Abstract
In this randomized, double-blinded Phase 2 trial, 30 patients with Leishmania panamensis cutaneous leishmaniasis were randomly allocated (1:1) to receive once daily topical treatment with WR 279,396 (15% paromomycin + 0.5% gentamicin) or Paromomycin Alone (15% paromomycin) for 20 days. The index lesion cure rate after 6 months follow-up was 13 of 15 (87%) for WR 279,396 and 9 of 15 (60%) for Paromomycin Alone (P = 0.099). When all treated lesions were included, the final cure rate for WR 279,398-treated patients was again 87%, but the final cure rate for Paromomycin Alone-treated patients was 8 of 15 (53.3%; P = 0.046). Both creams were well tolerated with mild application site reactions being the most frequent adverse event. The increased final cure rate in the WR 279,396 group in this small Phase 2 study suggests that the combination product may provide greater clinical benefit than paromomycin monotherapy against L. panamensis cutaneous leishmaniasis.
Introduction
Leishmania infection is endemic in 98 countries or territories, with more than 350 million people at risk. Published figures indicate an estimated incidence of 2 million new cases per year (0.5 million visceral leishmaniasis [VL] and 1.5 million of cutaneous leishmaniasis [CL]).1 In the United States (U.S.), CL has been reported in southern Texas along the Mexican border2 and in travelers returning from endemic areas.3 In the U.S. Military over 2,878 cases of CL has been parasitologically confirmed since April 2003 at the Leishmania Diagnostics Laboratory at the Walter Reed Army Institute for Research. Although CL ultimately self-cures, the infection can create substantial morbidity caused by the continued presence of a skin ulcer and the psychological impact of disfigurement4; there is no U.S. Food and Drug Administration (FDA) approved drug for CL in the United States, no available vaccines, and no chemoprophylaxis.
Several antileishmanial chemotherapeutic agents have been developed and evaluated for the treatment of VL,5 a life-threatening disease where the need for systemic therapy is compelling.6 An inherent difficulty with using anti-VL drugs for CL is that benefit may easily outweigh the toxicity of systemic agents for a fatal disease such as VL, but such toxicity may be harder to justify for a non-fatal disease such as CL.6 As a result, treatment of CL is unsatisfactory and is currently the major unmet medical need for the leishmaniases.
A new approach for CL treatment favored by the World Health Organization (WHO) and other experts is the use of a local treatment followed by parenteral treatment only if the local treatment fails or cannot be performed.7,8 This step-wise approach is intended to limit the risk of severe adverse events, increase compliance, facilitate CL treatment, and reduce cost while preserving efficacy. One prominent non-systemic treatment is the topical application of paromomycin-containing creams. To create a paromomycin cream that would be simultaneously effective, well tolerated, and improve healing, we developed a cream (WR 279,396) containing paromomycin sulfate 15% plus gentamicin sulfate 0.5% in a complex base to aid drug penetration. Gentamicin was included in the formulation because gentamicin augmented the effect of paromomycin in mouse models of CL, particularly against the New World species, Leishmania panamensis and Leishmania amazonensis.9 In a Phase 2 clinical trial in Tunisia,10 WR 279,396 was significantly more effective than vehicle control versus Leishmania major at the same study site at which a WHO topical formulation (paromomycin without gentamicin) was ineffective.11
In the course of developing WR 279,396, two studies of the pharmacokinetics (PKs) of this combination formulation and of Paromomycin Alone in the same complex base were performed in CL patients in Panama and Peru. The combined PK data from both sites is being reported in a separate publication (accepted for publication). Efficacy and safety data were secondary endpoints in these studies. Here, we report the efficacy and safety of WR 279,396 compared with Paromomycin Alone against CL in the Panama study.
Methods
Ethics.
The study was sponsored by the Office of the Surgeon General, Department of the Army, USA, and it is registered with ClinicalTrials.gov identifier NCT 01083576. The protocol was approved by the Panamanian National Committee of Bioethics for Research, Panama City, Panama, and by the Human Research Protections Office, U.S. Army Medical Research and Materiel Command, Ft. Detrick, Maryland.
The study complied with all applicable laws, rules, and regulations of the United States and Panama, and it was conducted in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines, the Belmont Principles, and the ethical principles that have their origins in the Declaration of Helsinki. The investigators adhered to the policies for protection of human subjects as prescribed in U.S. Army Regulation 70-25. Informed consent was obtained from all study participants and/or guardians before enrollment. Minors also provided assent.
Study patients.
Eligible patients were males or non-pregnant/non-lactating females; ≥ 5 years of age; with ≤ 10 lesions; and with one of these lesions (the index lesion) having the following characteristics: ulcerative, at least 1 cm, and < 5 cm in greatest diameter of lesion, including induration, and confirmed to contain Leishmania by culture or microscopic examination of lesion material. The reason to designate one lesion as the index lesion is that the response of at least that lesion would reflect the efficacy of treatment on an ulcer known to be caused by Leishmania. Subjects could not have signs of disseminated disease, against which a topical treatment would not be expected to be effective or recent treatment (within 8 weeks of starting study treatments) with a recognized antileishmanial, and were generally healthy otherwise.
Sample size.
