Abstract
Lymphatic filariasis (LF) in rural southeastern Nigeria is transmitted mainly by Anopheles spp. mosquitoes. Potential coinfection with Loa loa in this area has prevented use of ivermectin in the mass drug administration (MDA) strategy for LF elimination because of potential severe adverse L. loa-related reactions. This study determined if long-lasting insecticidal net (LLIN) distribution programs for malaria would interrupt LF transmission in such areas, without need for MDA. Monthly entomologic monitoring was conducted in sentinel villages before and after LLIN distribution to all households and all age groups (full coverage) in two districts, and to pregnant women and children less than five years of age in the other two districts. No change in human LF microfilaremia prevalence was observed, but mosquito studies showed a statistically significant decrease in LF infection and infectivity with full-coverage LLIN distribution. We conclude that LF transmission can be halted in southeastern Nigeria by full-coverage LLIN distribution, without MDA.
Introduction
The epidemiology of Loa loa infection has become critically important in the battle against neglected tropical diseases in sub-Saharan Africa.1–3 The concern about serious adverse events that are associated with ivermectin treatment in persons with heavy L. loa infection has slowed scale up of mass drug administration (MDA) programs for onchocerciasis and lymphatic filariasis (LF).4–8 An estimated 29.6 million persons at risk for LF also live in areas highly endemic for L. loa; these persons have not been reached under current MDA programmatic strategies.9 Most proposed solutions to the L. loa problem have focused on finding new chemotherapy regimens that have better safety profiles than ivermectin (for onchocerciasis) or the ivermectin/albendazole combination (for LF) in L. loa–coendemic areas.6,7,10–13
This paper reports the results of a four and a half year research program designed to determine if long-lasting insecticidal nets (LLINs) used under malaria programmatic strategies can interrupt LF transmission in which L. loa coendemicity has up to now prevented MDA. Because the same mosquitoes (Anopheles gambiae s.l. and Anopheles funestus) that transmit LF in rural west Africa also transmit malaria, it is hoped that the ongoing rapid scale-up of LLINs in Africa through the World Health Organization (WHO) Roll Back Malaria policy, with support from the Global Fund and many other large donors, will have an impact on LF transmission in addition to reducing the malaria burden.6,14–17 This research further develops the evidence base for LLINs impact on LF distribution, but in this instance, from the perspective that LLIN distribution as a sole intervention (without MDA) could be sufficient to halt LF transmission. If so, LLINs might substitute for MDA in the effort to eliminate LF in L. loa–coendemic areas of sub-Saharan Africa.
Materials and Methods
Study sites.
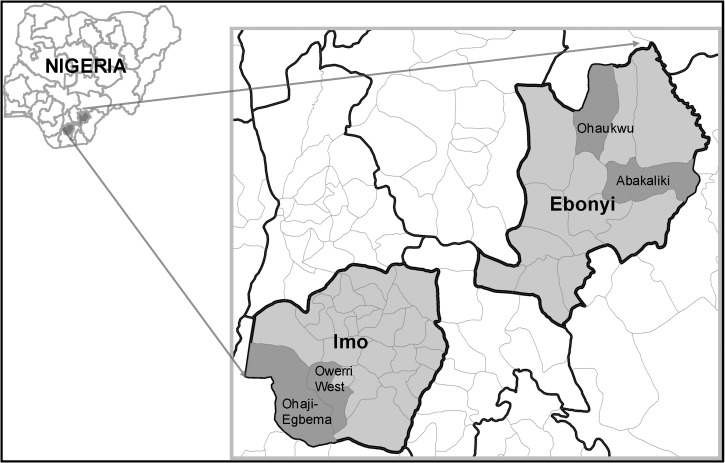
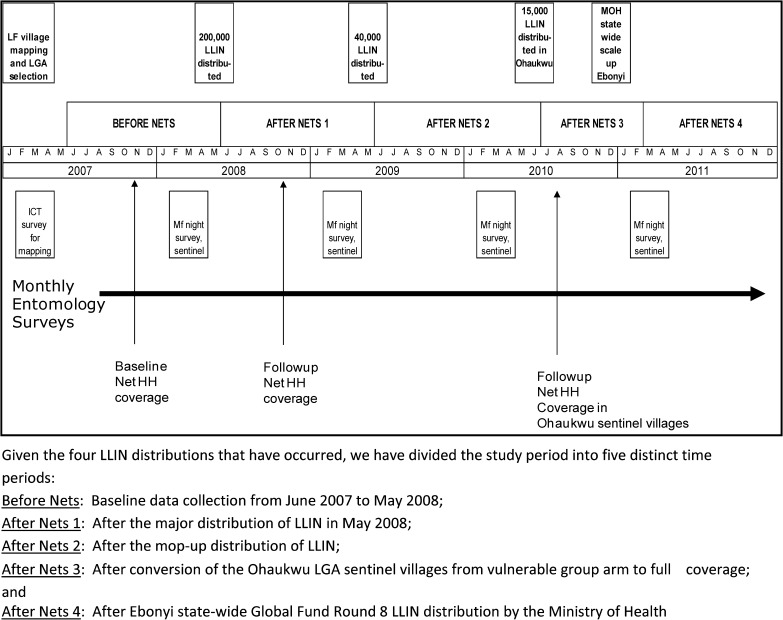
The study was conducted in two states (Ebonyi and Imo) located in southeastern Nigeria where LF, malaria, and L. loa are well known to occur (Figure 1 ). The research project had several phases: 1) Mapping of LF antigenemia at the district level (termed local government areas [LGAs] in Nigeria) to identify four high LF-prevalence LGAs; 2) Establishing baseline entomology and human microfilaremia prevalence in the four study LGAs; 3) Distributing LLINs LGA-wide, free of charge, at community level distribution points; 4) Documenting good LLIN coverage in the LGAs; and 5) Longitudinal entomologic and epidemiologic monitoring in sentinel villages to determine impact of LLINs on mosquitoes (abundance, LF infection, and LF infectivity rates) and human microfilaremia prevalence. The study began in January 2007 and lasted 55 months; a schematic diagram of study activities by month and year is shown in Figure 2 .
Figure 1.
Study local government areas (LGAs) in Imo and Ebonyi States, Nigeria.
Figure 2.
Detailed timeline of net distribution and surveys, Nigeria. LLIN = long-lasting insecticidal net.
History of LLIN distribution policy in Nigeria pertaining to the study.
Over the course of the study the Nigerian federal ministry of health (FMOH) malaria policy on LLINs evolved with respect to LLIN distribution targets, and with it so did our study design. When the study was launched in 2007, the FMOH policy was to provide free LLINs only to households with vulnerable groups (pregnant women and children less than five years of age). Because we believed that coverage of all households with sufficient nets would be needed to have substantial impact on LF transmission, our original design comprised two study arms, with two LGAs in each arm. The first arm had distribution of LLINs only to pregnant women and children less than five years of age (the FMOH malaria strategy at the time), and the second arm had full LLIN coverage intended to cover all sleeping spaces. The intent was to examine differential impact on LF transmission of vulnerable group LLIN coverage versus full LLIN coverage.
