Abstract
The aim of this study was to evaluate the accuracy of invasive and non-invasive tests for diagnosis of visceral leishmaniasis (VL) in a large series of human immunodeficiency virus (HIV)-infected patients. In this delayed-type cross-sectional study, 113 HIV-infected symptomatic patients were evaluated by an adjudication committee after clinical follow-up to establish the presence or absence of VL as the target condition (reference test). The index tests were recombinant K39 antigen-based immunochromatographic test (rK39), indirect fluorescent antibody test (IFAT), prototype kit of direct agglutination test (DAT-LPC), and real-time polymerase chain reaction (qPCR) in peripheral blood. Compared with parasitological test and adjudication committee diagnosis or latent class model analyses, IFAT and rk39 dipstick test presented the lowest sensitivity. DAT-LPC exhibited good overall performance, and there was no statistical difference between DAT-LPC and qPCR diagnosis accuracy. Real-time PCR emerges as a less invasive alternative to parasitological examination for confirmation of cases not identified by DAT.
Introduction
Concurrent visceral leishmaniasis (VL) and human immunodeficiency virus (HIV) infection have been reported in most areas of the world where the geographical distributions of the two infections overlaps. The disease is characterized by significantly lower cure rates and higher drug toxicity, relapse, and mortality rates than those rates for VL in non–HIV-infected individuals.1 The clinical diagnosis has many limitations, because features of VL can be easily mistaken for other febrile illnesses, such as tuberculosis, histoplasmose, enteric fever, and lymphoma.2,3 Cytopenia is frequent during the course of HIV infection and may result from several mechanisms.4 In addition, it is important to be alert for possible situations of coinfection where manifestations of VL are atypically present.5 Demonstration of Leishmania parasites in bone marrow aspirate or other biologic specimens, either by visualization or culture, is the most reliable diagnostic technique in the setting of HIV coinfection. However, invasive procedures require trained physicians, and microscopic examination is time-consuming. Although antileishmanial antibodies have high diagnostic value in immunocompetent patients,6 serological tests are less reliable for immunosuppressed individuals.7 There is some doubt whether one serological technique would be superior to the other for the VL diagnosis among HIV-infected patients and if there is difference in tests performance among global regions.7 Indirect fluorescent antibody test (IFAT) remains the routine serological test used by the public health services in Brazil, despite requiring fluorescence microscopes and relatively well-equipped laboratories. The direct agglutination test (DAT) offers high sensitivity and specificity and may be performed in laboratories with limited infrastructure.8–10 Similarly, the development of the rapid recombinant K39 antigen-based immunochromatographic tests (rK39) has brought a major improvement in the diagnosis of VL in non–HIV-infected patients in the field.8 Nevertheless, the paucity of data about these rapid tests in HIV-infected patients11,12 makes clear the need for more research before they are integrated in a diagnostic algorithm. In addition, in recent years, different molecular methods, particularly polymerase chain reaction (PCR), have successively been evaluated as a sensitive and specific alternative for the diagnosis of leishmaniasis,13 but the application of PCR outside Europe, in areas of high endemicity, is still poorly studied. The objective of the present study is to evaluate the diagnostic accuracy of spleen palpation and parasitological, molecular, and serological tests for the diagnosis of VL among HIV-infected symptomatic patients in a reference center in Brazil.
Patients and Methods
This study is part of a cohort involving Leishmania–HIV-coinfected patients in progress in Belo Horizonte, Minas Gerais, in a reference center in Brazil—Eduardo de Menezes Hospital, Fundação Hospitalar do Estado de Minas Gerais (HEM-FHEMIG). Minas Gerais state has a population of around 20 million people. Patients with infectious diseases from the capital Belo Horizonte and small cities of the state are referred by the Brazilian universal health system: the Sistema Único de Saúde. All patients with medical suspicion of VL were invited to participate. The study was planned as a delayed-type cross-sectional study, where clinical follow-up was used to enhance the validity of the reference standard (which we named target condition) defined by an adjudication committee. Patients were enrolled consecutively until the sample required had been reached.
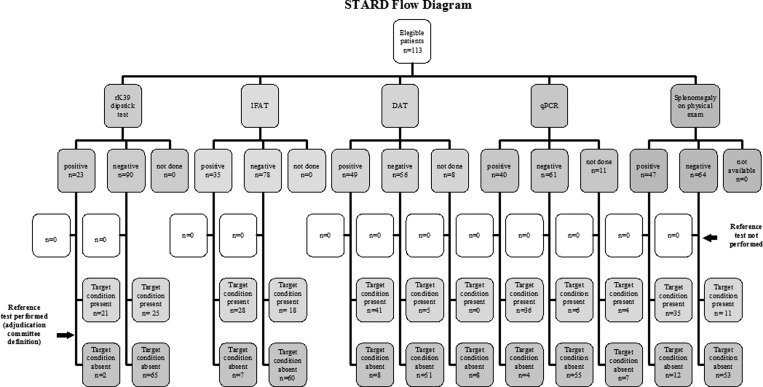
Approval for this study was obtained from the Ethical Review Boards of HEM-FHEMIG and from Centro de Pesquisas René Rachou (CPqRR), Fundação Oswaldo Cruz. Patients were included in the study only after appropriate informed consent was obtained. The flow diagram that describes the design of the study and the flow of patients according to Standards for Reporting of Diagnostic Accuracy (STARD) statement14 is presented in Figure 1.
Figure 1.
STARD flow diagram.
Clinical suspicion for VL was defined as a history of more than 14 days of fever or splenomegaly or cytopenia. Cytopenia was defined as a hemoglobin level < 11 g/dL and/or a white blood cell count < 3.5 × 109/L and/or a platelet count < 120 × 109/L. Patients with clinical suspicion were eligible to participate only if they had not received any treatment for VL. A questionnaire with clinical, epidemiologic, and demographic data was filled out for each patient at enrollment, and diagnostic procedures were performed when consented; bone marrow aspiration and a venous blood sample were taken. Serological techniques, direct smear examinations, and cultures of the bone marrow samples were carried out in separate services by different laboratory personnel who were not aware of results of the other tests. Regardless of the study or pending tests results, the decision to treat leishmaniasis was defined by hospital staff based on clinical and laboratory findings as well as the results of the parallel investigation for other diseases.
Adjudication committee definition.