As the study was primarily a PK evaluation that was not designed to compare the efficacy of the two topical creams, the selection of the number of subjects (15 in each arm) was based on the following objectives: 1) to obtain PK data which, when combined with PK data from a similarly designed Phase 2 study in Peru, would provide a collective body of data to determine the extent of systemic drug exposure; and, 2) to obtain sufficient data to have a preliminary estimation of the initial clinical cure rate as a basis for calculating sample sizes for a possible larger trial.
Study design.
This was a randomized, double-blinded, parallel-group study in which the PKs, efficacy, and safety of WR 279,396 and Paromomycin Alone creams were compared for the treatment of L. panamensis CL in Panama. After establishing eligibility for the study, a total of 30 patients were randomly assigned in 1:1 allocation to the two treatment groups in a blinded manner. To balance treatment assignments by age group, a permuted block randomization method was used to generate the treatment randomization within age groups. Subjects were stratified by age group: 5–11 years, 12–7 years, and ≥ 18 years of age. No more than 18 subjects could be randomized in any age range, so that there would be at least six subjects in each age stratum evaluable for the PK analysis.
Subjects were treated once daily for 20 days with the topical creams. Blood samples for PKs were collected during the first 20 days, local application site toxicity was assessed daily, and lesion sizes were measured five times during the treatment period. Subjects were followed at weekly intervals after completing treatment of safety and efficacy up to Day 63, and then had final follow-up visits at Days 100 and 168.
Study area.
This study was conducted between 16 February 2010 and 28 March 2011 on patients recruited from the area surrounding Panama City, Panama, where L. panamensis is prevalent.
Parasitology.
Lesion samples were obtained from ulcerative lesions by scraping and aspiration at baseline and 1 day after the end of therapy on Day 21. Specimens were smeared onto microscope slides, placed in culture medium, and analyzed at the Parasitology Laboratory of the Instituto Conmemorativo Gorgas de Estudios de la Salud (ICGES), Panamá City, Panamá. At the ICGES, proof of infection caused by leishmaniasis was documented through 1) the microscopic identification of Leishmania amastigotes by Giemsa staining of specimen slides; and/or 2) by the demonstration of motile Leishmania promastigotes in aspirate cultures. Whenever possible, Leishmania species identification (speciation) was also performed using polymerase chain reaction (PCR) analysis or isoenzyme analysis. For PCR, a sample of lesion scrapings from each subject was sent to the ICGES Parasitology Laboratory for analysis12,13; for isoenzyme analysis, only samples of cultures that were positive at the ICGES were sent for isoenzyme analysis14 to the Leishmania Diagnostics Laboratory, Walter Reed Army Institute of Research, Silver Spring, MD.
Drug administration.
WR 279,396 and Paromomycin Alone creams were manufactured by Teva Pharmaceuticals USA, Sellersville, PA, in accordance with Good Manufacturing Practice. For each patient, all lesions (i.e., the index lesion plus any non-index lesions) were treated topically once daily for 20 days. Before the first application, the lesions were cleaned with soap and water, debrided, and then dried. At each subsequent application of the investigational creams, the previous day's application and dressing were removed, the lesion sites were cleaned with soap, water, and sterile 0.9% saline, and then dried using sterile cotton gauze sponges, and redressed. A generous amount of cream was applied to each lesion by rubbing the cream into the lesion for about one minute before applying the dressing.
Study procedures.
Medical history, physical exam, vital signs, parasitology, and baseline clinical laboratory measurements (alanine aminotransferase, aspartate aminotransferase, glucose, sodium, potassium, creatinine, white blood cells, hemoglobin, and platelet count) were performed to establish patient eligibility for inclusion in the study. Females of childbearing potential had a pregnancy test. Serum creatinine was repeated at the end of treatment (Day 20), and parasitology was also repeated one day after completing treatment (Day 21). Adverse events were assessed at every visit during the study. Lesion measurements and vital signs were taken before treatment, five times during treatment, and at all follow-up visits. Lesions were photographed before treatment and at all follow-up visits.
Follow-up and toxicity evaluation.
Efficacy was assessed by measuring the CL lesion area (calculated according to the formula for an ellipse) at baseline, at the end of therapy on Day 20, and at follow-up on Days 28, 35, 42, 49, 56, 63, 100, and 168 (end of study). Application sites (CL lesions and surrounding area) were inspected daily during treatment of erythema and edema/swelling and any other clinical signs, and the patient was asked about pain using the Wong-Baker FACES pain rating scale.15 General questions about adverse events were also asked at each visit. Medications to treat side effects were recorded. Serum creatinine was measured at Day 20 to assess potential aminoglycoside-related renal toxicity.
Clinical endpoints criteria.
Final clinical cure was defined as (A and C) or (B and C), where A = patient had initial clinical cure (100% re-epithelialization of index lesion by nominal Day 63); B = patient had initial clinical improvement (> 50% re-epithelialization of index lesion by nominal Day 63 followed by 100% re-epithelialization of the index lesion on or before nominal Day 100; and C = patient had no relapse of index lesion. Relapse was defined as an index lesion meeting the criteria for initial clinical cure or initial clinical improvement that had any new ulceration (> 0 × 0 mm measurement) by nominal Day 168.
