Abstract
Current regulations stipulate a yellow fever (YF) booster every 10 years. We conducted a systematic review of the protective efficacy and duration of immunity of YF vaccine in residents of disease-endemic areas and in travelers to assess the need for a booster in these two settings and in selected populations (human immunodeficiency virus–infected persons, infants, children, pregnant women, and severely malnourished persons). Thirty-six studies and 22 reports were included. We identified 12 studies of immunogenicity, 8 of duration of immunity, 8 of vaccine response in infants and children, 7 of human-immunodeficiency virus–infected persons, 2 of pregnant women, and 1 of severely malnourished children. Based on currently available data, a single dose of YF vaccine is highly immunogenic and confers sustained life-long protective immunity against YF. Therefore, a booster dose of YF vaccine is not needed. Special considerations for selected populations are detailed.
Introduction
Yellow fever (YF) poses a considerable health care burden and a serious risk to residents of disease-endemic regions, non-immunized travelers entering disease-endemic areas, and persons moving within their own country from low-risk to high-risk regions.1–3 Because there is no effective treatment for YF, prevention through immunization is critical to lower morbidity and mortality.
The World Health Organization currently recommends two live attenuated YF vaccines: 1) strain 17D-204 and 2) strain 17DD. The combined strategy for YF control includes the Expanded Program on Immunization and preventive vaccination campaigns. Administration of YF vaccine is recommended for persons ≥ 9 months of age who are traveling to or living in high-risk areas.4 For travelers, International Health Regulations stipulate that the vaccination certificate for YF is valid beginning 10 day(s) after administration of the vaccine for primary vaccine recipients and requires a booster after 10 years.5 However, this recommendation has been challenged because many studies have suggested that the duration of immunity after YF vaccine may last for several years in as many as 80% of vaccines.6–13
We conducted a systematic review of the protective efficacy and duration of immunity after YF vaccination. The principal aim of this review was to assess the need for a booster dose every 10 years based on the efficacy profile and the available evidence on duration of immunity in residents of disease-endemic areas and in travelers. We also searched for reports of YF that developed in YF vaccinees post-vaccination and the time elapsed since they were immunized. We also explored the immunogenicity in specific groups in which a booster or a second dose may need to be considered.
Methods
Search strategy.
We used the EndNote X5 Software (Thomson Reuters, New York, NY). We systematically searched in two databases: PubMed (last search June 2012) and SCIELO (last search May 2012). The search was conducted in four languages: English, French, Portuguese, and Spanish and evaluated any study addressing the efficacy and/or duration of immunity after YF immunization. In PubMed, we used the Medical Subject Headings (MeSH) terms yellow fever vaccine, immunity, antibody formation, antibodies, NTAbs, travel, immunization, booster immunization, secondary immunizations, revaccination, human immunodeficiency virus, acquired immune deficiency Syndrome, HIV seropositivity, malnutrition, immunocompromised hosts, immunocompromised patient, pregnancy, infant, child, preschool, and aged. We performed MeSH term-pertinent combinations by using the Boolean operators AND or OR. The MeSH terms were also combined with relevant free terms as text word: efficacy, neutralization test, endemic, immunocompromised, and elderly.
In addition, we scanned the reference lists of all included papers and of all the articles that were excluded because of being review articles to identify additional relevant studies. We also used the words yellow fever, vaccine failure, and case report in the Google internet search engine. Furthermore, we obtained unpublished information through personal communication with an expert in the field (Dr. Thomas Monath).
Study selection and eligibility criteria.
All abstracts and full text articles were independently read by two reviewers to determine relevance with any disagreements resolved by consensus. We used inclusion and exclusion criteria to assess the full eligibility of the included studies. To be included in this review, studies could address either efficacy, duration of immunity, or both. Studies that assessed duration of immunity but had a shorter than 10-year-follow-up period after vaccination were excluded.
We did not exclude studies on the basis of the type of correlate of protection or test used to measure antibody persistence but the specific technique and cutoff titer were detailed for each individual study. We did not apply any study-design exclusion criteria; randomized controlled trials (RCTs), prospective and retrospective cohort studies, and other small observational studies were acceptable.
Data extraction.
Two authors revised all the potentially included abstracts and full-text articles, achieved consensus regarding eligibility, and extracted the data by using pre-designed data extraction sheets. These sheets were designed by the authors and detailed the study population (making a special remark on whether it was carried out in a disease-endemic region or in travelers), the type of YF vaccine used, the method used to determine antibody titers, and the time since last immunization for studies assessing duration of immunity.
Assessment of risk of bias.
We used the PRISMA guidelines to assess the risk of bias in individual studies and across studies. We did not use scales. Instead, we assessed the risk for different items separately. For RCTs, all three authors independently evaluated the quality of the study according to the Cochrane risk-of-bias approach. For the observational studies we used the GRADE approach. The authors were not biased with regard to journal, institution, or study results.
Results
Study selection process.
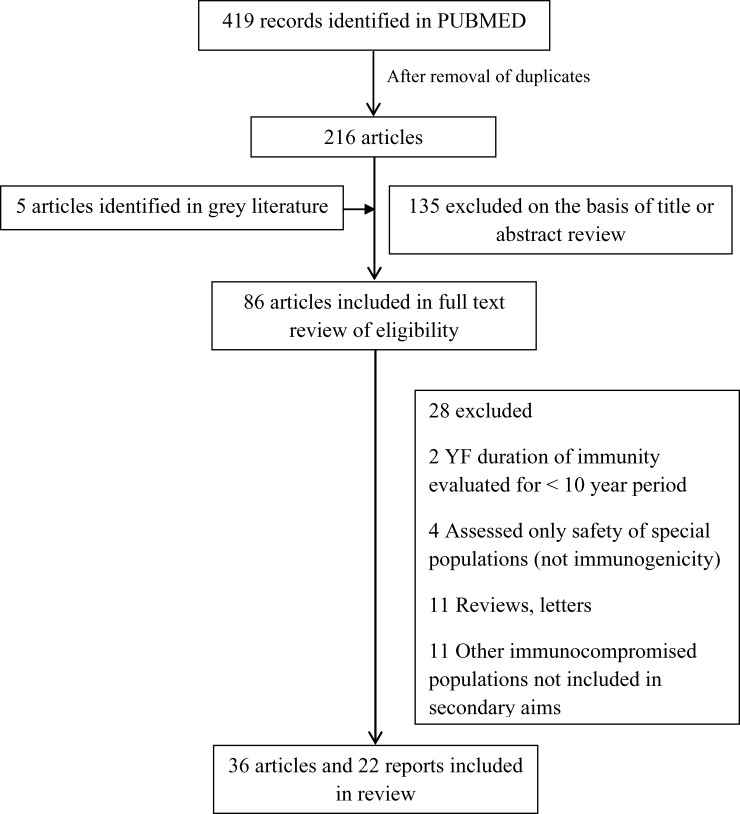
Electronic database and other searching identified 419 related studies. After removal of duplicates, we obtained 216 abstracts. According to title and abstract, 88 were selected for full text retrieval (Figure 1). All but two of the included papers identified in the SCIELO database were also found in PUBMED.
Figure 1.
Study selection flow diagram for vaccination against yellow fever (YF).
Thirty-six studies and 22 reports were included in this review because specific data on the included population groups of interest could be extracted: 12 studies in 11 articles of efficacy, 8 of duration of immunity, 8 of infants and children, 7 of vaccine response in persons infected with human immunodeficiency virus (HIV), 2 of pregnant women, and 1 of severely malnourished children. There was some overlapping in these categories, meaning that some studies of specific populations were also included in the efficacy or duration-of-immunity analysis. Of the 22 reports, 10 were vaccine-failure related (Table 1), 6 were reports of YF in unvaccinated travelers in the past 15 years (Table 2), 5 were related to neurologic complications reported in breastfeeding infants of vaccinated mothers,14–16 and 1 was a case report of an HIV-infected patient in whom meningoencephalitis developed after vaccination against YF. 17 Among included studies, 30 records were published in English, 3 in Spanish, 1 in French, and 2 in Portuguese.
Table 1.
