Abstract
Cholera outbreak following the earthquake of 2010 in Haiti has reaffirmed that the disease is a major public health threat. Vibrio cholerae is autochthonous to aquatic environment, hence, it cannot be eradicated but hydroclimatology-based prediction and prevention is an achievable goal. Using data from the 1800s, we describe uniqueness in seasonality and mechanism of occurrence of cholera in the epidemic regions of Asia and Latin America. Epidemic regions are located near regional rivers and are characterized by sporadic outbreaks, which are likely to be initiated during episodes of prevailing warm air temperature with low river flows, creating favorable environmental conditions for growth of cholera bacteria. Heavy rainfall, through inundation or breakdown of sanitary infrastructure, accelerates interaction between contaminated water and human activities, resulting in an epidemic. This causal mechanism is markedly different from endemic cholera where tidal intrusion of seawater carrying bacteria from estuary to inland regions, results in outbreaks.
Introduction
Cholera remains a major public health threat in developing countries where safe water and sanitation facilities are inadequately available. The World Health Organization (WHO) estimates that annually, about 3 to 5 million people are affected worldwide by cholera and over 100,000 cases result in death (http://www.who.int/mediacentre/factsheets/fs107/en/index.html accessed on 11/12/2012). Vibrio cholerae is a natural inhabitant of the aquatic environment,1 which with evidence of new biotypes emerging from its environmental reservoir,2,3 indicates that cholera bacteria cannot be eradicated. Because cholera outbreaks will continue to occur over time, the most effective means of controlling or preventing the disease is to minimize exposure to pathogenic strains and/or high concentrations of cholera bacteria. Cholera is one of the most prevalent water-related infections in many regions of the world, specifically in South Asia, sub-Saharan Africa, and Latin America. Observational records show that the vast majority of cholera outbreaks originate in coastal regions, indicating a strong association between environment and the disease.4–8 Despite significant advances in our knowledge of the metabolism, pathogenesis, and genomics of V. cholerae, we still cannot predict precisely when the next cholera epidemic will occur or the probability, timing, and/or location of an outbreak, all of which is essential if an effective intervention strategy is to be designed and implemented. The recent outbreak of cholera in Haiti has affected millions of people and is a classic result of having a limited understanding of the dynamics of disease and the relationship of V. cholerae, the causative agent of cholera, with its environment.
Microbiological and environmental understanding of cholera epidemics is based on studies conducted over the past 20 years in the Bengal Delta, unfortunately referred to as the native homeland of the disease, despite the ubiquity of cholera globally. The Bengal deltaic region has endemic cholera (endemic being defined as a region where recurrence and persistence of the disease has continued for at least 10 consecutive years in the WHO cholera database). The challenge is translating knowledge of the ecology, microbiology, and pathobiology of V. cholerae and the epidemiology of cholera into a predictive model applicable to regions where cholera outbreaks are sporadic but not endemic. With respect to medical treatment, oral rehydration therapy, within just a few decades, has reduced the mortality rate of cholera from over 30% to < 1% in endemic regions, notably Bangladesh and several sub-Saharan countries, e.g., South Africa, Congo, Mozambique, and Ghana. Examples of countries with non-endemic (or epidemic thereafter) cholera, that is, experiencing periodic and sudden outbreaks of cholera include Pakistan (2008) and Congo (2008), and most recently Haiti (2010). A significant difference between an endemic and epidemic region is the mortality rate, i.e., 1% or lower in an endemic region. In contrast, > 3% mortality has been recorded for epidemic regions over the last 5 years, including Zimbabwe (4.3% in 2008–09), Angola (4% in 2006–07), Nigeria (3.8% in 2010), and Sudan (3.3% in 2006–07).9 Unfortunately, rates of cholera in epidemic regions can exceed 6% (6.4% for Haiti in 2010 and 6% for Madagascar in 2000).9 A sharp contrast in mortality rates between epidemic and endemic regions exists, not because knowledge of how to treat cholera patients in an unprepared community is lacking, but instead understanding those environmental factors that contribute to outbreaks and/or major epidemics is needed. The objective of this study was to seek an understanding of the relationship between hydroclimatological processes and cholera in epidemic regions. The approach taken includes current knowledge of cholera and the effectiveness of interventions, employing surveillance data from endemic regions. Also taken into consideration is that V. cholerae is autochthonous to the aquatic environment globally. Finally, outbreaks of cholera in endemic regions show strong hydroclimatological influence, which is encouraging because those conditions can be monitored in epidemic regions. Within this context, seasonality was quantified as a mechanism of occurrence and from that set of data, a hydroclimatological explanation of the disease was developed providing a testable framework linking large-scale etiology of cholera in epidemic regions.