The protocol-specified primary efficacy endpoint was the number of subjects with an index lesion that exhibited final clinical cure. If the subject was withdrawn early from the study, this subject was then considered a treatment failure. The occurrence of new lesions was not considered a treatment failure, as the investigational drugs were administered directly to the lesion and no systemic effects were expected. Secondary endpoints included number of subjects where all baseline lesions that received treatment met the definition for final clinical cure and all lesions treated independently of the subject.
The safety endpoints were adverse events in general, application site reactions, and aminoglycoside renal toxicity determined by serum creatinine measurements at the end of therapy on Day 20.
Statistical analysis.
Statistical analyses were performed using SAS software, version 9.2 (SAS Institute, Inc., Cary, NC). The modified intention-to-treat (mITT) population, which included all randomized subjects who received at least one application of a study drug, was used for efficacy and safety analyses.
There were no formal hypotheses tested with respect to efficacy endpoints as the study was primarily a PK evaluation. It was not designed to compare the efficacy of the two topical creams, but rather to collect data to support the design of a possible Phase 3 trial. However, exploratory analyses were performed on efficacy endpoints to determine if the observed differences in the two study groups were statistically significant. The null hypothesis was that there was no difference in the primary and secondary efficacy endpoints between the 2 study groups. Final clinical cure rates were compared between the two study groups by two-sided uncorrected χ2 analysis.16 Although the sample size in this study was small (30 total subjects), as this study was “One Margin Fixed Design,” the χ2 analysis was considered more appropriate than the more conservative Fisher's exact test to explore the clinical endpoints.17 Baseline variables were explored statistically to identify differences that might have an impact on outcomes to be considered in possible future study designs.
Results
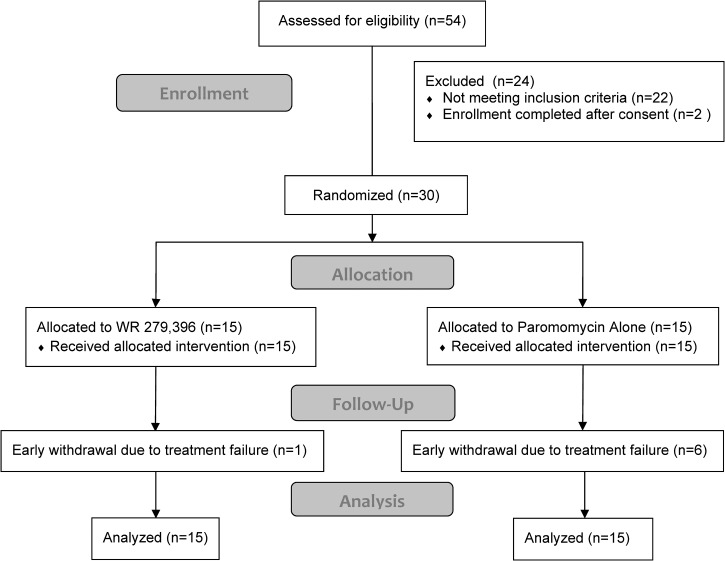
Of the 54 patients who were screened, 30 were eligible for the study and were randomized equally between the two treatment groups (Figure 1). Of the 24 subjects not eligible to participate in the study, 10 had lesions that were parasitologically negative, 5 had a single lesion that was < 1 cm, 2 had a single lesion > 5 cm, 2 had > 10 lesions, 2 had started antimonial therapy, 1 did not have an ulcerated lesion, and 2 started screening but recruitment ended. Six subjects in the Paromomycin Alone group and one subject in the WR 279,396 group were withdrawn from the study by the investigator before the final visit at Day 168 because of treatment failure. All of these subjects were included in the mITT analysis.
Figure 1.
Subject disposition. A total of 54 subjects were consented and screened of which 30 were randomized. All but one of the 30 subjects received 20 days application of study drug. One subject missed one day. Subjects who were withdrawn early from the study were withdrawn by the investigator as judged to be treatment failures: one subject in the WR 279,396 group and six subjects in the Paromomycin Alone group.
Most patients (80%) were male (Table 1). There were 17 adults, 7 patients aged 12–17 years, and 6 patients aged 5–11 years. There were a total of 64 lesions on the 30 patients, with most patients having two lesions; the index lesion and one other. Because index lesions were ulcerative by protocol, the majority of lesions were ulcerative. The mean lesion area was ∼175 mm2, although lesions ranged in area between 2 and 839 mm2. Forty-seven percent of parasites in index lesions were positive by culture and were speciated; all were L. panamensis.
Table 1.
Baseline characteristics of 30 subjects with cutaneous leishmaniasis (CL) enrolled in the study
| Characteristics | WR 279,396 (N = 15) | Paromomycin alone (N = 15) |
|---|---|---|
| Sex: N male (%) | 11 (73) | 13 (87) |
| Age: mean (SD) | 25 (16) | 24 (16) |
| Adults: N | 9 | 8 |
| Children (12–17 yr): N | 3 | 4 |
| Children (5–11 yr): N | 3 | 3 |
| Total number of lesions: N | 34 | 30 |
| Ulcerated lesions: N | 25 | 28 |
| Non-ulcerated lesions: N | 9 | 2 |
| Number of Lesions per subject: mean (SD) | 2.3 (1.7) | 2.0 (1.0) |
| Area of all lesions (mm2): mean (SD) | 149 (202) | 181 (207) |
| Area of index lesion (mm2): mean (SD) | 165 (152) | 215 (220) |
| Duration of disease before treatment (days): mean (SD) | 94 (97) | 68 (18) |
| Diagnosis of index lesion | ||
| Microscopy: N positive/N tested (%) | 14/15 (93) | 15/15 (100) |
| Culture: N positive/N tested (%) | 7/13 (54) | 12/13 (92) |
| Speciation by isoenzyme: | ||
| N positive for Leishmania panamensis/N tested (%) | 3/13 (23%) | 11/13 (85%) |
All but one subject received 20 days of daily drug application. One subject in the WR 279,396 group missed the Day 3 visit, but received the rest of the scheduled treatments.