Given the lack of a gold standard for the diagnosis of VL, an adjudication committee with four members (G.F.C., M.R.d.S, B.M.d.F.N., and A.R.) was formed and it decided by consensus after clinical follow-up about the presence or absence of the target condition under study (VL). This expert panel evaluated results of all tests available, including those tests performed for other diagnostic possibilities and outcomes observed at follow-up by reviewing the medical records 4 weeks after the last patient inclusion in the study. Patients were considered as having the target condition if clinical symptoms were judged to be caused by L. (L.) infantum.
Direct examination.
Six good-quality smears prepared from bone marrow aspirate and stained with Leishman stain were examined under an oil immersion light microscope for 45 min each time. Leishmania spp. bodies in the smear were confirmed independently by two experienced microscopists by detection of the standard parasite morphology: typical oval or elliptical cells bounded by a cytoplasmic membrane containing the nucleus and kinetoplast.
Leishmania culture.
The material of bone marrow aspirate (BMA) was immediately placed in culture tubes containing biphasic medium McNeal, Novy & Nicolle (NNN) and 500 μL Liver Infusion Tryptose (LIT) supplemented with 20% heat-inactivated fetal bovine serum (FBS; GIBCO/Invitrogen, Grand Island, NY) and streptomycin (50 μ/mL). The cultures were incubated at 26°C and weekly examined by microscopy (400× magnification) for the presence of promastigote of Leishmania spp. for a total of 30 days.
Real-time PCR.
Total DNA from peripheral blood of patients was extracted using the QIAamp DNA Blood Mini-Kit (Qiagen GMbH, Hilden, DE). Two independent assays for the detection and quantification of Leishmania spp. and human DNA were performed using the StepOnePlus Real-Time PCR System (Life Technologies, Carlsbad, CA). For the Leishmania assay, the target DNA was the small-subunit ribosomal RNA (SSU rRNA) gene, which is conserved among all Leishmania species. It consisted of the primers LEIS.U1 (5′-AAGTGCTTTCCCATCGCAACT-3′) and LEIS.L1 (5′-GACGCACTAAACCCCCTCCAA-3′), which were designed to amplify a 67-bp fragment and the fluorogenic probe LEIS.P1 (FAM 5′-CGGTTCGGTGTGTGGCGCC-3′TAMRA) as described by Wortmann and others.15 The protocol described by Gomes and others16 was applied. For the human assay, the ACTB reference gene was used as the target, and the primers Aco1 and Aco2,17 which generate 120-bp fragments, were used in this assay. The reaction mixtures contained 12.5 μL Syber Green PCR Master Mix 2X (Life Technologies), 0.1 μM each primer, and 3 μL DNA template in a final volume of 25 μL. The cycling parameters were universal, and the melting analysis was conducted based on the parameters of the StepOnePlus Real-Time PCR System. Standard curves were prepared for each assay using known quantities of pCR-4 TOPO vector (Life Technologies) containing the cloned human gene actin, beta (ACTB; 120 bp) and the 67-bp L. infantum SSU rRNA fragment. The recombinant plasmids were serially diluted 1:10 to create each standard curve. The quality parameters of the standard curves, including PCR efficiency, linear dynamic range, and correlation coefficient, were obtained by software analysis, and they were accurate and similar to the parameters obtained by previous studies from our group.16,18 The parasite load was expressed by the Leishmania DNA load (relative copy number of the 67-bp SSU rRNA fragment) normalized against the reference gene ACTB according to the work by Overbergh and others.19 ACTB copy numbers for the target samples were divided by the highest ACTB value obtained in the experiment, resulting in a correction factor used for normalization.
Serological tests.
The presence of L. infantum-specific antibodies was determined by three different methods: a rapid rK39 antigen-based immunochromatographic test dipstick test (Kalazar Detect), IFAT (IFI, Bio-Manguinhos), and a direct agglutination test using a prototype kit produced at Laboratório de Pesquisas Clínicas of CPqRR (DAT-LPC). The dipstick test was performed according to the manufacturer's instructions (InBios International, Inc., Seattle, WA); 20 μL serum were mixed with two drops of the buffer provided with the test and placed on a cellulose strip. Using the manufacturer's instructions, a test result was positive when two bands (a control band and a positive test band) appeared within 10 minutes. The test result was negative only if the control band appeared. The IFAT was carried out at the Parasitic Diseases Laboratory of the Fundação Ezequiel Dias using cultured promastigotes of L. major as the antigen. A cutoff value of 1:80 was used to establish a positive IFAT result. DAT-LPC was produced using freeze-dried antigen developed with L. (L.) infantum (MHOM/BR/2002/LPC-RPV) and prepared as described by Harith and others20 after the improvements recommended by Oliveira and others.21 First, the 10× concentrated physiologic solution (9% NaCl plus 1% sodium azide) was diluted 1:10 with type I water, and 5 mL were added into the antigen vial, which was carefully homogenized. Second, the 10× concentrated diluents solution (9% NaCl, 5 mM N-Acetyl Cystein [NAC], and 1% sodium azide) was diluted 1:10 with physiologic solution (0.9% NaCl). Sera were diluted in diluents solution, and a twofold dilution series was made from 1:100 to 1:102,400. Third, 50 μL DAT-LPC antigen suspension (concentration of 2 × 107 parasites/mL) were added to each well of a V-shaped microtiter plate (Greiner Bio-One, Americana, SP, Brazil) containing 50 μL diluted serum. After a minimum incubation of 4 hours at room temperature, the end titer was read as the dilution immediately before the well with a clear sharp-edged blue spot identical in size to the negative control.
Statistical analysis.