Reports of ten persons with yellow fever vaccine failure
| Age (years)/sex | Reference | Vaccine source | Time since immunization* | Date of disease onset | Outcome | Clinical presentation | Comments |
|---|---|---|---|---|---|---|---|
| 32/M† | Elliot74 | Vaccination on service personnel | 1 year 4 months | Jan 1942 | Died | Severe disease | In West Africa for 3.5 months before yellow fever developed |
| 35/M† | Elliot74 | Vaccination on service personnel | 1 year 3 months | Feb 1942 | Died | Severe disease | In West Africa for 14 months before yellow fever developed |
| 25/M† | Elliot74 | Vaccination on service personnel | 1 year 4 months | Feb 1942 | Recovered | Mild disease | In West Africa for 15.5 months before yellow fever developed |
| 39/M‡ | Ross and others75 | Not stated | 4 years 81 days | Jan 1952 | Died | Severe disease | Engaged in drilling operations in Uganda (Toro district) |
| 37/F‡ | Nolla-Salas and others76 | Travel clinic in Madrid (she had a valid international certificate) | 5 years | Oct 1988 | Recovered | Severe disease | In West Africa for 17 days before she had symptoms |
| 21/M | Tuboi and others77 | Evidence of prior vaccination in surveillance system | 8 months | 1998–2002§ | Recovered | Mild disease | No other data reported |
| 20/F | Tuboi and others77 | Evidence of prior vaccination in surveillance system | 5 years 2 months | 1998–2002§ | Recovered | Severe disease | No other data reported |
| 17/F | Tuboi an others77 | Evidence of prior vaccination in surveillance system | 1 year 6 months | 1998–2002§ | Recovered | Severe disease | No other data reported |
| 62/M | Tuboi and others58 | Evidence of prior vaccination in surveillance system | 1 year | 1998–2002§ | Died | Severe disease | No other data reported |
| 30/F | Tuboi and others58 | Evidence of prior vaccination in surveillance system | 5 months | 1998–2002§ | Died | Severe disease | No other data reported |
Time elapsed since vaccine was reported to onset of symptoms.
Soldier.
Traveler.
Reference does not specify date of disease onset.
Table 2.
Reports of fatal yellow fever in six unvaccinated travelers, 1996–2002
Study characteristics.
Included studies were conducted during 1947–2012. The range of the sample size was 2–1,440 persons, and the time since last immunization in the included studies addressing the duration of immunity ranged from 10 to 60 years. Among the 12 efficacy studies, 7 were conducted in disease-endemic regions and 5 evaluated vaccine performance among travelers or non-endemic settings. Of the eight studies addressing duration of immunity, two were historical studies conducted on military and navy personnel, one was conducted in a disease-endemic area, and the remaining five were conducted in travelers or persons from non-endemic areas.
Efficacy.
Correlates of protection.
Correlates of protection need to be considered to assess the protective efficacy of YF vaccine. There are no studies on humans. Therefore, the minimal protective level of neutralizing antibodies induced by 17D YF vaccine is estimated from dose-response studies in rhesus monkeys that were challenged after immunization with virulent YF virus.18,19 Based on the evidence of these studies, the Food and Drug Administration approved a log10 neutralization index (LNI) > 0.7 as a surrogate of protection against YF. However, the LNI assay requires an amount of serum suitable for animal studies or clinical trials but not for routine screening among humans. As a result, a plaque reduction neutralization test that uses a constant amount of virus and varying dilutions of serum has replaced the LNI. The 1:10 and 1:20 titers frequently used as cutoff titers have been estimated by extending the results of studies on passive immunization in hamsters, and available evidence on titers considered to be protective for other related viruses such as Japanese encephalitis virus.20–22 Overall, there is agreement in the assumption that a titer > 1:10 is associated with protective immunity, considering the paucity of YF cases in immunized persons.10,11,23
Protective immunity after YF vaccination.
Twelve studies in 11 articles6,10,25–33 addressed the efficacy of YF vaccine in terms of immunogenicity (Table 3). Seroconversion rates were consistently > 90% in 9 of 10 studies. Only one study reported a 75% seroconversion rate six months after a mass vaccination campaign.26
Table 3.
Efficacy of yellow fever vaccine*
| Reference | Sample size | Study Type | Setting | Vaccine | Method for correlates of protection | Results | Comments |
|---|---|---|---|---|---|---|---|
| Groot and Gast-Galvis25 | 330 | Obs | Endemic | YF 17D either subcutaneously or by escarification | NT (French neurotropic virus) mouse test and HI | Of 298 persons without pre-vaccination neutralizing antibodies, 282 (94.6%) were seropositive after immunization | |
| Groot and Gast-Galvis25 | 387 | Obs | Endemic | YF 17D by escarification | NT test in mice; survival rates of 4 of 6, 5 of 6, or 6 of 6 were strongly positive | Of 387 serum samples obtained post-vaccination, 363 (94%) were seropositive | Vaccination status was based on person's recall of having been immunized |
| Guerra and others26 | 173 | Obs | Endemic | YF 17D | NT test in mice; survival rates of 4 of 6, 5 of 6, or 6 of 6 were strongly positive | Of those vaccinated, 75% were seropositive, 17% were seronegative, and 7% showed an inconclusive result. The proportion of seropositivity attributable to vaccination, adjusted for age, was 86.8% (95% confidence interval = 70.6–94.0%) | Case-patients were first-grade students who had been vaccinated 6 months earlier during a mass immunization campaign in 1989 |
| Reinhardt and others6 | 17 | Obs | Not endemic | YF 17D | PRNT | 100% of 12 first-time vaccines seroconverted within 2 weeks | 12 YF vaccine-naive persons and 5 revaccinees |
| Monath and others33 | 1,440 | RCT | Not endemic | YF 17D (Arilvax and YF-VAX) | Efficacy endpoint: LNI ≥ 0.7 | Seroconversion occurred in 98.6% of persons in the ARILVAX group and 99.3% of those in the YF-VAX group | Statistically, ARILVAX was equivalent to YF-VAX (P = 0.001) |
| Vazquez and others27 | 21 | Obs | Endemic | YF 17 D | MAC-ELISA and ELISA inhibition method compared with PRNT | 16 (4%) of 17 vaccine-naive persons vaccinated seroconverted by MAC-ELISA; 14 (82%) of 7 vaccine-naive persons vaccinated seroconverted by ELISA inhibition method | 10.7% of the dengue-positive serum samples cross-reacted by MAC-ELISA with YF antigen |
| Tavares-Neto and others29 | 390 | Obs | Endemic | YF 17D | HI antibodies | Serologic conversion to YF reached 89.7% (130 of 145) among previously negative persons | Time since immunization was 3 months |
| Camacho and others28 | 1,087 | Placebo-controlled trial | Not endemic setting in Brazil | YF 17D, YF 17 DD (two seed lots) | Neutralizing yellow fever antibody titers | Seroconvertion rate > 98% among persons previously seronegative, and ≥ 90% of the total cohort of vaccinees, including those previously seropositive | No difference in adverse events or immunogenicity between 17D and 17DD vaccines |
| Belmusto and others30 | 1107 | RCT | Endemic setting (Sullana, Peru) | YF 17D (Arilvax and YF-VAX) | Efficacy endpoint: LNI ≥ 0.7 | 619 (94.9%) of 652 ARILVAX™ and 298 (90.6%) of 329 YF-VAX recipients seroconverted | Difference in seroconversion rate was most pronounced in the two youngest age groups (95.8% vs. 88.5% in children 9–18 months of age and 94.6% vs. 86.2% in children 18–36 months of age |
| Pfister and others31 | 304 | RCT | Not endemic | YF 17D from different manufacturers | PRNT NT titer ≥ 1:10 | Seroconvertion rate = 100% in each vaccine group | Males exhibited a higher antibody response than females |
| Suzano and others32 | 480 | Obs | Endemic | YF 17 DD | PRNT NT titer ≥ 1:10 | Seroconvertion rate = 98.2% | Vaccine applied during the first trimester does not appear to cause malformations, complications to the central nervous system, or adverse perinatal results |
| de Melo and others10 | 298 | Obs | Traveler | YF 17 DD | PRNT NT titer ≥ 1:20 | Protective humoral immune response developed in 100% of persons in prospective cohort (n = 238) | 238 vaccine-naive, 20 persons vaccinated 5 years ago, 20 persons vaccinated 10 years ago |
Obs = observational, YF = yellow fever; NT = neutralization test; PRNT = plaque reduction neutralization test; RCT = randomized controlled trial; LNI = log neutralization index; MAC-ELISA = IgM antibody capture-enzyme-linked immunosorbent assay; HI = hemaglutination inhibition test.