Data
Surveillance data for cholera in epidemic regions were obtained by collecting and processing historical climatological data from annual reports of the Meteorological Reporter to the Government of India, covering a time period of 1875 to 1900. In addition, cholera mortality observations were obtained from statistical statements appended to the Annual Reports of the Sanitary Commissioner to the Government of Punjab, 1875–1900. During the time period of our study, 1875–1900, there were no known disease interventions or mitigation strategies available against cholera for the general population. Therefore, in all likelihood pattern and seasonality in the time series are not affected by introduced controls. We focused on nine inland areas located in the Indus River Basin of Northern India and Pakistan (Figure 1). The Centers for Disease Control and Prevention (CDC) of the United States has defined outbreak and epidemics as a sudden increase in the number of cases of a disease above what is normally expected in that population in that area.10 Within this context, we analyzed global cholera occurrence time series from the WHO and determined that regions where the least continuous data are available be termed as endemic regions. Hence, the Pakistan region of Indus River Basin has been categorized as an epidemic region in this study. Details of methods and analysis are provided below.
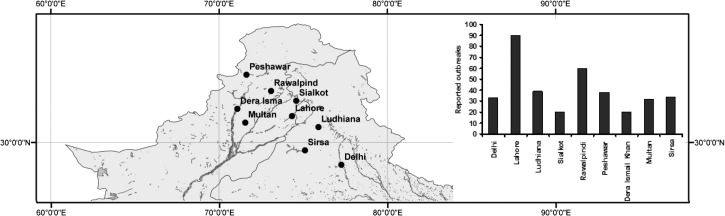
Figure 1.
Location of areas in the Indus River Basin where cholera outbreaks were reported from 1875 to 1900.
Results
Cholera in the Indus River Basin.
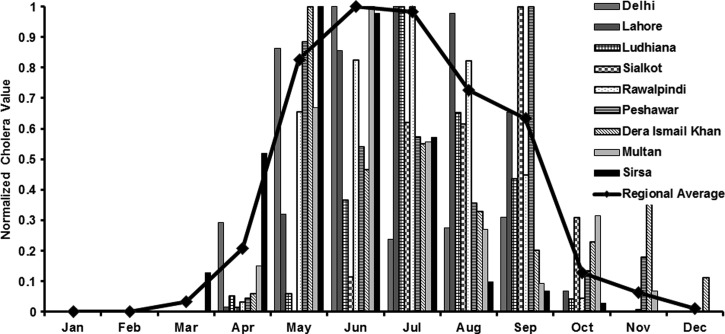
The Indus basin has a dense network of rivers and tributaries. The nine locations shown in Figure 1 were both major agricultural and industrial hubs during British rule over the Indian subcontinent. This geographical region typically receives heavy monsoonal rainfall from June through September and endures hot (air temperatures greater than 30°C) summer months. Out of 312 study-months, Lahore city recorded the highest number of cholera outbreaks followed by Rawalpindi and Peshawar (Figure 1: inset). Dera Ismail Khan had the lowest number of cholera outbreaks over a span of 26 years. Regionally, cholera outbreaks were reported for about 20% of the month-time of the study period. Furthermore, cholera in this region is primarily epidemic in nature, i.e., there is no periodic interannual reoccurrence of disease over time. Figure 2 shows seasonality in the monthly cholera time series for the nine locations. The disease outbreaks are generally reported over a range of months, typically from April through October.
Figure 2.
Seasonal cholera for nine locations in the Indus River Basin. The data were normalized between 0 and 1 for the purpose of comparison. The solid line represents the regional average of cholera.