Efficacy.
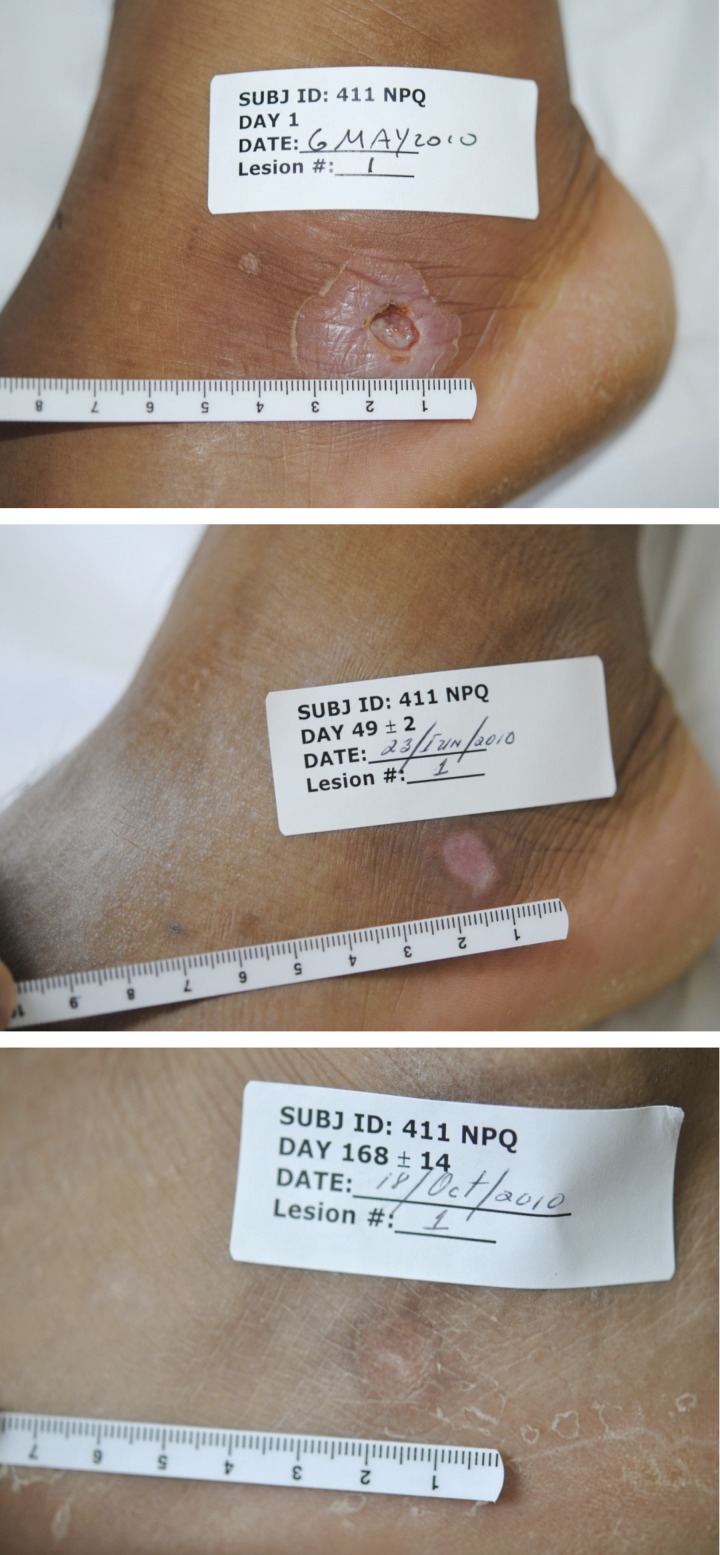
For the patients in the WR 279,396 group, the index lesion final clinical cure rate was 13 of 15 (87%) (Table 2). The all-lesions-per-patient cure rate was also 13 of 15 (87%). Because there were a total of 34 presenting lesions of which 32 cured, the per-lesion cure rate was 94%. For the two patients in the WR 279,396 group whose index lesions did not meet the protocol criteria for cure, one index lesion failed because it never re-epithelialized and one index lesion failed because of relapse. All non-index lesions on these patients also cured. The typical response of a lesion to treatment is shown in Figure 2.
Table 2.
Summary of efficacy endpoints
| Endpoint | WR 279,396 | Paromomycin alone | χ2 P value |
|---|---|---|---|
| Subjects with final clinical cure of the index lesion, N (%) | 13/15 (86.7)* | 9/15 (60.0)† | 0.099 |
| Subjects with final clinical cure of all lesions, N (%) | 13/15 (86.7) | 8/15 (53.3) | 0.046 |
| Final cure rate by–all lesions, n (%) | 32/34 (94.1) | 20/30 (66.7) | 0.005 |
One index lesion failed because it never re-epithelialized and one index lesion failed because of relapse.