We identified two large RCTs that used two YF 17 D vaccines (Arilvax® and YF-VAX®®) and LNI as the method to identify neutralizing antibodies. Belmusto-Worn and others reported seroconversion rates of 90.6–94.9% among 1,107 healthy children.30 Monath and others found seroconversion rates of 98.6–99.3% among 1,440 healthy adults by using the same two vaccines.33 After antibody kinetic studies, Monath also reported that protective levels of neutralizing antibodies were found in 90% of recipients within 10 days and in 99% within 30 days.34 Seroconversion rates among studies were similar regardless of vaccine substrain, manufacturer, assay used to measure neutralizing antibodies, or method of administration.28,30,33,35
Immunologic response to booster.
Early studies suggested enhanced antibody production following revaccination.36 Our search identified two studies that suggest that titers in revaccinees do not differ from those found in first-time vaccinees. Rosenzweig and others reported results on 9 of 24 persons who were revaccinated within eight years of primary immunization. The titers of revaccinees did not differ from those who had received only one dose.11 Another study monitored early and late events of immune system activation after primary and secondary YF vaccination in 17 healthy persons, 5 of whom had been vaccinated once at least ten years) earlier. The authors reported that revaccination was followed by a minor and transient increase in neutralizing antibodies that disappeared seven months after the primary challenge.6 In this study, all five revaccinees had neutralizing antibodies at a protective level before secondary immunization, suggesting that if primary vaccination was effective, then revaccination may not provide any additional benefit.
In contrast, when a pre-booster serologic result is low or negative, the efficacy of revaccination is well documented.24,37–39 This finding is true regardless of the reason for seronegativity: whether there was no seroconversion or the levels of neutralizing antibodies decreased below detectable levels over time. Hepburn and others conducted a retrospective study on YF vaccination among laboratory workers receiving annual serologic assessments. They found an appropriate immune response to booster (defined as a 4-fold increase in serologic titers) in 78% (646 of 829) of persons with low titers (≤ 1:10) versus 10% (8 of 79) in persons who had pre-vaccination titers > 1:40.24 In the study conducted by Bonnevie-Nielsen and others, only 1 of 10 persons who had received a dose of YF vaccine two years earlier had an antibody titer < 1:10. Seven days after revaccination, this person had protective neutralizing antibodies.39
Duration of immunity.
Eight studies addressed the duration of immunity ≥ 10 years after YF vaccination6–13 (Table 4). The percentage of persons with neutralizing antibodies at a protective level ranged from 74.5–100%.
Table 4.
Duration of immunity after vaccination with yellow fever vaccine*
| Reference | Sample size | Population | Vaccine/method | Time since last immunization | Results | Comments |
|---|---|---|---|---|---|---|
| Groot and Riberiro8 | 108 | Residents of Pouso Alegre, Brazil, where YF was not endemic | FNV/ intracerebral technique in weanling mice | 17 years | 76% had readily demonstrable neutralizing antibody to the FNV strain of YF virus (21% were partially positive) | Of a control group of 78 unimmunized persons from the same area, only one had neutralizing antibody, indicating that natural flaviviral infection had not contributed to the high rate of seropositivity in the vaccinees |
| Rosenzweig and others11 | 24 | Retiring Marine and Navy personnel | YF 17D/ neutralization test in suckling mice | 16–19 years | 100% had neutralizing antibodies. Mean LNI in group III = 2.6 (range = 16–19 years) | 9 persons were revaccinated within 8 years of testing. Titers did not differ from the ones who had received only one dose |
| Poland and others9 | 149 | Veterans of Second World War | YF 17 D, YF FNV/PRNT (titer = 2) and mouse protection test | 30–35 years | Neutralizing antibody persisted for > 30 years in 80.6% of veterans who had presumably been vaccinated | Difference related to branch of service: army personnel 60%; navy/air corp personnel 97% seropositive |
| Reinhardt and others6 | 17 | Healthy persons in Germany | YF 17 D/PRNT cutoff NT = 1:10 | 10 years | All 5 revaccinees had persistent neutralizing antibody before revaccination; mean titer = 1:72 | Revaccination was followed by a minor and transient (7 month) increase of neutralizing antibodies |
| Niedrig and others12 | 209 | Healthy persons in Berlin | YF 17 D/PRNT cut-off NT 1:10 | ≤ 38 years | NT titer > 1:10 decreases from 94% significant; positive results in the first year to 74.5% after 10 years | |
| Gómez and Ocazionez7 | 216 | 100 persons with documented vaccine and 116 that reported to have been YF vaccinated during a YF outbreak | YF17 D/75% PRNT | 3 months–24 years | YF-NT titers > 1:10 were found in 90% of persons with documented vaccination. In residents in a YF-endemic area, YF NT titers > 1.10 were found in 92.6% of adults and 69% of children; after ≥ 4 years of vaccination, 31.6% (6 of 19) of vaccinees had NT titers < 1:10 | Correlation between decrease of seroprotective YF NT titers frequency and increase in immunization time |
| Coulange and others13 | 84 | Elderly persons who attended a travel clinic | YF 17D/PRNT | Median time = 14 years (range = 11–60 years). | Antibody titer was at a protective level in 95.2% of persons | Serologic results were immunosuppressive therapy (19% of persons), cancer (32%), hemopathy (10.7%), HIV infection (3.6%), chronic hepatitis/chronic renal failure/dialysis (2.4%), autoimmune diseases (2.4%); in 29.8% of persons, age was the indication of serologic results |
| de Melo and others10 | 298 | Travelers | YF 17 DD/ MAC-ELISA and PRNT cutoff = 1:20 | 5–10 years | Neutralizing antibodies against YFV were detected in all 40 persons in the postvaccination group; 25% (5 of 20) of persons had PRNT titers < 1:10 five years postimmunization, whereas 35% (7 of 20) had a titer < 1:10 ten years after immunization | 238 vaccine-naive persons; 20 persons vaccinated 5 years ago, 20 persons vaccinated 10 years ago |
All studies were observational studies. YF = yellow fever; FNV = French neurotropic vaccine; LNI = log neutralization index; PRNT = plaque reduction neutralization test; NT = neutralization test; HIV = human immunodeficiency virus; MAC-ELISA = IgM antibody-capture enzyme-linked immunosorbent assay.
Poland and others found that neutralizing antibodies persisted > 30 years in 80.6% of veterans of the Second World War.9 Interestingly, they found that seropositivity was especially high in the subgroup of navy/air corp personnel (97% versus 60% for army personnel). Groot and Riberiro reported that 76% of 108 residents of a non-endemic YF region in Brazil had readily demonstrable neutralizing antibodies against the French neurotropic virus strain of YF virus (21% were partially positive).8 A year later, Rosenzweig and others conducted a retrospective study of 24 retiring Marine and Navy personnel, and found that 100% had neutralizing antibodies and an LNI of 2.6 after 16–19 years.11 Niedrig and others reported a lengthened duration of immunity to 40 years. They reported neutralizing antibody titers > 1:10 in 74.5% of 209 persons several years after immunization.12 A recent study reported neutralizing antibodies at a protective level in 95% of persons > 60 years of age and median time after immunization of 14 years (range = 11–60 years).13
Although there is evidence suggesting that YF immunity persist for life, with some frequency neutralizing antibodies show a time-dependent decrease.7,10,12,26 One study showed that neutralizing antibody titers > 1:10 decreased from 94% in the first year after vaccination to 75% 10 years later.12
Two studies reported neutralizing antibody titers < 1:10 10 years after immunization.7,10 De Melo and others10 found that 65% (13 of 20) had neutralizing antibody titers > 1:10 10 years after immunization, and Gomez and Ocazionez7 reported neutralizing antibody titers < 1:10 in 6 of 19 persons who had been vaccinated ≥ 4 years earlier. This latter sample was a small sample and data provided did not enable us to elucidate how many persons vaccinated ≥ 10 s earlier were included in this group.
Efficacy and duration of immunity in specific populations.