To estimate a sample size, we used the following strategy. Because IFAT is still widely used in Brazil, there is interest in its comparison with DAT. The software MedCalc version 9.4.2.022,23 calculated a sample size of 47 patients for the comparison of the areas under two receiver operating characteristic (ROC) curves (DAT and IFAT tests as continuous variable derived from the same cases). This estimation of sample size takes into account the significance level of 0.05 and power of 0.80 considering the hypothesized areas for IFAT and DAT ROC curves of 0.78 and 0.92,7 respectively. The software used requires hypothesized rank correlation coefficient in the positive group (abnormal cases) and the negative cases (normal cases). We estimated these values to be 0.46 and 0.05, respectively, a conservative estimation derived from a veterinary study.24 If we maintain all parameters and change correlation coefficients in the positive and negative groups to 0.81 and 0.05, respectively (an estimation derived from a human study),9 sample size needed would be 49 patients. Another comparison of interest is DAT versus PCR. The software calculated the required sample size of 99 patients for the comparison of the areas under DAT and PCR ROC curves22,23 derived from same cases. This estimate takes into account the significance level of 0.05 and power of 0.80 considering the hypothesize area for DAT and PCR ROC curves of 0.92 and 0.98, respectively.7 The hypothesized rank correlation coefficients in the positive and negative groups were estimated at 0.5 and 0.5, conservative estimates from Deborggraeve and others.25 Finally, a sample size of roughly 100 patients is needed for latent class analysis.26 Descriptive statistical analysis was performed in MedCalc software version 9.4.2.0. Categorical variables were analyzed by χ2 or Fisher exact tests. The distribution of continuous variables was compared by the Mann–Whitney test. Continuous variables were described by mean and SD or median with interquartile range (IR 25–75%). Analysis of variance (ANOVA) with Levene test for equality of variances was used for continuous variables with parametric distribution. Wilcoxon rank-sum test was performed for non-parametric variables. Sensitivity and specificity of index tests were estimated using two reference comparators: parasitological test and adjudication committee final diagnosis. The tests' performances were also estimated through latent class model (LCM) analyses obtained by using R Program Software. Latent class analysis is a mathematical modeling technique based on the idea that the true disease status for each patient is unknown and needs to be estimated from the data. It can be thought of as the analogue of factor analysis for categorical data. LCM attempts to model associations between observed categorical variables by assuming that a non-observed (latent) variable is determining these associations. In diagnostic test validation, the true disease status of an individual can be considered as a dichotomous latent variable with two categories: infected and not infected. Within a group of individuals with unknown disease status, for whom at least three independent diagnostic test results are available, LCM will model the probability of each combination of test results (or response patterns) conditional on the latent class. An estimate of disease prevalence, sensitivity, and specificity of all tests can be derived from the pattern of diagnostic test results as expected under the LCM.26
The sensitivity and specificity of different cutoff values of IFAT and DAT in predicting VL were determined by the construction of ROC curves. The area under curve (AUC) obtained from the ROC curve was used as a measure of global accuracy.
Results
One hundred seventy-eight patients, in which one of the differential diagnoses could be VL, were evaluated over a period of 2 years (from March of 2011 to February of 2013). Of this total, 115 patients were carriers of HIV. Two cases were excluded from the analysis: one patient did not fulfill the suspicion criteria, and another patient was excluded because of amphotericin B use before the diagnostic evaluation. The clinical characteristics of the 113 included patients are presented in Table 1; 24 patients (21.2%) had one or more VL episodes in the past, 67 patients (59%) had present or past opportunistic infection, and 70 patients (62%) were taking antiretroviral (ARV) therapy at study inclusion.
Table 1.
Demographic and clinical characteristics of 113 HIV-infected patients who underwent diagnostic testing for VL
| Variable | Patients |
|---|---|
| Sex (male:female) | 39:74 |
| Age (years); mean years + SD | 40; 42 ± 10.22 |
| Previous VL (%) | 24/113 (21.2%) |
| CD4 count median (25–75% interquartile range)* | 67 (37–164) cell/mm3 |
| PCR-HIV-1 load median (25–75% interquartile range)† | 34.176 (79–184.370) copies/mm3 |
| ARV therapy use | 70/113 (61.9%) |
| Fever more than 14 days | 81/113 (71.7%) |
| Cytopenia | 111/113 (98.2%) |
| Splenomegaly | 50/113 (44.2%) |
| Opportunist infection previously | 67/113 (59.3%) |
Available in 95 patients.
Available in 69 patients.
The diagnosis of VL was reached by parasitological confirmation in 41 of 113 patients: 38 patients (92.7%) by direct examination and 3 patients (7.3%) by culture of bone marrow specimen. Two patients did not allow the bone marrow aspiration for parasite examination. Other than these 41 patients, 6 patients were treated for leishmaniasis, including 2 patients who had not undergone the sample aspiration of bone marrow (totaling 47 patients treated for VL by the hospital staff); 5 of 6 patients were also considered by the adjudication committee as true VL cases (46 of 113 VL-suspected patients) on the basis of the overall case clinical picture, available tests, therapeutic response, and clinical follow-up data (details about these patients, including clinical picture, outcomes, and test results, are shown in Supplemental Table 1). By having the adjudication committee diagnosis as the reference test, the sensitivity and specificity of parasitological exam were 93.2% (95% confidence interval [95% CI] = 81.3–98.5) and 100% (95% CI = 94.6–100), respectively.
There were 72 VL-suspected patients with a negative parasitology, and of these 72 patients, 59 patients (82%) had at least one clinical assessment after 4 weeks of the study enrollment. The median follow-up time by reviewing the medical records was 38 weeks, and it ranged from 7 to 94 weeks. Through clinical follow-up, an alternative diagnosis (not VL) was confirmed in 19 patients (26.4%) by a histological, microbiological, or laboratorial exam. For 35 patients (48.6%), the alternative diagnosis was deemed most likely on clinical grounds. Nine cases (12.5%) remained without a conclusive diagnosis. The adjudication committee examined all parasitological negative cases, and the target condition (VL) was considered present in 5 of 72 patients.
The characteristics of VL patients with and without positive parasitological examination were compared, and no statistical differences in clinical and laboratory variables were found (data not shown). The clinical and laboratory characteristics of VL and non-VL cases according to adjudication committee definition are shown in Table 2. The most important clinical differences observed between the two groups were a higher splenomegaly rate (76% × 22%) and a lower leukocyte count median in the VL group. The 30-day mortality also differed between patients with and without VL diagnosis, and it was significantly higher in non-VL patients. It is probably related to the most frequent diagnosis in the non-VL group: disseminated tuberculosis, a very common condition reported in studies evaluating hospitalized HIV-infected patients and a disease with high mortality rate.27,28 The quantification of the parasite load by quantitative PCR (qPCR) exhibited great variability in each group, and there was no statistical difference between VL and non-VL patients. It should be emphasized that these results were obtained from only four qPCR-positive patients in the control group, which prevents the extrapolation of these findings.
Table 2.
