Abstract
French Guiana, the French territory most affected by human immunodeficiency virus (HIV) (1.3% of pregnant women), is also endemic for human T lymphotropic virus 1 (HTLV1). The objective of this study was to determine if the HTLV1/HIV coinfected patients had particular characteristics. All HIV-infected patients having a computerized medical file containing an HTLV1 serology were included: there were 1,333 HIV monoinfections and 76 HTLV1/VIH coinfections. The prevalence of HTLV1/HIV coinfections was 5.39%. Women (odds ratio [OR] = 1.91[1.13–3.24]), subjects > 40 years of age, and patients of Surinamese origin (OR = 2.65 [1.25–5.61]) were overrepresented among the coinfected. CD4 count at the time of diagnosis and viral loads were higher among coinfected patients. The clinical stage was not significantly different between the two groups. The number of CD4 cells was not higher among the coinfected, unlike most reports from the literature. Prevalence of HTLV1 among HIV-infected patients is high in French Guiana, and physicians seem to omit the prescription of serology for this potentially serious coinfection.
Introduction
Located in South America between Brazil and Suriname, French Guiana is the French territory with the worst human immunodeficiency virus (HIV) epidemic. With 1.3% of infected pregnant women,1 the epidemic is generalized according to UNAIDS criteria. However, 64% of new patients are migrants and sex work and crack use are suspected drivers of the epidemic2; the predominant mode of infection is heterosexual sex.3
French Guiana is also an area where the human T lymphotropic virus 1 (HTLV1) is endemic, concentrated in descendants of African slaves (notably Maroons).4 This retrovirus is responsible for, in about 5% of infected persons, adult T cell lymphoma/leukemia, HTLV1-associated spastic tropical paraparesis/myelopathy, other autoimmune manifestations, or certain opportunistic infections.5 It shares almost the same transmission routes as HIV: blood, sexual (a higher percentage from men to women), and vertical transmission mostly through maternal breastmilk.5 In French Guiana, HTLV1 is generally transmitted through breastfeeding and sexual relations.6 The HTLV1-HIV coinfections are frequent in endemic regions for these two retroviruses.
Although they present different viral cycles, the two viruses have the same cellular tropism: CD4 lymphocytes. In vitro, it was shown that HTLV1 provoked an upregulation of HIV,7 and conversely.8 In clinical studies, there is a frequent description of an increased CD4 count in coinfected patients, without any corresponding immunological benefit. However, given the number of discordant results,9 it was not conclusively shown that HTLV1 coinfection accelerated the evolution of HIV infection.
The only study performed on HTLV1/HIV in French Guiana showed lower survival in coinfected patients,10 but the number of patients was small (151 persons, of whom only 18 were coinfected) and only looked at survival.
In this study, the HIV parameters of two populations, coinfected and HIV-infected without HTLV1 were compared.
Methods
A retrospective comparative study was performed using a database collecting the eNADIS electronic patient file, an HIV-specific tool devised to the care of HIV patients and viral hepatitis patients. The database collected information on patients seen between January 1, 2000 and January 23, 2012. In French Guiana, most HIV patients are followed in three centers (one in Cayenne, one in Kourou, and one in Saint Laurent). The patients followed in metropolitan France usually do not live in French Guiana, but in metropolitan France. There are 30 patients that are sufficiently wealthy to take the plane every 3 months to get care in Paris, these patients are not in the eNADIS database. In Cayenne, one private practitioner follows < 100 patients who are not included in the eNADIS database. With over 75% of migrants among patients, loss to follow-up is high with over 20/100 person-years of patients lost to follow-up.3 French Guiana, a French territory, attracts numerous immigrants from South American countries and the Caribbean. Immigrants come in search of better socioeconomic prospects, or to flee conflicts, but usually not for medical reasons. Overall, 30% of the population is foreign.2
Because a large number of HTLV1 serology results were missing in the patient file, a preliminary stage was to collect the missing data from the main laboratories in French Guiana performing this serology. The missing data was then entered into eNADIS to complete the database.
