Abstract
Rickettsioses caused by Rickettsia felis are an emergent global threat. Historically, the northern region of the province of Caldas in Colombia has reported murine typhus cases, and recently, serological studies confirmed high seroprevalence for both R. felis and R. typhi. In the present study, fleas from seven municipalities were collected from dogs, cats, and mice. DNA was extracted and amplified by polymerase chain reaction (PCR) to identify gltA, ompB, and 17kD genes. Positive samples were sequenced to identify the species of Rickettsia. Of 1,341 fleas, Ctenocephalides felis was the most prevalent (76.7%). Positive PCR results in the three genes were evidenced in C. felis (minimum infection rates; 5.3%), C. canis (9.2%), and Pulex irritans (10.0%). Basic Local Alignment Search Tool (BLAST) analyses of sequences showed high identity values (> 98%) with R. felis, and all were highly related by phylogenetic analyses. This work shows the first detection of R. felis in fleas collected from animals in Colombia.
Introduction
Bacteria from the genus Rickettsia are obligate intracellular microorganisms transmitted by arthropods to vertebrate hosts, including man and domestic animals.1 R. felis infection in humans produces a disease known as flea-borne spotted fever (or cat flea typhus), which is an emergent and global threat.2 Despite reports of infection in almost 12 different species of fleas, 8 species of ticks, mites, and lice, the cat flea (Ctenocephalides felis felis) is currently the only arthropod associated with the biological transmission of this agent.3,4
R. felis has been identified in fleas and other arthropods from different countries in the Americas, including Argentina,5 Brazil,6–11 Canada,12 Chile,13 Costa Rica,14 Mexico,15 Panama,16 Peru,17 the United States,18–22 and Uruguay.23
The northern aspect of Caldas Province, Colombia, is an area that historically reports murine typhus cases to the public health authorities. Previous studies in this region confirmed (by indirect fluorescence assay [IFA]) cases of rickettsioses that were seropositive for anti-R. typhi immunoglobulin G (IgG) and IgM.24 Recently, we completed a transversal serological study in seven municipalities of this region and found seroprevalence of 25.2% and 17.8% against R. typhi and R. felis, respectively. A prospective arm of this study also corroborated different human infections with the aforementioned flea-borne rickettsial species.25
The aim of this work was to detect, by molecular methods, the presence of Rickettsia species in fleas collected from animals in the urban area of seven municipalities from Caldas Province, Colombia.
Materials and Methods
Geographical location.
Municipalities included in the study are listed in Tables 1 and 2 and highlighted in the map (Figure 1 and Supplemental Figure 1).
Table 1.
Fleas and numbers of pools positive for the Rickettsia genes gltA, ompB, and 17kD collected from animals in seven municipalities from Caldas, Colombia