Clinical and laboratory characteristics of patients with target condition (VL) present or absent according to adjudication committee definition
| Target condition present (N = 46) | Target condition absent (N = 67) | P value | |
|---|---|---|---|
| Age (years; mean ± SD) | 41.02 ± 10.8 | 40.0 ± 9.8 | 0.60 |
| Sex (male:female) | 11:35 | 28:39 | 0.07 |
| Anti-HVC presence | 2 (4.3%) | 6 (9.5%) | 0.44 |
| HbsAg presence | 0 | 2 (3.2%) | 0.31 |
| Illicit drug use | 16 (34.8%) | 27 (40.3%) | 0.58 |
| Alcohol abuse | 31 (67.4%) | 40 (59.7%) | 0.39 |
| Previous VL diagnosis | 20 (43.4%) | 4 (5.9%) | 0.00 |
| Previous opportunist infection | 31 (67.4%) | 36 (53.7%) | 0.17 |
| Previous schistosomiasis diagnosis | 3 (6.5%) | 7 (10.4%) | 0.05 |
| Comorbidities | 13 (28.3%) | 16 (23.8%) | 0.64 |
| CD4 + T cells count (25–75% IR) | 67 (6–864)* | 92 (5–483)† | 0.18 |
| HIV-PCR load (copies/mm3; 25–75% IR) | 5,000 (0–65,712)‡ | 78,132 (7,412 –316,650)§ | 0.01 |
| Fever | 28 (60.8%) | 53 (79.1%) | 0.06 |
| Cytopenia | 46 (100%) | 65 (97.0%) | 0.51 |
| Splenomegaly on physical exam | 35 (76%) | 15 (22.4%) | 0.00 |
| Death in 30 days | 4 (8.7%) | 8 (11.9%) | 0.02 |
| ARV therapy use | 32 (69.6%) | 34 (50.7) | 0.15 |
| ARV therapy regular use | 15/32 (46.8%) | 7/34 (20.6) | 0.01 |
| Hemoglobin (g/dL) | 8.2 ± 1.6 | 9.1 ± 2.4 | 0.04 |
| Leukocytes count (cell/L; 25–75% IR) | 2.0 (1.75–2.80) × 109 | 3.3 (1.75–4.20) × 109 | 0.005 |
| Platelets count (cell/L; 25–75% IR) | 114 (82.75–173.75) × 109 | 139 (90.50–244.50) × 109 | 0.233 |
| rK39 dipstick test positivity | 21/46 (45.6%) | 2/67 (3.0%) | 0.00 |
| IFAT positivity | 28/46 (60.9%) | 7/67 (10.4%) | 0.00 |
| DAT-LPC positivity | 41/46 (89%) | 8/59 (13.6%) | 0.00 |
| Leishmania qPCR in peripheral blood positivity | 36/42 (85.7%) | 4/60 (6.7%) | 0.00 |
| Median copies Leishmania qPCR in blood (25–75% IR) | 16,543 (108–70,209)¶ | 66,436 (5,066–36,479,000)∥ | 0.56 |
Anti-HVC = antibody to the hepatitis C virus; HbsAg = hepatitis B surface antigen.
Performed in 43 patients.
Performed in 52 patients.
Performed in 29 patients.
Performed in 40 patients.
Available in 35 patients.
Available in four patients.
Sensitivity, specificity, and 95% CIs of index tests were calculated using conventional formulas and the target condition or parasitological results as reference test or by LCM (Table 3). In all three analyses, rK39 dipstick test showed the lowest sensitivity and highest specificity. There was no statistical difference among performance presented by DAT-LPC and qPCR. The IFAT exhibited a poor performance, which was shown by sensitivity below 65% independent of reference test used.
Table 3.
Sensitivity, specificity, and 95% confidence interval presented by index tests using two reference tests and LCM analysis
| Index test | Parasitological test as reference test | Adjudication committee diagnosis as reference test | LCM analyses | |||
|---|---|---|---|---|---|---|
| Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | |
| rK39 dipstick test (Kalazar Detect) | 46.6 (30.7–62.6) | 97.1 (90.0–99.6) | 45.6 (30.9–61.0) | 97.0 (89.6–99.5) | 46.2 (31.3–61.7) | 98.4 (89.3–99.8) |
| IFAT | 61.0 (44.5–75.8) | 87.1 (77.0–94.0) | 60.9 (45.4–74.9) | 89.5 (79.6–95.7) | 61.5 (45.6–75.3) | 88.5 (77.8–94.4) |
| DAT-LPC | 87.8 (73.8–95.9) | 82.3 (70.5–90.8) | 89.1 (76.4–96.3) | 86.4 (75.0–93.9) | 89.7 (75.7–96.1) | 85.3 (74.0–92.1) |
| qPCR in peripheral blood | 87.2 (72.6–95.7) | 93.4 (84.0–98.1) | 85.7 (71.0–94.5) | 94.9 (85.8–98.9) | 84.6 (69.7–92.9) | 91.8 (81.8–96.6) |
| Splenomegaly on physical exam | 75.6 (59.7–87.6) | 79.4 (67.4–88.3) | 76.1 (61.2–87.4) | 81.5 (70.0–90.1) | 79.5 (64.0–89.4) | 80.3 (68.5–88.5) |
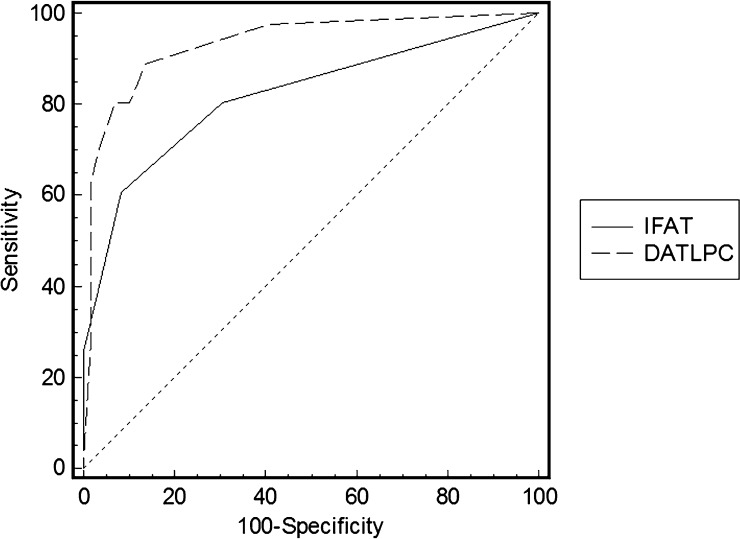
Figure 2 presents the performance of the DAT-LPC and IFAT in diagnosis of VL in HIV-infected patients using the adjudication committee definition as reference test. The accuracy of DAT-LPC based on the area under the ROC curve was 0.94 (SE = 0.026, 95% CI = 0.87–0.97, P = 0.0001), which means good overall performance. In turn, performance exhibited by IFAT was significantly lower (P = 0.006), with global measure of accuracy by AUC of 0.82 (SE = 0.043, 95% CI = 0.73–0.89, P = 0.001).