Inclusion criteria were patient followed for an HIV infection in one of the three main hospitals of French Guiana (Center Hospitalier Andrée Rosemon de Cayenne, Center Médico-Chirurgical de Kourou, and Center Hospitalier de l'Ouest Guyanais de Saint Laurent du Maroni), using the eNADIS file, with a result for HTLV1 serology.
The exclusion criterion was the absence of an HTLV1 serology after attempting to recover the results in the laboratories.
The eNADIS database and patient file system are in agreement with the French Law (Informatique et Libertés) and are declared at the Commission Nationale Informatique et Liberté. All data were anonymized and all patients gave written informed consent before the creation of the computerized patient file, which entails retrospective use of the data.
The data analysis was performed using Stata 8 (College Station, TX).
A bivariate analysis was first performed comparing different variables between the HIV-HTLV1 coinfected patients and the HIV without HTLV1 infection. The studied variables were sex, age, nationality, contamination mode, duration of infection, CDC stage, antiretroviral treatment, maximum viral load, viral load before treatment, viral load on treatment, CD4 count, CD4 nadir, CD4 count before treatment, CD4 count at the time of diagnosis, aspartate amino transferase/alanine amino transferase (ASAT ALAT), coinfections (hepatitis B and C, syphilis, toxoplasmosis), comorbidities (renal failure, malignancy, high blood pressure, stroke, hyperlipemia, cardiopathies), virological and/or immunological failure. The student's t test was used for Gaussian quantitative variables and Mann Whitney's test for non-Gaussian variables. The χ2 test, or Fisher's exact test were used for qualitative variables. For ordinal variables the linear trend χ2 test was used. For binary variables odds ratios (ORs) and their confidence intervals (CIs) were calculated.
Finally, a multivariate analysis was performed to obtain adjusted ORs for age, gender, country of residence, clinical stage, and antiretroviral treatment. Covariates were retained from the saturated model using the likelihood ratio test to obtain the most parsimonious model.
Results
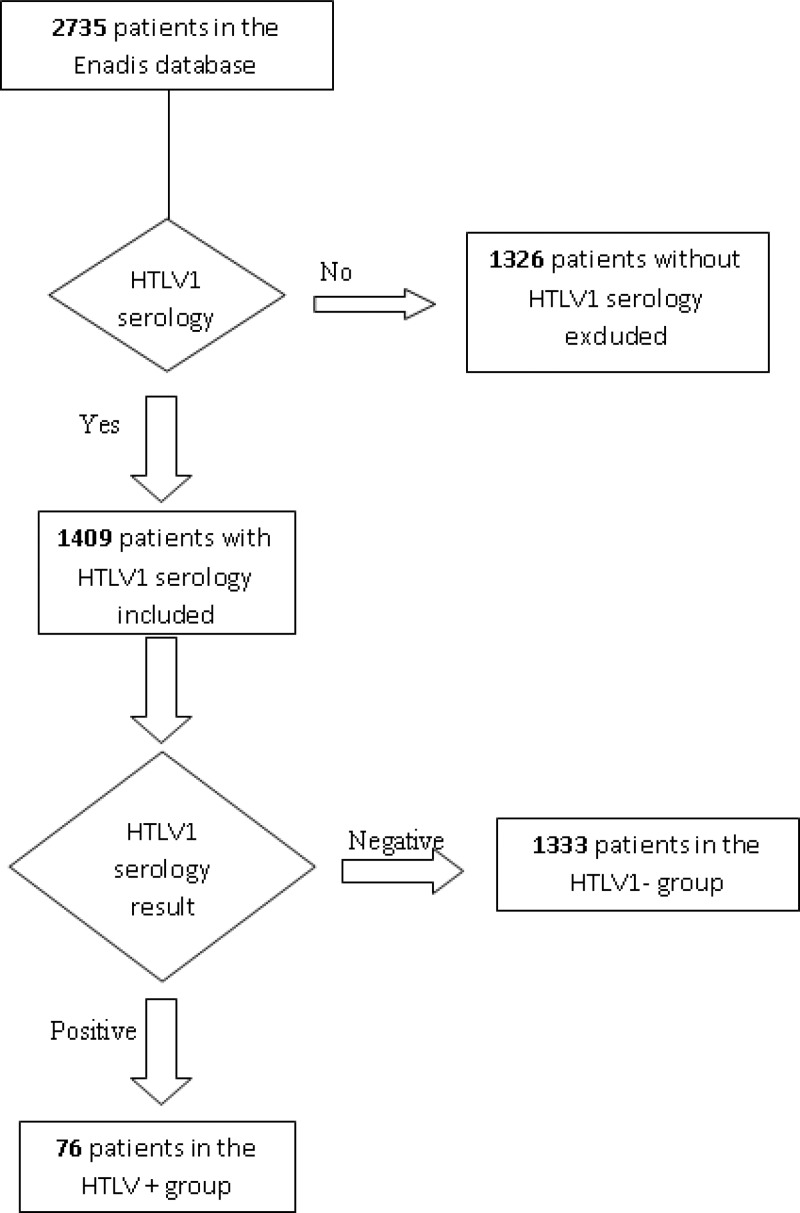
There were 1,333 patients HIV+/HTLV1− included in the single infection group and 79 patients HIV+/HTLV1+ included in the coinfected group. Patient characteristics are shown in Table 1 and the flow chart of patients analyzed is shown in Figure 1.
Table 1.
