Abstract
When the U.S. Agency for International Development (USAID) began to support national programs integrating their neglected tropical disease (NTD) program activities, the expected impact on individual disease-specific programs was unclear, particularly with respect to program financing and coverage. To assess this impact, data were collected by NTD program managers and their non-governmental organization (NGO) partners in Burkina Faso, Mali, and Uganda from 2 years prior and 2 years after their individual programs received funding for an integrated NTD program. Findings show that these countries experienced some increases in overall funding available for integrated NTD programs, an expansion of geographical coverage and of the number of persons treated, and the addition of treatments targeted at new diseases. What is not clear is whether these achievements can be sustained if there are decreases in external support in the future. Seeking increased government commitment or sustained external donor support should be a top priority.
Introduction
The control of neglected tropical diseases (NTDs) through preventive chemotherapy is a public health strategy recently advanced by the World Health Organization (WHO) that is based on a broad-scale distribution of medicine (“mass drug administration” [MDA]) to at-risk populations that now targets nearly 2 billion people.1 However, managers of previously individual, disease-specific NTD control programs (targeting lymphatic filariasis [LF], onchocerciasis, schistosomiasis, soil-transmitted helminthiasis [STH], and trachoma) expressed concern that because of a loss of autonomy—both programmatic and budgetary—their individual programs would be negatively impacted and progress toward disease-specific goals would be compromised.2 This recent discussion within the NTD community reflects the long-standing debate between proponents of horizontal approaches to health care delivery (promoting increased synergy and efficiency) and proponents of vertical approaches (promoting more individual program accountability).3,4
The drive toward integration of disease-specific NTD programs is fueled by two expectations: first, that integration of activities from multiple individual NTD programs will improve the efficiency with which funds are spent; and second, that the packaging of NTD interventions will result in increased overall funding as a result of achieving greater visibility.5–7 If these assumptions hold true, integrated NTD programs should 1) bring in more resources to treat NTDs, 2) reach more persons with more interventions, and 3) be more cost-efficient. This study tests the first two of these hypotheses at country level. An analysis of cost efficiency is beyond the scope of this work but is an important topic that should be addressed in future studies.
The U.S. Agency for International Development (USAID) was one of the first donors to fund implementation of large-scale, integrated approaches to treat NTDs, beginning its support in five “fast track” countries in 2006.8,9 Three of these countries—Burkina Faso, Mali, and Uganda—were selected to examine at national level how integration of NTDs would affect the funding and scaling up of disease-specific interventions. In 2006, all three countries were operating disease-specific programs for up to five diseases targeted by USAID's integrated NTD Control Program (managed by RTI International). Some effort toward integration had already been made, most notably between schistosomiasis and STH programs, but most of the NTD programs operated autonomously with independent planning, budgeting, training, drug distribution, monitoring and evaluation cycles, and staffing. Additionally, the three study countries had varying levels of achievement in addressing the NTDs: Mali had existing drug distribution programs for all five of the targeted diseases, Burkina Faso and Uganda had not yet begun treatment of trachoma with azythromycin, and the LF elimination program in Uganda had started treatment but was faltering because of a lack of funding.
Methods
The study sought to answer the following questions:
-
1)
Was there any change in the total amount of financial support available after NTDs were integrated?
-
2)
Was there any change in program coverage after NTDs were integrated?
Financial data and treatment data were collected by NTD program managers and non-governmental organization (NGO) partners according to a structured protocol in Burkina Faso, Mali, and Uganda for 2 years before integration (2005, 2006) and 2 years post-integration (2007, 2008). In all three countries, each program manager met with study investigators to review and interpret the data and to discuss their experiences regarding integration. Data on budgets was also collected from the Department of Finance in each country.
Financial support.
Data on program budgets were collected by source and type of funding (disease-specific funding from three sources: “government,” referring to in-country government budgets; “internal,” referring to funding received from in-country sources other than the government; and “external,” referring to funding received from organizations based outside the country; and integrated funding from one source: “NTD Control Program”). The data were also collected by activity (training, mapping, social mobilization/education/advocacy, drug distribution including supervision, adverse reaction monitoring, surveillance/monitoring and evaluation/laboratory services, program administration, and non-MDA costs such as morbidity management, vector control, surgery, hygiene education, and environmental improvements). Disease mapping funds were excluded from the analysis because they constitute “a one-time expense” that is highly variable from year to year and disease to disease.
