Abstract
West Nile virus (WNV) causes sporadic outbreaks of human encephalitis in Phoenix, Arizona. To identify amplifying hosts of WNV in the Phoenix area, we blood-sampled resident birds and measured antibody prevalence following an outbreak in the East Valley of metropolitan Phoenix during summer, 2010. House sparrow (Passer domesticus), house finch (Haemorhous mexicanus), great-tailed grackle (Quiscalus mexicanus), and mourning dove (Zenaida macroura) accounted for most WNV infections among locally resident birds. These species roost communally after early summer breeding. In September 2010, Culex vector-avian host contact was 3-fold greater at communal bird roosts compared with control sites, as determined by densities of resting mosquitoes with previous vertebrate contact (i.e., blood-engorged or gravid mosquitoes). Because of the low competence of mourning doves, these were considered weak amplifiers but potentially effective free-ranging sentinels. Highly competent sparrows, finches, and grackles were predicted to be key amplifying hosts for WNV in suburban Phoenix.
Introduction
West Nile virus (WNV; Flavivirus, Flaviviridae) is a mosquito-borne arbovirus known for outbreaks of neurologic disease and death among people, horses, and birds in temperate regions of North America.1 Human cases of WNV-attributed neurologic illness in Arizona (southwest USA) have been reported annually since 2003.2 The second largest outbreak in Arizona occurred during the period June–August 2010 in the East Valley of metropolitan Phoenix, located in Maricopa County.3 The East Valley consists of seven towns and cities located east of Phoenix, including Apache Junction, Chandler, Gilbert, Mesa, Queen Creek, Tempe, and other unincorporated areas. The geographically and temporally focused epidemic presented an opportunity to evaluate the vectors and vertebrate amplifying hosts of WNV in suburban metropolitan Phoenix. This study describes investigations of the avian hosts of WNV after the outbreak, in September–October 2010. Specific objectives included 1) measuring the WNV antibody prevalence in common bird species, 2) estimating the relative number of infections among these birds during the current outbreak, and 3) calculating the relative importance of these birds as amplifying hosts. Additionally, mosquitoes were collected at communal bird roost sites to evaluate whether communally roosting bird species may play an important role as the source of bloodmeals for Culex mosquitoes and whether these nocturnal bird congregations possibly serve as highly focused transmission foci for infecting Culex vectors with WNV.
Methods
Study area.
Because many of the human cases were clustered in the East Valley region of metropolitan Phoenix (Maricopa County Health Department, unpublished data), we chose bird sampling sites and mosquito collection sites in the East Valley region (Figure 1). Land use within our study region (an area ∼100 km2 south of U.S. highway 60 and north of state highway 202) includes: about 70% low-density residential property (< 5 dwelling units per acre) characterized by xeric landscaping around residences and small-acreage “horse properties” with grass lawns and pastures that are flood-irrigated twice each month; 15% monoculture agriculture, citrus groves, and dairy cattle production; 10% parkland, man-made lakes and ponds, and desert scrub habitat; and 5% urban town centers, industrial parks, and shopping centers.4 While scouting the region for bird and mosquito sampling locations, sites with large aggregations (i.e., 50+) of communally roosting birds were noted and three of these were targeted as mosquito collection sites. Matched comparison sites were similar in all respects except that communally roosting birds were absent.
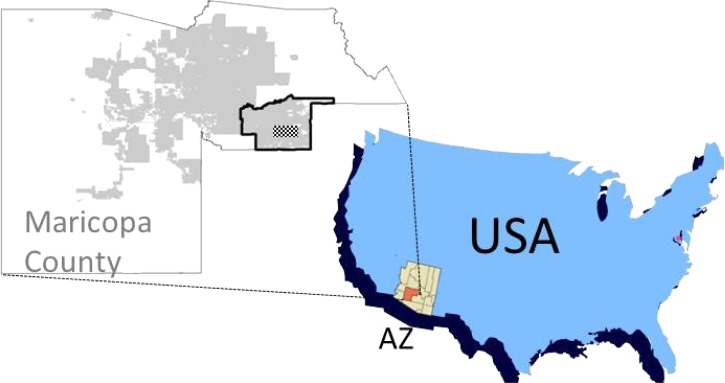
Figure 1.
The East Valley of Maricopa County, Arizona. Avian study sites were located in the stippled area within the East Valley (outlined in black). The gray-shaded area is urban and suburban sections of metropolitan Phoenix. The inset shows the position of Maricopa County relative to the State of Arizona in the southwest USA.
Bird sampling.
Animals were handled in this study following guidelines of the Public Health Service and National Research Council, and approved by the Institutional Animal Care and Use Committee of the Centers for Disease Control and Prevention (CDC), Division of Vector Borne Diseases, and as authorized by the Arizona Department of Conservation and the U.S. Fish and Wildlife Service. Six locations were selected for mist netting of free-ranging birds based on observations of high bird densities. Bird capture and sampling occurred 14–21 September and 27–29 October 2010, in suburban (four residential horse properties), agricultural (dairy cattle feedlot adjacent to citrus groves), and mesquite shrub forest habitats. Birds (except for mallard, which were held captive at one of the sites) were captured using mist nets (Avinet, Inc., Dryden, NY, various mesh sizes) during the first 2 or last 2 hr of daylight. During these periods, nets were monitored constantly to ensure that birds were not entangled for more than a few minutes. Birds were handled using disposable latex gloves. Blood samples were obtained via jugular venipuncture using sterile, disposable 26-g and 27-g subcutaneous needles attached to 1-mL tuberculin syringes. A maximum of 0.65 mL of whole blood or 1% of a bird's mass, determined using a 100-g precision spring scale (Avinet, Inc.), was obtained, whichever volume was smaller, and placed into Microtainer serum separator tubes (Becton, Dickinson and Company, Franklin Lakes, NJ). After 15–60 minutes at ambient temperature, samples were placed on wet ice until centrifuged for separation of serum. After centrifugation, specimens were stored frozen at −20°C to −30°C. Captured birds were each marked with a uniquely numbered aluminum leg band (provided by the U.S. Geological Survey Bird Banding Laboratory, Patuxent, MD). Data collected for each bird included species, sex (categories included male, female, unknown), age (categories included hatch-year, after-hatch-year, second-year, after-second-year, etc.),5 location, band number, and specimen number for the blood sample. Recaptured birds and birds weighing < 10 g were released without sampling.
