Abstract
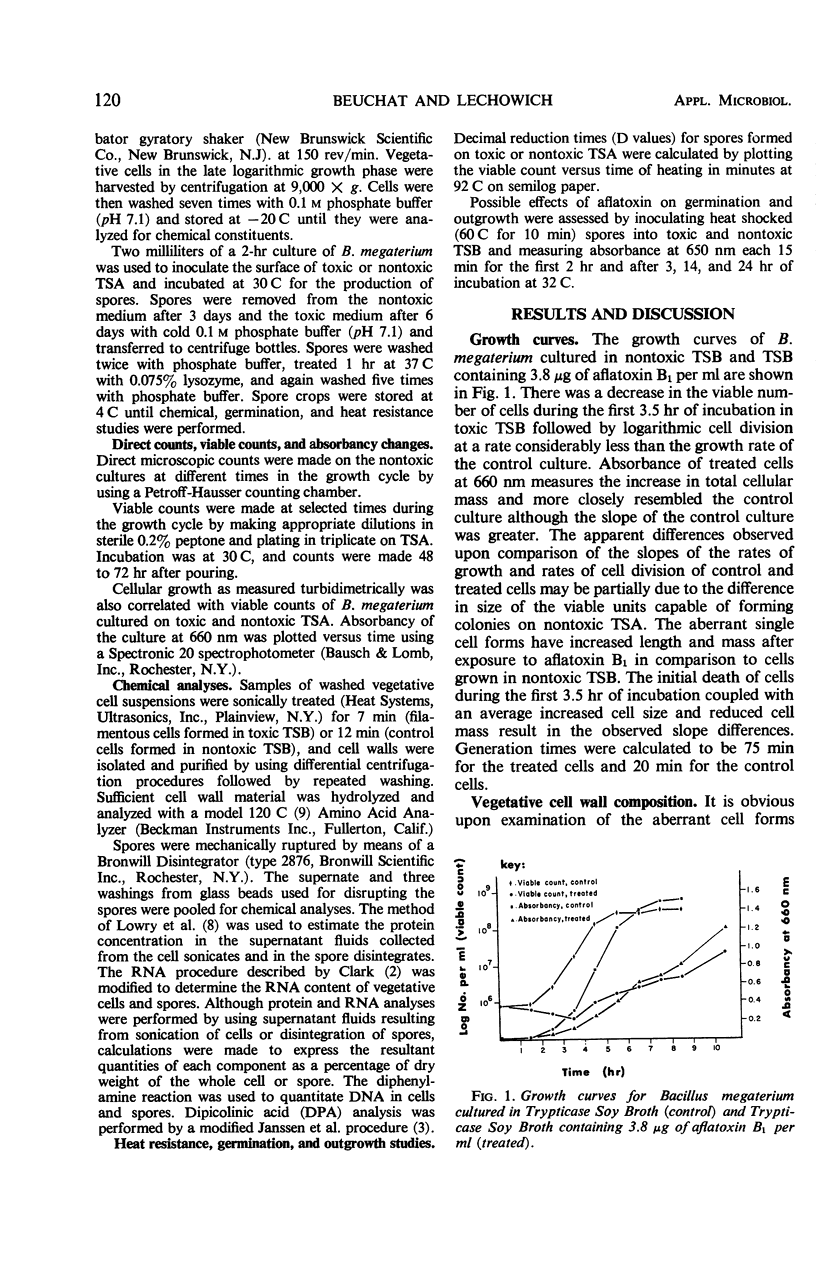
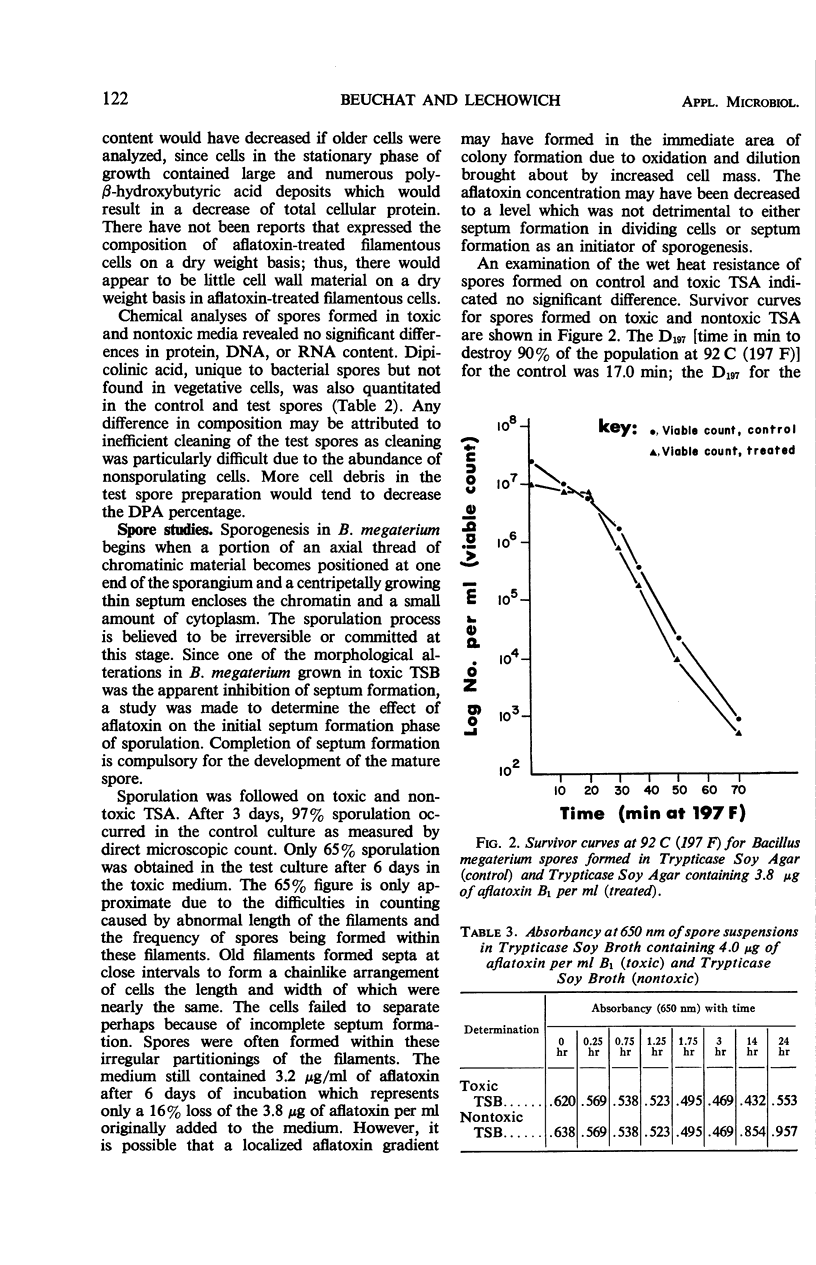
Bacillus megaterium NRRL B-1368 cells and spores were produced on Trypticase Soy Broth (TSB) and Agar (TSA) containing 3.8 μg of aflatoxin B1 per ml, analyzed for selected chemical constituents, and compared to cells and spores of B. megaterium produced on nontoxic Trypticase Soy Media. There was an initial 30% kill of cells after inoculation into toxic TSB and during the first 3.5 hr of incubation followed by a logarithmic growth phase in which the generation time was 75 min as compared to 20 min for the control culture. Chemical analyses revealed an increase in protein, deoxyribonucleic acid (DNA), and ribonucleic acid (RNA) on both a per cell basis and a per cent dry weight basis when B. megaterium was grown in toxic TSB. There was a concurrent decrease in the total amounts of cellular protein, DNA, and RNA synthesized in toxic TSB. Amino acid analyses of control and test cell walls showed little, if any, difference in cell wall composition. About 97% sporulation of B. megaterium occurred after 3 days on nontoxic TSA although 6 days were required to attain 65% sporulation on toxic TSA. Germination of spores was not inhibited by 4.0 μg of aflatoxin per ml but outgrowth was. No significant differences were observed in the heat resistance, protein, DNA, RNA, or dipicolinic acid content of spores formed on toxic TSA and nontoxic TSA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burmeister H. R., Hesseltine C. W. Survey of the sensitivity of microorganisms to aflatoxin. Appl Microbiol. 