Healthy persons rarely fail to develop neutralizing antibodies after YF vaccination. In controlled clinical trials, the primary failure rate is generally approximately 1%.33 However, certain host factors have been associated with a reduced immunologic response or safety concerns following vaccination.40–42 We explored available evidence for the following persons: infants and children, HIV-infected persons, pregnant women, and severely malnourished persons.
Infants and children.
Eight included studies addressed the immunogenic response to YF vaccine in infants and children. Two old and small studies found no significant difference between children and adults regarding neutralizing antibodies or duration of immunity five years after primary vaccination.43,44 However, more recent studies have not supported these observations.7,26,30,45–48 Children may not develop an immunologic response as effectively as adults or may lose immunity more rapidly. However, these studies have methodologic limitations, including use of an intraperitoneal protection test for young mice, which was later found to be less sensitive than newer techniques or the use of old vaccination records to recruit persons.
Human immunodeficiency virus.
Four retrospective observational studies reported a good immunologic response in HIV patients with CD4 cell counts > 200 cells/mm3 and variable viral load.49–52 The seroconversion rates were high (92–100%) although the number of persons included in the analysis was small in three of the studies (2, 12, and 14 respectively).
Receveur and others reported two persons with CD4 cell counts > 500 cells/mm3 and viral load < 20,000 whose vaccination was followed by a good immune response.49 In both patients, a decrease in CD4 cell count of approximately 200 occurred in the first month after vaccination without any disease manifestation and with a steady recovery. The retrospective study conducted by Tattevin and others also showed favorable efficacy results for a 17DYF vaccine in HIV-infected patients with CD4 cell counts > 200 cells/mm3.50 The 12 included persons had a mean CD4 cell count of 561 cells/mm3 (range = 240–1,300 cells/mm3) and a mean viral load of 5,477 (range = 20–31,100). Pistone and others evaluated neutralizing antibodies 23 HIV-infected patients in France and reported that 93% (13 of 14) of patients without baseline immunity had successful seroconversion. However, time to seroconversion was prolonged; only 2 of the 5 patients tested within five weeks had neutralizing antibodies.51 The recent study of Sidibe and others52 was conducted in a YF-endemic area in Mali and reported that 92% (76 of 83) of HIV patients had neutralizing antibody titers > 1:20 nine months after a mass immunization campaign.
In contrast to this data, Sibially and others found that only 3 (17%) of 18 HIV-infected children in Abidjan, Cote d'Ivoire had adequate levels of neutralizing antibodies compared with 42 (74%) of 57 controls matched for age, sex, and nutritional status.53 An important limitation of these findings is that immunogenicity in the HIV-uninfected children was lower than expected, which suggested that vaccine antigenicity, storage, or administration were suboptimal. Furthermore, there were no data on the level of immunosupression for the HIV-infected children. Veit and others investigated a larger cohort of 102 HIV-infected patients in Switzerland after vaccination with 17DYF vaccine. At one year post-vaccination, significantly fewer HIV-infected patients had protective neutralizing antibodies, and their titers were significantly lower than HIV-uninfected persons.54 In this study, the median CD4 cell count was 537 cells/mm3 (range = 11–1,730 cells/mm3), and the viral load was undetectable in 41 of 102 HIV-infected patients.
Regarding duration of immunity, there is evidence suggesting that neutralizing antibodies decrease quicker in HIV-infected persons. In the HIV cohort study in Switzerland, 11 of 65 patients who initially showed protective responses also had non-protective neutralizing antibodies within five years of primary vaccination. During the first decade after vaccination, the rate of non-protective response for HIV-positive recipients was 23%, which was twice that for HIV-negative recipients.54
Two recent and well-designed studies suggest that viral load inversely correlates with the immune response to YF vaccine: the lower the viral load at the time of vaccination, the stronger the immune response. Veit and others reported that higher levels of neutralizing antibodies during the first year after immunization were associated with undetectable HIV RNA levels at the time of vaccination.54 Moreover, a prospective cohort showed that among 240 patients immunized after HIV diagnosis, neutralizing antibody titers < 1:10 were associated only with detectable plasma HIV RNA at immunization.55 Conversely, Sidibe and others52 found that suppressed HIV RNA was associated with adequate immune titers.
Our search did not identify any study addressing the response to YF vaccine booster in HIV-infected patients. However, one study showed that a booster effect was noted in only 3 of 9 patients with baseline immunity.51
Pregnancy.
Only two studies addressed the immunogenicity of YF vaccine in pregnant women.32,56 These studies reported contrasting results; they showed high seroconversion rates in women vaccinated early in their pregnancy versus low seropositivity after vaccination in their third trimester.
Suzano and others reported results of the inadvertent immunization of 480 pregnant women during a mass vaccination campaign in Brazil.32 These women received vaccine at a mean of 5.7 weeks of gestation. In the six weeks after vaccination, 98.2% had neutralizing antibodies. In contrast, a study conducted in Nigeria found that pregnant women had significantly lower levels of neutralizing antibodies than non-pregnant women of childbearing age, male students, and the general population.56 Only 38.6% had neutralizing antibodies compared with 81.5–93.7% of the other groups. In this study, 88% of immunizations had taken place during the third trimester.56 No evidence was found regarding duration of immunity in women who were pregnant at the time of vaccination.
Severe malnutrition.
Only one small study showed that protein malnutrition was associated with impaired antibody response to YF vaccine and reported that only 1 of 8 persons with kwashiorkor seroconverted after vaccination compared with 5 of 6 controls.57,58 The role of cellular immunity in this population was not explored. Our search could not identify any report that addressed the duration of immunity to YF vaccine in malnourished children.
Risk of bias.
Three of 12 studies included in the efficacy analysis were RCTs. One was a placebo-controlled trial and the remaining eight, along with all studies included in the duration-of-immunity analysis, were observational studies. Two of the RCTs were similar in design and quality of methods. Both studies used stratified randomization to ensure equal distribution of vaccination by age group and sex and allocation concealment as a safeguard against selection bias. All study staff who dealt with persons in the study, as well as co-investigators, laboratory personnel, and assessors of adverse events, remained blinded to prevent detection bias. All three RCTs completely reported results of outcomes described in the methods section. Moreover, reported outcomes from RCTs did not significantly differ from their register forms (ClinicalTrials.gov). Therefore, we could not detect any selective reporting. The placebo-controlled trial included in the efficacy analysis was open-label, randomized, comparator-controlled, parallel group. Regarding observational studies, all clearly defined their eligibility criteria, and these criteria, along with outcome assessment, were applied equally to all participants. In relation to loss to follow-up in cohorts and in RCTs, the loss was always < 15%. No studies were excluded because of poor quality of methods or high risk of bias.
Discussion
This systematic review aimed to assess the need for a vaccine booster every 10 years based on the protective efficacy of YF vaccine in terms of immunogenicity and the duration of immunity after vaccination in residents of disease-endemic areas and in travelers, as well as selected populations.
Efficacy.
Although the effectiveness of YF vaccine in humans has not been formally tested in controlled clinical trials, several observations attest to its effectiveness: “the reduction of laboratory-associated infections in immunized workers, the fact that jungle YF in Brazil and other South American countries occurs only in unimmunized persons, that immunization during outbreaks results in rapid disappearance of cases, and the fact that populations with high vaccine coverage have experienced a marked reduction in YF incidence despite continued human exposure to the enzootic cycle.”59 Included studies showed variation regarding the assay used to identify neutralizing antibodies. Early studies used mouse protection tests either with either intracerebral or intraperitoneal techniques. Later studies replaced tests in mice with tissue culture neutralization tests. The lack of a standardized test makes it difficult to compare efficacy data from multiple studies. However, seroconversion rates seem to be similar across studies, suggesting that it may not be significantly biased by differences in test method.
Most studies showed a consistently high immunogenic response to YF vaccine, which attests to its high efficacy. In the study showing a seroconversion rate as low as 75%, operational failures were considered by the authors, although they could not confirm that external factors such as vaccine storage, handling, or administration were the cause of the lower rate. In addition, it is uncertain whether reports of vaccine failure correspond to persons who failed to show immunity to a properly administered vaccine or received a vaccine that had deteriorated because of improper cold chain handling, storage, or use. Evidence from some studies that showed up to 26% seronegativity in vaccinees after mass immunization campaigns7,60 emphasizes the need of routine systematic monitoring by health services of neutralizing antibodies after campaigns to ensure adequate primary vaccine coverage. For this to be possible, it is necessary to develop a tool for rapid and low-cost diagnosis, which is not available.