In 2009, however, the FMOH changed its policy to one of free LLIN distribution to all households (each household to receive two LLINs). We immediately altered our study design by closing the vulnerable group arm (e.g., converting to full coverage). Unfortunately, both study and FMOH LLIN resources were insufficient to provide a rapid scale-up of LLINs throughout the vulnerable group study arm and we were only able to convert one of the two LGAs (Ohaukwu LGA in Ebonyi State) to full coverage during 2010–2011. The change in FMOH policy, however, gave us an opportunity to observe the entomologic impact of converting the Ohaukwu study arm from vulnerable group to full coverage. The conversion to full coverage in Ebonyi State was completed in February 2011 with the completion of the FMOH mass LLIN distribution campaign in that state. Full-coverage LLIN distribution in Imo State (to close the final vulnerable group LGA of Owerri West) did not occur until August 2012, after the study had ended.
Mapping of LF.
A LF mapping exercise took place in the two L. loa–endemic states in early 2007 to identify the four LGAs that were to be the focus of the study. The objective was to identify LGAs in which LF was most endemic. Because onchocerciasis occurs in parts of Ebonyi and Imo States,18,19 only the 12 LGAs that did not have hyper/mesoendemic onchocerciasis were selected for mapping. Thus, any LGAs where mass ivermectin drug administration for onchocerciasis was being conducted were excluded from the study.
For mapping and LGA selection, 50 villages were surveyed in the 12 LGAs (3–5 villages per LGA). In each of these villages, after obtaining consent from village leaders and in community meetings, prevalence of LF circulating antigen20,21 was determined by using WHO LF village mapping guidelines among a convenience sample of 100 permanent residents, all > 15 years of age, with the sample equally divided between men and women.22 One hundred microliters of blood (measured by using a calibrated capillary tube) were obtained by finger puncture, then transferred to the pad on the whole blood immunochromatographic test (ICT) kit card (ICT; AmRad, Frenchs Forest, New South Wales, Australia, currently produced as NOW® ICT Filariasis Kits; Inverness Medical Professional Diagnostics, Princeton, NJ). The test was then run per the manufacturer's instructions.
A total of 4,986 persons (99.7%) of a targeted 5,000 persons were tested in the mapping exercise (the mapping teams were unable to achieve a sample of 100 persons in all 50 villages). Ebonyi State was found to be the most LF endemic; it had a mean LF antigen prevalence in resident adults of 29.6% (village prevalence range = 0–88%). Imo State was much less endemic; it had a mean LF antigen prevalence of 4.3% (village prevalence range = 0–36%). After a review of the data, four LGAs (two per state) were chosen on the basis of having the highest mean ICT prevalences in their respective states. Within the four study LGAs, 12 sentinel villages were selected from those that had been in the mapping surveys based on their having high baseline antigen rates in their LGA, good road access throughout the year, good security, and community willingness to participate in a multiyear longitudinal study. The study LGAs in Ebonyi State were Ohaukwu (sentinel village LF antigen prevalence = 44%) and Abakaliki LGAs (sentinel village LF antigen prevalence = 44%), and in Imo State were Ohaji-Egbema (sentinel village LF antigen prevalence = 9%) and Owerri West LGAs (sentinel village LF antigen prevalence = 17%) (Figure 1). Respective mapping exercise–derived LF antigen prevalence, by sentinel village and LGA, are shown in Table 1.
Table 1.
Human lymphatic filariasis epidemiology baseline in 12 sentinel villages in four selected LGAs, 2007 lymphatic filariasis antigenemia (ICT) and 2008 baseline microfilaremia results, Nigeria*
| State | LGA | Village | No. persons examined by ICTs | % ICT positive | No. persons examined for mf | % mf positive |
|---|---|---|---|---|---|---|
| Ebonyi | Ohaukwu | Orijuruafor | 100 | 32 | 269 | 5.6 |
| Okpochiri | 100 | 43 | 239 | 4.2 | ||
| Ndagu Obu | 100 | 57 | 254 | 11.0 | ||
| Sub-total | 300 | 44 | 762 | 7.0 | ||
| Abakaliki | Obegu Ibom | 100 | 36 | 184 | 7.1 | |
| Mgababeluzor | 100 | 88 | 222 | 4.5 | ||
| Okaria Echida | 100 | 8 | 187 | 2.7 | ||
| Sub-total | 300 | 44 | 593 | 4.7 | ||
| Imo | Owerri West | Mboke | 100 | 36 | 136 | 0.7 |
| Umuokpo | 100 | 10 | 315 | 0.3 | ||
| Umunjo | 100 | 5 | 111 | 0.0 | ||
| Sub-total | 300 | 17 | 562 | 0.4 | ||
| Ohaji-Egbema | Opuoma | 100 | 3 | 272 | 0.0 | |
| Etioha | 100 | 20 | 301 | 0.3 | ||
| Umukene | 100 | 5 | 169 | 0.0 | ||
| Sub-total | 300 | 9 | 742 | 0.1 | ||
| Total | 1,200 | 29 | 2,659 | 3.2 | ||
LGAs = local government areas; ICT = immunochromatographic test; mf = microfilariae.
Longitudinal surveillance of LF in 12 sentinel villages: monthly anopheline entomology.
Houses in each sentinel community were numbered and a 30-household sample was randomly chosen for monthly monitoring. Two full-time entomology teams (one in each state) conducted the entomology monitoring protocol. The state team visited each sentinel village in its two study LGAs once a month for two days to conduct pyrethrum knockdown spray catches in one room in each of the 30 households, beginning in June 2007 in Ohaukwu sentinel villages, expanding to include Owerri West and Abakaliki sentinel villages in July 2007, and reaching Ohaji-Egbema sentinel villages in September 2007. Indoor-resting mosquitoes were collected as described.23,24 The dead and dying mosquitoes that were collected were immediately placed in Petri dishes containing moist tissue, and the dishes were kept in coolers with wet towels until dissections were performed, always on the day of collection and usually in the same village. Each mosquito was identified as An. gambiae s.l., An. funestus, other Anopheles spp., Culex spp., Mansonia spp., Aedes spp., or other, and separated into head, thorax, and abdomen on a glass slide under a binocular dissecting microscope. Each of these was teased open in a drop of normal saline. The slide preparation was then examined at 100×under a compound microscope by a trained microscopist who noted the presence or absence of filarial larval stages (L1–3). Data on each dissected mosquito, including household of collection, were entered into a log book and later entered into Excel (Microsoft, Redmond, WA). Entomology data were analyzed for key indicators of abundance and infection rates at baseline and then after LLINs were distributed. Infected mosquitoes were defined as having any larval stage of Wuchereria bancrofti (L1, L2, or L3). Infective mosquitoes were defined as those harboring L3. Results were expressed as the absolute number of infected and infective mosquitoes observed, as well as infected or infective rates (infected mosquitoes/mosquitoes dissected×100, infective mosquitoes/mosquitoes dissected×100).