1966 May;14(3):403–404. doi: 10.1128/am.14.3.403-404.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- King A. M., Nicholson B. H. The interaction of aflatoxin B1 with polynucleotides and its effect on ribonucleic acid polymerase. Biochem J. 1969 Oct;114(4):679–687. doi: 10.1042/bj1140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lillehoj E. B., Ciegler A. Aflatoxin B1 binding and toxic effects on Bacillus megaterium. J Gen Microbiol. 1968 Dec;54(2):185–194. doi: 10.1099/00221287-54-2-185. [DOI] [PubMed] [Google Scholar]
- Lillehoj E. B., Ciegler A., Hall H. H. Aflatoxin B 1 uptake by Flavobacterium aurantiacum and resulting toxic effects. J Bacteriol. 1967 Jan;93(1):464–471. doi: 10.1128/jb.93.1.464-471.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj E. B., Ciegler A. Inhibition of deoxyribonucleic acid synthesis in Flavobacterium aurantiacum by aflatoxin B1. J Bacteriol. 1967 Sep;94(3):787–788. doi: 10.1128/jb.94.3.787-788.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wragg J. B., Ross V. C., Legator M. S. Effect of aflatoxin B1 on the deoxyribonucleic acid polymerase of Escherichia coli. Proc Soc Exp Biol Med. 1967 Aug-Sep;125(4):1052–1055. doi: 10.3181/00379727-125-32274. [DOI] [PubMed] [Google Scholar]