Four index lesions did not meet efficacy criteria for lesion re-epithelialization at designated time points: one on Day 63, two on Day 100, and one patient was removed by the investigator on Day 35 because the lesion had doubled in size. For two other patients, the index lesion re-epithelialized on Day 63, but had evidence of infiltration at that time presumably caused by continued parasitic infection.
Figure 2.
Example of response to treatment with WR 279,396. The subject presented with a deep ulcerous lesion surrounded by a large area of induration on the foot. At 1 week after completing treatment (Day 28), the induration had resolved and the ulcer was nearly completely cured. The ulcer completely cured by Day 42 and remained cured for the duration of follow-up.
For the patients in the Paromomycin Alone group, the index lesion final clinical cure rate was 9 of 15 (60%, P = 0.099 versus the WR 279,396 group) (Table 2). The all-lesions-per-subject cure rate was 8 of 15 (53.3%, P = 0.046 versus the WR 279,396 group). Because there were a total of 30 presenting lesions of which 20 cured, the per-lesion cure rate was 66.7% (P = 0.005 versus the WR 279,396 group). For the six patients in the Paromomycin Alone group whose index lesions did not show a final clinical cure, one patient was removed by the investigator on Day 35 because the lesion had doubled in size (Table 2), one failed to show at least 50% re-epithelialization on Day 63, and two had not 100% re-epithelialized on Day 100. For the remaining two who did not cure, the index lesion re-epithelialized on Day 63, but had evidence of infiltration at that time, presumably caused by continued parasitic infection. One patient also had a non-index lesion that did not meet the protocol defined criteria for cure.
In addition, although this did not contribute to the failure rate, a new cutaneous lesion arose on two subjects (one subject in each of the two study groups). These new lesions were treated for 20 days with the same treatment to which each subject had been randomized. Both new lesions cured.
When repeat parasitological testing was performed on Study Day 21 (1 day after treatment completion), 15 of 15 (100%) of index lesions in the WR 279,396 group were negative for parasites by both smear and culture examination. In the Paromomycin Alone group, 13 of 14 (92.9%) were negative.
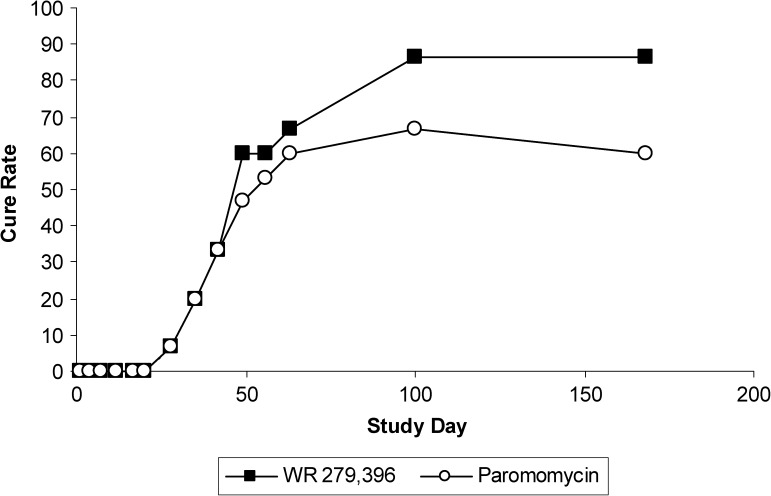
For index lesions that cured, median time to cure was almost identical in the two treatment groups: 49 days for the WR 279,396 group and 48 days for the Paromomycin Alone group (Figure 3).
Figure 3.
Mean percentage cure rate over time for the index lesion. Index lesions start to completely re-epithelialize at Day 28 and continue to heal until about Day 100 after which time, if subjects had a lesion that did not cure; the subject was taken off study and offered other treatment. At Day 49 the curves for the two treatment groups start to separate with lesions treated with WR 279,396 ultimately reaching a higher final cure rate than that for the Paromomycin Alone group.
Two adult subjects, one in each of the two treatment groups, developed erythema secondary to an erosive lesion in the nostril and turbinate during the trial; both were grade 1 (mild). In the first subject, an erosive lesion in the left nostril was observed at Study Day 62 and a second lesion was observed at Day 132. In the second subject, an erosive lesion was observed in the right inferior turbinate at Day 63. These lesions were biopsied for pathology, culture, and PCR. Although no lesion showed evidence of Leishmania parasites by microscopy or culture, lesions from both patients were positive by PCR. Both subjects were removed from the study (therefore they were considered treatment failures) and were treated with meglumine antimoniate off-study.
Safety.
For both treatment groups, essentially all patients had mild adverse events and less than half of the patients had moderate adverse events (Table 3).
Table 3.