Baseline anopheline entomologic indices determined during June 2007–May 2008 by sentinel village, LGA, and state are shown in Table 2. A total of 9,502 mosquitoes were captured (infection rate = 2.4% and infective rate = 0.5%). There was considerable variance among the LGAs, and mosquito abundance (number caught per 30 rooms for 2 nights a month in each of 3 sentinel sites in each LGA) ranged from 3,871 for the year (Ohaukwu) to 254 (Ohaji-Egbema), infection rate ranged from 4.8% (Ohaukwu) to 0.8% (Ohaji-Egbema), and infective rate ranged from 0.9% (Ohaukwu) to 0% (Ohaji-Egbema and Owerri West). Baseline entomologic findings indicated that the highest force of LF transmission was in Ohaukwu.
Table 2.
Lymphatic filariasis entomology baseline (before nets, Anopheles only) in 12 sentinel villages in four selected LGAs, Nigeria*
| State | LGA | Village | No. dissected | Any larval form, % infected | Third-stage larvae in heads, % infective |
|---|---|---|---|---|---|
| Ebonyi | Ohaukwu | Orijuruafor | 958 | 1.8 | 0.6 |
| Okpochiri | 608 | 2.6 | 0.7 | ||
| Ndagu Obu | 2,305 | 6.6 | 1.0 | ||
| Sub-total | 3,871 | 4.8 | 0.9 | ||
| Abakaliki | Obegu Ibom | 1,280 | 1.0 | 0.4 | |
| Mgababeluzor | 1,024 | 0.9 | 0.3 | ||
| Okaria Echida | 600 | 0.5 | 0.2 | ||
| Sub-total | 2,904 | 0.9 | 0.3 | ||
| Imo | Owerri West | Mboke | 542 | 1.3 | 0.0 |
| Umuokpo | 608 | 0.7 | 0.0 | ||
| Umunjo | 312 | 0.6 | 0.0 | ||
| Sub-total | 1,462 | 0.9 | 0.0 | ||
| Ohaji-Egbema | Opuoma | 254 | 0.4 | 0.0 | |
| Etioha | 462 | 0.9 | 0.0 | ||
| Umukene | 549 | 0.9 | 0.0 | ||
| Sub-total | 1,265 | 0.8 | 0.0 | ||
| Total | 9,502 | 2.4 | 0.5 | ||
LGAs = local government areas.
Longitudinal surveillance of LF in the 12 sentinel villages: annual human microfilaremia prevalence determinations.
Nocturnal blood surveys to determine sentinel village LF microfilaremia prevalence were conducted in each of the 12 sentinel villages at the same time of year (February–March) on four occasions (Figure 2): 1) immediately before distribution of LLINs (2008), and then annually thereafter (2009, 2010, and 2011). Surveys were based on convenience samples of permanent village residents. Each year the team would explain LF, L. loa, the study, and the purpose of the nightlong survey to obtain permission first from the village chief and his council, and then again during a village wide health education and mobilization session. At the end of the meeting, persons of all ages were asked to come on an agreed upon night at 9:00 pm. On the night of the survey, between 10:00 pm and 2:00 am, residents ≥ 2 years of age who came for examination had their age and sex recorded and a finger puncture blood specimen was collected by a technician. Using disposable calibrated capillary tubes, we obtained 60 μL of blood, which was used to prepare thick blood films. The slides were air-dried and returned to the laboratory at Carter Center headquarters in Owerri for staining with Giemsa and qualitative examination for W. bancrofti microfilariae (mf) by trained microscopists. Technicians were well trained in distinguishing the unsheathed mf of Mansonella perstans (also prevalent in this part of Nigeria) from those of sheathed W. bancrofti. All positive slides were confirmed by another microscopist, and 10% of negative readings were reread by another microscopist as a standard quality control measure. Results were expressed as crude mf prevalence (number mf slide positive/number examined×100) and were not age adjusted.
Baseline mf prevalence determined during February–March 2008 by sentinel village and study LGA is shown in Table 1. Overall 2,659 persons were tested and 3.2% were mf positive. As with the baseline mosquito infection rates (Table 2), there was considerable variation among the LGAs (prevalence ranging from 0.1% in Ohaji-Egbema LGA to 7.0% in Ohaukwu LGA). Prevalence in the sentinel villages ranged from 0% to 11.0%. As with entomologic indices, Ohaukwu LGA had the highest baseline mf values.
Distribution of long-lasting LLINs.
The four LGAs were stratified by state before being randomized (by picking numbers from a hat) to each LLIN study arm (full-coverage or vulnerable group) to ensure that the highest LF transmission areas in Ebonyi were not in the same arm. It was not possible to randomize by village or area smaller than the LGA because the mechanism of net distribution operated LGA wide. Ohaukwu LGA in Ebonyi State (the LGA with the greatest force of transmission) was allocated to the vulnerable group (LLINs only to households with children less than five years of age and/or pregnant women) arm, as was Owerri West in Imo State. Abakaliki LGA (Ebonyi State) and Ohaji-Egbema (Imo State) were assigned to the full-coverage LLIN strategy (intended to cover all sleeping spaces). During April–June 2008 (Figure 2), 200,000 LLINs (deltamethrin-impregnated PermaNets®; Vestergaard Frandsen, Lausanne, Switzerland) were distributed free of charge in more than 950 villages to 99,397 households in all areas of the four LGAs except urban Abakaliki. Distribution took place at the village level in preannounced fixed distribution points (school yards, health centers, churches, local market places). In the vulnerable group LGAs, villagers presented to their assigned village distribution point and reported the number of children less than five years of age and pregnant women in the household; a net was given for each child less than five years of age and each pregnant woman. In the full-coverage LGAs, villagers came to their distribution point and reported the total number of persons residing within their household. The nets were distributed based on a simple algorithm relating the number of persons in a household with the theoretical number of sleeping spaces in that household (as derived from the baseline 2007 household survey described below). In both arms, households whose request entitled them to more than three LLINs required additional verification from either the village chief or a personal household check by the distributors.
After the 2008 distribution we determined that we needed to distribute 40,000 additional LLINs because, by chance, the largest LGAs (Abakaliki and Ohaji-Egbema) had been randomized to receive full LLIN coverage, and the 200,000 nets were not enough to meet the 2008 needs of the four study LGAs. To address this shortage, we purchased and supplied an additional 40,000 nets in a mop up operation one year later during May–June 2009 (Figure 2) that focused primarily on the two full-coverage LGAs.
As mentioned above, with the change in FMOH policy to full LLIN coverage in late 2009, we ended the vulnerable group arm. Unfortunately, because of limited LLIN availability in 2010 we only were able to distribute 15,000 donated PermaNets® in June and July of that year to convert the three sentinel villages in (SVs) Ohaukwu LGA to full LLIN coverage (Figure 2). Coverage of the entire Ohaukwu LGA had to wait until the distribution of Global Fund Round 8 nets throughout Ebonyi state during January–February 2011 (Figure 2). Because Imo State did not distribute its Global Fund Round 8 LLINs until August 2012, its vulnerable group LGA of Owerri West could not be converted to full-household coverage during the study. Health education messages on how to properly hang and use the LLINs were given during the distribution process and at least annually thereafter throughout the study.
Determining the change in household LLIN coverage in LGAs.
We assessed the success of the distribution by conducting cluster surveys to determine household ownership (% of households with ≥ 1 net) and mean number of nets per household. These were assessed in standardized household interviews by trained staff members. The surveys reported were conducted before (November–December 2007) and a few months after (October–December 2008) the completion of the major LLIN distribution (Figure 2).
