10.1128/JVI.02132-08. [Erratum, J Virol 2009;83:6974.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haaland RE, Hawkins PA, Salazar-Gonzalez J, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5(1):e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bar KJ, Li H, Chamberland A, et al. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J Virol. 2010;84:6241–6247. doi: 10.1128/JVI.00077-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masharsky AE, Dukhovlinova EN, Verevochkin SV, et al. A substantial transmission bottleneck among newly and recently HIV-1-infected injection drug users in St Petersburg, Russia. J Infect Dis. 2010;201:1697–1702. doi: 10.1086/652702. [DOI] [PubMed] [Google Scholar]
- 38.Fischer W, Ganusov VV, Giorgi EE, et al. Transmission of single HIV-1 genomes and dynamics of early immune escape revealed by ultra-deep sequencing. PLoS One. 2010;5(8):e12303. doi: 10.1371/journal.pone.0012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kramer HB, Lavender KJ, Qin L, et al. Elevation of intact and proteolytic fragments of acute phase proteins constitutes the earliest systemic antiviral response in HIV-1 infection. PLoS Pathog. 2010;6(5):e1000893. doi: 10.1371/journal.ppat.1000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gasper-Smith N, Crossman DM, Whitesides JF, et al. Induction of plasma (TRAIL), TNFR-2, Fas ligand, and plasma microparticles after human immunodeficiency virus type 1 (HIV-1) transmission: implications for HIV-1 vaccine design. J Virol. 2008;82:7700–7710. doi: 10.1128/JVI.00605-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borrow P, Bhardwaj N. Innate immune responses in primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:36–44. doi: 10.1097/COH.0b013e3282f2bce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med. 2007;204:3027–3036. doi: 10.1084/jem.20070695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansasuta P, Dong T, Thananchai H, et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol. 2004;34:1673–1679. doi: 10.1002/eji.200425089. [DOI] [PubMed] [Google Scholar]
- 45.Martin MP, Gao X, Lee JH, et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet. 2002;31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 46.Tomaras GD, Yates NL, Liu P, et al. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J Virol. 2008;82:12449–12463. doi: 10.1128/JVI.01708-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 48.Moore PL, Crooks ET, Porter L, et al. Nature of nonfunctional envelope proteins on the surface of human immunodeficiency virus type 1. J Virol. 2006;80:2515–2528. doi: 10.1128/JVI.80.5.2515-2528.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollara J, Landucci G, Trac C, et al. Early appearance of ADCC- and ADCVI-mediating antibody responses against autologous HIV-1 transmitted/founder virus. AIDS Res Hum Retroviruses. 2010;26:A-12. abstract. [Google Scholar]
- 50.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichterfeld M, Yu XG, Cohen D, et al. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS. 2004;18:1383–1392. doi: 10.1097/01.aids.0000131329.51633.a3. [DOI] [PubMed] [Google Scholar]
- 52.Goonetilleke N, Liu MK, Salazar-Gonzalez JF, et al. The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med. 2009;206:1253–1272. doi: 10.1084/jem.20090365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrari G, Korber BT, Turnbull E, et al. High magnitude and multifunctionality epitope-specific CD8+ T-cell responses are associated with selection of escape mutants in acute HIV infection. AIDS Res Hum Retroviruses. 2010;26:A-5. abstract. [Google Scholar]
- 54.Hersperger AR, Pereyra F, Nason M, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6(5):e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leslie AJ, Pfafferott KJ, Chetty P, et al. HIV evolution: CTL escape mutation and reversion after transmission. Nat Med. 2004;10:282–289. doi: 10.1038/nm992. [DOI] [PubMed] [Google Scholar]
- 56.Phillips AN. Reduction of HIV concentration during acute infection: independence from a specific immune response. Science. 1996;271:497–499. doi: 10.1126/science.271.5248.497. [DOI] [PubMed] [Google Scholar]