Figure 2.
Performance of the DAT-LPC and IFAT in diagnosis of VL in HIV-infected patients.
Discussion
To our knowledge, no study so far in the Americas has addressed VL diagnostics by a comparative assessment of serological and molecular tests in HIV-infected patients. Strengths of this study are that it includes symptomatic patients living in an endemic area and uses delayed-type cross-sectional design.
The population studied here presents an unexpected predominance of women, which is different from other published series. We have no clear explanation for this result. Although both acquired immunodeficiency syndrome (AIDS) and VL predominantly affect men, it has recently changed. The spread of AIDS among women has been observed in almost all continents, and women account for 48% of cases worldwide.29 In Brazil, the sex ratio clearly shows this trend: in 1986, there was a 15:1 male to female case ratio, and in 2009, this ratio was 1.6:1.30 Also, the recent urban behavior of VL may have contributed, because the predominance of men over women in exposure to Leishmania spp. caused by the labor activity in the field no longer exists. Regarding the clinical aspects, the high prevalence of palpable spleen and severe leukopenia among true VL cases are the most significant aspects that may increase clinical suspicion of VL.
A widely documented problem hampering diagnosis research in VL is the absence of a true gold standard to classify with certainty patients who have the target condition. Thus, the published sensitivity and specificity estimates of tests might be biased to some extent depending on the controls and reference test used. Microscopic examination and cultures of bone marrow, lymph node, and spleen aspiration are the conventional diagnostic procedures. Parasitology for VL is very specific, but unless spleen aspirates can be taken, its sensitivity is less than 90%.31 When a reference test with suboptimal sensitivity for case ascertainment is used, true VL cases are missed and therefore, included in the group of controls. They will generate a false-positive result in any new test that one wishes to evaluate (assuming that this new test is 100% sensitive). For those cases, the new test is actually right, whereas the reference test is wrong, and the specificity of the new test will, thus, be systematically underestimated.32 For these problems, the term reference standard has been introduced. This term acknowledges the absence of a gold standard and refers to the best available method for classifying patients as having the target condition. Consensus diagnosis definition by an adjudication committee using follow-up information is an attractive alternative if a generally accepted reference standard does not exist and multiple sources of information have to be interpreted in a judicious way to reach a diagnosis.33 Another strategy used to deal with the lack of a perfect gold standard was LCM analysis.
Parasitological test proved to be the most sensitive test for diagnosing VL in HIV-infected patients in our series. The sensitivity of direct examination performed on slides from bone marrow aspirate in our study was higher than the 70% reported in immunocompetent patients.31 This observation can strengthen the hypothesis of higher Leishmania spp. parasitemia in HIV-infected patients, which has already been suggested by other authors.13,34 Although this fact legitimizes the test as a good diagnostic strategy, obtaining the material requires an invasive and painful technique. Furthermore, several factors interfere with the positivity rates of the method. Bone marrow aspiration should be carried out by trained individuals, and performance of a correct technical procedure is vital to its sensitivity. Finally, the expertise and persistence of the microscopist are also factors of utmost importance influencing the final performance of the test.
Serologic tests have a high diagnostic value for VL diagnosis in immunocompetent patients,35,36 but their value is limited in HIV-infected patients.7 Our serology results confirm the low sensitivity of all tests, except DAT-LPC, for VL diagnosis. Although rK39 dipstick has an ideal format for use in the field, because it is a rapid and simple test not requiring extensive training of the operator, its lower sensitivity limits its use for VL diagnosis among HIV-infected patients. Unsatisfactory results also have been observed in Africa,8,37 and one possible explanation would be the high prevalence of HIV coinfection, which is often unrecognized.
DAT is a well-validated test for serodiagnosis of VL, and it has been used for the last two decades in immunocompetent individuals, combining high levels of intrinsic validity with ease of use.8,38 In the present study, DAT-LPC was highly sensitive and found to be a suitable alternative to screening VL in HIV-infected patients. Of 46 patients considered VL cases by adjudication committee, 41 patients were identified by DAT-LPC (89%). Of five unidentified VL cases, the diagnosis could be done by both parasitological examination and blood qPCR Leishmania in four cases. The ability to make most VL diagnoses without the need for specialized equipment or invasive procedures makes this test a major advance for peripheral healthcare facilities. Eight patients had a positive DAT-LPC and were considered non-VL cases by adjudication committee. In two patient, qPCR Leishmania was also positive in peripheral blood, and one patient had presented VL years before and was under prophylactic use of amphotericin B. Despite the fact that the target condition was also considered absent in this case, the patient had chronic abnormalities, such as thrombocytopenia and splenomegaly, which are consistent with a recently recognized form of VL among HIV-infected patients: a chronic active form.39 These observations suggest that some patients with considered false-positive results of DAT-LPC were truly infected with Leishmania spp., although Leishmania was not the etiologic agent of the disease under investigation. A fourth patient was empirically treated for VL and disseminated mycobacteriosis with no therapeutic response. This patient had very advanced immunosuppression. He discontinued the use of all medications and died in a few months without confirmed etiological diagnosis. As suggested by others,36 the specificity of the DAT, like any other diagnostic test, depends to a certain extent on the choice of the control group. When control groups are composed of non-symptomatic people or patients with other confirmed diseases, the specificity of the test tends to be higher than when only clinical suspects are studied, which is the case here.