| Municipality | Number of fleas collected | Total number of fleas (number of pools) | Number of positive pools (MIR; %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dogs (276) | Cats (63) | Rats (6) | Mice (7) | gltA | ompB | 17kD | ||||||
| Aguadas | ||||||||||||
| C. felis | 117 | 9 | – | – | 126 | (15) | 14 | (11.1) | 14 | (11.1) | 12 | (9.5) |
| C. canis | 38 | – | – | – | 38 | (5) | 4 | (10.5) | 3 | (7.9) | 4 | (10.5) |
| P. irritans | 5 | – | – | – | 5 | (1) | – | – | – | |||
| X. cheopis | 1 | – | – | 1 | (1) | – | – | – | ||||
| Aranzazu | ||||||||||||
| C. felis | 62 | 53 | – | – | 115 | (15) | 13 | (11.3) | 13 | (11.3) | 11 | (9.6) |
| C. canis | 36 | 2 | – | – | 38 | (6) | 4 | (10.5) | 4 | (10.5) | 3 | (7.9) |
| P. irritans | 6 | – | – | – | 6 | (1) | 1 | (16.7) | 1 | (16.7) | 1 | (16.7) |
| X. cheopis | – | – | – | – | – | (–) | – | – | – | |||
| Filadelfia | ||||||||||||
| C. felis | 154 | 72 | – | – | 226 | (31) | 8 | (3.5) | 6 | (2.7) | 6 | (2.7) |
| C. canis | 7 | 4 | – | – | 11 | (3) | 3 | (27.3) | 3 | (27.3) | 1 | (9.1) |
| P. irritans | 2 | – | – | – | 2 | (1) | 1 | (50) | – | – | ||
| X. cheopis | 1 | – | 10 | – | 11 | (1) | – | – | – | |||
| La Merced | ||||||||||||
| C. felis | 81 | 27 | – | – | 108 | (15) | 5 | (4.6) | – | 4 | (3.7) | |
| C. canis | 1 | – | – | – | – | (–) | – | – | – | |||
| P. irritans | – | – | – | – | – | (–) | – | – | – | |||
| X. cheopis | – | – | – | – | – | (–) | – | – | – | |||
| Neira | ||||||||||||
| C. felis | 267 | 16 | – | – | 283 | (35) | 30 | (10.6) | 28 | (9.9) | 21 | (7.4) |
| C. canis | 87 | 10 | 1 | – | 98 | (12) | 10 | (10.2) | 10 | (10.2) | 10 | (10.2) |
| P. irritans | 13 | 1 | – | – | 14 | (3) | – | – | – | |||
| X. cheopis | – | – | – | – | – | (0) | – | – | – | |||
| Pácora | ||||||||||||
| C. felis | 33 | 14 | – | – | 47 | (6) | 6 | (12.8) | 5 | (10.6) | 4 | (8.5) |
| C. canis | 66 | 4 | – | – | 70 | (9) | 9 | (12.9) | 8 | (11.4) | 7 | (10.0) |
| P. irritans | 1 | – | – | – | 1 | (1) | – | – | – | |||
| X. cheopis | – | – | – | – | – | (–) | – | – | – | |||
| Salamina | ||||||||||||
| C. felis | 93 | 30 | – | – | 123 | (15) | 12 | (9.8) | 8 | (6.5) | 6 | (4.9) |
| C. canis | 5 | 1 | – | – | 6 | (2) | 2 | (33.3) | 1 | (16.7) | 1 | (16.7) |
| P. irritans | 3 | 1 | 3 | – | 7 | (3) | 1 | (14.3) | – | – | ||
| X. cheopis | – | – | – | 4 | 4 | (1) | – | – | – | |||
| Total | 1,341 | (182) | 123 | (9.2) | 104 | (7.8) | 91 | (6.8) | ||||
Table 2.
Total fleas collected by host and in all locations
| Total fleas collected by host | Total fleas collected (all municipalities) | ||||
|---|---|---|---|---|---|
| Dogs | Cats | Rats | Mice | ||
| C. felis | 807 | 221 | – | – | 1,028 |
| C. canis | 240 | 21 | 1 | – | 262 |
| P. irritans | 30 | 2 | 3 | – | 35 |
| X. cheopis | 2 | – | 10 | 4 | 16 |
| Total | 1,079 | 244 | 14 | 4 | 1,341 |
Figure 1.
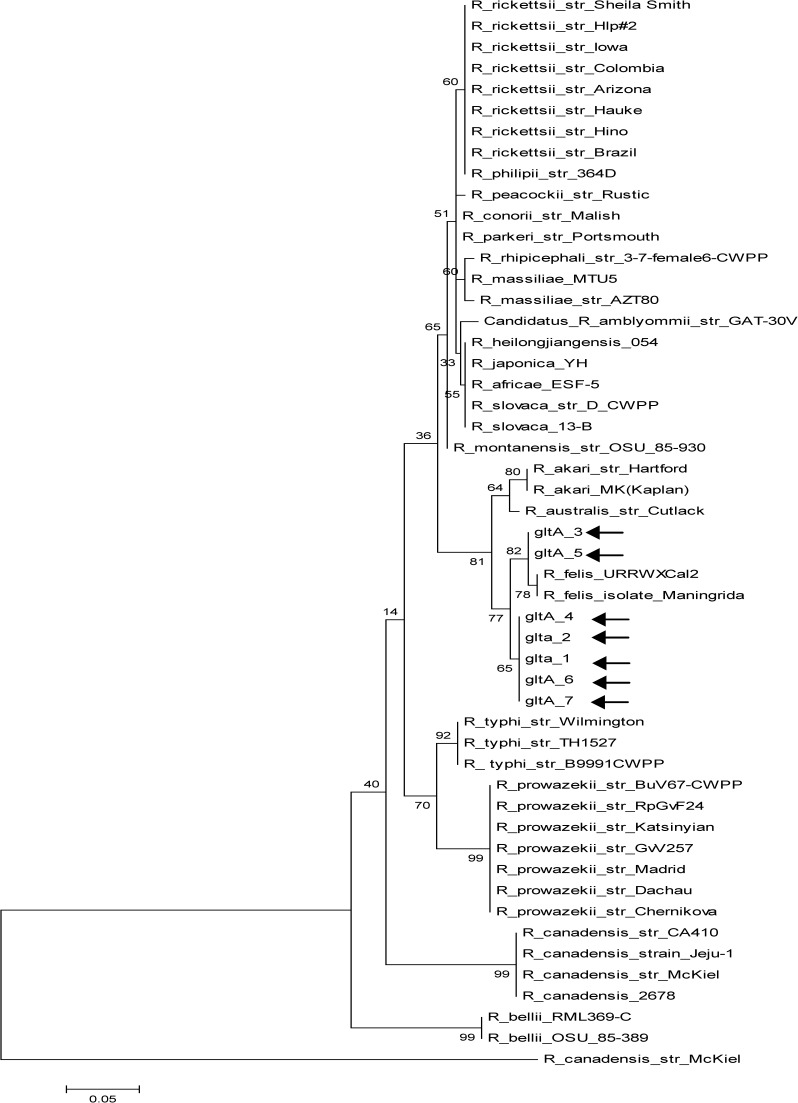
Molecular phylogenetic analysis by maximum likelihood method for the gltA gene (arrows point to study samples).