| Variable | HTLV− (N = 1,333) | HTLV1+ (N = 76) |
|---|---|---|
| Age in years: mean (standard deviation) | 44.05 (12.99) | 51.87 (14.29) |
| Sex: count (percent) | ||
| Male | 638 (47.86%) | 28 (36.84%) |
| Female | 695 (52.14%) | 48 (63.16%) |
| Infection duration in years: median (interquartile range) | 7 (8) | 6 (9) |
| Country of origin: count (percent) | ||
| Brazil | 83 (6.95%) | 2 (2.99%) |
| France | 346 (28.98%) | 17 (25.37%) |
| Guyana | 123 (10.3%) | 7 (10.45%) |
| Haiti | 397 (33.25%) | 17 (25.37%) |
| Caribbean other than Haiti | 23 (1.93%) | 3 (4.48%) |
| Surinam | 199 (28.36%) | 19 (28.36%) |
| Others | 23 (1.93%) | 2 (2.99%) |
| Contamination mode | ||
| Heterosexual sex | 1,199 (94.93%) | 71 (98.61%) |
| Homosexual sex | 47 (3.72%) | 1 (1.39%) |
| Mother to child | 10 (0.79%) | 0 |
| Intravenous (transfusion or intravenous drug use) | 7 (0.55%) | 0 |
| CDC clinical stage: count (percent) | ||
| A | 737 (55.29%) | 41 (53.92%) |
| B | 182 (13.65%) | 8 (10.53%) |
| C | 414 (31.06%) | 27 (35.53%) |
HTLV = human T lymphotropic virus; CDC = Centers for Disease Control and Prevention.
Figure 1.
Flow chart of patients included in the study.
In this population, the proportion of coinfected patients was 5.4%, the sex ratio was 0.9, the mean age was 44.5 years (SD 13.2) (Table 2). The most frequent nationalities were Haitians (29.4%), French (25.8%), and Surinamese (15.5%). The main contamination mode was sexual transmission (90.1%). The median duration of infection was 7 years (interquartile range = 8 years), 55.2% of patients were asymptomatic (CDC stage A), 31.3% had acquired immunodeficiency syndrome (AIDS) (CDC stage C), and 80.4% were on antiretrovirals (Table 1).
Table 2.
Distribution of age, viral load, CD4 count at the time of diagnosis, nadir CD4, and ASAT in a population of HIV+ French Guianese patients*
| HTLV1– (N = 1333) | HTLV1+ (N = 76) | P | |
|---|---|---|---|
| Age in years: mean (standard deviation) | 44.05 (12.99) | 51.87 (14.29) | P < 0.0001† |
| Viral load with treatment in copies/mL: median (interquartile range) | 30 (208) | 47 (7580) | P = 0.0178‡ |
| CD4 count at the time of the diagnosis in number/mm3: median (interquartile range) | 316 (364.7) | 376 (363.2) | P = 0.02‡ |
| Nadir CD4 in number/mm3: médiane (interquartile range) | 159.7 (236) | 196.6 (237.7) | P = 0.15‡ |
| ASAT in IU/L: median (interquartile range) | 23 (13) | 27 (12) | P = 0.02‡ |
HTLV = human T lymphotropic virus; ASAT = aspartate amino transferase; HIV = human immunodeficiency virus.