We selected 15 clusters (census enumeration areas [EAs]) per LGA (60 clusters in all 4 LGAs) from census lists of EAs in each LGA. It was important purposively to include the sentinel sites in these surveys, but also to survey the rest of the LGA to ensure representation. First, the EAs that comprised each sentinel site village (which varied in number) were identified and removed from the list of each LGA. Twelve clusters (EAs) were selected per LGA by systematic selection with random start from this non-sentinel EA list. One EA was then selected randomly from the list of EAs in each sentinel site, i.e., an additional 3 EAs per LGA. For EA selection, it was assumed that all EAs were roughly of equal size (i.e., they were not selected as proportional to population size). All households in the EA were visited for the survey over the course of one day. If the EAs (in either non-sentinel or sentinel villages) were discovered by the survey team to be too large (too many households to do in one day), they were segmented according to an algorithm based on number of households in the EA using UNICEF MICS sampling protocol (UNICEF 2006; http://www.childinfo.org/files/Multiple_Indicator_Cluster_Survey_Manual_2005.pdf) and a randomly selected segment was surveyed.
Data analysis.
Household survey data were collected in a paper format and then double-entered by trained data entry staff using EpiInfo for Windows (CDC public domain software: http://wwwn.cdc.gov/epiinfo/html/downloads.htm). The EpiInfo Data Compare routine was used to check for data entry accuracy. Entomology data (by mosquito and by household) were entered directly into Excel spreadsheets. Data were entered by mosquito species and infection status, then checked by cross-tabulation for data inconsistency. Data were cleaned and then converted for analysis into Microsoft Access 2007, STATA 9, and STATA 11 (StataCorp LP, College Station, TX). Appropriate weighting was included in the analysis to account for selection probability in the sentinel and non-sentinel EAs within each LGA, and within EA if they were segmented.
Ethical approval.
The comprehensive protocol, including consent forms, was approved by the Imo and Ebonyi State Ministries of Health, Imo State University, and by the Emory University Institutional Review Board (Emory Institutional Review Board Protocol no. 5533).
Results
LLIN coverage.
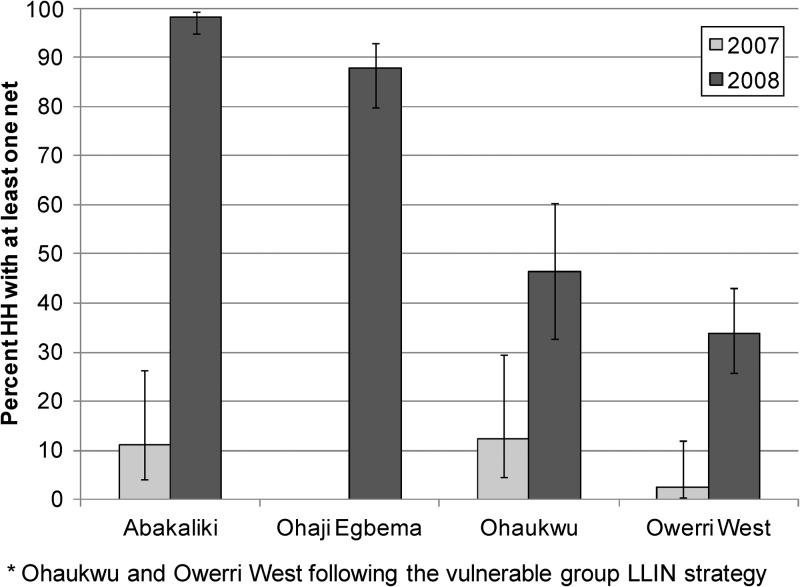
Household LLIN ownership by LGA before (2007) and after (2008) the major LLIN campaign is shown in Figure 3. The 2007 values are based on a cluster survey that encompassed 968 households with 5,197 residents. Only 72 nets were found, with only 6% of households owning at least one net. Ohaji-Egbema in Imo State had no nets in any of the sampled households. Ohaukwu in Ebonyi State had the highest baseline household ownership; 12.4% of households had at least one net (average = 0.17 nets/household). The 2008 post-LLIN distribution data were based on a cluster survey that covered 1,078 households with 5,200 residents. Significant increases in household ownership of at least one net were demonstrated in both study arms. The two full coverage LGAs demonstrated an increase in household ownership from 3.3% to 92.0% (a 28-fold increase from baseline), with an average of 1.9 LLINs/household. The two vulnerable group LGAs increased overall household LLIN ownership from 8.9% to 42.4% of all households (five-fold increase from baseline), with an average of 1.5 LLINs in households having a vulnerable group member (overall average = 0.6 LLINs/household).
Figure 3.
Overall household net ownership, pre- long-lasting insecticidal net (LLIN) distribution (2007) and post-distribution (2008), by study local government area, Nigeria. Error bars indicate 95% confidence intervals.
After distribution of LLINs in the three sentinel villages of Ohaukwu in 2010 (Figure 2), a household survey in November 2010 showed an 83.6% of households LLINs owned at least one net in these villages (average = 1.4 LLINs/household).
Entomology.
Over the 55-month period (June 2007–October 2011) a total of 31,134 mosquitoes were collected, of which 29,945 (96.2%) were dissected (Table 3). Overall, 410 mosquitoes were infected (1.37% of the dissected mosquitoes), and 0.20% had infective (L3) stage larvae. Anopheles spp. mosquitoes were by far the most common mosquito (24,574 dissected, 82.1%), and 92.7% of those being An. gambiae s.l. Although Anopheles spp. mosquitoes represented 82.1% of dissections, they provided 97.8% of LF infections (401 of 410 infected mosquitoes). The next most frequent genus dissected was Culex spp. (14.2%) followed by Mansonia spp. (3.1%). Each of these contributed just over 1% of infections and was considered to be inconsequential vectors of LF in the area. The remainder of the entomologic analysis focused on dissections of Anopheles spp. mosquitoes.
Table 3.
Mosquito species caught, infected and infective, Nigeria, June 2007–December 2011
| Mosquito | No. dissected | % Dissected | No. infected | % Infected (by genus) | No. infected | % Infective (by genus) |
|---|---|---|---|---|---|---|
| Anopheles* | 24,574 | 82.1 | 401 | 1.63 | 56 | 0.23 |
| Culex | 4,343 | 14.5 | 5 | 0.12 | 2 | 0.05 |
| Mansonia | 941 | 3.1 | 4 | 0.43 | 1 | 0.11 |
| Aedes | 87 | 0.3 | 0 | 0.00 | 0 | 0.00 |
| Total | 29,945 | 100.0 | 410 | 1.37 | 59 | 0.20 |
92.7% An. gambiae s.l. and 7.3% An. funestus.