Interestingly, four of the studies evaluated vaccine performance in the context of mass vaccination campaigns25,26,29,32 The seroconversion rates in these studies ranged from 89.7% to 98.2%. Moreover, Tavares-Neto and others29 reported a seroconversion rate of 94% (363 of 387) after a vaccination campaign in a remote region of Brazil, which was characterized by its difficult access, minimally trained personnel, and limited resources. These findings, although not absolute, suggest that YF vaccine is effective even in precarious field conditions. Nonetheless, clear recommendations should be provided by manufacturers to minimize operational failure, and investigators should document measures taken to minimize the influence of external and operational factors in field prospective studies.
Results of all 12 studies that evaluated efficacy in terms of neutralizing antibodies reflected humoral immunity. However, it has been demonstrated that CD4+ and CD8+ T cells increase during the first 14 days after YF vaccination, before production of neutralizing antibodies, which suggests activation of the cellular immune system.6,61 These findings have had authors hypothesize that vaccinees without detectable neutralizing antibodies could also be protected by cellular immunity. As a consequence, studies focusing exclusively on neutralizing antibodies may underestimate YF vaccine protective efficacy.
Overall, any given population is considered to be protected if the percentage of immune persons reaches 60–80%. This level has proven to be sufficient to prevent risk of outbreaks.62 Even if there is some evidence that shows a decrease in neutralizing antibodies over time, the percentage of the population with protective titers at the end of the follow-up period was consistently > 60% in all studies. Moreover, the included studies show that seroconvertion rates after immunization are high (usually > 90%) and they remain > 75% several years after primary vaccination. This finding further emphasizes that a single dose of YF vaccine confers life-long protective immunity. Furthermore, some evidence suggests that if a booster were to be given to a given population, most persons would show only a minor or transient increase in neutralizing antibodies because most of them would be already immune and immunologic response to booster correlates inversely with the amount of preexisting antibodies. Finally, the most recent reports of vaccine failure come from secondary information sources of passive surveillance systems, which may be biased. This situation emphasizes the need for active surveillance in regions at high risk.
Populations in disease-endemic areas are adequately protected against YF infection because of herd immunity,63 which indicates that the high level of protection is a direct consequence of the high percentage of immune persons within that population. Thus, because of herd immunity, these populations maintain their protective immunity for several years. Given the limited availability of YF vaccines, vaccination in at-risk regions should be directed towards ensuring good primary vaccination, rather than to providing booster doses.
Regarding travelers, it should be noted that reported YF cases are almost exclusively related to unimmunized travelers during outbreaks. Therefore, if a single dose of YF vaccine prevents outbreaks, travelers should also be safe. In the past 15 years, incidence of YF cases among travelers has been low, and there have not been any reports in the past 10 years. Nonetheless, there is a need to follow-up the impact of removing the 10-year booster dose stipulated by international health regulations for travelers.
Duration of immunity.
Historical studies are valuable regarding duration of immunity. Most of them are retrospective studies, including cohorts of specific populations with specific characteristics: they received a single vaccine dose in the past during periods when vaccination was required for defined groups, they lived in areas to which YF was not endemic, and they did not travel to areas where immunization is required because they received their primary vaccination.
Overall, the included studies demonstrate long-lasting immunity after use of YF vaccine: as long as 40 years in as many as 80% of vaccinees. Some authors have argued that because there are no known cases of YF infection in persons who had been vaccinated and showed a documented, appropriate, initial response, these findings support the hypothesis that protection may be life-long.33
The two studies that reported neutralizing antibody titers < 1:10 ten years after immunization were small retrospective studies that used either randomly selected persons from immunization records or self-reported vaccinees. With the data provided, it was not possible to determine whether persons with neutralizing antibody titers < 1:10 belonged to the group of documented vaccinees or the group of self-reported vaccines (Table 4).
Specific groups.
Surveillance in disease-endemic countries and clinical studies can identify specific risk groups that could benefit from a second dose or booster dose.
Human immunodeficiency virus.
It seems clear that the level of immunosupression plays a key role in the immune response in HIV patients.64,65 Vaccination against YF may be offered to asymptomatic HIV-infected persons with CD4+ cell counts ≥ 200 cells/mm3 who require vaccination, but further studies are needed to provide recommendations for severely immunocompromised HIV patients, including those with symptomatic HIV or CD4+ cell counts < 200 cells/mm3.
Patients infected with HIV more often have non-protective neutralizing antibodies and experience a more rapid decrease in titers during follow-up. Accordingly, it is recommended that patients who are not receiving highly active antiretroviral therapy and who have low CD4+ cell counts preferably postpone receipt of YF vaccine until the plasma HIV RNA level is undetectable to attain a more vigorous vaccine response. Current recommendations need to be readdressed because a 10-year interval between vaccine doses seems to be too long for the HIV population. Moreover, the 10-day interval after vaccination recommended for the general population before exposure may be too short for this group.
Infants and children.
Infants and children represent one of the main populations in which YF vaccine is indicated in disease-endemic areas.30,66,67 During outbreaks, children are usually greatly affected.68,69 Since 1988, the United Nations Children's Fund/World Health Organization Technical Group on Immunization in Africa has recommended routine childhood immunization against YF. Nonetheless, a disparity persists between at-risk countries and countries with immunization programs, and few countries have achieved coverage levels > 80%. In South America, YF vaccine has also been included in childhood immunization programs, although most of them tend to focus on Amazonian jungle regions, leaving urban areas at risk of YF outbreaks. In addition, in South America, immunization strategies and vaccine-coverage rates vary considerably. Some regions of Brazil and Bolivia have achieved vaccine coverage rates > 70%, whereas some disease-endemic areas have only reached 30%.3
Some evidence suggests that children have a lower immunologic response to YF vaccine than adults. Interestingly, a recent pediatric trial found that the difference in seroconversion rate (compared with the rates reported in the analogous trial in adults) was most pronounced in the two youngest age groups (9–18 and 18–36 months of age) which is exactly the age at which childhood immunization programs recommend YF immunization.30 This finding needs further investigation.
Pregnancy.
Pregnancy constitutes an important host factor in relation to immunity. Pregnant women are more prone to acquire severe forms of disease,70 and pregnancy has been associated with failure to respond immunologically to certain vaccines such as hepatitis B vaccine.71 This finding is why it is important to evaluate efficacy of YF vaccine in this group.
Most identified studies involving pregnant women were designed to assess congenital infection or structural defects resulting fromimmunization.32,72,73 Fortunately, two studies provided data on seroconversion rates. The results varied, depending on the trimester in which vaccine was administered.
In light of current evidence, vaccination is recommended if indicated for pregnant women traveling to disease-endemic areas if travel cannot be avoided or postponed. Pregnant women at high risk of YF should be counseled regarding the benefits and potential risks of vaccination so that they can make an informed decision about immunization.
Future Lines of Research
This review shows that there is a scarcity of high-quality evidence regarding YF vaccine immunogenicity and duration of immunity. Accordingly, it is important to outline the areas that need future research. An important aspect for further exploration is the cellular immune response after vaccination against YF. Available evidence points towards an early protective role of T cells, but these findings need to be confirmed.
Antibody titers of children immunized during routine infant immunization programs should be adequately assessed in prospective studies many years post-vaccination. These studies should assess the immunologic response to YF vaccine while controlling possible confounding factors such as severe malnourishment, parasitic disease, and anemia, which are frequently encountered in disease-endemic regions.
For children, the question of protective immunity against YF after co-administration with measles, mumps, rubella and meningococcal and/or polio vaccines is of great importance. This issue will be a critical question that needs to be addressed. Further investigations are needed to assess the relationship between seroconversion and the trimester of pregnancy in which vaccine was administered, as well as the duration of immunity after seroconversion.
Additional data on safety and immunogenicity should be obtained for HIV patients (adults and children), especially for those with advanced infection. Prospective studies should determine the best timing for YF booster in persons infected with HIV through the evaluation of antibody titers 5 and 10 years after primary vaccination. Time to seroconversion and the role of viral load in the development of a good immune response also need to be explored.