Financial input—not economic—was evaluated. The purpose of this study is to assess changes in financial support as an indicator of funding available for activities related to program implementation. Therefore, the following contributions were not included: the enormous in-kind drug donations by pharmaceutical companies, in-kind contributions made by endemic country governments including salaries and office space, and volunteer drug distributors' time. Programs would not be possible without these vital contributions, however, the assumption was made that these remained constant before and after integration.
Budget data were collected instead of cost data, because the study sought to understand the amount of funding available to programs pre- and post-integration.
Program coverage.
To measure changes in program reach, data on the total number of treatments delivered (defined as the number of treatments given in the form of tablets/syrup/ointment for each of the targeted NTDs) and districts targeted (by disease) was collected. As a measure of program quality, data on treatment coverage (calculated as the number of people treated divided by the number of eligible people in areas targeted for treatment) was also collected.
Results And Discussion
Each country experienced differing degrees of “integration.” Activities that were integrated in all three countries included: program planning, field supervision of drug distributions, training, and transport for field activities. In Burkina Faso, the NTD programs also conducted joint social mobilization and development of integrated information, education, and communication materials. In Mali, it was noted that because drug distributions were coordinated over a several week period (instead of scattered throughout the year) and because trainings were integrated, community drug distributors were now the same for all of the diseases.
The total amount of funding available to NTD programs and total number of persons treated for the different diseases is presented by country and by year in Figures 1 and 2 and discussed below.
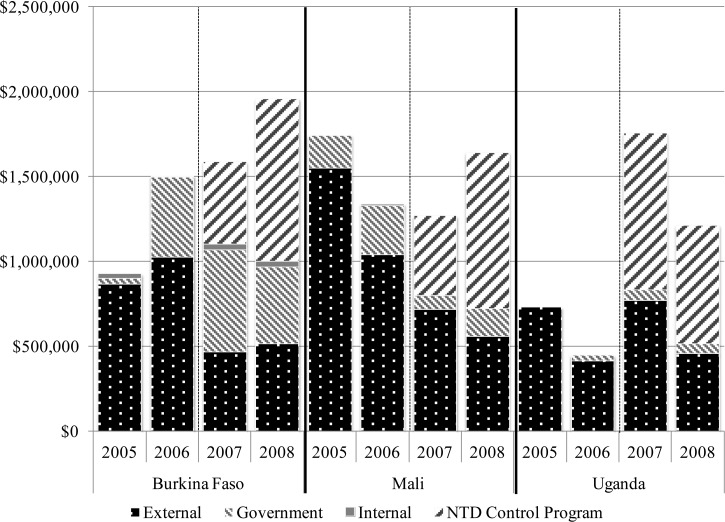
Figure 1.
Neglected tropical disease (NTD) program financial support, by country and source.
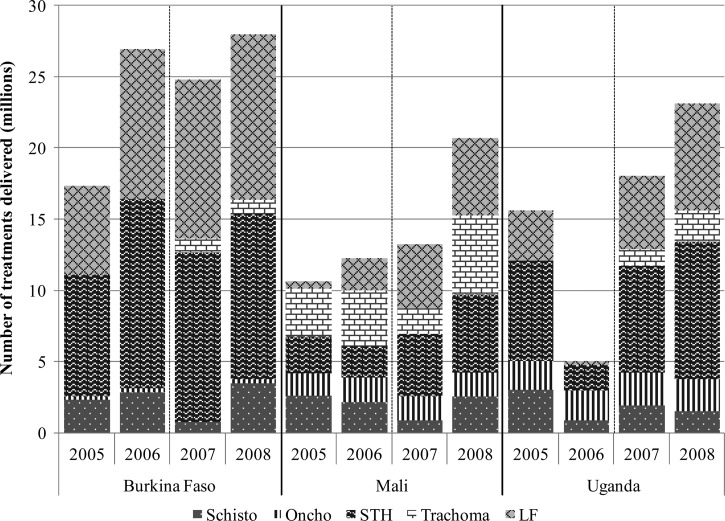
Figure 2.
Treatments delivered, by disease.
Burkina Faso.
In Burkina Faso, an increase in funding for NTDs and an expansion of treatments to include trachoma was observed.
Before the NTD Control Program's engagement with Burkina Faso, there were pre-existing government-funded programs for LF and onchocerciasis and an STH and schistosomiasis program with external funds available only through 2006. In 2007, there was an initial decrease in total disease-specific funding (27%) that was offset by the addition of NTD integration funding. By 2008, total available funds had increased by 30%. Of note, this included an increase in government support earmarked for the NTDs following integration. The STH/schistosomiasis program, which was largely externally funded before NTD integration, experienced a 57% decrease in disease-specific external funding in 2007 post-integration (largely because of expiration of prior grant support); NTD Control Program funds therefore largely replaced program funding for STH and schistosomiasis, ensuring sustained benefits.