Serologic testing.
Serum samples were heat-inactivated for 20 minutes at 56°C. Serum samples diluted 1:10 in BA-1 (Hank's M-199 salts, 1% bovine serum albumin, 350 mg/L sodium bicarbonate, 100 units/mL penicillin, 100 mg/L streptomycin, 1 mg/L amphotericin B in 0.05 M Tris, pH 7.6) were screened for detection of flavivirus-neutralizing antibodies using a WNV plaque-reduction neutralization test (PRNT).6 Any of these samples that neutralized a challenge dose of ∼100 pfu of WNV (strain NY99-4132) by at least 80% were further tested at 1:10 dilution for St. Louis encephalitis virus (SLEV)-neutralizing antibodies using strain TBH-28, and at a 1:40 dilution for WNV-neutralizing antibodies by 80% PRNT. Samples that were either positive for SLEV-neutralizing antibodies or negative for WNV-neutralizing antibodies were further tested by titrating in duplicate at serial 2-fold dilutions to determine comparative titers for the two closely related flaviviruses. Reciprocal titers that were within 4-fold of each other for both WNV and SLEV were considered undifferentiable and characterized as “flavivirus antibody positive.”
Estimates of relative abundance, number of avian infections, and mosquito inoculation index.
The Tres Rios (Phoenix) Christmas Bird Counts of 2008–2012 were used to estimate the mean relative abundance, A, of 33 permanent resident bird species.7 To determine the relative number of avian infections for each of these bird species, we took the product of relative abundance and seroprevalence, with the latter used as a surrogate for infection rate. We used the modified mosquito inoculation index (M′)8,9 to assess the relative importance of four abundant resident bird species as amplifying hosts of WNV. This equation is
For the population-based measure, P, we used A. Antibody seroprevalence data, S, were used in place of I because in the absence of pathogen-attributed mortality, seroprevalence is equal to infection rate (I). There have been relatively few reports of WNV-attributed avian mortality in Arizona in recent years. Thus, our equation for mosquito inoculation index is
For vertebrate reservoir competence index (C) values,10 we used data published for Mexican great-tailed grackles and house sparrows infected with WNV from northern Mexico,11 California house finches infected with WNV isolated from California (Reisen WK and Worwa G, unpublished data), and California mourning doves infected with WNV from New York (Brault AC, unpublished data). These values are derived experimentally from the duration and infectiousness of viremia and describe a species' innate potential for infecting mosquitoes. This calculation of the mosquito inoculation index relies on data accumulated for bird infections that occurred retrospectively, with the assumption that most of these infections occurred during the outbreak period.
We also calculated a prospective mosquito inoculation index from mosquito feeding index values derived from Culex quinquefasciatus collected in early August and 6 weeks later in September, 2010, for Cx. quinquefasciatus, following the method described by Kent and others12:
where B is the feeding index, which is calculated by the number of bloodmeals derived from the vertebrate species of interest, divided by the total number of bloodmeals collected.
Mosquito collection and processing.
To determine the proportion of mosquito bloodmeals from Culex tarsalis and Cx. quinquefasciatus taken from different bird species, blood-engorged mosquitoes were collected in CDC resting traps,13 modified to collect mosquitoes in a collection tube with an updraft suction configuration to avoid damaging mosquitoes. These traps were placed within shaded vegetation near or beneath dense congregations of communally roosting birds, and in habitat-matched control sites. At each mosquito collection site, host-seeking mosquitoes were collected for comparison in CO2-baited CDC light traps with the light removed. Mosquitoes were trapped at each site for a period of ∼4 days (in September only). Mosquitoes were removed daily from traps each morning, and frozen on dry ice. Collections were sorted by trap type and location and transferred frozen to the laboratory for identification and sorting into species-specific pools for virus detection assays, or for bloodmeal identification assays for individual mosquitoes that contained bloodmeals scored as < 50% digested by visual criteria (less than half of the abdomen containing eggs). Abdomens from the engorged mosquitoes were separated from the mosquito carcasses and triturated by agitation in a microeppendorf tube containing 0.2 mL phosphate buffered saline and a zinc-coated ball bearing. Mosquito pools of up to 50 adult females were also triturated by agitation, using 1 mL of BA-1and an iron-coated ball bearing.
Bloodmeal identification.
Individual bloodmeals were identified by polymerase chain reaction amplification of the mitochondrial CO I gene and/or cytochrome B gene and nucleotide sequencing following previously described methods.12
Virus detection.
Mosquito homogenates were tested for the presence of WNV RNA following previously published protocols.14 Homogenates were screened with a primer-probe set detecting genome positions 10668 (WN3′-NC forward), 10770 (WN3′-NC reverse), and 10691 (WN3′-NC probe). Samples positive for WNV RNA (with a cycle threshold ≤ 37.0) were confirmed with primer-probe sets detecting genome positions 1160 (WNVENV-forward), 1229 (WNVENV-reverse), and 1186 (WNVENV-probe).