ACKNOWLEDGMENTS
We thank Dr. Thomas Monath for providing valuable information and advice, Dr. D. Freedman for reviewing and providing valuable opinions on the manuscript, and R. Porudominsky for help with data collection.
Disclaimer: The opinions expressed in this publication are those of the authors and do not necessarily represent the decisions, policies, or views of the World Health Organization.
Footnotes
Disclosure: Eduardo Gotuzzo is an Advisory Board member and consultant for Pfizer and will participate in a study conducted by Sanofi Pasteur on the epidemiology of meningococcal infections.
Authors' addresses: Eduardo Gotuzzo and Erika Cordóva, Instituto de Medidina Tropical Alexander von Humbolt, Lima, Peru, E-mails: eduardo.gotuzzo@upch.pe and erika.cordova@upch.pe. Sergio Yactayo, Control of Epidemic Diseases, and Pandemic and Epidemic Diseases, World Health Organization, Geneva, Switzerland, E-mail: yactayos@who.int.
References
- 1.Jentes ES, Poumerol G, Gershman MD, Hill DR, Lemarchand J, Lewis RF, Staples JE, Tomori O, Wilder-Smith A, Monath TP. Informal WHO Working Group on Geographic Risk for Yellow Fever The revised global yellow fever risk map and recommendations for vaccination, 2010: consensus of the Informal WHO Working Group on Geographic Risk for Yellow Fever. Lancet Infect Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- 2.Wilson ME, Chen LH, Barnett ED. Yellow fever immunizations: indications and risks. Curr Infect Dis Rep. 2004;6:34–42. doi: 10.1007/s11908-004-0022-5. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on medicine and health in the tropics, Marseille, France, Monday 12 September 2005. Vaccine. 2007;25:2758–2765. doi: 10.1016/j.vaccine.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 5.Wilder-Smith A, Hill DR, Freedman DO. The revised International Health Regulations (2005): impact on yellow fever vaccination in clinical practice. Am J Trop Med Hyg. 2008;78:359–360. [PubMed] [Google Scholar]
- 6.Reinhardt B, Jaspert R, Niedrig M, Kostner C, L'Age-Stehr J. Development of viremia and humoral and cellular parameters of immune activation after vaccination with yellow fever virus strain 17D: a model of human flavivirus infection. J Med Virol. 1998;56:159–167. doi: 10.1002/(sici)1096-9071(199810)56:2<159::aid-jmv10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Gomez SY, Ocazionez RE. Yellow fever virus 17D neutralizing antibodies in vaccinated Colombian people and unvaccinated ones having immunity against dengue [in Spanish] Rev Salud Publica (Bogota) 2008;10:796–807. doi: 10.1590/s0124-00642008000500012. [DOI] [PubMed] [Google Scholar]
- 8.Groot H, Riberiro RB. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 year(s) after vaccination with 17D vaccine. Bull World Health Organ. 1962;27:699–707. [PMC free article] [PubMed] [Google Scholar]
- 9.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 year(s) after immunization with 17D yellow fever vaccine. Bull World Health Organ. 1981;59:895–900. [PMC free article] [PubMed] [Google Scholar]
- 10.de Melo AB, da Silva Mda P, Magalhaes MC, Gonzales Gil LH, Freese de Carvalho EM, Braga-Neto UM, Bertani GR, Marques ET, Jr, Cordeiro MT. Description of a prospective 17DD yellow fever vaccine cohort in Recife, Brazil. Am J Trop Med Hyg. 2011;85:739–747. doi: 10.4269/ajtmh.2011.10-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenzweig EC, Babione RW, Wisseman CL., Jr Immunological studies with group B arthropod-borne viruses. IV. Persistence of yellow fever antibodies following vaccination with 17D strain yellow fever vaccine. Am J Trop Med Hyg. 1963;12:230–235. [PubMed] [Google Scholar]
- 12.Niedrig M, Lademann M, Emmerich P, Lafrenz M. Assessment of IgG antibodies against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop Med Int Health. 1999;4:867–871. doi: 10.1046/j.1365-3156.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 13.Coulange Bodilis H, Benabdelmoumen G, Gergely A, Goujon C, Pelicot M, Poujol P, Consigny PH. Long term persistence of yellow fever neutralizing antibodies in elderly persons [in French] Bull Soc Pathol Exot. 2011;104:260–265. doi: 10.1007/s13149-011-0135-7. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Transmission of yellow fever vaccine virus through breast-feeding–Brazil, 2009. MMWR Morb Mortal Wkly Rep. 2010;59:130–132. [PubMed] [Google Scholar]
- 15.Kuhn S, Twele-Montecinos L, MacDonald J, Webster P, Law B. Case report: probable transmission of vaccine strain of yellow fever virus to an infant via breast milk. CMAJ. 2011;183:E243–E245. doi: 10.1503/cmaj.100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anonymous Meeting of the Global Advisory Committee on Vaccine Safety, December 2010. Wkly Epidemiol Rec. 2011;86:38–43. [PubMed] [Google Scholar]
- 17.Kengsakul K, Sathirapongsasuti K, Punyagupta S. Fatal myeloencephalitis following yellow fever vaccination in a case with HIV infection. J Med Assoc Thai. 2002;85:131–134. [PubMed] [Google Scholar]
- 18.Mason RA, Tauraso NM, Ginn RK, O'Brien TC, Trimmer RW. Yellow fever vaccine. V. Antibody response in maonkeys inoculated with graded doses of the 17D vaccine. Appl Microbiol. 1972;23:908–913. doi: 10.1128/am.23.5.908-913.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25:539–544. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Staples JE, Gershman M, Fischer M. Centers for Disease Control and Prevention Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 21.Julander JG, Trent DW, Monath TP. Immune correlates of protection against yellow fever determined by passive immunization and challenge in the hamster model. Vaccine. 2011;29:6008–6016. doi: 10.1016/j.vaccine.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–5211. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 23.Monath TP. Yellow fever vaccine. Expert Rev Vaccines. 2005;4:553–574. doi: 10.1586/14760584.4.4.553. [DOI] [PubMed] [Google Scholar]
- 24.Hepburn MJ, Kortepeter MG, Pittman PR, Boudreau EF, Mangiafico JA, Buck PA, Norris SL, Anderson EL. Neutralizing antibody response to booster vaccination with the 17d yellow fever vaccine. Vaccine. 2006;24:2843–2849. doi: 10.1016/j.vaccine.2005.12.055. [DOI] [PubMed] [Google Scholar]
- 25.Groot H, Gast-Galvis A. Observations on the 17 D virus vaccine against yellow fever, applied by cutaneous scarification [in Spanish] Rev Invest (Guadalajara) 1965;5:5–25. [PubMed] [Google Scholar]
- 26.Guerra HL, Sardinha TM, da Rosa AP, Lima e Costa MF. Effectiveness of the yellow fever vaccine 17D: an epidemiologic evaluation in health services [in Spanish] Rev Panam Salud Publica. 1997;2:115–120. [PubMed] [Google Scholar]
- 27.Vazquez S, Valdes O, Pupo M, Delgado I, Alvarez M, Pelegrino JL, Guzmán MG. MAC-ELISA and ELISA inhibition methods for detection of antibodies after yellow fever vaccination. J Virol Methods. 2003;110:179–184. doi: 10.1016/s0166-0934(03)00128-9. [DOI] [PubMed] [Google Scholar]
- 28.Camacho LA, Freire Mda S, Leal Mda L, Aguiar SG, Nascimento JP, Iguchi T, Lozana Jde A, Farias RH. Collaborative Group for the Study of Yellow Fever Vaccines Immunogenicity of WHO-17D and Brazilian 17DD yellow fever vaccines: a randomized trial. Rev Saude Publica. 2004;38:671–678. doi: 10.1590/s0034-89102004000500009. [DOI] [PubMed] [Google Scholar]