Fleas.
Fleas were collected manually or by hair combing from owned dogs and cats. They were also collected from synanthropic rats and mice in seven municipalities from the north of Caldas between 2010 and 2011. All specimens were conserved in 70% ethanol and further classified by current morphological keys.26,27
DNA extraction.
We produced pools of one to seven fleas from the same species, host, and site of sampling as pooling criteria. Each pool was dried in 70% ethanol in a bath at 70°C and subsequently cut into small fragments on sterile filter paper. The pieces were macerated in 40 μL (1×) phosphate-buffered saline solution (PBS) and stored at −20°C. We used a commercial kit (DNeasy Blood and Tissue; QIAGEN Inc., Valencia, CA) for DNA extraction with the addition of 400 μL guanidine-thiocyanic acid (DNAzol; Invitrogen™, Life Technologies Corp., Grand Island, NY) for tissue lysis. All extracted samples were evaluated in a Nanodrop 2000 Spectrophotometer (Thermo Fisher Scientific Inc., Walthman, MA) for DNA concentration and purity, and they were conserved at −20°C for additional analyses.
Molecular detection.
DNA of each pool was amplified by conventional polymerase chain reaction (PCR) with specific primers for gltA (CS78–CS323; 401 bp)28 Rickettsia gene, and all positive samples were further confirmed with primers for ompB (120M59-120.807; 862 bp)29 and 17kD (17kD1–17kD2; 434 bp)30 genes. For each reaction, a positive control (R. rickettsii DNA, infected Vero cells, and Sheila Smith strain) and a negative control (water) were added to the procedure. PCR reactions were performed in a C1000 Thermal Cycler (Bio-Rad Lab., Hercules, CA) with the original conditions reported for each set of primers mentioned above; 10 μL of the PCR products were separated in a 2.0% agarose gel stained with SYBRsafe (Invitrogen™, Life Technologies Corp., Grand Island, NY) and examined in an ultraviolet transilluminator.
Sequencing and analyses.
Samples selected for sequencing were PCR-amplified using a proofreading Taq polymerase system (Expand High Fidelity PLUS PCR System; Roche, Pleasanton, CA) for each of the three pair of primers described above. Thereafter, all products were purified with a commercial kit (Wizard SV Gel and PCR Clean-Up System; Promega Corp., Madison, WI) and sequenced (3500 Genetic Analyzer; Applied Biosystems®, Life Technologies Corp., Grand Island, NY). The nucleotide chromatograms were edited with BIOEDIT software (http://www.mbio.ncsu.edu/bioedit/bioedit.html), and sequences were compared with other available Rickettsia sequences from GenBank using the BLAST tool.31