Student's t test.
Mann Whitney's test.
Bivariate analysis showed no significant difference regarding gender, nationality, HIV infection duration, contamination mode, clinical stage, antiretroviral treatment, virologic and immunologic failure, and coinfections with hepatitis B, hepatitis C, syphilis, and toxoplasmosis. In the HTLV1-HIV coinfected group, age was significantly higher (an average of 7 years older, P < 0.0001) and the OR for diabetes was 2.2 (95 CI = 1.0–4.34), P = 0.02 (Table 3). For other comorbidities (renal failure, malignancy, etc.), for CD4 count, nadir CD4, maximum viral load, viral load before treatment, CD4 count before treatment, and alanine amino transferase, there were no significant differences. In the HTLV1-HIV coinfected group, the viral load on treatment was higher (median HTLV1+ = 47 copies/mL, interquartile range = 7,580, median HTLV1− = 30 copies/mL, interquartile range = 208, P = 0.02), the CD4 count at the time of diagnosis was higher (median HTLV1+ = 376 CD4/mm3, interquartile range = 363, median HTLV1− = 316, interquartile range = 365, P = 0.02), and ASAT were higher (median HTLV1+ = 27 UI/L, interquartile range = 12, median HTLV1− = 23, interquartile range = 13, P = 0.02) (Table 2).
Table 3.
Odds Ratio for antiretroviral treatment, diabetes, and cardiopathies in a population of HIV+ French Guianese patients*
| Variable | HTLV1+ N (%) | HTLV1−N (%) | Odds Ratio (95% confidence interval) | P |
|---|---|---|---|---|
| Antiretroviral treatment | 1.61 (0.36–1.1) | 0.069 | ||
| Yes | 55 (72.37) | 1078 (80.87) | ||
| No | 21 (27.63) | 255 (19.13) | ||
| Diabetes | 2.18 (1–4.34) | 0.02 | ||
| Yes | 11 (14.47) | 96 (7.2) | ||
| No | 65 (85.53) | 1237 (92.80) | ||
| Cardiopathies | 2.84 (0.53–9.97) | 0.08 | ||
| Yes | 3 (3.95) | 19 (1.43) | ||
| No | 73 (96.05) | 1314 (98.57) |
HIV = human immunodeficiency virus.
Multivariate analysis (Table 4) adjusting for age showed that diabetes was not more frequent among coinfected patients than in HIV patients without HTLV1. In the final model women (OR = 1.9, 95 CI = 1.13–3.24, P = 0.02), Surinamese nationals, and older patients (starting 41 years of age) were overrepresented in the coinfected group (Table1). Similarly, CD4 counts > 500/mm3 at the time of diagnosis (OR = 2.3, 95 CI = 1.04–5.25, P = 0.04). And viral load > 1,000 copies were more frequent in the coinfected group than in HIV patients without HTLV1. Table 5 shows the proportion of missing data.
Table 4.