Molecular diagnosis exploiting PCR combines several advantages; it is minimally invasive, has a high sensitivity and specificity, and is capable of identifying relapses and reinfections in treated VL patients. The sensitivity exhibited by qPCR (based on SSUrRNA target and performed on blood samples) not overcame, but it was as good as the parasitological test. This finding is beneficial, because the use of a test with blood means that invasive diagnostic procedures can be avoided. Previous studies with HIV-infected patients have shown that the sensitivity of PCR for the diagnosis of VL ranges from 76% to 100%.13,34,39–42 However, two points need to be noted. First, PCR is not one technique but a method encompassing a number of techniques depending on a variety of factors. Therefore, a direct comparison between results from studies using different PCR should not be made. The choice of different PCR targets should be guided by the aim to which it is directed. In fact, the PCR assay based on kinetoplast amplification is probably the most sensitive, because this molecular target is present in about 10,000 copies per parasite.43 However, the heterogeneity of kinetoplast minicircles can be a problem for accurate quantification.13 Moreover, the high sensitivity of the kinetoplast target might be a double-edged sword when used as a tool to verify the therapeutic response. For that reason, we chose the SSU rRNA gene of the parasite as the target sequence. The goal of our group was to evaluate a test that could be applied in not only diagnosis but also, monitoring of HIV-infected patients after VL treatment (there is a cohort in progress). Second, another point to note is that the estimates of sensitivity and specificity should be carefully interpreted according to sample selection criterion. Studies using sera from healthy controls in non-endemic areas or patients with other confirmed infectious diseases might overestimate the specificity of the test. In our series, 6% qPCR positivity (4 of 67 patients) in the control group was observed. Two of the patients were treated for other conditions (one patient had pulmonary tuberculosis, and one patient had secondary syphilis associated with vitamin B12 deficiency) and evolved with complete remission of fever, splenomegaly, and cytopenia. The third patient had VL years before. After the initial study evaluation, he had complete improvement of cytopenia with the reintroduction of the ARV therapy that he had interrupted. The favorable evolution without specific treatment of VL suggests that these three patients were asymptomatic carriers of Leishmania spp. The fourth patient had disseminated cryptococcosis and died in few days because of neurological complications. In this case, it was not possible to undoubtedly attest that Leishmania spp. infection was not liable for some of his symptoms.
In conclusion, our results show that, in an urban setting in Brazil, unlike other serological techniques, DAT is a suitable tool for the VL diagnosis among HIV-infected patients. DAT is a simple, inexpensive technique with reasonable specificity and sensitivity. It uses very little serum, can also use plasma, and is performed at room temperature. Nevertheless, the laboratory infrastructure needed to carry out DAT is much simpler than IFAT, because no sophisticated equipment is required. We reiterate that DAT rather than rk39 strip test or IFAT can be routinely applied for VL diagnosis in HIV-infected patients.
Despite high sensitivity and specificity of the qPCR based on SSU rRNA target, qPCR diagnosis may have limitations in VL diagnosis. Real-time PCR diagnosis alone cannot differentiate between asymptomatic and symptomatic VL infections. Thus, this method would be used only for the confirmation of suspected cases of active disease and exclude other diagnoses.
No currently available method for VL diagnosis exhibits all the desirable characteristics of high sensitivity and specificity in addition to ease of use and low cost. This finding emphasizes the importance of associating serology, parasitological, and molecular methods to reach higher positivity rates in diagnosis. Using DAT as a screening test followed by parasitological tests only in DAT-negative cases, confirmation of VL might be possible in nearly 100% of patients, and invasive tests will be done in no more than 11% of the patients. Molecular tests, including tests based on SSU rRNA target and performed in peripheral blood, represent a non-invasive alternative for VL confirmation in settings with adequate laboratory infrastructure.
Supplementary Material
ACKNOWLEDGMENTS
This study was undertaken in healthcare facilities of Eduardo de Menezes Hospital, and the authors thank all staff for their support and assistance. The Ethical Review Boards of Centro de Pesquisas René Rachou (CPqRR-FIOCRUZ) and Hospital Eduardo de Menezes (HEM-FHEMIG), Belo Horizonte, Minas Gerais, Brazil, approved the study in agreement with Resolution 357/05 of the National Health Council of the Ministry of Health, which regulates research involving human subjects in Brazil. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Belo Horizonte, Minas Gerais, Brazil (Grant APQ-01562-11 to A.R.); FAPEMIG also provided scientific initiation scholarship for the undergraduate students A.L.P.d.M. and B.F.P. Financial support was also available from PDTIS-FIOCRUZ (Rede de Plataformas Tecnológicas do Programa de Desenvolvimento Tecnológico em Insumos para Saúde) [RID06] and National Counsel of Technological and Scientific Development (CNPq) Grant 311641/2009-1. A.R. is a research fellow of CNPq (304881/2009-0).
Authors' addresses: Gláucia Fernandes Cota, Eduardo de Menezes Hospital, Fundação Hospitalar do Estado de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, and Laboratório de Pesquisas Clínicas, Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz, Belo Horizonte, Minas Gerais, Brazil, E-mail: glauciacota@uol.com.br. Marcos Roberto de Sousa, Post Graduation Program in Adult Health Sciences, Universidade Federal de Minas Gerai, Belo Horizonte, Minas Gerais, Brazil, E-mail: sousa.mr@uol.com.br. Betânia Mara de Freitas Nogueira and Andréa Laender Pessoa de Mendonça, Eduardo de Menezes Hospital, Fundação Hospitalar do Estado de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil, E-mails: betaniamfn@hotmail.com and andrea.laender@yahoo.com.br. Luciana Inácia Gomes, Edward Oliveira, Tália Santana Machado Assis, Bruna Fernandes Pinto, Juliana Wilke Saliba, and Ana Rabello, Laboratório de Pesquisas Clínicas, Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz, Belo Horizonte, Minas Gerais, Brazil, E-mails: lgomes@cpqrr.fiocruz.br, edwardjo@cpqrr.fiocruz.br, talia@cpqrr.fiocruz.br, bruna.fernandes@cpqrr.fiocruz.br, julianawilke@yahoo.com.br, and ana@cpqrr.fiocruz.br.