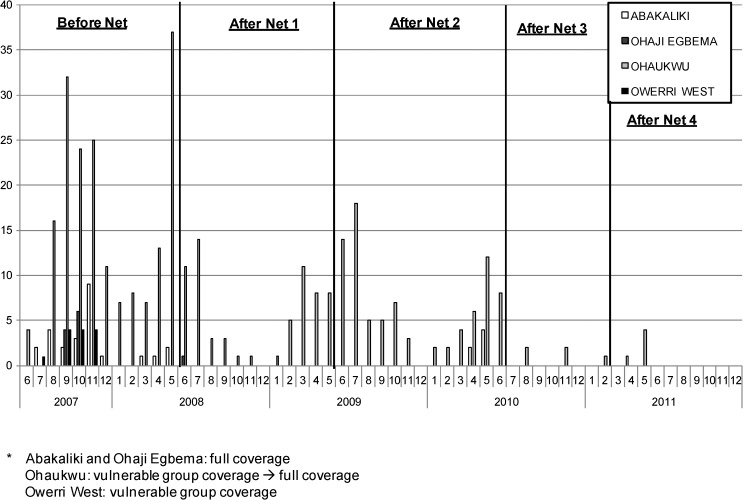
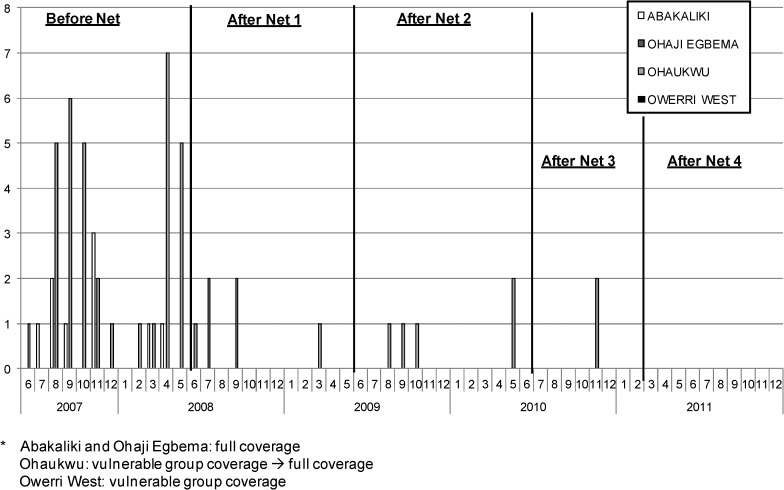
The number of infected and infective Anopheles spp. mosquitoes identified in dissections, by month, LGA, and the five LLIN distribution intervals (before nets, and after nets 1, 2, 3, and 4) are shown in Figures 4 and 5 . Infection and infectivity decreased over the period of the study. The greatest numbers of infections were found before LLINs were distributed. Evidence of mosquito infection ended promptly in both LGAs in Imo State: Ohaji-Egbema (full coverage) and Owerri West (vulnerable group coverage) after the initial LLIN distribution in 2008. The vulnerable group distribution strategy in Owerri West seemed sufficient to stop LF infection in mosquitoes, but the low baseline mosquito infection rates made reliable conclusions of the impact of LLINs on transmission difficult to discern. Abakaliki (full coverage) had no infective mosquitoes detected after the initial LLINs were distributed in 2008, but continued to show sporadic infected mosquitoes until after the mop up LLIN exercise in 2009 (After Nets 2). Ohaukwu was most interesting. Originally allocated to the vulnerable group arm in 2008 and converted to full coverage beginning in 2010, Ohaukwu had at baseline the highest force of infection of the four LGAs (based on number of vector mosquitoes collected, infected, and infective, and prevalence of mf in the human population). There was entomologic evidence of ongoing transmission after LLIN distribution to the vulnerable groups (the period during 2008–mid 2010), but transmission abated with conversion to full coverage in the sentinel villages (After Nets 3). Statewide mass LLIN distribution (After Nets 4) in 2011 afforded all of Ohaukwu LGA full coverage, and was associated with the end of detectable infection/infectivity in mosquitoes collected in the sentinel villages by April 2011.
Figure 4.
Number of Anopheles spp. mosquitoes infected (all lymphatic filariasis larval stages) by month and local government area, Nigeria.
Figure 5.
Number of Anopheles spp. mosquitoes infective (lymphatic filariasis third-stage larvae) by month and local government area, Nigeria.
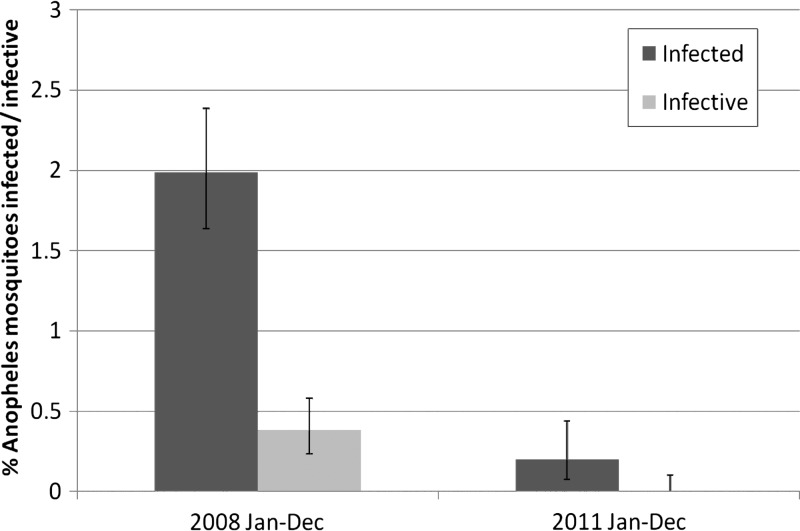
A statistically significant decrease > 90% in mosquito infection rates and mosquito infectivity rates for the calendar years 2008 and 2011 for the three LGAs that had achieved full coverage LLIN before (Abakaliki and Ohaji-Egbema) or by (Ohaukwu) 2011 is shown in Figure 6. The upper 95% confidence interval for all larval stages was < 0.5%. No L3 were detected in the study after May 2011, which indicated a 100% decrease between calendar years 2008 and 2011 (P = 0.001).
Figure 6.
Anopheles spp. mosquito infective rates (all larval stages and third-stage larval stages only) in Ohaukwu, Abakaliki and Ohaji/Egbema, local government area, Nigeria, 2008 and 2011.
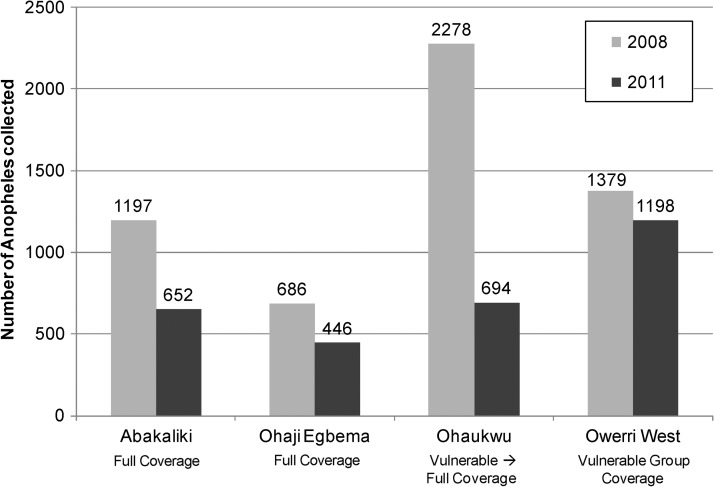
Mosquito abundance for 2008 and 2011 for all four LGAs is shown in Figure 7. Reductions in abundance were observed in the three LGAs that had full-household LLIN coverage. In contrast, Owerri West, where only vulnerable groups received LLINs, had essentially unchanged mosquito abundance during 2008–2011.