Adverse events
| WR 279,396 (N = 15) | Paromomycin (N = 15) | |
|---|---|---|
| Summary of overall adverse events - total number (%) of subjects: | ||
| Mild | 15 (100) | 14 (93) |
| Moderate | 6 (40) | 4 (27) |
| Severe | 1 (7) | 0 (0) |
| Summary of application site reactions - total number (%) of subjects: | ||
| Application site erythema | 3 (20) | 2 (13) |
| Application site edema | 2 (13) | 3 (20) |
| Application site pain | 1 (7) | 5 (33) |
The one severe adverse event was a migraine headache in the WR 279,396 group that was not considered to be drug related. The most frequently observed treatment-related adverse events were application site reactions, with edema being reported in 13.3% of subjects in the WR 279,396 group and 20% in the Paromomycin Alone group. Erythema was reported in 20.0% of subjects in the WR 279,396 group and 13.3% in the Paromomycin Alone group. Application site pain was noted more frequently in the Paromomycin Alone group (33.3%) than in the WR 279,396 group (6.7%). Contact dermatitis reactions (caused by the gauze and tape of the dressing) occurred in roughly the same number of subjects in both groups, 6 (40.0%) for the WR 279,396 group and 8 (53.3%) for the Paromomycin Alone group.
Discussion
Two topical antileishmanial formulations, WR 279,396 (paromomycin 15% plus gentamicin 0.5%) in a complex cream base, and Paromomycin Alone (15% paromomycin in the same complex cream base, were each administered to 15 subjects with uncomplicated CL in Panama. The index lesion (the lesion confirmed to be caused by Leishmania) and all other lesions were treated once daily for 20 days. Although the primary purpose of this trial was PKs, the need to provide effective, well-tolerated treatment of New World CL makes the efficacy and safety data also of interest.
The protocol-defined primary efficacy endpoint was final clinical cure of the index lesion (at least 50% reduction in the index lesion area by Day 63, continued or complete re-epithelialization by Day 100, and no relapse by the end of the trial on Day 168). This compound definition is in accord with clinical practice. Treatment of New World CL is viewed as failing if the lesion is not appreciably smaller at early time points and not completely healed at 6 months7; the cure rates for all of the clinical endpoints were greater in the WR 279,396 group than the Paromomycin Alone group. As expected from a topical formulation, there were no systemic toxicities, and drug-related adverse events were limited to application site reactions. Hypersensitivity reactions were also observed. These were attributed to the tape that had been used in the dressings overlying treatment application sites.
Topical treatment with WR 279,396 can be compared with topical treatment with Paromomycin Alone in this study, to topical treatment with paromomycin 15% plus methylbenzethonium chloride (MBCL) 12% in the literature, and to treatment with vehicle/no treatment in the literature. In this study, WR 279,396 trended toward superiority versus Paromomycin Alone on the basis of the index lesion cure rate, and was statistically superior to Paromomycin Alone on the basis of per patient and per-lesion cure rate. Although the gentamicin component that differentiates WR 279,396 from Paromomycin Alone is not known as an antileishmanial agent, Daneshvar and others18–20 succeeded in producing attenuated Leishmania by culturing promastigotes under pressure of gentamicin. Promastigotes of the attenuated lines could enter but not survive in macrophages derived from murine bone marrow, and mice inoculated subcutaneously with attenuated parasites did not develop CL skin lesions. In lesions treated with WR 279,396, it may be that parasites' constant exposure to the gentamicin component of WR 279,396 leads to their attenuation, thereby increasing their vulnerability to both paromomycin treatment, and host immune mechanisms.
Paromomycin 15% plus MBCL 12% has not been evaluated alone against L. panamensis. However, the combination of this topical formulation with short courses of parenteral antimony was tested in an L. panamensis-endemic area of Colombia, and it did not augment the cure rate compared with short courses of antimony alone.21 In contrast, Paromomycin 15% plus MBCL 12% was tested in Guatemala (infecting species L. braziliensis and L. mexicana), where it was very effective (86% of patients were cured) compared with vehicle control (39% of patients were cured).22
The mITT and per protocol cure rates for this formal study were identical, and can be compared with the per protocol cure rate for L. panamensis treated with placebo/no-treatment in investigator-initiated studies. The WR 279,396 cure rate in this study was considerably higher than published placebo/no-treatment cure rates of 33–36% reported for L. panamensis in Colombia.23,24 The index lesion cure rate of 87% for WR 279,396 also compares favorably with cure rates after treatment with the antimonial Glucantime for L. panamensis in Colombia of 36–81% in three different studies after the same 6-month follow-up period.24–26 However, the cure rate of WR279,396 in L. panamensis observed in this study will be confirmed in a Phase 3 study (N = 300), planned to start in Panama in Spring 2013.
Topical treatments are being developed for uncomplicated CL where the less tolerable systemic antileishmanial therapies may not have an acceptable benefit-risk profile. Patients with uncomplicated CL present with diagnostically confirmed CL, but lesion location and infecting Leishmania species are such that the parasite has little or no potential to disseminate to the mucosal tissue or elsewhere. Entry criteria for patients in our studies of WR 279,396 include patients with < 10 lesions and no evidence of systemic dissemination to mucosal tissue by qualified physician examination.
Compared with systemic treatments, important advantages of all topical or local treatments for CL are 1) their failure to generate clinically meaningful systemic drug exposure, and 2) their relatively low risk for systemic side effects. Topical or local interventions may, however, generate considerable application site reactions, depending on the formulation. For example, topical paromomycin 15% formulated with MBCL 12% causes unacceptable stinging27; intralesional injections of antimony are painful28 and particularly problematic for children and patients with facial lesions; the ThermoMed device requires anesthetics to dull the blistering pain that results from intense heating of the skin29; and cryotherapy creates edema and bullae, which necessitate 1–2 weeks of saline compresses and antibiotic creams.29 In contrast, both WR 279,396 and Paromomycin Alone were formulated to promote drug penetration into the dermis while avoiding substantial local toxicity. With WR 279,396, any application site reactions are typically mild and have yet to cause a patient to withdraw from treatment.