References
- 1.Alvar J, Aparicio P, Aseffa A, Den Boer M, Canavate C, Dedet JP, Gradoni L, Ter Horst R, Lopez-Velez R, Moreno J. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev. 2008;21:334–359. doi: 10.1128/CMR.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang MR, Taira CL, Paniago AM, Taira DL, Cunha RV, Wanke B. Study of 30 cases of histoplasmosis observed in the Mato Grosso do Sul State, Brazil. Rev Inst Med Trop Sao Paulo. 2007;49:37–39. doi: 10.1590/s0036-46652007000100007. [DOI] [PubMed] [Google Scholar]
- 3.Hot A, Schmulewitz L, Viard JP, Lortholary O. Fever of unknown origin in HIV/AIDS patients. Infect Dis Clin North Am. 2007;21:1013–1032. doi: 10.1016/j.idc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Dikshit B, Wanchu A, Sachdeva RK, Sharma A, Das R. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disord. 2009;9:5. doi: 10.1186/1471-2326-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo R, Laguna F, Lopez-Velez R, Medrano FJ, Rosenthal E, Cacopardo B, Nigro L. Visceral leishmaniasis in those infected with HIV: clinical aspects and other opportunistic infections. Ann Trop Med Parasitol. 2003;97((Suppl 1)):99–105. doi: 10.1179/000349803225002570. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava P, Dayama A, Mehrotra S, Sundar S. Diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2011;105:1–6. doi: 10.1016/j.trstmh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cota GF, de Sousa MR, Demarqui FN, Rabello A. The diagnostic accuracy of serologic and molecular methods for detecting visceral leishmaniasis in HIV infected patients: meta-analysis. PLoS Negl Trop Dis. 2012;6:e1665. doi: 10.1371/journal.pntd.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chappuis F, Rijal S, Soto A, Menten J, Boelaert M. A meta-analysis of the diagnostic performance of the direct agglutination test and rK39 dipstick for visceral leishmaniasis. BMJ. 2006;333:723. doi: 10.1136/bmj.38917.503056.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedras MJ, de Gouvea Viana L, de Oliveira EJ, Rabello A. Comparative evaluation of direct agglutination test, rK39 and soluble antigen ELISA and IFAT for the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2008;102:172–178. doi: 10.1016/j.trstmh.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Schallig HD, Canto-Cavalheiro M, da Silva ES. Evaluation of the direct agglutination test and the rK39 dipstick test for the sero-diagnosis of visceral leishmaniasis. Mem Inst Oswaldo Cruz. 2002;97:1015–1018. doi: 10.1590/s0074-02762002000700015. [DOI] [PubMed] [Google Scholar]
- 11.ter Horst R, Tefera T, Assefa G, Ebrahim AZ, Davidson RN, Ritmeijer K. Field evaluation of rK39 test and direct agglutination test for diagnosis of visceral leishmaniasis in a population with high prevalence of human immunodeficiency virus in Ethiopia. Am J Trop Med Hyg. 2009;80:929–934. [PubMed] [Google Scholar]
- 12.Goswami RP, Rahman M, Guha SK. Utility of K39 strip test in visceral leishmaniasis (VL) and HIV co-infected patients: an early report from eastern India. J Assoc Physicians India. 2007;55:154–155. [PubMed] [Google Scholar]
- 13.Antinori S, Calattini S, Longhi E, Bestetti G, Piolini R, Magni C, Orlando G, Gramiccia M, Acquaviva V, Foschi A, Corvasce S, Colomba C, Titone L, Parravicini C, Cascio A, Corbellino M. Clinical use of polymerase chain reaction performed on peripheral blood and bone marrow samples for the diagnosis and monitoring of visceral leishmaniasis in HIV-infected and HIV-uninfected patients: a single-center, 8-year experience in Italy and review of the literature. Clin Infect Dis. 2007;44:1602–1610. doi: 10.1086/518167. [DOI] [PubMed] [Google Scholar]
- 14.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. The Standards for Reporting of Diagnostic Accuracy Group. Croat Med J. 2003;44:639–650. [PubMed] [Google Scholar]
- 15.Wortmann G, Sweeney C, Houng HS, Aronson N, Stiteler J, Jackson J, Ockenhouse C. Rapid diagnosis of leishmaniasis by fluorogenic polymerase chain reaction. Am J Trop Med Hyg. 2001;65:583–587. doi: 10.4269/ajtmh.2001.65.583. [DOI] [PubMed] [Google Scholar]
- 16.Gomes LI, Gonzaga FM, de Morais-Teixeira E, de Souza-Lima BS, Freire VV, Rabello A. Validation of quantitative real-time PCR for the in vitro assessment of antileishmanial drug activity. Exp Parasitol. 2012;131:175–179. doi: 10.1016/j.exppara.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Musso O, Sommer P, Drouet E, Cotte L, Neyra M, Grimaud JA, Chevallier M. In situ detection of human cytomegalovirus DNA in gastrointestinal biopsies from AIDS patients by means of various PCR-derived methods. J Virol Methods. 1996;56:125–137. doi: 10.1016/0166-0934(95)01892-1. [DOI] [PubMed] [Google Scholar]
- 18.dos Santos Marques LH, Gomes LI, da Rocha IC, da Silva TA, Oliveira E, Morais MH, Rabello A, Carneiro M. Low parasite load estimated by qPCR in a cohort of children living in urban area endemic for visceral leishmaniasis in Brazil. PLoS Negl Trop Dis. 2012;6:e1955. doi: 10.1371/journal.pntd.0001955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 20.Harith AE, Kolk AH, Kager PA, Leeuwenburg J, Muigai R, Kiugu S, Kiugu S, Laarman JJ. A simple and economical direct agglutination test for serodiagnosis and sero-epidemiological studies of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1986;80:583–586. doi: 10.1016/0035-9203(86)90149-5. [DOI] [PubMed] [Google Scholar]
- 21.Oliveira E, Saliba SW, Saliba JW, Rabello A. Validation of a direct agglutination test prototype kit for the diagnosis of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 2013;107:243–247. doi: 10.1093/trstmh/trt004. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 23.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 24.Vercammen F, Berkvens D, Le Ray D, Jacquet D, Vervoort T. Development of a slide ELISA for canine leishmaniasis and comparison with four serological tests. Vet Rec. 1997;141:328–330. doi: 10.1136/vr.141.13.328. [DOI] [PubMed] [Google Scholar]