Phylogenetic analyses.
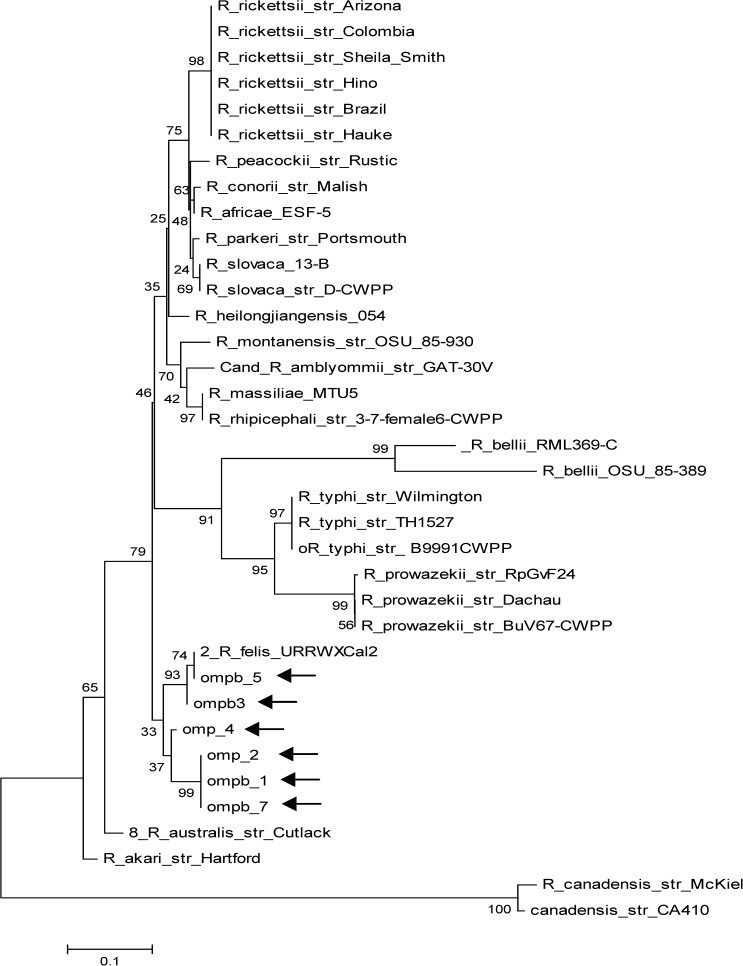
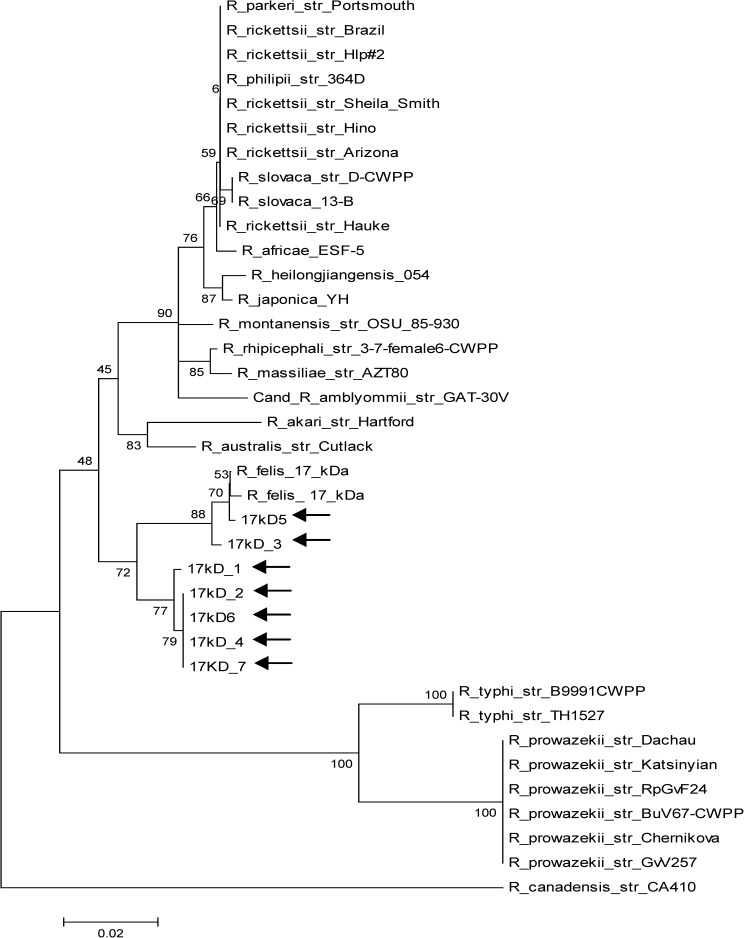
Evolutionary history was inferred by using the maximum likelihood method based on the Tamura–Nei model.32 Trees for each of the three genes (gltA, ompB, and 17kD) were obtained by inference from 1,000 replicates (Figures 1–3
Figure 2.
Molecular phylogenetic analysis by maximum likelihood method for the ompB gene (arrows point to study samples).
). All evolutionary analyses were conducted in MEGA 5.32
Figure 3.
Molecular phylogenetic analysis by maximum likelihood method for the 17kD gene (arrows point to study samples).
Ethical guidelines.
This study was approved by the Pontificia Universidad Javeriana ethical committee and complies with the National Research Council guidelines.
Results
A total of 1,341 fleas were collected in all seven localities. C felis was the most prevalent species, with 1,028 (76.7%) specimens, followed by C. canis (262; 19.5%), Pulex irritans (35; 2.6%), and Xenopsylla cheopis (16; 1.2%); 1,079 (80.5%) of these fleas were from dogs, 244 (18.2%) fleas were from cats, 14 (1%) fleas were from rats, and 4 (0.3%) fleas were from mice (Tables 1 and 2).
A total of 182 pools was produced and included in the amplification. Minimum infection rates (MIRs) were calculated as the percentage of a ratio between the total number of flea pools positive for R. felis and the total number of fleas tested.33 We made this assessment with the assumption that only one flea from each positive pool was positive for the Rickettsia gene analyzed. A total of 123 (MIR; 9.2%) pools yielded PCR products of the expected size for gltA, 104 (7.8%) pools yielded PCR products of the expected size for ompB, and 91 (6.8%) pools yielded PCR products of the expected size for 17kD gene (Tables 1 and 2); 79 (5.9%) pools were positive for the three genes evaluated from all species with the exception of X. cheopis. P. irritans showed the highest MIR for all genes (10.0%) followed by C. canis (9.2%) and C. felis (5.3%). Additionally, the results of positive samples by flea species and host showed that C. canis collected from dogs was the species with the largest proportion of positive pools for gltA (27/37; 73%), ompB (26/37; 70%), and 17kD (24/37; 65%) genes. Valid PCR reactions showed amplicons of the expected size in the positive control and no products in the negative control.