In conclusion, new topical therapies containing paromomycin with or without gentamicin may offer an effective low toxicity alternative to the current standard of care pentavalent antimonials for the treatment of uncomplicated CL in Panama.
ACKNOWLEDGMENTS
Acknowledgments for contribution to the conduct of the study at the Gorgas Memorial Institute: Yaravi Suarez (administrative), Aracely Miranda (Dr. Calzada's assistant), Lourdes Kirshenbaum (study coordinator), Manuel Mann Yi (laboratory and pharmacokinetics), and Salomon Puga (general clinical support). Contributors from USAMMDA to the review of the manuscript include Jeanne Norwood and for the monitoring of the study are Melissa Askin, Lorena DeRienzo, and Ekaterina Anderson. Contributing to the study design and execution from USAMMDA is Carl Nielsen.
Disclaimer: The opinions or assertions contained herein are the private views of the authors, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense.
Footnotes
Financial support: This study was funded by the U.S. Army Medical Materiel Development Activity, U.S. Army Medical Research and Materiel Command.
Authors' addresses: Néstor Sosa, Zeuz Capitán, Javier Nieto, Melissa Nieto, José Calzada, and Hector Paz, Instituto Conmemorativo Gorgas de Estudios de la Salud, Panamá City, Panamá, E-mails: drnososa@gmail.com, zcapitan@gmail.com, nietdom@gmail.com, je_calzada@yahoo.com, and hpazdata@yahoo.com. Carmenza Spadafora, Instituto de Investigaciones Cientificas y Servicios de Alta Tecnologia AIP (INDICASAT AIP), City of Knowledge, Panama City, Panama. Mara Kreishman-Deitrick, Karen Kopydlowski, Diane Ullman, and William F. McCarthy, U.S. Army Medical Materiel Development Activity, Fort Detrick, MD, E-mails: mara.kreishmandeitrick.mil@mail.mil, karen.m.kopydlowski.mil@mail.mil, diane.r.ullman.civ@mail.mil, and william.f.mccarthy1.civ@mail.mil. Janet Ransom, Jonathan Berman, and Charles Scott, Fast-Track Drugs & Biologics, LLC, North Potomac, MD, E-mails: jransom@fasttrackresearch.com, jberman@fasttrackresearch.com, and cscott@fasttrackresearch.com. Max Grogl, Walter Reed Army Institute of Research, Silver Spring, MD, E-mail: max.grogl@gmail.com.
References
- 1.WHO Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March 2010. WHO Technical Report Series. 2010:949. [Google Scholar]
- 2.University of Texas Southwestern Medical Center Dermatologists Identify North Texas Leishmaniasis Outbreak. 2008. http://www.utsouthwestern.edu/utsw/cda/dept353744/files/411952.html New Release DALLAS—September 14, 2007. Available at. Accessed 15 August 2012.
- 3.Pehoushek JE, Quinn DM, Crum WP. Cutaneous leishmaniasis in soldiers returning from deployment to Iraq. J Am Acad Dermatol. 2004;51:S125–S128. doi: 10.1016/j.jaad.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Bern C, Maguire JH, Alvar J. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl Trop Dis. 2008;2:e313. doi: 10.1371/journal.pntd.0000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundar S, Jha TK, Thakur CP, Sinha PK, Bhattacharya SK. Injectable paromomycin for visceral leishmaniasis in India. N Engl J Med. 2007;356:2571–2581. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- 6.Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez U, Pinart M, Rengifo-Pardo M, Macaya A, Alvar J, Tweed JA. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004834.pub2. CD004834. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez U, Pinart M, Reveiz L, Alvar J. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD005067.pub3. CD005067. [DOI] [PubMed] [Google Scholar]
- 9.Grogl M, Schuster BG, Ellis WY, Berman JD. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (Aminosidine) and gentamicin. J Parasitol. 1999;85:354–359. [PubMed] [Google Scholar]
- 10.Ben Salah A, Buffet PA, Morizot G, Ben Massoud N, Zâatour A, Ben Alaya N, Haj Hamida NB, El Ahmadi Z, Downs MT, Smith PL, Dellagi K, Grögl M. WR279,396, a third generation aminoglycoside ointment for the treatment of Leishmania major cutaneous leishmaniasis: a phase 2, randomized, double blind, placebo controlled study. PLoS Negl Trop Dis. 2009;3:e432. doi: 10.1371/journal.pntd.0000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ben Salah A, Zakraoui H, Zaatour A, Ftaiti A, Zaafouri B, Garraoui A, Olliaro PL, Dellagi K, Ben Ismail R. A randomized, placebo-controlled trial in Tunisia treating cutaneous leishmaniasis with paromomycin ointment. Am J Trop Med Hyg. 1995;53:162–166. doi: 10.4269/ajtmh.1995.53.162. [DOI] [PubMed] [Google Scholar]