Figure 7.
Reduction in Anopheles spp. mosquitoes collected, by local government area in first and last years of collections, and by local government area and study group (January–December 2008 and January–December 2011), Nigeria. Error bars indicate 95% confidence intervals.
Nocturnal microfilaremia.
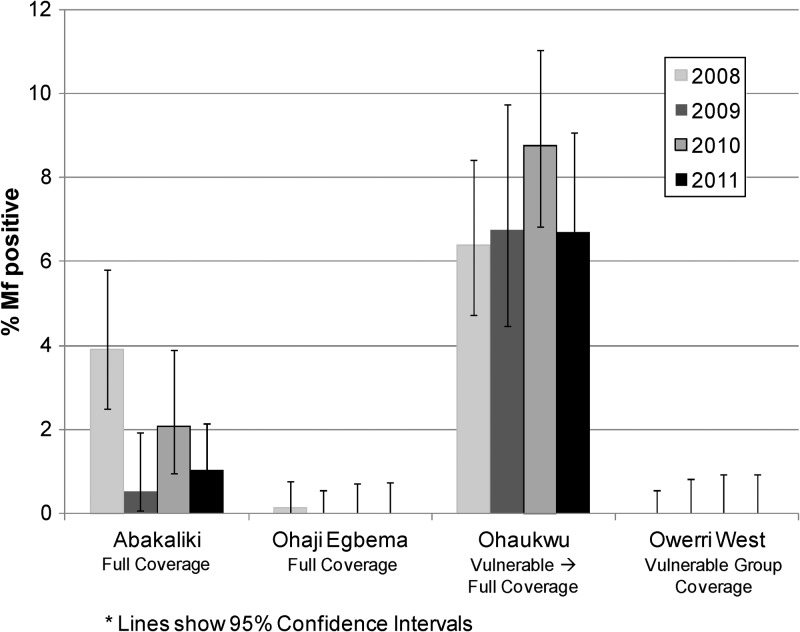
Nocturnal mf prevalence in the sentinel villages for the period 2008–2011 is shown in Figure 8. Values showed little change, with the exception of Abakaliki (full coverage) where there was a suggestion of a downward trend. Microfilaremia in Ohaukwu (which converted from vulnerable group to full coverage in 2010) was unchanged compared with the 2008 baseline.
Figure 8.
Prevalence of lymphatic filariasis microfilariae in blood, by year and local government area, Nigeria. Error bars indicate 95% confidence intervals.
Discussion
Based on 55 months of entomologic studies in 360 households in sentinel village sites, we concluded that LLINs alone can reduce mosquito LF infective rates to a point where L3 are no longer detectable by dissection, thereby halting transmission. Pedersen and others estimated the transmission threshold as being below an overall mosquito larval infection rate of 0.65%.14 We found infection rates to be less than this value by the end of the study (Figure 6). The sentinel villages studied were examples of a worst case LF scenario to be faced by a stand-alone LLIN intervention in Imo and Ebonyi States. If LF transmission is so influenced by LLINs in these disease-endemic situations, one could argue that LF transmission has been halted by the malaria program throughout the two states in the study.
The most intensely LF endemic of the sentinel villages (e.g., those having the highest force of transmission) were in Ohaukwu LGA in Ebonyi State. Interestingly, this finding was in the face of having the highest baseline LLIN household ownership of the study, albeit ownership was only 12.4% of households. Randomization in 2008 put this LGA into the vulnerable group arm in which LLINs were given only to children less than five years of age and pregnant women. It was clear by 2009 that transmission continued despite LLIN delivery to vulnerable groups (and 40% of households). When the FMOH changed its national policy to full-household coverage, we made Ohaukwu priority for conversion to full LLIN coverage. The resultant decrease in mosquito infection rates after Ohaukwu reached 83% household ownership gave the most convincing evidence in support of a conclusion that, in the worst case transmission scenarios, only full coverage LLIN interventions are sufficient to stop LF transmission.
Hawley and others have shown that full-household coverage LLIN distribution needs to be executed across a broad geographic zone to obtain a mass killing effect against the vectors.25 Therefore, we provided LLINs not only to a few sentinel villages, but across an entire LGA zone to provide the spatial scope/scale needed to establish an LLIN vector kill zone in which mosquito flight ranges, additional breeding sites, and unprotected microfilaremic persons would not influence our measurements of program impact on mosquito abundance and LF infection in sentinel sites. The wisdom of this approach was again demonstrated in Ohaukwu, where it was not the limited full coverage LLIN distribution to the sentinel villages in 2010, but rather the distribution to all households in the LGA in the 2011 state-wide campaign that finally ended all dissection-detectible evidence of LF transmission.
The observed entomologic reduction of LF transmission occurred despite relatively unchanged mf prevalence in the communities, which would be expected given the persistence of LF infection for many years. One important way that LLINs stop LF transmission is by providing a barrier between the infected (microfilaremic) human carrier and the mosquito vector. Continued human infection indicated the potential for resumption of transmission if there was a drop in LLIN ownership/use (and/or the failure to replace worn out nets as necessary). Given the potential for the entomologic situation to revert to its baseline parameters, LF transmission by the end of the study would best be described as suppressed rather than interrupted. The current challenge is to maintain sufficient LLIN ownership and use for a period of time necessary for all or most adult W. bancrofti worms to die or lose their ability to produce microfilariae, perhaps ≥ 6 years (the estimated reproductive life of the worms).10,26,27
We did not offer treatment to mf-positive persons. One reason was because of the potential for serious adverse events from ivermectin or diethylcarbamazine in an area with concomitant L. loa infection, where mass treatment cannot be offered under current MDA guidelines.6 We could have offered ivermectin/albendazole treatment to LF-infected persons identified in the nocturnal surveys after first performing another (daytime) blood smear to ensure that the person did not have concomitant L. loa infection. However, LF treatment in such persons does not clearly benefit the person because ivermectin and albendazole do not provide a single-dose cure, nor are they known to prevent the development of lymphedema or (in men) hydrocele. The WHO strategy for the use of these medicines in MDA is for the altruistic purpose of decreasing community mf levels to a point low enough where mosquitoes are not infected and new human infections cease.10 This strategy was accomplished (more safely) by distributing LLINs. Provisional WHO guidelines are being considered that may enable us to safely offer albendazole monotherapy as MDA in these communities in the near future.6
Our 2007 baseline LF endemicity studies in sentinel villages offered an opportunity to make two observations. First, we found that sentinel village LF antigenemia (measured by ICT tests in 100 adults) did not correlate as well as might be expected with mf prevalence obtained several months later in the same villages from a separate sample comprised of all age groups (Table 1). The sentinel village of Mgbabeluzor in Abakaliki LGA had the highest antigenemia (88% prevalence) but was ranked fourth by microfilaremia (4.5%). Ndagu Obu in Ohaukwu LGA had twice the microfilaremia prevalence as Mgbabeluzor (11%), but had an antigenemia prevalence (57%) that was 35% lower. Second, a key WHO indicator of transmission is a microfilaremia prevalence > 1%.28,29 We found baseline mf prevalence ascertained in blood slide surveys in 2008 was below this threshold in all sentinel villages in Imo State, despite their having baseline antigenemia rates of up to 20% (Table 1). Although no sentinel villages in Imo State had baseline mf > 1% (Table 1), three of the six had Anopheles spp. mosquito infection rates (Table 2) greater than the transmission threshold of 0.65% set by Pedersen and others.14
For LLINs to work effectively, they need to be appropriately hung, kept in good repair, and routinely used in a proper manner by everyone. Adult males have been identified in some studies as having lower routine use of LLINs.30,31 They often see malaria and malaria fever as serious for children, but simply a nuisance for themselves. We found that gearing our health education messages to include the idea that using nets helps men avoid getting hydrocele and large legs made a greater impression on adult males than the message of prevention of fever in Plateau State, Nigeria (Patterson A, unpublished data). We believe that influencing LLIN use through an LF health education message can also help lower malaria transmission rates because adults with asymptomatic gametocytemia may be important infectors of mosquitoes in some epidemiologic settings.