- 57.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 59.Liu J, O’Brien KL, Lynch DM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2009;457:87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330:1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daar ES, Pilcher CD, Hecht FM. Clinical presentation and diagnosis of primary HIV-1 infection. Curr Opin HIV AIDS. 2008;3:10–15. doi: 10.1097/COH.0b013e3282f2e295. [DOI] [PubMed] [Google Scholar]
- 62.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 63.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 64.Ma ZM, Stone M, Piatak M, Jr., et al. High specific infectivity of plasma virus from the pre-ramp-up and ramp-up stages of acute simian immunodeficiency virus infection. J Virol. 2009;83:3288–3297. doi: 10.1128/JVI.02423-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198:687–693. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 66.Pinkerton SD. How many sexually-acquired HIV infections in the USA are due to acute-phase HIV transmission? AIDS. 2007;21:1625–1629. doi: 10.1097/QAD.0b013e32826fb6a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Idem. Probability of HIV transmission during acute infection in Rakai, Uganda. AIDS Behav. 2008;12:677–684. doi: 10.1007/s10461-007-9329-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jacquez JA, Koopman JS, Simon CP, Longini IM., Jr. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994;7:1169–1184. [PubMed] [Google Scholar]
- 69.Koopman JS, Jacquez JA, Welch GW, et al. The role of early HIV infection in the spread of HIV through populations. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:249–258. doi: 10.1097/00042560-199703010-00009. [DOI] [PubMed] [Google Scholar]
- 70.Kretzschmar M, Dietz K. The effect of pair formation and variable infectivity on the spread of an infection without recovery. Math Biosci. 1998;148:83–113. doi: 10.1016/s0025-5564(97)10008-6. [DOI] [PubMed] [Google Scholar]
- 71.Coutinho FA, Lopez LF, Burattini MN, Massad E. Modelling the natural history of HIV infection in individuals and its epidemiological implications. Bull Math Biol. 2001;63:1041–1062. doi: 10.1006/bulm.2001.0257. [DOI] [PubMed] [Google Scholar]
- 72.Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS. 2004;18:1311–1320. doi: 10.1097/00002030-200406180-00010. [DOI] [PubMed] [Google Scholar]
- 73.Hayes RJ, White RG. Amplified HIV transmission during early-stage infection. J Infect Dis. 2006;193:604–606. doi: 10.1086/499606. [DOI] [PubMed] [Google Scholar]
- 74.Abu-Raddad LJ, Longini IM., Jr. No HIV stage is dominant in driving the HIV epidemic in sub-Saharan Africa. AIDS. 2008;22:1055–1061. doi: 10.1097/QAD.0b013e3282f8af84. [DOI] [PubMed] [Google Scholar]
- 75.Powers K, Kamanga G, Mapanje C, et al. Efficient detection of acute HIV infection through targeted HIV RNA screening in a Malawian STI clinic; Vienna, Austria. Presented at the XVIII International AIDS Conference; July 18–23, 2010; abstract. [Google Scholar]
- 76.Salomon JA, Hogan DR. Evaluating the impact of antiretroviral therapy on HIV transmission. AIDS. 2008;22(Suppl 1):S149–S159. doi: 10.1097/01.aids.0000327636.82542.87. [DOI] [PubMed] [Google Scholar]
- 77.Pinkerton SD, Abramson PR. Implication of increased infectivity in early-stage HIV infection: application of a Bernoulli-process model of HIV transmission. Eval Rev. 1996;20:516–540. doi: 10.1177/0193841X9602000502. [DOI] [PubMed] [Google Scholar]
- 78.Miller WC, Rosenberg NE, Rutstein SE, Powers KA. Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS. 2010;5:277–282. doi: 10.1097/COH.0b013e32833a0d3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brenner BG, Roger M, Routy JP, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195:951–959. doi: 10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 80.Morris L, Binley JM, Clas BA, et al. HIV-1 antigen-specific and -nonspecific B cell responses are sensitive to combination antiretroviral therapy. J Exp Med. 1998;188:233–245. doi: 10.1084/jem.188.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bonsignori M, Moody MA, Parks RJ, et al. HIV-1 envelope induces memory B cell responses that correlate with plasma antibody levels after envelope gp120 protein vaccination or HIV-1 infection. J Immunol. 