Additional statistical analyses.
We calculated 95% confidence intervals (CIs) for seroprevalence proportions using the Wilson score method (S-PLUS 6.1 Professional software, Insightful, Inc., Seattle, WA). Seroprevalence proportions were compared using the Fisher exact test or the Pearson χ2 test. For multiple comparisons, Bonferroni adjustments were applied. Ninety-five percent CIs for relative abundances of birds were calculated from the standard deviations around the means for a sample size of four survey counts for each species. The methods of Zou and Donner were used to construct CIs around species-specific estimates of the relative number of infected birds and mosquito inoculation index values.15 Mosquito infection rates and their CIs were calculated using the maximum likelihood estimate as applied to mosquito pools of variable sample sizes.16
Results
A total of 303 birds (representing 17 species) were captured, of which 300 were blood sampled. Antibodies derived from WNV infections were confirmed in 144 samples from 14 species (Table 1). The eight most frequently sampled (N ≥ 7) species were house sparrow (51.4% WN-seropositive), mourning dove (44.9%), brown-headed cowbird (17.5%), Inca dove (75.0%), mallard (41.2%), red-winged blackbird (77.8%), bronzed cowbird (28.6%), and great-tailed grackle (85.7%). In addition, six samples (from three mourning doves, an Inca dove, and two mallards) tested positive for undifferentiated flavivirus-neutralizing antibodies, and one house sparrow tested positive for SLEV-neutralizing antibodies. Seropositivity was evaluated separately for two age classes: “hatch-year” (< 1 year of age) and “after-hatch-year” (≥ 1 year of age). The differences in these rates among species tested individually failed significance tests at α = 0.05 except for the mourning dove (two-sided P = 0.006), which had a lower seroprevalence among hatch-year birds.
Table 1.
Birds sampled in the East Valley of Maricopa County, AZ, during September and October 2010 after an outbreak of human West Nile neuroinvasive disease and detection of West Nile virus-neutralizing antibodies (by species and age class*)
| Bird species Latin name | Hatch-year | After-hatch-year | All ages | |||
|---|---|---|---|---|---|---|
| No. WN pos. (N) | prop. (95% CI) | No. WN pos. (N) | prop. (95% CI) | No. WN pos. (N) | prop. (95% CI) | |
| Mallard† Anas platyrhynchos | 4 (8) | 0.50 (0.22–0.79) | 3 (8) | 0.38 (0.14–0.69) | 7 (17) | 0.41 (0.27–0.64) |
| American Kestrel† Falco sparverius | – | NT | – | NT | 1 (1) | 1.00 (0.21–1.00) |
| Inca Dove Columbina inca | 6 (12) | 0.50 (0.25–0.75) | 12 (12) | 1.00 (0.76–1.00) | 18 (24) | 0.75 (0.55–0.88) |
| Mourning Dove† Zenaida macroura | 19 (54) | 0.35 (0.24–0.48) | 11 (14) | 0.79 (0.52–0.92) | 31 (69) | 0.45 (0.34–0.57) |
| White-winged Dove Zenaida asiatica | 0 (1) | 0.00 (0.00–0.79) | 1 (2) | 0.50 (0.09–0.91) | 1 (3) | 0.33 (0.06–0.79) |
| Eurasian Collared-Dove Streptopelia decaocto | 0 (3) | 0.00 (0.00–0.56) | 1 (1) | 1.00 (0.21–1.00) | 1 (4) | 0.25 (0.05–0.67) |
| Northern Mockingbird Mimus polyglottos | – | NT | 1 (1) | 1.00 (0.21–1.00) | 1 (1) | 1.00 (0.21–1.00) |
| Curve-billed Thrasher Toxostoma curvirostre | – | NT | 2 (2) | 1.00 (0.34–1.00) | 2 (2) | 1.00 (0.34–1.00) |
| Abert's Towhee Pipilo alberti | – | NT | 0 (1) | 0.00 (0.00–0.79) | 0 (1) | 0.00 (0.00–0.79) |
| European Starling Sturnus vulgaris | 0 (1) | 0.00 (0.00–0.79) | – | NT | 0 (1) | 0.00 (0.00–0.79) |
| Red-winged Blackbird Agelaius phoeniceus | 4 (4) | 1.00 (0.51–1.00) | 3 (5) | 0.60 (0.23–0.88) | 7 (9) | 0.78 (0.45–0.94) |
| Yellow-headed Blackbird X. xanthocephalus | 0 (2) | 0.00 (0.00–0.66) | – | NT | 0 (2) | 0.00 (0.00–0.66) |
| Great-tailed Grackle Quiscalus mexicanus | 5 (6) | 0.83 (0.44–0.97) | 1 (1) | 1.00 (0.21–1.00) | 6 (7) | 0.86 (0.49–0.97) |
| Bronzed Cowbird Molothrus aeneus | 2 (7) | 0.29 (0.08–0.64) | – | NT | 2 (7) | 0.29 (0.08–0.64) |
| Brown-headed Cowbird Molothrus ater | 6 (30) | 0.20 (0.10–0.37) | 1 (10) | 0.10 (0.02–0.40) | 7 (40) | 0.18 (0.09–0.32) |
| House Finch Haemorhous mexicanus | 3 (3) | 1.00 (0.44–1.00) | 2 (2) | 1.00 (0.34–1.00) | 5 (5) | 1.00 (0.57–1.00) |
| House Sparrow Passer domesticus | 42 (82) | 0.51 (0.41–0.62) | 13 (25) | 0.52 (0.34–0.70) | 55 (107) | 0.51 (0.42–0.61) |
Hatch-year is < 1 year of age; After-hatch-year is ≥ 1 year of age.