Adjusted odds ratio* in a population of HIV+ French Guyanese patients
| Variable | HTLV1+ N (%) | HTLV1− N (%) | Adjusted odds ratio* (95% confidence interval) | P |
|---|---|---|---|---|
| Sex | ||||
| Male | 28 (36.84) | 638 (47.86) | 1 | |
| Female | 48 (63.16) | 695 (52.14) | 1.91 (1.13–3.24) | 0.02 |
| Age | ||||
| < 31 years | 6 (7.89) | 191 (14.33) | 1 | |
| 31–40 years | 9 (11.84) | 383 (28.73) | 0.96 (0.33–2.80) | 0.944 |
| 41–50 years | 23 (30.26) | 347 (26.03) | 3.64 (1.41–9.40) | 0.008 |
| 51–60 years | 16 (21.05) | 273 (20.48) | 4.88 (1.74–13.67) | 0.003 |
| > 60 years | 22 (28.95) | 139 (10.43) | 14.83 (5.30–41.5) | < 0.001 |
| Nationality | ||||
| French | 17 (22.37) | 346 (25.96) | 1 | |
| Brazilian | 2 (2.63) | 83 (6.23) | 0.47 (0.1–2.14) | 0.326 |
| Guyanian | 7 (9.21) | 123 (9.23) | 1.48 (0.58–3.82) | 0.415 |
| Haitian | 17 (22.37) | 397 (29.78) | 0.69 (0.33–1.42) | 0.311 |
| Caribbean but Haitian | 3 (3.95) | 23 (1.73) | 1.94 (0.47–7.99) | 0.356 |
| Others | 2 (2.63) | 23 (1.73) | 2.12 (0.42–10.62) | 0.360 |
| Surinamese | 19 (25) | 199 (14.93) | 2.65 (1.25–5.61) | 0.011 |
| Unknown | 9 (11.84) | 139 (10.43) | 1.21 (0.47–2.7) | 0.798 |
| Viral Load | ||||
| > 30,000 copies/mL | 12 (15.79) | 152 (11.4) | 2.56 (1.20–5.45) | 0.015 |
| 10,000–30000 | 8 (10.53) | 46 (3.45) | 5.68 (2.22–14.55) | < 0.001 |
| 1,000–9,999 | 11 (14.47) | 100 (7.5) | 3.27 (1.48–7.24) | 0.003 |
| 400–999 | 2 (2.63) | 53 (3.98) | 1.05 (0.23–4.74) | 0.946 |
| 50–399 | 8 (10.53) | 179 (13.43) | 1.17 (0.51–2.69) | 0.718 |
| < 50 | 28 (36.84) | 640 (48.01) | 1 | |
| Unknown | 7 (9.21) | 163 (12.23) | 1.62 (0.67–3.95) | 0.284 |
| CD4 count at the time of diagnosis | ||||
| < 200 CD4/mm3 | 21 (27.63) | 230 (17.25) | 1 | |
| 200–349 | 15 (19.74) | 196 (14.7) | 2.5 (0.98–5.18) | 0.057 |
| 350–499 | 7 (9.21) | 181 (13.58) | 0.92 (0.33–2.56) | 0.871 |
| > 500 | 11 (14.47) | 295 (22.13) | 2.34 (1.04–5.25) | 0.040 |
| Unknown | 22 (28.95) | 431 (32.33) | 1.58 (0.72–3.45) | 0.253 |
Adjusted using a logistic regression model. The saturated model included sex, age, nationality, viral load, CD4 count at the time of diagnosis, treatment, cardiopathies, diabetes, nadir CD4, and aspartate amino transferase (ASAT) as explanatory variables, the most parsimonious model was obtained using the Likelihood ratio test.
Table 5.
Proportion of missing data in HTLV- and HTLV-groups*
| Variable | Missing data in HTLV1− group: N (%) | Missing data in HTLV1+ group: N (%) |
|---|---|---|
| Country of origin | 139 (10.43) | 9 (11.84) |
| Contamination mode | 70 (5.25) | 4 (5.26) |
| Viral load | 163 (12.23) | 7 (9.21) |
| CD4 count | 155 (11.63) | 7 (9.21) |
| Hepatitis B | 86 (6.45) | 4 (5.26) |
| Hepatitis C | 107 (8.03) | 4 (5.26) |
| Toxoplasmosis | 159 (11.93) | 10 (13.16) |
| Syphilis | 424 (31.81) | 24 (31.58) |
| Virologic failure | 323 (29.96) | 21 (38.18) |
| Immunologic failure | 343 (31.82) | 19 (34.55) |
HTLV = human T lymphotropic virus.