As a result of integration and increased availability of funds, the Ministry of Health built on the already existing platforms and began MDA for trachoma in 2007. Program coverage remained strong, with treatment coverage rates of over 80% for each of the diseases. Although it appears the number of treatments delivered for schistosomiasis decreased between 2006 and 2007, this was a result of the alternate year treatment schedule implemented for schistosomiasis control.
Mali.
In Mali, sustained funding and a significant increase in treatments delivered were observed after NTD integration.
Before the start of the NTD control program in Mali, there were independent MDA programs for all of the diseases targeted, but with external funding already decreasing and set to expire in 2006. The 40% decrease in total disease-specific funding from 2006 to 2007 was partially offset by the introduction of integration funding for NTDs, and overall funding increased by 23% between 2006 and 2008, approaching the 2005 funding level. Although there was concern that government contributions decreased in 2007, they rose again in 2008.
The total number of treatments delivered increased from just over 14 million in 2006 to nearly 21 million by 2008. These increases are mostly accounted for by the rapid scale-up of the LF and trachoma MDA programs (Figure 2), which maintained high treatment coverage rates of over 80%. On the other hand, the schistosomiasis control program only scaled up treatments to an additional 400,000 persons from 2006 to 2008, with program coverage rates of 40% in 2007 and 58% in 2008. These rates were attributed by program managers to logistical problems associated with changing delivery strategies and the necessity of treating during the rainy seasons because of delayed arrival of drugs. On the other hand, the schistosomiasis program coverage of school age children remained high, increasing from 81% in 2006 to 100% in 2008.10
Uganda.
In Uganda, a significant increase in funding, rapid scale-up of already established programs, and the addition of treatments for trachoma were observed.
In Uganda, unlike the other two countries, there was an increase in disease-specific funding in 2007, including notably an increase in government contributions. With the inclusion of the NTD Control Program funding for integration, there was therefore a 171% increase in overall funding available for the NTDs between 2006 and 2008 (Figure 1). Though the level of government support remained the same for the 2 years following integration, the amount contributed was relatively low and the Ugandan NTD programs remained largely reliant on external sources of funding both before and after integration.
Additional funding enabled treatment of LF to resume and rapidly scale up. As a result of scaling up LF treatment and Uganda's Child Days Plus strategy, treatment coverage for STH also significantly increased, with the WHO reporting 88.6% national coverage of all school-age children in 2008 compared with 29.5% in 2006.11 Mapping for trachoma began in 2006, enabling the start of a new mass treatment program for trachoma in 2007. Treatment coverage for trachoma started low at 49% in 2007, though it improved marginally to 63% in 2008.
Conclusions
There is no evidence in the three countries studied that progress toward reaching disease-specific goals has been compromised following integration. Burkina Faso and Uganda experienced an increased availability of NTD funding, whereas Mali experienced a decrease; all three countries, however, maintained or increased the number of people treated. The introduction of new funds for the integrated control of NTDs ensured short-term sustainability of programs during the study years, enabled the scaling up of treatment delivery (especially for LF in Mali and Uganda, and for trachoma in Mali), and leveraged existing platforms to launch new programs (trachoma elimination in Burkina Faso and Uganda).
These results also highlight the fragility of the NTD programs, so heavily reliant on external funding. In both Burkina Faso and Mali, the cessation of earlier external funding was just able to be offset by the arrival in 2007 and 2008 of support from the NTD Control Program. In Uganda, the LF elimination program missed a treatment round before the NTD Control Program as a result of inadequate funding. Longer gaps in funding resulting in delayed program implementation may cause a loss of previous gains made toward achieving the elimination and control of these diseases. Although government financial support was generally maintained after integration, it represents a very small proportion of the total funds available for program implementation activities. This shows the need for a longer term sustainability plan: whether this is best achieved through increased government commitment or increased donor support from a wider array of donors is up for debate.
Footnotes
Financial support: This study is made possible by the generous support of the American people through the USAID. The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. The authors are grateful to the Bill and Melinda Gates Foundation for their financial support of the study.