There are several weaknesses to this study. First, it was impossible to conduct the study blindly; the LLIN arm (full coverage or vulnerable group) to which the LGAs and sentinel sites belonged were obvious to the survey teams. Second, in addition to their having high baseline antigen rates in their LGA, the 12 sentinel sites were picked based on their having good road access throughout the year, good security, and community willingness to participate in a multiyear longitudinal study. Obviously, this was required to accomplish monthly entomologic surveys, but may have introduced a bias. Last, the frequent visitation of the study teams to the same sentinel sites could have enhanced the knowledge about LF and its linkage with mosquitoes, and as a result, greater use of the LLINs in those areas.
It has been recognized that in Anopheles spp. mosquito–driven LF transmission systems, the combination of MDA and LLINs is likely to be more effective than the MDA alone.16,27 However, the results reported here demonstrate that LLINs alone can halt LF transmission. The clear implication is that the LF elimination strategy could rely on LLINs where L. loa prevents MDA. Thinking more broadly, the remarkable scale-up of LLIN delivery for malaria control in sub-Saharan Africa (where household ownership is estimated to have increased from 3% in 2000 to 50% in 2011)32 has the potential to have had a dramatic impact on LF transmission in many LF-endemic countries, despite the slow scale-up of MDA in Nigeria, Ethiopia, and the Democratic Republic of Congo.4,5,33 Integrated partnerships between LF and malaria personnel is strongly encouraged by the WHO integrated vector management initiative.4,6,17,34 A recommendation from this study is to enhance mutual understanding of program goals between the malaria and LF communities, and identify strategic synergies and opportunities to maximize and sustain community-wide LLIN delivery and use.
ACKNOWLEDGMENTS
We thank Dr. Ngozi Njepuome (former director of public health at the FMOH in Abuja) for assistance during the study; Josephine Obiezu and Njideka Theresa Okpala for data entry; D. Pam, Kenneth Akubue, Gloria Mbanu, Dr. Austin Amaechi, Helen Uduji, Chinyere Okoro, and Rev Sister MaryFlora Ezeabikwa for assisting with entomology field work; Adaku Echebima, Gift Opara, Mgbodichi Onyia, Rita Otozi for providing malaria focal persons in the LGA studies; and Dr. Jeremiah Ngondi for assisting with data management and analysis, particularly with the bed net ownership surveys.
Footnotes
Financial support: This study was supported by the Bill and Melinda Gates Foundation. A generous donation by Vestergaard Frandsen of 15,000 PermaNets® enabled us to quickly convert the vulnerable group arm to full LLIN coverage in the sentinel villages of Ohaukwu LGA in Ebonyi State in 2010.
Authors' addresses: Frank O. Richards, Patricia M. Graves, Lindsay Rakers, Aryc Mosher, Amy Patterson, and Masayo Ozaki, Malaria, River Blindness, Lymphatic Filariasis, and Schistosomiasis Programs, The Carter Center, Atlanta, GA, E-mails: frich01@emory.edu, pgraves.work@gmail.com, lrakers@emory.edu, awmoshe@emory.edu, aepatte@emory.edu, and masayo.ozaki@gmail.com. Emmanuel Emukah, Chidiebere Njoku, Kenrick Nwodu, and Andrew Obasi, The Carter Center, Owerri, Imo State, Nigeria, E-mails: emukahe@yahoo.com, feb4real@yahoo.com, kenrome@yahoo.com, and andyobasi2@yahoo.com. Omeni Nkwocha, Ministry of Health, Owerri, Imo State, Nigeria, E-mail: occnten@yahoo.com. Lawrence Nwankwo, Ministry of Health, Ebonyi, Ebonyi State, Nigeria, E-mail: eb4rbm@yahoo.com. Bertram E. B. Nwoke and Chinyere N. Ukaga, Imo State University, PMB 2000, Owerri, Nigeria, E-mails: bebndie@yahoo.com and chinyukaga@yahoo.com. Emmanuel S. Miri, The Carter Center, Jos, Nigeria, E-mail: emmamiri@yahoo.com.
References
- 1.Twum-Danso NA. Loa loa encephalopathy temporally related to ivermectin administration reported from onchocerciasis mass treatment programs from 1989 to 2001: implications for the future (review) Filaria J. 2003;2((Suppl 1)):S7. doi: 10.1186/1475-2883-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown MJ. Report of a scientific working group on serious adverse events following Mectizan® treatment of onchocerciasis in Loa loa endemic areas 28–30 May 2002. Filaria J. 2003;2((Suppl 1)):S2. doi: 10.1186/1475-2883-2-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Addiss D, Rheingans R, Twum-Danso NAY, Richards F. A framework for decision-making for mass distribution of Mectizan® in areas endemic for Loa loa. Filaria J. 2003;2((Suppl 1)):S9–S18. doi: 10.1186/1475-2883-2-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization Meeting of the International Task Force for Disease Eradication (on elimination of LF and onchocerciasis in Africa), April 2011. Wkly Epidemiol Rec. 2011;86:341–351. [Google Scholar]
- 5.World Health Organization Global programme to eliminate lymphatic filariasis: progress report 2010. Wkly Epidemiol Rec. 2011;86:377–388. [PubMed] [Google Scholar]
- 6.World Health Organization Provisional Strategy for Interrupting Lymphatic Filariasis Transmission in Loiasis-Endemic Countries. 2012. http://apps.who.int/iris/bitstream/10665/75139/3/WHO_HTM_NTD_PCT_2012.6_eng.pdf Available at.