These species had one bird of undetermined age.
NT = not tested; CI = confidence interval.
The relative abundance of resident bird species was estimated based on more than 83,500 observation records during Christmas Bird Counts of 33 species of birds that are present year-round in the outbreak region. Six species comprised more than 80% of these records: mourning dove (38.4%), house finch (5.3%), rock pigeon (13.9%), European starling (7.5%), house sparrow (8.0%), and great-tailed grackle (7.4%).
Only four of the six abundant resident bird species were blood sampled. By taking the product of relative abundance and seroprevalence, we estimated the relative number of WNV infections that had occurred among these four species. Infected mourning doves were 2-fold more frequent than infected great-tailed grackles, between 2- and 3-fold more frequent than house finches, and more than 3-fold more frequent than house sparrows (Table 2). However, the mourning dove was the least important among these four species as a WNV-amplifying host, as determined by calculating a retrospective mosquito inoculation index. This index measures the relative contribution of vertebrate host species to the population of infectious vectors, and ranked great-tailed grackle and house finch as most important, and then house sparrow and finally mourning dove was relatively unimportant (Table 2).
Table 2.
Calculation of the relative number of West Nile virus (WNV) infections and the modified mosquito inoculation index among four abundant species of birds in the study area in the East Valley of Maricopa County, AZ
| Species | Relative abundance* A (95% CI) | WNV antibody prevalence S (95% CI) | Estimated relative no. of infections = A × S (95% CI) | Competence C (95% CI) | Mosquito inoculation index = A × S2 × C (95% CI) |
|---|---|---|---|---|---|
| Mourning dove† | 63.9 (60.5–67.3) | 0.35 (0.24–0.48) | 22.5 (16.2–33.4) | 0.096 (0.012–0.18) | 0.8 (0.3–7.1) |
| House finch | 8.8 (3.8–13.8) | 1.00 (0.57–1.00) | 8.8 (3.8–18.2) | 1.19 (0.77–1.61) | 10.4 (4.3–24.4) |
| House sparrow | 11.4 (9.1–13.6) | 0.51 (0.42–0.61) | 5.9 (4.4–7.7) | 1.12 (0.43–1.81) | 3.4 (1.8–9.7) |
| Great-tailed grackle | 13.3 (8.8–17.8) | 0.86 (0.49–0.97) | 11.4 (7.4–21.5) | 1.28 (0.49–2.07) | 12.5 (2.8–24.8) |
Calculated as birds per party-hr.
Only infection rate for hatch-year mourning doves is used for this analysis because statistical analysis infers that numerous adult doves were already seropositive from transmission in previous years. For the other species, the infection rate for all ages is used.
CI = confidence interval.
In mid-September, mosquitoes were collected with the objective of understanding the host selection patterns for Culex quinquefasciatus and Culex tarsalis mosquitoes, the two suspected vectors that were present in the study area, with respect to communal bird roosts. Overall, 2,211 adult mosquitoes (of six species) were collected, of which 1,332 were adult females. Most of these mosquitoes were also tested for active WNV infections, and three isolates were made, two from host-seeking Cx. quinquefasciatus and one from host-seeking Cx. tarsalis (Table 3). To test the hypothesis that mosquitoes were contacting vertebrate hosts preferentially at communal bird roosts, we compared density of engorged mosquitoes resting at communal roost sites versus habitat-matched control sites. All roost sites were in suburban habitat, typically in oleander (Nerium oleander) hedgerows (house sparrows) or ornamental bamboo thickets (mixed species roosts containing doves, finches, grackles, blackbirds, cowbirds, and starlings). Resting Cx. quinquefasciatus and Cx. tarsalis mosquitoes were 25-fold and 5-fold more dense, respectively, at the communal roost sites compared with the control sites (Table 4). Considering only mosquitoes with recent vertebrate contact (gravid and partially gravid mosquitoes still digesting a recent bloodmeal), these differences were 33-fold for Cx. quinquefasciatus, and 3-fold for Cx. tarsalis (Table 4). The density of host-seeking Cx. quinquefasciatus mosquitoes, collected in CO2-baited traps, was similar at the roost sites compared with control sites with no communal roost. More host-seeking Cx. tarsalis were captured at the control sites than at the roost sites, but the difference was not statistically significant (P = 0.16, student's t-test) (Table 5).
Table 3.
Host-seeking and resting adult female mosquitoes collected in the East Valley of Maricopa County, AZ, during September 2012, as part of an evaluation of vertebrate hosts of West Nile virus*
| Species | Behavior | Location | Total | Total tested | Pools tested | Positive pools† | Infection rate (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| Roost | Non-roost | |||||||
| Aedes aegypti | Host-seeking | 34 | 11 | 45 | ||||
| Resting | 4 | 0 | 4 | |||||
| Combined | 49 | 48 | 2 | 0 | 0.0 (0.0–33.1) | |||
| Aedes vexans | Host-seeking | 0 | 2 | 2 | ||||
| Resting | 0 | 0 | 0 | |||||
| Combined | 2 | 0 | 0 | NA | NA | |||
| Anopheles franciscanus | Host-seeking | 0 | 0 | 0 | ||||
| Resting | 2 | 1 | 3 | |||||
| Combined | 3 | 2 | 2 | 0 | 0.0 (0.0–657.6) | |||
| Culex species | Host-seeking | 1 | 3 | 4 | ||||
| Resting | 0 | 0 | 0 | |||||
| Combined | 4 | 4 | 2 | 0 | 0.0 (0.0–391.8) | |||
| Cx. quinquefasciatus | Host-seeking | 57 | 68 | 125 | ||||
| Resting | 348 | 8 | 356 | |||||
| Combined | 481 | 481 | 140 | 2 | 4.3 (0.8–14.1) | |||
| Cx. tarsalis | Host-seeking | 11 | 68 | 79 | ||||
| Resting | 118 | 13 | 131 | |||||
| Combined | 210 | 210 | 57 | 1 | 4.7 (0.3–22.4) | |||
| Psorophora columbiae | Host-seeking | 36 | 546 | 582 | ||||
| Resting | 1 | 0 | 1 | |||||
| Combined | 583 | 583 | 21 | 0 | 0.0 (0.0–5.7) | |||
Collections were categorized according to the presence or absence of a nocturnal communal bird roost.