- 25.Deborggraeve S, Boelaert M, Rijal S, De Doncker S, Dujardin JC, Herdewijn P, Buscher P. Diagnostic accuracy of a new Leishmania PCR for clinical visceral leishmaniasis in Nepal and its role in diagnosis of disease. Trop Med Int Health. 2008;13:1378–1383. doi: 10.1111/j.1365-3156.2008.02154.x. [DOI] [PubMed] [Google Scholar]
- 26.Boelaert M, Aoun K, Liinev J, Goetghebeur E, Van der Stuyft P. The potential of latent class analysis in diagnostic test validation for canine Leishmania infantum infection. Epidemiol Infect. 1999;123:499–506. doi: 10.1017/s0950268899003040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogoina D, Obiako RO, Muktar HM, Adeiza M, Babadoko A, Hassan A, Bansi I, Iheonye H, Iyanda M, Tabi-Ajayi E. Morbidity and mortality patterns of hospitalised adult HIV/AIDS patients in the era of highly active antiretroviral therapy: a 4-year retrospective review from Zaria, northern Nigeria. AIDS Res Treat. 2012;2012(940580) doi: 10.1155/2012/940580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lins TB, Soares Ede M, dos Santos FM, Mandacaru PM, Pina T, de Araujo Filho JA. Mycobacterium tuberculosis and human immunodeficiency virus coinfection in a tertiary care hospital in Midwestern Brazil. Infez Med. 2012;20:108–116. [PubMed] [Google Scholar]
- 29.World Health Organization . Global Health Sector Strategy on HIV/AIDS 2011–2015. Geneva: World Health Organization; 2011. http://whqlibdoc.who.int/publications/2011/9789241501651_eng.pdf Available at. Accessed February 20, 2013. [Google Scholar]
- 30.Brasil, Ministério da Saúde Departamento de DST, Aids e Hepatites Virais. AIDS/DST. Boletim Epidemiológico. 2011;8:1–160. http://www.aids.gov.br/sites/default/files/anexos/publicacao/2011/50652/boletim_aids_2011_final_m_pdf_26659.pdf Available at. Accessed February 20, 2013. [Google Scholar]
- 31.Zijlstra EE, Ali MS, el-Hassan AM, el-Toum IA, Satti M, Ghalib HW, Kager PA. Kala-azar: a comparative study of parasitological methods and the direct agglutination test in diagnosis. Trans R Soc Trop Med Hyg. 1992;86:505–507. doi: 10.1016/0035-9203(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 32.Boelaert M, Rijal S, Regmi S, Singh R, Karki B, Jacquet D, Chappuis F, Campino L, Desjeux P, Le Ray D, Koirala S, Van der Stuyft P. A comparative study of the effectiveness of diagnostic tests for visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:72–77. [PubMed] [Google Scholar]
- 33.Rutjes AW, Reitsma JB, Coomarasamy A, Khan KS, Bossuyt PM. Evaluation of diagnostic tests when there is no gold standard. A review of methods. Health Technol Assess. 2007;11:iii–51. doi: 10.3310/hta11500. [DOI] [PubMed] [Google Scholar]
- 34.Bossolasco S, Gaiera G, Olchini D, Gulletta M, Martello L, Bestetti A, Bossi L, Germagnoli L, Lazzarin A, Uberti-Foppa C, Cinque P. Real-time PCR assay for clinical management of human immunodeficiency virus-infected patients with visceral leishmaniasis. J Clin Microbiol. 2003;41:5080–5084. doi: 10.1128/JCM.41.11.5080-5084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. WHO Technical Report Series No. 949. Geneva: World Health Organization; 2010. [Google Scholar]
- 36.Chappuis F, Rijal S, Singh R, Acharya P, Karki BM, Das ML, Bovier PA, Desjeux P, Le Ray D, Koirala S, Loutan L. Prospective evaluation and comparison of the direct agglutination test and an rK39-antigen-based dipstick test for the diagnosis of suspected kala-azar in Nepal. Trop Med Int Health. 2003;8:277–285. doi: 10.1046/j.1365-3156.2003.01026.x. [DOI] [PubMed] [Google Scholar]
- 37.Boelaert M, El-Safi S, Hailu A, Mukhtar M, Rijal S, Sundar S, Wasunna M, Aseffa A, Mbui J, Menten J, Desjeux P, Peeling RW. Diagnostic tests for kala-azar: a multi-centre study of the freeze-dried DAT, rK39 strip test and KAtex in East Africa and the Indian subcontinent. Trans R Soc Trop Med Hyg. 2008;102:32–40. doi: 10.1016/j.trstmh.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Ritmeijer K, Melaku Y, Mueller M, Kipngetich S, O'Keeffe C, Davidson RN. Evaluation of a new recombinant K39 rapid diagnostic test for Sudanese visceral leishmaniasis. Am J Trop Med Hyg. 2006;74:76–80. [PubMed] [Google Scholar]
- 39.Bourgeois N, Lachaud L, Reynes J, Rouanet I, Mahamat A, Bastien P. Long-term monitoring of visceral leishmaniasis in patients with AIDS: relapse risk factors, value of polymerase chain reaction, and potential impact on secondary prophylaxis. J Acquir Immune Defic Syndr. 2008;48:13–19. doi: 10.1097/QAI.0b013e318166af5d. [DOI] [PubMed] [Google Scholar]
- 40.Cruz I, Canavate C, Rubio JM, Morales MA, Chicharro C, Laguna F, Jimenez-Mejias M, Sirera G, Videla S, Alvar J. A nested polymerase chain reaction (Ln-PCR) for diagnosing and monitoring Leishmania infantum infection in patients co-infected with human immunodeficiency virus. Trans R Soc Trop Med Hyg. 2002;96((Suppl 1)):S185–S189. doi: 10.1016/s0035-9203(02)90074-x. [DOI] [PubMed] [Google Scholar]
- 41.Piarroux R, Gambarelli F, Toga B, Dumon H, Fontes M, Dunan S, Quilici M. Interest and reliability of a polymerase chain reaction on bone-marrow samples in the diagnosis of visceral leishmaniasis in AIDS. AIDS. 1996;10:452–453. doi: 10.1097/00002030-199604000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Campino L, Cortes S, Pires R, Oskam L, Abranches P. Detection of Leishmania in immunocompromised patients using peripheral blood spots on filter paper and the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2000;19:396–398. doi: 10.1007/s100960050503. [DOI] [PubMed] [Google Scholar]
- 43.Nuzum E, White F, 3rd, Thakur C, Dietze R, Wages J, Grogl M, Berman J. Diagnosis of symptomatic visceral leishmaniasis by use of the polymerase chain reaction on patient blood. J Infect Dis. 1995;171:751–754. doi: 10.1093/infdis/171.3.751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.