- 29.Tavares-Neto J, Freitas-Carvalho J, Nunes MR, Rocha G, Rodrigues SG, Damasceno E, Darub R, Viana S, Vasconcelos PF. Serologic survey for yellow fever and other arboviruses among inhabitants of Rio Branco, Brazil, before and three months after receiving the yellow fever 17D vaccine [in Portuguese] Rev Soc Bras Med Trop. 2004;37:1–6. doi: 10.1590/s0037-86822004000100001. [DOI] [PubMed] [Google Scholar]
- 30.Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, Pastor-Cauna G, Echevarria C, Laguna-Torres VA, Samame BK, Baldeon ME, Burnas JP, Olson JG, Bedford P, Kitchener S, Monath TP. Randomized, double-blind, phase III, pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (Arilvax and YF-VAX) in healthy infants and children in Peru. Am J Trop Med Hyg. 2005;72:189–197. [PubMed] [Google Scholar]
- 31.Pfister M, Kursteiner O, Hilfiker H, Favre D, Durrer P, Ennaji A, L'Age-Stehr J, Kaufhold A, Herzog C. Immunogenicity and safety of BERNA-YF compared with two other 17D yellow fever vaccines in a phase 3 clinical trial. Am J Trop Med Hyg. 2005;72:339–346. [PubMed] [Google Scholar]
- 32.Suzano CE, Amaral E, Sato HK, Papaiordanou PM. Campinas Group on Yellow Fever Immunization during Pregnancy The effects of yellow fever immunization (17DD) inadvertently used in early pregnancy during a mass campaign in Brazil. Vaccine. 2006;24:1421–1426. doi: 10.1016/j.vaccine.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 33.Monath TP, Nichols R, Archambault WT, Moore L, Marchesani R, Tian J, Shope RE, Thomas N, Schrader R, Furby D, Bedford P. Comparative safety and immunogenicity of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in a phase III multicenter, double-blind clinical trial. Am J Trop Med Hyg. 2002;66:533–541. doi: 10.4269/ajtmh.2002.66.533. [DOI] [PubMed] [Google Scholar]
- 34.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1:11–20. doi: 10.1016/S1473-3099(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 35.Niedrig M, Kursteiner O, Herzog C, Sonnenberg K. Evaluation of an indirect immunofluorescence assay for detection of immunoglobulin M (IgM) and IgG antibodies against yellow fever virus. Clin Vaccine Immunol. 2008;15:177–181. doi: 10.1128/CVI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wisseman CL, Jr, Sweet BH. Immunological studies with group B arthropod-borne viruses. III. Response of human subjects to revaccination with 17D strain yellow fever vaccine. Am J Trop Med Hyg. 1962;11:570–575. [PubMed] [Google Scholar]
- 37.Omilabu SA, Adejumo JO, Olaleye OD, Fagbami AH, Baba SS. Yellow fever haemagglutination-inhibiting, neutralising and IgM antibodies in vaccinated and unvaccinated residents of Ibadan, Nigeria. Comp Immunol Microbiol Infect Dis. 1990;13:95–100. doi: 10.1016/0147-9571(90)90521-t. [DOI] [PubMed] [Google Scholar]
- 38.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, García-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnevie-Nielsen V, Heron I, Monath TP, Calisher CH. Lymphocytic 2′,5′-oligoadenylate synthetase activity increases prior to the appearance of NTAbs and immunoglobulin M and immunoglobulin G antibodies after primary and secondary immunization with yellow fever vaccine. Clin Diagn Lab Immunol. 1995;2:302–306. doi: 10.1128/cdli.2.3.302-306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bruyand M, Receveur MC, Pistone T, Verdiere CH, Thiebaut R, Malvy D. Yellow fever vaccination in non-immunocompetent patients [in French] Med Mal Infect. 2008;38:524–532. doi: 10.1016/j.medmal.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 41.Thomas RE, Lorenzetti DL, Spragins W, Jackson D, Williamson T. Active and passive surveillance of yellow fever vaccine 17D or 17DD-associated serious adverse events: systematic review. Vaccine. 2011;29:4544–4555. doi: 10.1016/j.vaccine.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 42.Duchet Niedziolka P, Launay O, Salmon Ceron D, Consigny PH, Ancelle T, Van der Vliet D, Lortholary O, Hanslik T. pour fe groupe GEVACCIM Antiviral immunization of immunocompromised adults, literature review [in French] Rev Med Interne. 2008;29:554–567. doi: 10.1016/j.revmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Dick GW, Smithburn KC. Immunity to yellow fever 6 year(s) after vaccination. Am J Trop Med Hyg. 1949;29:57–61. doi: 10.4269/ajtmh.1949.s1-29.57. [DOI] [PubMed] [Google Scholar]
- 44.Anderson CR, Gast-Galvis A. Immunity to yellow fever five year(s) after vaccination. Am J Hyg. 1947;45:302–304. doi: 10.1093/oxfordjournals.aje.a119138. [DOI] [PubMed] [Google Scholar]
- 45.Fox JP, Fonseca Da Cunha J, Kossobudzki SL. Additional observations on the duration of humoral immunity following vaccination with the 17D strain of yellow fever virus. Am J Hyg. 1948;47:64–70. doi: 10.1093/oxfordjournals.aje.a119186. [DOI] [PubMed] [Google Scholar]
- 46.Veras MA, Flannery B, de Moraes JC, da Silva Teixeira AM, Luna EJ. Vaccine Coverage Survey 2007 Group Yellow fever vaccination coverage among children in Brazilian capitals. Vaccine. 2010;28:6478–6482. doi: 10.1016/j.vaccine.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 47.Lopes Ode S, Guimaraes SS, de Carvalho R. Studies on yellow fever vaccine. III. Dose response in volunteers. J Biol Stand. 1988;16:77–82. doi: 10.1016/0092-1157(88)90034-0. [DOI] [PubMed] [Google Scholar]
- 48.Stefano I, Sato HK, Pannuti CS, Omoto TM, Mann G, Freire MS, Yamamura AM, Vasconcelos PF, Oselka GW, Weckx LW, Salgado MF, Noale LF, Souza VA. Recent immunization against measles does not interfere with the sero-response to yellow fever vaccine. Vaccine. 1999;17:1042–1046. doi: 10.1016/s0264-410x(98)00320-x. [DOI] [PubMed] [Google Scholar]
- 49.Receveur MC, Thiebaut R, Vedy S, Malvy D, Mercie P, Bras ML. Yellow fever vaccination of human immunodeficiency virus-infected patients: report of 2 cases. Clin Infect Dis. 2000;31:7–8. doi: 10.1086/314031. [DOI] [PubMed] [Google Scholar]
- 50.Tattevin P, Depatureaux AG, Chapplain JM, Dupont M, Souala F, Arvieux C, Poveda JD, Michelat C. Yellow fever vaccine is safe and effective in HIV-infected patients. AIDS. 2004;18:825–827. doi: 10.1097/00002030-200403260-00020. [DOI] [PubMed] [Google Scholar]
- 51.Pistone T, Verdiere CH, Receveur MC, Ezzedine K, Lafon ME, Malvy D. Immunogenicity and tolerability of yellow fever vaccination in 23 French HIV-infected patients. Curr HIV Res. 2010;8:461–466. doi: 10.2174/157016210793499277. [DOI] [PubMed] [Google Scholar]
- 52.Sidibe M, Yactayo S, Kalle A, Sall AA, Sow S, Ndoutabe M, Perea W, Avokey F, Lewies RF, Veit O. Immunogenicity and safety of yellow fever vaccine among 115 HIV-infected patients after a preventive immunisation campaign in Mali. Trans R Soc Trop Med Hyg. 2012;106:437–444. doi: 10.1016/j.trstmh.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Sibailly TS, Wiktor SZ, Tsai TF, Cropp BC, Ekpini ER, Adjorlolo-Johnson G, Gnaore G, De Cock KM, Greenberg AE. Poor antibody response to yellow fever vaccination in children infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1997;16:1177–1179. doi: 10.1097/00006454-199712000-00015. [DOI] [PubMed] [Google Scholar]