The two positive pools for Cx. quinquefasciatus were collected at non-roost locations. The positive pool for Cx. tarsalis was collected at a roost location. The roost contained doves and grackles.
CI = confidence interval; NA = not applicable.
Table 4.
Density of resting adult female Culex quinquefasciatus and Culex tarsalis mosquitoes at three avian communal roost sites versus three habitat-matched control sites*
| Culex species | Communal roost (N = 56) | Control (N = 28) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fully engorged | Partially engorged & gravid | Empty | All | Fully engorged | Partially engorged & gravid | Empty | All | |
| Cx. quinquefasciatus | 1.07 | 1.23 | 2.93 | 5.23 | 0.00 | 0.07 | 0.14 | 0.21 |
| Cx. tarsalis | 0.23 | 0.71 | 1.04 | 1.98 | 0.07 | 0.21 | 0.11 | 0.39 |
Data are expressed as the mean number per CDC resting-trap night.
N = sample size of resting trap-nights.
Table 5.
Density of host-seeking adult female Culex quinquefasciatus and Culex tarsalis mosquitoes at three avian communal roost sites versus two habitat-matched control sites*
| Culex species | Communal roost (N = 13) | Control (N = 6) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fully engorged | Partially engorged & gravid | Empty | ALL | Fully engorged | Partially engorged & gravid | Empty | ALL | |
| Cx. quinquefasciatus | 0.00 | 0.00 | 4.46 | 4.46 | 0.00 | 0.00 | 4.33 | 4.33 |
| Cx. tarsalis | 0.00 | 0.00 | 0.85 | 0.85 | 0.00 | 0.17 | 5.00 | 5.17 |
Data are expressed as the mean number per CO2-baited CDC trap-night.
N = sample size of trap-nights.
Mosquito abdomens containing fresh blood (up to 50% digested) were individually assayed for bloodmeal identification. Bloodmeals were identified for 35 of 38 Cx. tarsalis, and 113 of 119 Cx. quinquefasciatus. As expected, most (94% for Cx. quinquefasciatus, 90% for Cx. tarsalis) of the bloodmeals identified pertained to communally roosting bird species (Table 6). To assess the relative importance of bird species as WNV-amplifying hosts at the communal roosts, the Cx. quinquefasciatus feeding index values and vertebrate reservoir competence index values were used to calculate prospective mosquito inoculation index values. This analysis, which ignores the immune status of birds, predicted that within the three communal bird roosts that we sampled for mosquitoes, sparrows would infect 11-fold more Cx. quinquefasciatus mosquitoes than house finches, more than 200-fold more than great-tailed grackles, and more than 5,000-fold more than mourning doves (Table 7). However, because house finches and grackles sampled in this study were mostly immune, mainly house sparrows and mourning doves were available to amplify WNV.
Table 6.
Vertebrate bloodmeal identification among engorged mosquitoes, by vertebrate species and collection habitat, East Valley of Maricopa County, AZ, September 2010
| Habitat | Bird species | Culex quinquefasciatus (N = 113) | Culex tarsalis (N = 35) |
|---|---|---|---|
| Roost | House sparrow | 66 | 15 |
| House finch | 19 | 3 | |
| Great-tailed grackle | 4 | 0 | |
| Brown-headed cowbird | 4 | 2 | |
| Red-winged blackbird | 1 | 0 | |
| European starling | 1 | 0 | |
| Curve-billed thrasher* | 3 | 2 | |
| House wren* | 0 | 1 | |
| Inca dove* | 1 | 0 | |
| Mourning dove | 3 | 2 | |
| Eurasian collared-dove | 1 | 1 | |
| White-winged dove | 0 | 2 | |
| Chicken | 7 | 1 | |
| Human | 3 | 0 | |
| Non-roost | House sparrow | 1 | 0 |
| House finch | 0 | 1 | |
| White-winged dove | 0 | 1 | |
| Mourning dove | 0 | 1 | |
| Domestic turkey | 0 | 1 | |
| Human | 0 | 2 |
These species are solitary roosters.
Table 7.
Calculation of mosquito inoculation index of selected bird species using their respective Culex quinquefasciatus feeding index values (derived from mosquito collections at communal bird roosts) and vertebrate reservoir competence index values*
| Bird species | Feeding index B (95% CI) | Vertebrate reservoir competence index C (95% CI) | Mosquito inoculation index M = B2C (95% CI) |
|---|---|---|---|
| House sparrow | 0.589 | 1.12 | 3885.5 |
| (0.497–0.676) | (0.43–1.81) | (2158.1–10521.5) | |
| House finch | 0.170 | 1.19 | 343.9 |
| (0.111–0.250) | (0.77–1.16) | (139.2–833.9) | |
| Great-tailed grackle | 0.036 | 1.28 | 16.6 |
| (0.014–0.088) | (0.49–2.07) | (2.4–126.2) | |
| Mourning dove | 0.027 | 0.096 | 0.7 |
| (0.009–0.076) | (0.012–0.180) | (0.07–13.2) |
95% CI = 95% confidence interval.