2009;183:2708–2717. doi: 10.4049/jimmunol.0901068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McElrath MJ, Haynes BF. Induction of immunity to human Immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haynes B, Liao HX, Tomaras GD. Is developing an HIV-1 vaccine possible? Curr Opin HIV AIDS. 2010;5:362–367. doi: 10.1097/COH.0b013e32833d2e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mayer KH, Venkatesh KK. Antiretroviral therapy as HIV prevention: status and prospects. Am J Public Health. 2010;100:1867–1876. doi: 10.2105/AJPH.2009.184796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cohen MS, Gay C, Kashuba AD, Blower S, Paxton L. Narrative review: antiretroviral therapy to prevent the sexual transmission of HIV-1. Ann Intern Med. 2007;146:591–601. doi: 10.7326/0003-4819-146-8-200704170-00010. [DOI] [PubMed] [Google Scholar]
- 86.Karim QA, Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gay CL, Cohen MS. Antiretrovirals to prevent HIV infection: pre- and postexposure prophylaxis. Curr Infect Dis Rep. 2008;10:323–331. doi: 10.1007/s11908-008-0052-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pre-exposure prophylaxis (PrEP) for HIV prevention: promoting safe and effective use in the United States. Atlanta: Centers for Disease Control and Prevention; http://www.cdc.gov/hiv/prep. [Google Scholar]
- 90.Garcia-Lerma JG, Cong ME, Mitchell J, et al. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3000391. 14ra4. [DOI] [PubMed] [Google Scholar]
- 91.Hightow LB, MacDonald PD, Pilcher CD, et al. The unexpected movement of the HIV epidemic in the Southeastern United States: transmission among college students. J Acquir Immune Defic Syndr. 2005;38:531–537. doi: 10.1097/01.qai.0000155037.10628.cb. [DOI] [PubMed] [Google Scholar]
- 92.Golden MR, Dombrowski JC, Wood RW, Fleming M, Harrington RD. A controlled study of the effectiveness of public health HIV partner notification services. AIDS. 2009;23:133–135. doi: 10.1097/QAD.0b013e32831fb52f. [DOI] [PubMed] [Google Scholar]
- 93.Brown LB, Miller WC, Kamanga G, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56:437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pettifor A, Macphail C, Corneli A, et al. Continued high risk sexual behavior following diagnosis with acute HIV infection in South Africa and Malawi: implications for prevention. AIDS Behav. 2010 Oct 27; doi: 10.1007/s10461-010-9839-0. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fidler S, Fox J, Porter K, Weber J. Primary HIV infection: to treat or not to treat? Curr Opin Infect Dis. 2008;21:4–10. doi: 10.1097/QCO.0b013e3282f428bf. [DOI] [PubMed] [Google Scholar]
- 96.Bell SK, Little SJ, Rosenberg ES. Clinical management of acute HIV infection: best practice remains unknown. J Infect Dis. 2010;202(Suppl 2):S278–S288. doi: 10.1086/655655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moir S, Buckner CM, Ho J, et al. B cells in early and chronic HIV infection: evidence for preservation of immune function associated with early initiation of antiretroviral therapy. Blood. 2010;116:5571–5579. doi: 10.1182/blood-2010-05-285528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chun TW, Justement JS, Moir S, et al. Decay of the HIV reservoir in patients receiving antiretroviral therapy for extended periods: implications for eradication of virus. J Infect Dis. 2007;195:1762–1764. doi: 10.1086/518250. [DOI] [PubMed] [Google Scholar]
- 99.Archin N, Cheema M, Sackmann R, Sugarbaker A, Ngo L, Kuruc J. Correlation of peak and duration of viremia with resting CD4+ T cell infection in acute HIV infection; San Francisco. Presented at the 17th Conference on Retroviruses and Opportunistic Infections; February 16–19 2010; abstract. [Google Scholar]
- 100.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304:321–333. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 101.Murray JM, Emery S, Kelleher AD, et al. Antiretroviral therapy with the integrase inhibitor raltegravir alters decay kinetics of HIV, significantly reducing the second phase. AIDS. 2007;21:2315–2321. doi: 10.1097/QAD.0b013e3282f12377. [DOI] [PubMed] [Google Scholar]
- 102.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–731. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]