Seven pools were selected for sequencing. All of them were positive for the three genes and represented the most prevalent positive flea species in each municipality. None of the pools from La Merced met these criteria and consequently, were not included.
Sequence homology > 98% to the R. felis URRWXCal2 (complete genome) and other related sequences was obtained and is summarized in Table 3. Phylogenetic trees show the high homology between all sample sequences and other R. felis isolates in the three genes evaluated, and they confirm the monophyletic position of this species and its closeness with the R. akari group.
Table 3.
Pools of fleas selected for sequencing
| Sample number (identification) | Origin | Flea species | Host | Sequence homology (accession number) |
|---|---|---|---|---|
| 1 (AP-7) | Aguadas | C. felis | Dog | > 98%; R. felis URRWXCal2 complete genome (CP000053); Rickettsia sp. cf1 and cf5 17kDa antigen gene (AY953286); Rickettsia sp. R14 outer membrane protein B (ompB) gene (HM370113); R. felis clone Ar3 outer membrane protein B (ompB) gene (GQ385243) |
| 2 (AZP-14) | Aranzazu | P. irritans | Dog | |
| 3 (AZP-19) | Aranzazu | C. felis | Cat | |
| 4 (PP-6) | Pácora | C. canis | Dog | |
| 5 (SP-8) | Salamina | C. felis | Dog | |
| 6 (FP-31) | Filadelfia | C. felis | Cat | |
| 7 (NP-16) | Neira | C. felis | Dog |
Discussion
The results reported herein corroborate, for the first time in Colombia, the presence of R. felis in fleas collected from animals. The proportion of positive pools (for the three genes) of cat fleas (54/132; 41%) is consistent with similar studies in different countries from all continents, confirming the worldwide distribution of this emergent pathogen.2–4 However, the MIR reported here for C. felis (5.3%) is lower than MIRs reported in other countries, like Brazil (14.3%),34 Taiwan (8.2%),35 and the United States (13.3%).33 We must emphasize that these rates correspond to MIRs, because they were calculated with the assumption that only one flea from each positive pool was positive for the Rickettsia gene analyzed; therefore, positive pools were not further corroborated to obtain the results for individual fleas, because we had limited resources.
Some studies have identified prevalence in wild-caught cat fleas ranging from 1% to 100%. Other studies found infection proportions that are similar to the proportions reported here: 22.6% in Argentina,5 36% in Brazil,10 18% in Canada,12 17.5% in France,36 15% in New Zealand,37 35% in Panama,16 18.8% in Taiwan,35 and 41% in Uruguay.23
Other flea species with confirmed positive results for R. felis in this study were C. canis and P. irritans. Proportionally with C. felis, the sample sizes of both species were lower; however, 24 (MIR; 9.2%) and 1 (10%) samples of C. canis and P. irritans were positive for the three genes, respectively, and revealed higher MIR than C. felis. In the scientific literature, few reports of infection by this bacterium in these flea species have been published. In Latin America and the Caribbean, for example, only Brazil10 and Uruguay23 have described infection in C. canis, and there has been no reported infection in P. irritans.37,38 For the latter flea species, we only identified published reports from the Democratic Republic of the Congo39 and the United States.40 Additional studies should investigate the potential role of both flea species in the epidemiology of flea-borne spotted fever in the geographical area of this study.
None of the X. cheopis collected in this study showed positive PCR products for gltA, ompB or 17kD genes. However, this study was limited in the number of oriental rat fleas collected; this limitation was because of the low number of sampled rats and mice. Future research in this geographical area would increase the number of rodents (synanthropic and wild) to better understand the ecoepidemiological role of these mammals in flea-borne rickettsioses (murine typhus and R. felis rickettsioses).
A recent study in the same geographical area showed high seroprevalence for R. typhi and R. felis (25.2% and 17.8%, respectively).25 The finding is in agreement with the rickettsial infection identified in fleas of the same region in this study. The absence of R. typhi in our sample is probably because of the very small number of X. cheopis (the main vector for this bacterium)41 collected in this study. With a larger sample of X. cheopis and other flea species, it would be of interest to test for the presence of coinfection with R. felis and R. typhi in the same positive fleas, because it was previously reported in experimental works42 and under natural conditions.22,43,44
The analysis of the sequence electropherograms showed lack of ambiguity in the nucleotide assignments for all samples included. Because the PCR products were derived from pools in which several members could be positive, the clean sequence reads suggest that the amplified fragments were highly conserved and/or that only one flea in each pool was positive for a single Rickettsia.
In summary, by providing evidence of appropriate arthropod vectors and their infection by Rickettsia, our results further corroborate the endemicity of flea-borne rickettsioses in the north of Caldas Province, Colombia.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Claudia Cuervo for bioinformatical assistance.