Discussion
The finding that house sparrow, house finch, and mourning dove are frequently infected and therefore are potentially important amplification hosts mirrors similar findings from southern California and southern New Mexico.17–19 Although great-tailed grackle was not implicated as a key host in neighboring states, it was found to be the most important WNV-amplifying host in Guatemala.9
Because of the mobile nature of birds, it is difficult to assess their relationship with spatiotemporally restricted WNV transmission with precision. Our evaluation of their past infection with WNV took place several weeks after the period of intense transmission resulting in human disease. An important benefit of this delayed assessment of bird exposure is that it is cumulative for the preceding period when WNV was being transmitted. An assessment during the outbreak period would yield an incomplete picture of past infections. An important drawback of this retrospective approach is that the species composition of the avian populations present during the outbreak may have changed during the time interval leading up to their sampling. Certain common summering species (for example, western kingbird [Tyrannus verticalis]) have migrated out of the region by September. Some resident populations have shifted. For example, during the fall months, the local population of mourning dove becomes inflated with the arrival of migrants from the north. Some common species in October, such as white-crowned sparrow (Zonotrichia leucophrys), are strictly winter residents, and are completely absent during the summer. Because of these uncertainties among bird populations, we targeted abundant permanent resident species with populations suspected to be stable in the East Valley region. The stability of these populations justified our use of winter survey data from the Christmas Bird Count to estimate the relative abundance of permanent resident species such as great-tailed grackle, house finch, and house sparrow. Interestingly, < 5% of the bloodmeals identified in our study and one conducted earlier in the summer pertained to bird species that were not permanently resident in the Phoenix suburban area.20
The retrospective and prospective measurements of the mosquito inoculation index values for common bird species in suburban Phoenix produced markedly different results. This index expresses the relative number of infectious Cx. quinquefasciatus mosquitoes that would be derived from feeding on available susceptible amplifying hosts. With the retrospective approach that is based on bird species abundance and seroprevalence, the measurement applies broadly to a large area because the seroprevalence values observed reflect a wide scale in time and space. In this case, the infections probably took place over a 4–5-month period. Thus, the benefit of retrospective measurement is that its interpretation may apply to birds throughout the outbreak area. However, this broad timescale presents a limitation as well because birds are mobile, and therefore their exposure status may reflect time spent beyond the outbreak area. The effect of sampling birds that had traveled from beyond the outbreak area will dilute the apparent importance of that species as an amplifying host. This may be the case for mourning dove in our study, as many of these were seronegative, and may have arrived to the sampling area after the outbreak period. This is less of a concern for species like house finch and great-tailed grackle because their seroprevalences were extremely high, and thus these birds were likely local residents exposed to WNV over a period of months, and less likely to have come from other areas where WNV had not been active. House sparrow had a moderate level of seroprevalence like mourning dove; however, it is a sedentary species, and therefore sparrows probably had not dispersed much within the previous 6 months.21
A second limitation of the retrospective mosquito inoculation index calculation was the use of seroprevalence as a surrogate for the infection rate. If WNV infections were resulting in undetected avian mortality, the infection rates are then underestimated, and the estimated importance of the affected species as an amplifier is underestimated. Although avian mortality had not been reported by the surveillance system in Maricopa County, we did find one dead house sparrow, which tested positive for WNV infection (data not shown). If sparrow mortality was as high as reported in some experimental infection studies with the NY99 strain of WNV (67%),22 the true infection rate would then have been ∼77% (not 51%), and the relative number of infectious mosquitoes inoculated by feeding on house sparrows would more than double from 3.4 to 7.6.8 The other three species would not have been affected by unreported mortality because mourning dove is not expected to succumb to WNV infection at a high rate,22 and the infection rates used for great-tailed grackle and house finch were so high (87.5% and 100%, respectively), that factoring in mortality would have very little or no effect. Of interest, the count for house finch from the Christmas Bird Count declined by almost 50% in the winter after the 2010 outbreak. An experimental infection study of house finch in California observed more than 60% mortality following infection with the New York 1999 strain of WNV.23 These considerations suggest that avian mortality associated with WNV infection may be occurring in the Phoenix area, and therefore avian mortality surveillance may be useful for early detection of WNV activity in Phoenix. Although the number of dead birds found positive for WNV in the United States has diminished during the course of the North American epidemic, this may be largely a result of less aggressive reporting and testing.24
The prospective mosquito inoculation index calculation was based on the feeding index data for Cx. quinquefasciatus. This assessment of the amplification potential of different bird species is based not on events over the past several months, but rather on the current situation at the locations where engorged mosquitoes were collected. In this case, it represents the amplification potential of birds at communal bird roosts in September assuming that every bird is susceptible. The interpretation then would be that if all the birds present at the communal roost sites where mosquitoes are feeding were equally susceptible to WNV infection, an introduction of WNV would then initiate amplification primarily by sparrows, followed to a much smaller extent by house finches, and to a negligible extent by grackles and mourning doves. Because we did not detect WNV in any of the engorged mosquitoes, we could not detect evidence of active transmission. The high proportions of immune birds in the communal roosts may have conferred herd immunity, thereby preventing further amplification of WNV.