Discussion
The prevalence of HTLV1/HIV coinfections in this study was 5.39%, which is higher than the prevalence observed in Martinique (3.36%).11 The eNadis database in French Guiana included 1,326 patients without HTLV1 serology, which is 48.48% of all patients, despite considerable efforts to collect results of serologies, whereas the database in Martinique only had 3.58% of missing results.11 This probably leads to a selection bias, which could have inflated the proportion of positive HTLV1 tests (symptomatic patients with HTLV1 or belonging to a high risk group more likely to be tested, and positive results are more likely to be entered in the database than the default: negative).
As in most studies on HTLV1, here we observed that women and older persons were more at risk of HTLV1.5,10,12 Surinamese nationals appeared more infected with HTLV1. This is explained by the epidemiology of HTLV1 in French Guiana where the maroon populations are most affected with this infection,6,13 living mostly on the Maroni river, which marks the border with Suriname. It is noteworthy that Haitian nationals, or other nationalities from the Caribbean, were not more at risk, although HTLV1 prevalence is high in these countries.12
There was no difference regarding some risk factors described in other studies such as intravenous drug use.14,15 However, in contrast with the countries were those studies that took place (Brazil, United States),14 and French Guiana transmission is essentially sexual or maternofetal and intravenous drug use is marginal.13,16
The clinical stage was not significantly different between the two groups. In the literature, there are conflicting data with some reporting more advanced disease, whereas other studies did not find any difference.9,15
The HIV infection duration and the proportion on antiretroviral treatment, which could have been confounders, were equivalent in both groups.
Other coinfections (hepatitis B, hepatitis C, syphilis, and toxoplasmosis) were not more frequent in the HTLV1-HIVcoinfections. A previous study found increased hepatitis C prevalence in HTLV1-HIV coinfected patients,14 but then again this study took place in a North American population where intravenous drug use is more frequent than in French Guiana.
After adjusting for age there were no more comorbidities such as diabetes, cardiopathies, or renal failure in the HTLV1-HIV coinfected patients than in the HIV patients without HTLV1.
Malignancies were not more frequent in the HTLV1 coinfected group, however this may be caused by a lack of statistical power.
It was surprising to find no difference in CD4 counts between groups because it is a finding that is often reported elsewhere.10,14,15,17–21 Only the CD4 count at the time of diagnosis was significantly higher in the HTLV1-HIV coinfected patients, this after adjusting for gender, a potential confounder.
The viral load was significantly higher in coinfected patients. Conflicting results are found in the literature regarding this variable,9,22 but certain authors have suggested looking at this variable rather than CD4 counts to reflect the activity of the HIV infection.17
In this study, the ASAT level was higher in HTLV1-HIV coinfected patients. There was no more hepatitis, or antiretroviral treatments in this group. In addition, a study showed that liver enzymes were higher in patients with hepatitis C virus (HCV)/HTLV1 coinfections than single HCV infections.23 However, the difference observed has no clinical significance (4 units) and both medians are well below the upper limit of the normal range. During multivariate analysis, using a cutoff of 50 units, there was no difference observed between HTLV1-HIV coinfections and HIV. It is thus plausible that the statistical difference observed during bivariate analysis for liver enzymes is purely caused by chance.
The main limitation of this study is its retrospective design, leading to numerous missing variables. Indeed, the thoroughness of data entry varies between physicians. We also regret the absence of data on opportunistic infections (caused by HIV or HTLV1), hemoglobin, CD8 counts, and CD4%, which could have given additional information on the interactions between HTLV1 and HIV. Finally, in the absence of longitudinal data, no data on survival was available.
Despite these drawbacks, this study is interesting because it includes a large number of patients (76 coinfections, 1,333 HIV without HTLV1). Moreover, it shows a high prevalence of HTLV1 in HIV patients in French Guiana, a reminder that this serology should be part of the normal initial investigations in an HIV patient. A longitudinal study on HTLV1-HIV coinfections in French Guiana, and possibly other regional centers, would be useful to complete the present results.