Disclaimer: The authors declare no conflict of interests during the execution of this work.
Footnotes
Financial support: This study was supported by Pontificia Universidad Javeriana Grant PY003532.
Authors' addresses: Alejandro Ramírez-Hernández, Grupo ParasitologíaVeterinaria, Universidad Nacional de Colombia, Ciudad Universitaria, Bogotá, Colombia, E-mail: aramirezhe@unal.edu.co. Viviana Montoya, Alejandra Martínez, Marcela Mercado, and Marylin Hidalgo, Grupo de Enfermedades Infecciosas, Departamento de Microbiología, Pontificia Universidad Javeriana, Bogotá, Colombia, E-mails: pmontoya@javeriana.edu.co, amartinezr@javeriana.edu.co, mmercado@javeriana.edu.co, and hidalgo.m@javeriana.edu.co. Jorge E. Pérez, Laboratorio de Microbiología, Facultad de Ciencias de la Salud, Universidad de Caldas, Manizales, Colombia, E-mail: labmicro@ucaldas.edu.co. Alberto de la Ossa and Carolina Vélez, Dirección Territorial de Salud de Caldas, Manizales, Colombia, E-mails: tomasossa@hotmail.com and carovelez@hotmail.com. Gloria Estrada, Departamento de Bacteriología, Universidad Católica de Manizales, Manizales, Colombia, E-mail: gestrada19@gmail.com. María I. Correa, Laura Duque, Juan S. Ariza, and Cesar Henao, Departamento de Medicina Veterinaria y Zootecnia, Universidad de Caldas, Manizales, Colombia, E-mails: mariaico00@hotmail.com, lalitaduque7@hotmail.com, juanariza1709@hotmail.com, and cesar.a.velez@hotmail.com. Gustavo Valbuena, Department of Pathology, University of Texas Medical Branch, Galveston, TX, E-mail: gvalbuen@utmb.edu.
Reprint requests: Marylin Hidalgo, Grupo Enfermedades Infecciosas, Departamento de Microbiología, Pontificia Universidad Javeriana, Cra. 7 No 43-82, Bogotá, Colombia, E-mail: hidalgo.m@javeriana.edu.co.
References
- 1.Raoult D, Roux V. Rickettsioses as paradigms of new or emerging infectious diseases. Clin Microbiol Rev. 1997;10:694–719. doi: 10.1128/cmr.10.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Osorio CE, Zavala-Velazquez JE, Arias Leon JJ, Zavala-Castro JE. Rickettsia felis as emergent global threat for humans. Emerg Infect Dis. 2008;14:1019–1023. doi: 10.3201/eid1407.071656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdad MY, Stenos J, Graves S. Rickettsia felis, an emerging flea-transmitted human pathogen. Emerg Health Threats J. 2011;4:1–7. doi: 10.3402/ehtj.v4i0.7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46:723–736. doi: 10.1603/033.046.0402. [DOI] [PubMed] [Google Scholar]
- 5.Nava S, Perez-Martinez L, Venzal JM, Portillo A, Santibanez S, Oteo JA. Rickettsia felis in Ctenocephalides felis from Argentina. Vector Borne Zoonotic Dis. 2008;8:465–466. doi: 10.1089/vbz.2007.0243. [DOI] [PubMed] [Google Scholar]
- 6.Oliveira KA, Oliveira LS, Dias CC, Silva A, Jr, Almeida MR, Almada G, Bouyer DH, Galvao MA, Mafra C. Molecular identification of Rickettsia felis in ticks and fleas from an endemic area for Brazilian Spotted Fever. Mem Inst Oswaldo Cruz. 2008;103:191–194. doi: 10.1590/s0074-02762008000200011. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira RP, Galvao MA, Mafra CL, Chamone CB, Calic SB, Silva SU, Walker DH. Rickettsia felis in Ctenocephalides spp. fleas, Brazil. Emerg Infect Dis. 2002;8:317–319. doi: 10.3201/eid0803.010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horta MC, Pinter A, Cortez A, Soares RM, Gennari SM, Schumaker TTS, Labruna MB. Rickettsia felis (Rickettsiales: Rickettsiaceae) in Ctenocephalides felis felis (Siphonaptera: Pulicidae) in the State of Sao Paulo, Brazil. Arq Bras Med Vet Zootec. 2005;57:321–325. [Google Scholar]
- 9.Cardoso LD, Freitas RN, Mafra CL, Neves CV, Figueira FC, Labruna MB, Gennari SM, Walker DH, Galvao MA. Characterization of Rickettsia spp. circulating in a silent peri-urban focus for Brazilian spotted fever in Caratinga, Minas Gerais, Brazil. Cad Saude Publica. 2006;22:495–501. doi: 10.1590/s0102-311x2006000300004. [DOI] [PubMed] [Google Scholar]
- 10.Horta MC, Chiebao DP, de Souza DB, Ferreira F, Pinheiro SR, Labruna MB, Schumaker TT. Prevalence of Rickettsia felis in the fleas Ctenocephalides felis felis and Ctenocephalides canis from two Indian villages in Sao Paulo Municipality, Brazil. Ann N Y Acad Sci. 2006;1078:361–363. doi: 10.1196/annals.1374.071. [DOI] [PubMed] [Google Scholar]