Authors' addresses: Pamela J. Hooper, Dominique Kyelem, Brian Chu, and Rebecca Mann Flueckiger, Lymphatic Filariasis Support Center, Task Force for Global Health - LF Support Center, Decatur, GA, E-mails: phooper@taskforce.org, dkyelem@taskforce.org, bchu@taskforce.org, and rmann@taskforce.org. Kathryn L. Zoerhoff, Ambrose Onapa, and Scott Torres, RTI International - Center for International Health, Washington, DC, E-mails: kzoerhoff@rti.org, kwibale1@yahoo.co.uk, and storres@rti.org. Sanoussi Bamani, Alain Brice Paré, and Mamadou Oumar Traore, Ministère de la Santé - Programme National de Lutte contre la Cécité, Bamako, Mali, E-mails: pnlcsmb@orangemali.net, alainbricepare@yahoo.fr, and traoremot@yahoo.fr. Windtaré Roland Bougma, Ministère de la Santé - Programme National d'Elimination de la Filariose Lymphatique, Ouagadougou, Burkina Faso, E-mail: wrolandbougma@yahoo.fr. Fiona Fleming, Imperial College London - Schistosomiasis Control Initiative, London, UK, E-mail: f.fleming@imperial.ac.uk. Marjon Tuinsma, Helen Keller International - HKI-Mali, Bamako, Mali, E-mail: mtuinsma@hki.org. Mary Linehan, United States Agency for International Development - Office of Health, Jakarta, Indonesia, E-mail: marylinehan609@gmail.com. Margaret Baker, Georgetown University - Department of International Health, Washington, DC, E-mail: mcb93@georgetown.edu.
References
- 1.World Health Organization . Preventive Chemotherapy in Human Helminthiasis: Coordinated Use of Antihelminthic Drugs in Control Interventions: A Manual for Health Professionals and Programme Managers. Geneva: World Health Organization; 2006. http://whqlibdoc.who.int/publications/2006/9241547103_eng.pdf Available at. Accessed February 9, 2012. [Google Scholar]
- 2.Kabatereine NB, Malecela M, Lado M, Zaramba S, Amiel O, Kolaczinski JH. How to (or not to) integrate vertical programmes for the control of major neglected tropical diseases in sub-Saharan Africa. PLoS Negl Trop Dis. 2010;4:e755. doi: 10.1371/journal.pntd.0000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira Cruz V, Kurowski C, Mills A. Delivery of priority health services: searching for synergies within the horizontal vertical debate. J Int Dev. 2003;15:67–86. [Google Scholar]
- 4.Victora CG, Hanson K, Bryce J, Vaughan JP. Achieving universal coverage with health interventions. Lancet. 2004;364:1541–1548. doi: 10.1016/S0140-6736(04)17279-6. [DOI] [PubMed] [Google Scholar]
- 5.Molyneux DH, Hotez PJ, Fenwick A. “Rapid-impact interventions:” how a policy of integrated control for Africa's neglected tropical diseases could benefit the poor. PLoS Med. 2005;2:e336. doi: 10.1371/journal.pmed.0020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brady MA, Hooper PJ, Ottesen EA. Projected benefits from integrating NTD programmes in sub-Saharan Africa. Trends Parasitol. 2006;22:285–291. doi: 10.1016/j.pt.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Lammie PJ, Fenwick A, Utzinger J. A blueprint for success: integration of neglected tropical disease control programmes. Trends Parasitol. 2006;22:313–321. doi: 10.1016/j.pt.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Linehan M, Hanson C, Weaver A, Baker M, Kabore A, Zoerhoff KL, Sankara D, Torres S, Ottesen EA. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: proving the feasibility at national scale. Am J Trop Med Hyg. 2011;84:5–14. doi: 10.4269/ajtmh.2011.10-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson C, Weaver A, Zoerhoff KL, Kabore A, Linehan M, Doherty A, Engels D, Savioli L, Ottesen EA. Integrated implementation of programs targeting neglected tropical diseases through preventive chemotherapy: identifying best practices to roll out programs at national scale. Am J Trop Med Hyg. 2012;86:508–513. doi: 10.4269/ajtmh.2012.11-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dembélé M, Bamani S, Dembélé R, Traoré MO, Goita S, Traoré MN, Sidibe AK, Sam L, Tuinsma M, Toubali E, MacArthur C, Baker SK, Zhang Y. Implementing preventive chemotherapy through an Integrated National Neglected Tropical Disease Control Program in Mali. PLoS Negl Trop Dis. 2012;6:e1574. doi: 10.1371/journal.pntd.0001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Preventive Chemotherapy (PCT) Databank 2013. http://www.who.int/neglected_diseases/preventive_chemotherapy/databank/en/index.html Available at. Accessed August 1, 2012.