- 12.de Brujin MH, Labrada LA, Smyth AJ, Santrich C, Barker DC. A comparative study of diagnosis by the polymerase chain reaction and by current clinical methods using biopsies from Colombian patients with suspected leishmaniasis. Trop Med Parasitol. 1993;44:201–207. [PubMed] [Google Scholar]
- 13.Vergel C, Walker J, Saravia NG. Amplification of human DNA by primers targeted to Leishmania kinetoplast DNA and post-genome considerations in the detection of parasites by a polymerase chain reaction. Am J Trop Med Hyg. 2005;72:423–429. [PubMed] [Google Scholar]
- 14.Reithinger R, Dujardin JC. Molecular diagnosis of leishmaniasis: current status and future applications. J Clin Microbiol. 2007;45:21–25. doi: 10.1128/JCM.02029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garra G, Singer AJ, Taira BR, Chohan J, Cardoz H, Chisena E, Thode HC., Jr Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50–54. doi: 10.1111/j.1553-2712.2009.00620.x. [DOI] [PubMed] [Google Scholar]
- 16.Campbell I. Chi-squared and Fisher-Irwin tests of two-by-two tables with small sample recommendations. Stat Med. 2007;26:3661–3675. doi: 10.1002/sim.2832. [DOI] [PubMed] [Google Scholar]
- 17.Lydersen S, Fagerland MW, Laake P. Recommended tests for association in 2×2 tables. Stat Med. 2009;28:1159–1175. doi: 10.1002/sim.3531. [DOI] [PubMed] [Google Scholar]
- 18.Daneshvar H, Burchmore R, Hagan P, Phillips RS. Leishmania major H-line attenuated under pressure of gentamicin, induces a Th1 response which protects susceptible BALB/c mice against infection with virulent L. major. Parasitology. 2009;136:1243–1250. doi: 10.1017/S0031182009990679. [DOI] [PubMed] [Google Scholar]
- 19.Daneshvar H, Hagan P, Phillips RS. Leishmania mexicana H-line attenuated under pressure of gentamicin, potentiates a Th1 response and control of cutaneous leishmaniasis in BALB/c mice. Parasite Immunol. 2003;25:589–596. doi: 10.1111/j.0141-9838.2004.00671.x. [DOI] [PubMed] [Google Scholar]
- 20.Daneshvar H, Molaei MM, Kamiabi H, Burchmore R, Hagan P, Stephen Phillips R. Gentamicin-attenuated Leishmania infantum: cellular immunity production and protection of dogs against experimental canine leishmaniasis. Parasite Immunol. 2010;32:722–730. doi: 10.1111/j.1365-3024.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 21.Soto J, Fuya P, Herrera R, Berman J. Topical paromomycin/methylbenzethonium chloride plus parenteral meglumine antimonate as treatment for American cutaneous leishmaniasis: controlled study. Clin Infect Dis. 1998;26:56–58. doi: 10.1086/516267. [DOI] [PubMed] [Google Scholar]
- 22.Arana BA, Mendoza CE, Rizzo NR, Kroeger A. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with paromomycin plus methylbenzethonium chloride ointment in Guatemala. Am J Trop Med Hyg. 2001;65:466–470. doi: 10.4269/ajtmh.2001.65.466. [DOI] [PubMed] [Google Scholar]
- 23.Soto-Mancipe J, Grogl M, Berman JD. Evaluation of pentamidine for the treatment of cutaneous leishmaniasis in Colombia. Clin Infect Dis. 1993;16:417–425. doi: 10.1093/clind/16.3.417. [DOI] [PubMed] [Google Scholar]
- 24.Velez I, Agudelo S, Hendrickx E, Puerta J, Grogl M, Modabber F, Berman J. Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomized, controlled trial. Ann Intern Med. 1997;126:232–236. doi: 10.7326/0003-4819-126-3-199702010-00010. [DOI] [PubMed] [Google Scholar]
- 25.Martinez S, Marr JJ. Allopurinol in the treatment of American cutaneous leishmaniasis. N Engl J Med. 1992;326:741–744. doi: 10.1056/NEJM199203123261105. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Jaramillo P, Rincon MY, Garcia RG, Silva SY, Smith E, Kampeerapappun P, García C, Smith DJ, López M, Vélez ID. A controlled, randomized-blinded clinical trial to assess the efficacy of a nitric oxide releasing patch in the treatment of cutaneous leishmaniasis by Leishmania (V.) panamensis. Am J Trop Med Hyg. 2010;83:97–101. doi: 10.4269/ajtmh.2010.09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garnier T, Croft SL. Topical treatment for cutaneous leishmaniasis. Curr Opin Investig Drugs. 2002;3:538–544. [PubMed] [Google Scholar]
- 28.El-Sayed M, Anwar AE. Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. J Eur Acad Dermatol Venereol. 2009;24:335–340. doi: 10.1111/j.1468-3083.2009.03417.x. [DOI] [PubMed] [Google Scholar]
- 29.Willard RJ, Jeffcoat AM, Benson PM, Walsh DS. Cutaneous leishmaniasis in soldiers from Fort Campbell, Kentucky returning from Operation Iraqi Freedom highlights diagnostic and therapeutic options. J Am Acad Dermatol. 2005;52:977–987. doi: 10.1016/j.jaad.2005.01.109. [DOI] [PubMed] [Google Scholar]