- 54.Veit O, Niedrig M, Chapuis-Taillard C, Cavassini M, Mossdorf E, Schmid P, Bae HE, Litzba N, Staub T, Hatz C, Furrer H. Swiss HIV Cohort Study Immunogenicity and safety of yellow fever vaccination for 102 HIV-infected patients. Clin Infect Dis. 2009;48:659–666. doi: 10.1086/597006. [DOI] [PubMed] [Google Scholar]
- 55.Pacanowski J, Lacombe K, Campa P, Dabrowska M, Poveda JD, Meynard JL, Poirot JL, Fonquernie L, Girard PM. Plasma HIV-RNA is the key determinant of long-term antibody persistence after yellow fever immunization in a cohort of 364 HIV-infected patients. J Acquir Immune Defic Syndr. 2012;59:360–367. doi: 10.1097/QAI.0b013e318249de59. [DOI] [PubMed] [Google Scholar]
- 56.Nasidi A, Monath TP, Vandenberg J, Tomori O, Calisher CH, Hurtgen X, Munube GR, Sorungbe AO, Okafor GC, Wali S. Yellow fever vaccination and pregnancy: a four-year prospective study. Trans R Soc Trop Med Hyg. 1993;87:337–339. doi: 10.1016/0035-9203(93)90156-k. [DOI] [PubMed] [Google Scholar]
- 57.Brown RE, Katz M. Failure of antibody production to yellow fever vaccine in children with kwashiorkor. Trop Geogr Med. 1966;18:125–128. [PubMed] [Google Scholar]
- 58.Anonymous Effects of malnutrition on smallpox and yellow fever vaccination. Nutr Rev. 1967;25:108–110. doi: 10.1111/j.1753-4887.1967.tb05593.x. [DOI] [PubMed] [Google Scholar]
- 59.Monath TP, Gershman M, Barret AD. Yellow fever. In: Plotkin S, Orenstein W, editors. Vaccines. Sixth Edition. Philadelphia, PA: W. B. Saunders; 2012. pp. 870–968. [Google Scholar]
- 60.Groot H, Kerr JA, Sanmartin C, Vidales H. Antibodies to yellow fever and other arthropod-borne viruses in human residents of San Vicente de Chucuri, Santander, Colombia. Am J Trop Med Hyg. 1959;8:175–189. doi: 10.4269/ajtmh.1959.8.175. [DOI] [PubMed] [Google Scholar]
- 61.Gaucher D, Therrien R, Kettaf N, Angermann BR, Boucher G, Filali-Mouhim A, Moser JM, Mehta RS, Drake DR, III, Castro E, Akondy R, Rinfret A, Yassine-Diab B, Said EA, Chouikh Y, Cameron MJ, Clum R, Kelvin D, Somogyi R, Greller LD, Balderas RS, Wilkinson P, Pantaleo G, Tartaglia J, Hadad EK, Sékaly RP. Yellow fever vaccine induces integrated multilineage and polyfunctional immune responses. J Exp Med. 2008;205:3119–3131. doi: 10.1084/jem.20082292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Anonymous Yellow fever fact sheet. Wkly Epidemiol Rec. 2010;85:33–36. [PubMed] [Google Scholar]
- 63.Fox JP, Elveback L, Scott W, Gatewood L, Ackerman E. Herd immunity: basic concept and relevance to public health immunization practices. Am J Epidemiol. 1995;141:187–197. doi: 10.1093/oxfordjournals.aje.a117420. [DOI] [PubMed] [Google Scholar]
- 64.Goujon CT, Feuillie V, Coulaud P, Dupont B, Sansonetti P. Good tolerance and efficacy of yellow fever vaccine among carriers of human immunodeficiency virus. J Travel Med. 1995;2 [Google Scholar]
- 65.Pacanowski JC, Dabrowska M, Lacombe K. Washington, DC: 2008. Antibody response and safety of yellow fever vaccination in HIV infected patients. Proceedings of the 48th Annual International Conference on Antimicrobial Agents and Chemotherapy, October 25–28, 2008. [Google Scholar]
- 66.Anonymous Expanded programme on immunization (EPI). Inclusion of yellow fever vaccine in the EPI. Wkly Epidemiol Rec. 1996;71:181–185. [PubMed] [Google Scholar]
- 67.Monath TP. Yellow fever as an endemic/epidemic disease and priorities for vaccination. Bull Soc Pathol Exot. 2006;99:341–347. [PubMed] [Google Scholar]
- 68.Hanson H. Observations on the age and sex incidence of deaths and recoveries in the yellow fever epidemic in the department of Lambayeque, Peru, in 1921. Am J Trop Med. 1929;9:233–239. [Google Scholar]
- 69.Monath TP, Craven RB, Adjukiewicz A, Germain M, Francy DB, Ferrara L, Samba EM, N'Jie H, Cham K, Fitzgerald SA, Crippen PH, Simpson DI, Bowen ET, Fabiyi A, Salaun JJ. Yellow fever in the Gambia, 1978–1979: epidemiologic aspects with observations on the occurrence of Orungo virus infections. Am J Trop Med Hyg. 1980;29:912–928. doi: 10.4269/ajtmh.1980.29.912. [DOI] [PubMed] [Google Scholar]
- 70.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm MR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. Novel Influenza A (H1N1) Pregancy Working Group H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 71.Ingardia CJ, Kelley L, Steinfeld JD, Wax JR. Hepatitis B vaccination in pregnancy: factors influencing efficacy. Obstet Gynecol. 1999;93:983–986. doi: 10.1016/s0029-7844(98)00563-8. [DOI] [PubMed] [Google Scholar]
- 72.Nishioka Sde A, Nunes-Araujo FR, Pires WP, Silva FA, Costa HL. Yellow fever vaccination during pregnancy and spontaneous abortion: a case-control study. Trop Med Int Health. 1998;3:29–33. doi: 10.1046/j.1365-3156.1998.00164.x. [DOI] [PubMed] [Google Scholar]
- 73.Cavalcanti DP, Salomao MA, Lopez-Camelo J, Pessoto MA. Campinas Group of Yellow Fever Immunization during Pregnancy Early exposure to yellow fever vaccine during pregnancy. Trop Med Int Health. 2007;12:833–837. doi: 10.1111/j.1365-3156.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 74.Elliot M. Yellow fever in the recently inoculated. Trans R Soc Trop Med Hyg. 1944;38:231–236. [Google Scholar]
- 75.Ross RW, Haddow AJ, Raper AB, Trowell HC. A fatal case of yellow fever in a European in Uganda. East Afr Med J. 1953;30:1–11. [PubMed] [Google Scholar]
- 76.Nolla-Salas J, Saballs-Radresa J, Bada JL. Imported yellow fever in vaccinated tourist. Lancet. 1989;334:1275. doi: 10.1016/s0140-6736(89)91877-1. [DOI] [PubMed] [Google Scholar]
- 77.Tuboi SH, Costa ZG, da Costa Vasconcelos PF, Hatch D. Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans R Soc Trop Med Hyg. 2007;101:169–175. doi: 10.1016/j.trstmh.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 78.McFarland JM, Baddour LM, Nelson JE, Elkins SK, Craven RB, Cropp BC, Chang GJ, Grindstaff AD, Craig AS, Smith RJ. Imported yellow fever in a United States citizen. Clin Infect Dis. 1997;25:1143–1147. doi: 10.1086/516111. [DOI] [PubMed] [Google Scholar]
- 79.Barros ML, Boecken G. Jungle yellow fever in the central Amazon. Lancet. 1996;348:969–970. doi: 10.1016/s0140-6736(05)65392-5. [DOI] [PubMed] [Google Scholar]
- 80.Bae HG, Drosten C, Emmerich P, Colebunders R, Hantson P, Pest S, Parent M, Schmitz H, Warnat MA, Niedrig M. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J Clin Virol. 2005;33:274–280. doi: 10.1016/j.jcv.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 81.Anonymous Yellow fever, 1998–1999. Wkly Epidemiol Rec. 2000;75:322–328. [PubMed] [Google Scholar]
- 82.Colebunders R, Mariage JL, Coche JC, Pirenne B, Kempinaire S, Hantson P, Van Gompel A, Niedrig M, Van Esbroeck M, Bailey R, Drosten C, Schmitz H. A Belgian traveller who acquired yellow fever in the Gambia. Clin Infect Dis. 2002;35:e113–e116. doi: 10.1086/344180. [DOI] [PubMed] [Google Scholar]
- 83.Centers for Disease Control and Prevention Fatal yellow fever in a traveler returning from Amazonas, Brazil, 2002. JAMA. 2002;287:2499–2500. doi: 10.1001/jama.287.19.2499-jwr0515-3-1. [DOI] [PubMed] [Google Scholar]