Our prospective mosquito inoculation index calculations are limited in value because of the geographic bias presented by the communal roosts, which are highly localized and may not have been present in the summer during the outbreak period. Godsey and others20 also identified bloodmeals from Culex mosquitoes collected 6 weeks earlier at random locations throughout the outbreak region during the first week of August. These data provide an alternative opportunity to retrospectively assess the important WNV amplifiers among the bird population. Of nine bird species identified as bloodmeal hosts for Cx. quinquefasciatus within the outbreak area, three stood out as potentially important amplifiers, according to our formula used for assessing prospective amplification potential. These were primarily house sparrow, followed by mourning dove, and finally house finch. Other species, such as northern mockingbird and curve-billed thrasher contributed negligibly. Thus, house sparrow yet again emerges as the primary amplifying host involved in this outbreak.
From the mosquito feeding index data that we collected and from the earlier work,20 doves and chicken are clearly selected as hosts by Culex mosquitoes in suburban Phoenix. The extraordinarily high number of dove infections would indicate doves as candidate free-ranging bird sentinels. Pigeons and collared-doves are frequently held in captivity, and thus would be amenable to use as captive sentinels. Considering the high exposure rates of doves and grackle, it was surprising that very few bloodmeals from these species were identified in September. One explanation is that mosquitoes feed on these birds earlier in the season when they exhibit weaker defenses against mosquito bites as nestlings and brooding adults. This hypothesis is supported for doves (but not grackle) by the bloodmeal identifications from August, when the mourning dove feeding index of Cx. quinquefasciatus reached 0.33 (N = 39), compared with 0.03% (N = 113) in September (one-tailed Fisher's exact test P < 0.001).20 However, no great-tailed grackle bloodmeals were identified in August.
The focus of the entomological collections in August was to identify the vector for human infections. Culex quinquefasciatus appeared to be the primary vector because of the human feeding index of 0.21 in the outbreak region, and a higher vector index than Cx. tarsalis.20 Vector index measures the density of host-seeking infected mosquitoes.25 Our data also produced a slightly higher vector index for Cx. quinquefasciatus compared with Cx. tarsalis; however, the importance of Cx. tarsalis as a vector is elevated by its high vector competence compared with Cx. quinquefasciatus.26 Furthermore, if we apply the Kilpatrick vector risk index incorporating data on comparative vector competence and human feeding index, we calculate that in September, risk of WNV transmission to humans by Cx. tarsalis was ∼4-fold greater than by Cx. quinquefasciatus in our study (data not shown).27 Interestingly, although we found human bloodmeals among both of these vectors, we noticed that Cx. tarsalis was more likely than Cx. quinquefasciatus to be found host seeking away from large concentrations of nocturnally roosting birds. This suggests that the communally roosting birds may actually play a significant zooprophylactic role for humans, steering potentially infectious Cx. quinquefasciatus away from humans more so than Cx. tarsalis. Clearly, both of these Culex vectors become infected and feed on people, and thus both had potential to cause human infections in the East Valley.
Perhaps most notable among our observations is that the key vector–vertebrate host interactions were highly focal and centered on spatially clustered vertebrate hosts (i.e., nocturnal communal roosts), at least during the waning weeks of the WNV outbreak in Phoenix. Many questions about the role of communally roosting birds in arbovirus amplification persist, including how the public health system can harness this communal roosting behavior ultimately to reduce burden of arboviral disease. Neighborhoods with high densities of house sparrow and house finch should expect a higher risk of WNV transmission because of the WNV-amplifying potential of these two species, in particular, in suburban sections of the Phoenix area.
ACKNOWLEDGMENTS
Numerous private property owners and the Town of Gilbert granted permission for field studies. Field assistance was provided by Steve Baty, Anne Justice-Allen, Jamie Feld, Rebecca Levy, and Tricia Wadleigh. Laboratory assistance was provided by Jason Velez. Guidance for statistical analyses was provided by Brad Biggerstaff. W. K. Reisen and G. Worwa provided unpublished data.
Disclaimer: The statements and opinions expressed in this article are those of the authors and do not necessarily represent official policy of the Centers for Disease Control and Prevention or the U.S. Government or the State of Arizona.
Footnotes
Financial support: This work was funded by the Centers for Disease Control and Prevention.
Authors' addresses: Nicholas Komar, Nicholas A. Panella, Ginger R. Young, and Aaron C. Brault, Centers for Disease Control and Prevention - Arbovirus Diseases Branch, Fort Collins, CO, E-mails: nck6@cdc.gov, nap4@cdc.gov, gyoung527@gmail.com, and acbrault1@mac.com. Craig E. Levy, Arizona Department of Health Services - Epidemiology, Phoenix, AZ, E-mail: CraigLevy@mail.maricopa.gov.
Reprint requests: Nicholas Komar, CDC-DVBD-ADB, 3156 Rampart Road, Fort Collins, CO 80521, Tel: 970-221-6400, E-mail: NKomar@cdc.gov.
References
- 1.Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Arbonet. 2011. http://www.cdc.gov/ncidod/dvbid/westnile/surv&control_archive.htm Available at. Accessed April 13, 2012.
- 3.Gibney KB, Colborn J, Baty S, Bunko Patterson AM, Sylvester T, Briggs G, Stewart T, Levy C, Komatsu K, MacMillan K, Delorey MJ, Mutebi J-P, Fischer M, Staples JE. Modifiable risk factors for West Nile virus infection during an outbreak - Arizona, 2010. Am J Trop Med Hyg. 2012;86:895–901. doi: 10.4269/ajtmh.2012.11-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Town of Gilbert General Plan Land Use Map. 2010. http://www.gilbertaz.gov/areamaps/pdf/GeneralPlan0710.pdf Available at. Accessed February 1, 2011.