- 7.Malecela-Larazo M, Twum-Danso N. The lymphatic filariasis (LF) research forum: towards developing a strategic plan for research to support the global program to eliminate LF (GPELF): program implementation. Am J Trop Med Hyg. 2004;71:16–19. [Google Scholar]
- 8.Ottesen EA. Lymphatic filariasis: treatment, control and elimination. Adv Parasitol. 2006;61:395–441. doi: 10.1016/S0065-308X(05)61010-X. [DOI] [PubMed] [Google Scholar]
- 9.Zouré HG, Wanji S, Noma M, Amazigo UV, Diggle PJ, Tekle AH, Remme JH. The geographic distribution of Loa loa in Africa: results of large-scale implementation of the rapid assessment procedure for loiasis (RAPLOA) PLoS Negl Trop Dis. 2011;5:e1210. doi: 10.1371/journal.pntd.0001210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother. 2005;6:179–200. doi: 10.1517/14656566.6.2.179. [DOI] [PubMed] [Google Scholar]
- 11.Gyapong JO, Twum-Danso NA. Editorial: global elimination of lymphatic filariasis: fact or fantasy? Trop Med Int Health. 2006;11:125–128. doi: 10.1111/j.1365-3156.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 12.Kyelem D, Biswas G, Bockarie MJ, Bradley MH, El-Setouhy M, Fischer PU, Henderson RH, Kazura JW, Lammie PJ, Njenga SM, Ottesen EA, Ramaiah KD, Richards FO, Weil GJ, Williams SA. Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. Am J Trop Med Hyg. 2008;79:480–484. [PMC free article] [PubMed] [Google Scholar]
- 13.Alleman MM, Twum-Danso NA, Thylefors BI. The Mectizan® Donation Program: highlights from 2005. Filaria J. 2006;5:11. doi: 10.1186/1475-2883-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen EM, Stolk WA, Laney SJ, Michael E. The role of monitoring mosquito infection in the global programme to eliminate lymphatic filariasis. Trends Parasitol. 2009;25:319–327. doi: 10.1016/j.pt.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 15.Bockarie MJ, Pedersen EM, White GB, Michael E. Role of vector control in the global program to eliminate lymphatic filariasis. Annu Rev Entomol. 2009;54:469–487. doi: 10.1146/annurev.ento.54.110807.090626. [DOI] [PubMed] [Google Scholar]
- 16.Pichon G. Limitation and facilitation in the vectors and other aspects of the dynamics of filarial transmission: the need for vector control against Anopheles-transmitted filariasis. Ann Trop Med Parasitol. 2002;96((Suppl 2)):S143–S152. doi: 10.1179/000349802125002509. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization WHO position statement on integrated vector management to control malaria and lymphatic filariasis. Wkly Epidemiol Rec. 2011;86:121–127. [PubMed] [Google Scholar]
- 18.Emukah EC, Osuoha E, Miri ES, Onyenama J, Amazigo U, Obijuru C, Osuji N, Ekeanyanwu J, Amadiegwu S, Korve K, Richards F. A longitudinal study of impact of repeated mass ivermectin treatment on clinical manifestations of onchocerciasis in Imo State, Nigeria. Am J Trop Med Hyg. 2004;70:556–561. [PubMed] [Google Scholar]
- 19.Gutman J, Emukah E, Okpala N, Okoro C, Obasi A, Miri ES, Richards FO., Jr Effects of annual mass treatment with ivermectin for onchocerciasis on the prevalence of intestinal helminths. Am J Trop Med Hyg. 2010;83:534–541. doi: 10.4269/ajtmh.2010.10-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weil GJ, Lammie PJ, Weiss N. The ICT Filariasis Test: a rapid-format antigen test for diagnosis of bancroftian filariasis. Parasitol Today. 1997;13:401–404. doi: 10.1016/s0169-4758(97)01130-7. [DOI] [PubMed] [Google Scholar]
- 21.Weil GJ, Ramzy RM. Diagnostic tools for filariasis elimination programs. Trends Parasitol. 2007;23:78–82. doi: 10.1016/j.pt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization . Operational Guidelines for Rapid Mapping of Bancroftian Filariasis in Africa. Geneva: World Health Organization; 2000. [Google Scholar]
- 23.Richards FO, Jr, Pam DD, Kal A, Gerlong GY, Onyeka J, Sambo Y, Danboyi J, Ibrahim B, Terranella A, Kumbak D, Dakul A, Lenhart A, Rakers L, Umaru J, Amadiegwu S, Withers PC, Jr, Mafuyai H, Jinadu MY, Miri ES, Eigege A. Significant decrease in the prevalence of Wuchereria bancrofti infection in anopheline mosquitoes following the addition of albendazole to annual, ivermectin-based, mass treatments in Nigeria. Ann Trop Med Parasitol. 2005;99:155–164. doi: 10.1179/136485905X19838. [DOI] [PubMed] [Google Scholar]
- 24.Richards FO, Eigege A, Miri ES, Kal A, Umaru J, Pam D, Rakers LJ, Sambo Y, Danboyi J, Ibrahim B, Adelamo SE, Ogah G, Goshit D, Oyenekan OK, Mathieu E, Withers PC, Saka YA, Jiya J, Hopkins DR. Epidemiological and entomological evaluations after six years or more of mass drug administration for lymphatic filariasis elimination in Nigeria. PLoS Negl Trop Dis. 2011;5:e1346. doi: 10.1371/journal.pntd.0001346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hawley WA, Phillips-Howard PA, Ter Kuile FO, Terlouw DJ, Vulule JM, Ombok M, Nahlen BL, Gimnig JE, Kariuki SK, Kolczak MS, Hightower AW. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68:121–127. [PubMed] [Google Scholar]
- 26.Duerr HP, Dietz K, Eichner M. Determinants of the eradicability of filarial infections: a conceptual approach. Trends Parasitol. 2005;21:88–96. doi: 10.1016/j.pt.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 27.Michael E, Malecela-Lazaro MN, Simonsen PE, Pedersen EM, Barker G, Kumar A, Kazura JW. Mathematical modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–234. doi: 10.1016/S1473-3099(04)00973-9. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Sixth meeting of the Technical Advisory Group on the Global Elimination of Lymphatic Filariasis, Geneva, Switzerland, 20–23 September 2005. Wkly Epidemiol Rec. 2005;80:401–408. [PubMed] [Google Scholar]
- 29.World Health Organization Report on the mid-term assessment of microfilaraemia reduction in sentinel sites of 13 countries of the Global Programme to Eliminate Lymphatic Filariasis. Wkly Epidemiol Rec. 2004;79:358–365. [PubMed] [Google Scholar]
- 30.Baume CA, Marin MC. Intra-household mosquito net use in Ethiopia, Ghana, Mali, Nigeria, Senegal, and Zambia: Are nets being used? Who in the household uses them? Am J Trop Med Hyg. 2007;77:963–971. [PubMed] [Google Scholar]
- 31.Tsuang A, Lines J, Hanson K. Which family members use the best nets? An analysis of the condition of mosquito nets and their distribution within households in Tanzania. Malar J. 2010;9:211. doi: 10.1186/1475-2875-9-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization . World Malaria Report 2011. Geneva: World Health Organization, (WHO/HTM/GMP/2011.1); 2011. http://www.who.int/malaria/world_malaria_report_2011/en/ Available at. [Google Scholar]
- 33.World Health Organization Global programme to eliminate lymphatic filariasis: progress report 2011. Wkly Epidemiol Rec. 2012;87:346–356. [PubMed] [Google Scholar]
- 34.World Health Organization . Progress Report 2000–2009 and Strategic Plan 2010–2020 of the Global Programme to Eliminate Lymphatic Filariasis. Geneva: World Health Organization; 2011. [Google Scholar]