Footnotes
Authors' addresses: Elise Gouhier and Philippe Abboud, Centre Hospitalier de Cayenne, Departement des Maladies Infectieuses, Cayenne, French Guiana, E-mails: elisegouhier@hotmail.com and philippe.abboud@ch-cayenne.fr. Emilie Gaubert-Maréchal, Centre Hospitalier de Cayenne – COREVIH, Cayenne, French Guiana, E-mail: emilie.gaubert-maréchal@ch-cayenne.fr. Pierre Couppié, Centre Hospitalier de Cayenne, Cayenne, French Guiana, Service de Dermatologie, Cayenne, French Guiana, E-mail: couppie.pierre@voila.fr. Mathieu Nacher, CH Cayenne, Rue des Flamboyants, Cayenne, E-mail: mathieu.nacher@ch-cayenne.fr.
References
- 1.Nacher M, Huber F, El Guedj M, Vaz T, Magnien C, Djossou F, Randrianjohany A, Alvarez F, Couppié P. Risk factors for death among patients in French Guiana: 1996–2005. HIV Med. 2007;8:472–474. doi: 10.1111/j.1468-1293.2007.00492.x. [DOI] [PubMed] [Google Scholar]
- 2.Nacher M, Vantilcke V, Parriault MC, Van Melle A, Hanf M, Labadie G, Romeo M, Adriouch L, Carles G, Couppié P. What is driving the HIV epidemic in French Guiana? Int J STD AIDS. 2010;21:359–361. doi: 10.1258/ijsa.2010.009570. [DOI] [PubMed] [Google Scholar]
- 3.Nacher M. Activity Report of HIV Patient Care in French Guiana in 2007. 2007. www.corevih.org Available at. Accessed June 27, 2013.
- 4.Kazanji M, Gessain A. Human T-cell lymphotropic virus types I and II (HTLV-I/II) in French Guiana: clinical and molecular epidemiology. Cad Saude Publica. 2003;19:1227–1240. doi: 10.1590/s0102-311x2003000500002. [DOI] [PubMed] [Google Scholar]
- 5.Verdonck K, Gonzalez E, Van Dooren S, Vandamme AM, Vanham G, Gotuzzo E. Human T-lymphotropic virus 1: recent knowledge about an ancient infection. Lancet Infect Dis. 2007;7:266–281. doi: 10.1016/S1473-3099(07)70081-6. [DOI] [PubMed] [Google Scholar]
- 6.Plancoulaine S, Gessain A, Joubert M, Tortevoye P, Jeanne I, Talarmin A, de Thé G, Abel L. Detection of a major gene predisposing to human T lymphotropic virus type I infection in children among an endemic population of African origin. J Infect Dis. 2000;182:405–412. doi: 10.1086/315741. [DOI] [PubMed] [Google Scholar]
- 7.Casoli C, Pilotti E, Bertazzoni U. Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression. AIDS Rev. 2007;9:140–149. [PubMed] [Google Scholar]
- 8.Beilke MA, Traina-Dorge VL, Sirois M, Bhuiyan A, Murphy EL, Walls JM, Fagan R, Winsor EL, Kissinger PJ. Relationship between human T lymphotropic virus (HTLV) type 1/2 viral burden and clinical and treatment parameters among patients with HIV type 1 and HTLV-1/2 coinfection. Clin Infect Dis. 2007;44:1229–1234. doi: 10.1086/513428. [DOI] [PubMed] [Google Scholar]
- 9.Brites C, Sampalo J, Oliveira A. HIV/human T-cell lymphotropic virus coinfection revisited: impact on AIDS progression. AIDS Rev. 2009;11:8–16. [PubMed] [Google Scholar]
- 10.Sobesky M, Couppie P, Pradinaud R, Godard MC, Alvarez F, Benoit B, Carme B, Lebeux P. Coinfection with HIV and HTLV-I infection and survival in AIDS stage. French Guiana Study. GECVIG (Clinical HIV Study Group in Guiana) Presse Med. 2000;29:413–416. [PubMed] [Google Scholar]
- 11.Cabié A. Activity Report of the COREVIH Martinique. 2012. http://www.sfls.aei.fr/ckfinder/userfiles/files/BAO-CoreVIH/bao8/RA_2011_COREVIH_Martinique.pdf Available at. Accessed June 27, 2013.