- 5.Pyle P. Identification Guide to North American Birds, Part I Columbidae to Ploceidae. Bolinas, CA: Slate Creek Press; 1997. [Google Scholar]
- 6.Beaty B, Calisher CH, Shope RE. Arboviruses. In: Lennette EH, Lennette DA, Lennette ET, editors. Viral, Rickettsial, and Chlamydial Infections. Seventh edition. Washington, DC: American Public Health Association; 1995. pp. 189–212. [Google Scholar]
- 7.National Audubon Society The Christmas Bird Count Historical Results. 2010. http://www.christmasbirdcount.org Available at. Accessed April 2, 2012.
- 8.Komar N, Panella NA, Langevin SA, Brault AC, Amador M, Edwards E, Owen JC. Avian hosts for West Nile virus in St. Tammany Parish, Louisiana 2002. Am J Trop Med Hyg. 2005;73:1031–1037. [PubMed] [Google Scholar]
- 9.Morales-Betoulle ME, Komar N, Panella NA, Alvarez D, López MR, Betoulle J-L, Sosa SM, Müller ML, Kilpatrick AM, Lanciotti RS, Johnson BW, Powers AM, Cordón-Rosales C. Ecology of West Nile virus in a tropical ecosystem in Guatemala. Am J Trop Med Hyg. 2011;88:116–126. doi: 10.4269/ajtmh.2012.12-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komar N, Dohm DJ, Turell MJ, Spielman A. Eastern equine encephalitis in birds: relative competence of European starlings (Sturnus vulgaris) Am J Trop Med Hyg. 1999;60:387–391. doi: 10.4269/ajtmh.1999.60.387. [DOI] [PubMed] [Google Scholar]
- 11.Guerrero-Sánchez S, Cuevas-Romero S, Nemeth NM, Trujillo-Olivera MT, Worwa G, Dupuis A, Brault AC, Kramer LD, Komar N, Estrada-Franco JG. West Nile virus infection of birds, Mexico. Emerg Infect Dis. 2011;17:2245–2252. doi: 10.3201/eid1712.110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kent RJ, Juliusson L, Weissman M, Evans S, Komar N. Seasonal blood feeding behavior of Culex tarsalis (Diptera: Culicidae) in Weld County, Colorado, 2007. J Med Entomol. 2009;46:380–390. doi: 10.1603/033.046.0226. [DOI] [PubMed] [Google Scholar]
- 13.Panella NA, Kent RJ, Biggerstaff BJ, Komar N. A novel trap for collecting resting mosquitoes. J Am Mosq Control Assoc. 2011;27:323–325. doi: 10.2987/09-5900.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage H, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou GY, Donner A. Construction of confidence limits about effect measures: a general approach. Stat Med. 2008;27:1693–1702. doi: 10.1002/sim.3095. [DOI] [PubMed] [Google Scholar]
- 16.Biggerstaff BJ. PooledInfRate, version 3.0: a Microsoft Excel Add-In to compute prevalence estimates from pooled samples. Fort Collins, CO: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 17.Molaei G, Cummings RF, Su T, Armstrong PM, Williams GA, Cheng ML, Webb JP, Andreadis TG. Vector-host interactions governing epidemiology of West Nile virus in Southern California. Am J Trop Med Hyg. 2010;83:1269–1282. doi: 10.4269/ajtmh.2010.10-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vuong HB, Caccamise DF, Remmenga M, Creamer R. Ecological associations of West Nile virus and avian hosts in an arid environment. In: Paul E, editor. Emerging Avian Disease: Studies in Avian Biology. Vol. 42. Berkeley, CA: University of California Press; 2012. pp. 3–22. [Google Scholar]
- 19.Reisen WK, Lothrop HD, Wheeler SS, Kennsington M, Gutierrez A, Fang Y, Garcia S, Lothrop B. Persistent West Nile virus transmission and the apparent displacement of St. Louis encephalitis virus in southeastern California, 2003–2006. J Med Entomol. 2008;45:494–508. doi: 10.1603/0022-2585(2008)45[494:pwnvta]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godsey MS, Jr, Burkhalter K, Young G, Delorey M, Smith K, Townsend J, Levy C, Mutebi JP. Entomological investigations during an outbreak of West Nile virus disease in Maricopa County, Arizona, 2010. Am J Trop Med Hyg. 2012;87:1125–1131. doi: 10.4269/ajtmh.2012.11-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anderson TR. Biology of the Ubiquitous House Sparrow: From Genes to Populations. New York: Oxford University Press; 2006. [Google Scholar]
- 22.Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang Y, Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75:480–485. [PubMed] [Google Scholar]
- 24.Roth D, Henry B, Mak S, Fraser M, Taylor M, Li M, Cooper K, Furnell A, Wong Q, Morshed M. British Columbia West Nile Virus Surveillance Team West Nile virus range expansion into British Columbia. Emerg Infect Dis. 2010;16:1251–1258. doi: 10.3201/eid1608.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gujral IB, Zielinski-Gutierrez EC, LeBailly A, Nasci R. Behavioral risks for West Nile virus disease, northern Colorado, 2003. Emerg Infect Dis. 2007;13:419–425. doi: 10.3201/eid1303.060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reisen WK, Barker CM, Fang Y, Martinez VM. Does variation in Culex (Diptera: Culicidae) vector competence enable outbreaks of West Nile virus in California? J Med Entomol. 2008;45:1126–1138. doi: 10.1603/0022-2585(2008)45[1126:dvicdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Kilpatrick AM, Kramer LD, Campbell SR, Alleyne EO, Dobson AP, Daszak P. West Nile virus risk assessment and the bridge vector paradigm. Emerg Infect Dis. 2005;11:425–429. doi: 10.3201/eid1103.040364. [DOI] [PMC free article] [PubMed] [Google Scholar]