- 11.Horta MC, Labruna MB, Pinter A, Linardi PM, Schumaker TT. Rickettsia infection in five areas of the state of Sao Paulo, Brazil. Mem Inst Oswaldo Cruz. 2007;102:793–801. doi: 10.1590/s0074-02762007000700003. [DOI] [PubMed] [Google Scholar]
- 12.Kamrani A, Parreira VR, Greenwood J, Prescott JF. The prevalence of Bartonella, Hemoplasma, and Rickettsia felis infections in domestic cats and in cat fleas in Ontario. Can J Vet Res. 2008;72:411–419. [PMC free article] [PubMed] [Google Scholar]
- 13.Labruna MB, Ogrzewalska M, Moraes-Filho J, Lepe P, Gallegos JL, Lopez J. Rickettsia felis in Chile. Emerg Infect Dis. 2007;13:1794–1795. doi: 10.3201/eid1311.070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hun L, Troyo A, Taylor L, Barbieri AM, Labruna MB. First report of the isolation and molecular characterization of Rickettsia amblyommii and Rickettsia felis in Central America. Vector Borne Zoonotic Dis. 2011;11:1395–1397. doi: 10.1089/vbz.2011.0641. [DOI] [PubMed] [Google Scholar]
- 15.Zavala-Velazquez JE, Zavala-Castro JE, Vado-Solis I, Ruiz-Sosa JA, Moron CG, Bouyer DH, Walker DH. Identification of Ctenocephalides felis fleas as a host of Rickettsia felis, the agent of a spotted fever rickettsiosis in Yucatan, Mexico. Vector Borne Zoonotic Dis. 2002;2:69–75. doi: 10.1089/153036602321131869. [DOI] [PubMed] [Google Scholar]
- 16.Bermudez CS, Zaldivar AY, Spolidorio MG, Moraes-Filho J, Miranda RJ, Caballero CM, Mendoza Y, Labruna MB. Rickettsial infection in domestic mammals and their ectoparasites in El Valle de Anton, Cocle, Panama. Vet Parasitol. 2011;177:134–138. doi: 10.1016/j.vetpar.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 17.Blair PJ, Jiang J, Schoeler GB, Moron C, Anaya E, Cespedes M, Cruz C, Felices V, Guevara C, Mendoza L, Villaseca P, Sumner JW, Richards AL, Olson JG. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J Clin Microbiol. 2004;42:4961–4967. doi: 10.1128/JCM.42.11.4961-4967.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams SG, Sacci JB, Jr, Schriefer ME, Andersen EM, Fujioka KK, Sorvillo FJ, Barr AR, Azad AF. Typhus and typhuslike rickettsiae associated with opossums and their fleas in Los Angeles County, California. J Clin Microbiol. 1992;30:1758–1762. doi: 10.1128/jcm.30.7.1758-1762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevenson HL, Labruna MB, Montenieri JA, Kosoy MY, Gage KL, Walker DH. Detection of Rickettsia felis in a New World flea species, Anomiopsyllus nudata (Siphonaptera: Ctenophthalmidae) J Med Entomol. 2005;42:163–167. doi: 10.1093/jmedent/42.2.163. [DOI] [PubMed] [Google Scholar]
- 20.Reeves WK, Loftis AD, Sanders F, Spinks MD, Wills W, Denison AM, Dasch GA. Borrelia, Coxiella, and Rickettsia in Carios capensis (Acari: Argasidae) from a brown pelican (Pelecanus occidentalis) rookery in South Carolina, USA. Exp Appl Acarol. 2006;39:321–329. doi: 10.1007/s10493-006-9012-7. [DOI] [PubMed] [Google Scholar]
- 21.Hawley JR, Shaw SE, Lappin MR. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg. 2007;9:258–262. doi: 10.1016/j.jfms.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eremeeva ME. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2008;14:1613–1615. doi: 10.3201/eid1410.080571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Venzal JM, Perez-Martinez L, Felix ML, Portillo A, Blanco JR, Oteo JA. Prevalence of Rickettsia felis in Ctenocephalides felis and Ctenocephalides canis from Uruguay. Ann N Y Acad Sci. 2006;1078:305–308. doi: 10.1196/annals.1374.056. [DOI] [PubMed] [Google Scholar]
- 24.Hidalgo M, Salguero E, de la Ossa A, Sanchez R, Vesga JF, Orejuela L, Valbuena G. Murine typhus in Caldas, Colombia. Am J Trop Med Hyg. 2008;78:321–322. [PubMed] [Google Scholar]
- 25.Hidalgo M, Montoya V, Martinez A, Mercado M, De la Ossa A, Velez C, Estrada G, Perez JE, Faccini-Martinez AA, Labruna MB, Valbuena G. Flea-borne rickettsioses in the north of Caldas province, Colombia. Vector Borne Zoonotic Dis. 2013;13:289–294. doi: 10.1089/vbz.2012.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wall R, Shearer D. Veterinary Ectoparasites: Biology, Pathology and Control. Oxford, UK: Blackwell Science; 2001. [Google Scholar]