- 12.Césaire R, Lagathu G, Lézin A. HTLV1 and associated diseases in the French Antilles and French Guiana. Revue Francophone des Laboratoires. 2005;2005:35–44. [Google Scholar]
- 13.Plancoulaine S, Buigues RP, Murphy EL, van Beveren M, Pouliquen JF, Joubert M, Rémy F, Tuppin P, Tortevoye P, de Thé G, Moreau JP, Gessain A. Demographic and familial characteristics of HTLV-1 infection among an isolated, highly endemic population of African origin in French Guiana. Int J Cancer. 1998;76:331–336. doi: 10.1002/(sici)1097-0215(19980504)76:3<331::aid-ijc8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Beilke MA, Theall KP, Megan O, Clayton JL, Benjamin SM, Winsor EL, Kissinger PJ. Clinical outcomes and disease progression among patients coinfected with HIV and human T lymphotropic virus types 1 and 2. Clin Infect Dis. 2004;39:256–263. doi: 10.1086/422146. [DOI] [PubMed] [Google Scholar]
- 15.Schechter M, Harrison LH, Halsey NA, Trade G, Santino M, Moulton LH, Quinn TC. Coinfection with human T-cell lymphotropic virus type I and HIV in Brazil. Impact on markers of HIV disease progression. JAMA. 1994;271:353–357. [PubMed] [Google Scholar]
- 16.Oni T, Djossou F, Joubert M, Heraud JM. Awareness of mother-to-child transmission of human T-cell lymphotropic virus (HTLV) type I through breastfeeding in a small group of HTLV-positive women in Maripasoula and Papaichton, French Guiana. Trans R Soc Trop Med Hyg. 2006;100:715–718. doi: 10.1016/j.trstmh.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 17.Schechter M, Moulton LH, Harrison LH. HIV viral load and CD4+ lymphocyte counts in subjects coinfected with HTLV-I and HIV-1. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;15:308–311. doi: 10.1097/00042560-199708010-00010. [DOI] [PubMed] [Google Scholar]
- 18.Brites C, Alencar R, Gusmao R, Pedroso C, Netto EM, Pedral-Sampaio D, Badaró R. Co-infection with HTLV-1 is associated with a shorter survival time for HIV-1-infected patients in Bahia, Brazil. AIDS. 2001;15:2053–2055. doi: 10.1097/00002030-200110190-00023. [DOI] [PubMed] [Google Scholar]
- 19.Gudo ES, Bhatt NB, Bila DR, Abreu CM, Tanuri A, Savino W, Silva-Barbosa SD, Jani IV. Co-infection by human immunodeficiency virus type 1 (HIV-1) and human T cell leukemia virus type 1 (HTLV-1): does immune activation lead to a faster progression to AIDS? BMC Infect Dis. 2009;9:211. doi: 10.1186/1471-2334-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scapellato PG, Bottaro E, Brieschke MT, Scapellato JL, Dato A, Intile AD, Davaro R, Vidal GI. CD4 cell count among HIV-infected patients with an AIDS-defining disease: higher count in patients coinfected than in those not coinfected with human T-cell lymphotropic virus type I. J Acquir Immune Defic Syndr. 2003;33:279–280. doi: 10.1097/00126334-200306010-00028. [DOI] [PubMed] [Google Scholar]
- 21.Bessinger R, Beilke M, Kissinger P, Jarrott C, Tabak OF. Retroviral coinfections at a New Orleans HIV outpatient clinic. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;14:67–71. doi: 10.1097/00042560-199701010-00011. [DOI] [PubMed] [Google Scholar]
- 22.Harrison LH, Quinn TC, Schechter M. Human T cell lymphotropic virus type I does not increase human immunodeficiency virus viral load in vivo. J Infect Dis. 1997;175:438–440. doi: 10.1093/infdis/175.2.438. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso DF, Souza FV, Fonseca LA, Duarte AJ, Casseb J. Influence of human T-cell lymphotropic virus type 1 (HTLV-1) infection on laboratory parameters of patients with chronic hepatitis C virus. Rev Inst Med Trop Sao Paulo. 2009;51:325–329. doi: 10.1590/s0036-46652009000600003. [DOI] [PubMed] [Google Scholar]