- 27.Furman P, Catts D. Order Siphonaptera. In: Furman P, Catts D, editors. Manual of Medical Entomology. 4th Ed. Cambridge, UK: Cambridge University Press; 1982. pp. 138–157. [Google Scholar]
- 28.Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, Popov V, Gennari SM, Walker DH. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol. 2004;42:90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Evol Microbiol. 2000;50:1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- 30.Webb L, Carl M, Malloy DC, Dasch GA, Azad AF. Detection of murine typhus infection in fleas by using the polymerase chain reaction. J Clin Microbiol. 1990;28:530–534. doi: 10.1128/jcm.28.3.530-534.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 32.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abramowicz KF, Wekesa JW, Nwadike CN, Zambrano ML, Karpathy SE, Cecil D, Burns J, Hu R, Eremeeva ME. Rickettsia felis in cat fleas, Ctenocephalides felis parasitizing opossums, San Bernardino County, California. Med Vet Entomol. 2012;26:458–462. doi: 10.1111/j.1365-2915.2012.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gehrke FS, Gazeta GS, Souza ER, Ribeiro A, Marrelli MT, Schumaker TT. Rickettsia rickettsii, Rickettsia felis and Rickettsia sp. TwKM03 infecting Rhipicephalus sanguineus and Ctenocephalides felis collected from dogs in a Brazilian spotted fever focus in the State of Rio De Janeiro/Brazil. Clin Microbiol Infect. 2009;15((Suppl 2)):267–268. doi: 10.1111/j.1469-0691.2008.02229.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsai KH, Lu HY, Huang JH, Wang PJ, Wang HC, Huang CG, Wu WJ, Shu PY. Rickettsia felis in cat fleas in Taiwan. Vector Borne Zoonotic Dis. 2009;9:561–563. doi: 10.1089/vbz.2008.0076. [DOI] [PubMed] [Google Scholar]
- 36.Gilles J, Just FT, Silaghi C, Pradel I, Lengauer H, Hellmann K, Pfister K. Rickettsia felis in fleas, France. Emerg Infect Dis. 2008;14:684–686. doi: 10.3201/eid1404.071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly PJ, Meads N, Theobald A, Fournier PE, Raoult D. Rickettsia felis, Bartonella henselae, and B. clarridgeiae, New Zealand. Emerg Infect Dis. 2004;10:967–968. doi: 10.3201/eid1005.030986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Labruna MB, Mattar S, Nava S, Bermudez CS, Venzal JM, Dolz G, Abarca K, Romero L, De Sousa R, Oteo JA, Zavala-Castro JE. Rickettsiosis in Latin America, Caribbean, Spain and Portugal. Rev MVZ Córdoba. 2011;16:2435–2457. [Google Scholar]
- 39.Sackal C, Laudisoit A, Kosoy M, Massung R, Eremeeva ME, Karpathy SE, Van Wyk K, Gabitzsch E, Zeidner NS. Bartonella spp. and Rickettsia felis in fleas, Democratic Republic of Congo. Emerg Infect Dis. 2008;14:1972–1974. doi: 10.3201/eid1412.080610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azad AF, Radulovic S, Higgins JA, Noden BH, Troyer JM. Flea-borne rickettsioses: ecologic considerations. Emerg Infect Dis. 1997;3:319–327. doi: 10.3201/eid0303.970308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bitam I, Dittmar K, Parola P, Whiting MF, Raoult D. Fleas and flea-borne diseases. Int J Infect Dis. 2010;14:e667–e676. doi: 10.1016/j.ijid.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 42.Noden BH, Radulovic S, Higgins JA, Azad AF. Molecular identification of Rickettsia typhi and R. felis in co-infected Ctenocephalides felis (Siphonaptera: Pulicidae) J Med Entomol. 1998;35:410–414. doi: 10.1093/jmedent/35.4.410. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J, Soeatmadji DW, Henry KM, Ratiwayanto S, Bangs MJ, Richards AL. Rickettsia felis in Xenopsylla cheopis, Java, Indonesia. Emerg Infect Dis. 2006;12:1281–1283. doi: 10.3201/eid1208.060327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramowicz KF, Rood MP, Krueger L, Eremeeva ME. Urban Focus of Rickettsia typhi and Rickettsia felis in Los Angeles, California. Vector Borne Zoonotic Dis. 2010;11:979–984. doi: 10.1089/vbz.2010.0117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.