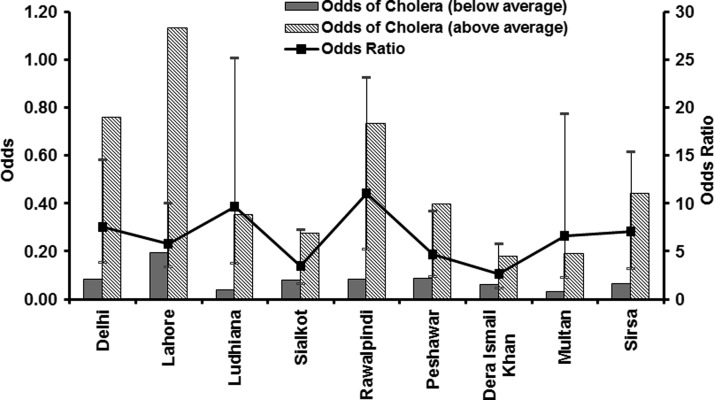
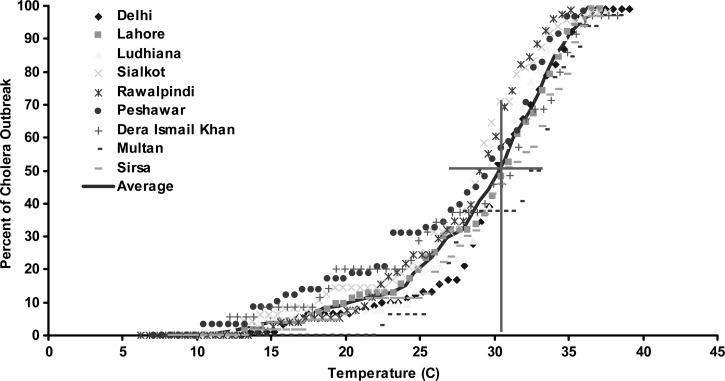
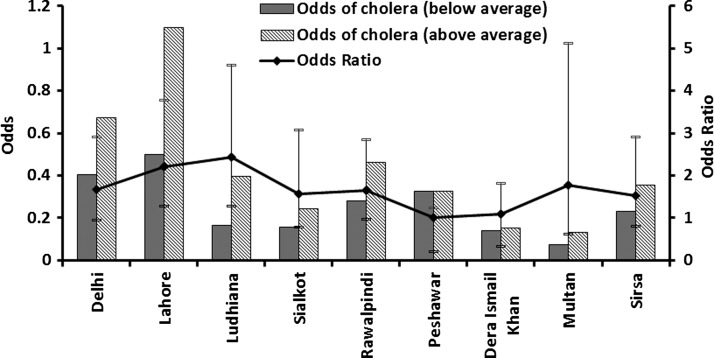
We hypothesize that, in epidemic cholera regions, elevated air temperatures create environmental conditions favorable for bacterial growth and, when followed by above normal rainfall in combination with appropriate transmission mechanisms such as poor availability of safe water11 and destruction of sanitation infrastructures aiding in mixing of overflowing sewers with flood waters,12 result in an epidemic of cholera. As a result of inherent structure (zeros in the time series) of the cholera mortality time series of our study, correlation between the air temperature and cholera mortality time series is essentially non-existent. Hence, we calculated odds of occurrence of cholera during above and below average air temperatures, as well as corresponding odds ratios (ORs) with appropriate confidence intervals (CIs). Figure 3 shows that the odds of a cholera occurring were significantly higher when the temperature was above climatological average over the previous 2 months. Cholera in Lahore, one of the major population centers of its time, showed highest odds of cholera incidence when the air temperature was elevated during the preceding months. We then calculated the ORs for cholera (Figure 3 ) and found that, on an average, across nine regions, the chances of an epidemic increased 6-fold if the air temperature was above climatological average during 2 months preceding the disease outbreak. Thresholds for air temperature and likelihood of an outbreak of cholera were then determined. Percentage of cholera outbreaks over a range of observed air temperatures for all locations was calculated and plotted against temperature. Figure 4 shows that about 50% or more cholera outbreaks occurred when the air temperature is > 31°C, approximately one standard deviation from average air temperature over the previous 2 months (solid line). However, air temperature alone will not cause an epidemic, unless accompanied by appropriate transmission mechanisms such as poor water quality and lack of sanitation infrastructure, as well as rainfall. Given the inadequate water and sanitation infrastructures during the time period of our study data for North India, rainfall provides the most appropriate mechanism for spread of cholera through cross-contamination of water, with the population having to rely on surface water for daily usage. The effect of rainfall is shown in Figure 5, i.e., the odds of occurrence of the disease during above and below normal rainfall and following months of above average air temperature at the nine locations. The odds for rainfall were calculated after isolating the months with above average air temperature, which accounted for 91% of the total data cholera outbreaks data points. This is complementary evidence that cholera outbreaks have a strong association with warm air temperature. In fact, the odds of a cholera outbreak occurring is 1.5 times greater than for below average precipitation (Figure 5).
Figure 3.
Odds of cholera outbreaks during above average and below average temperature and corresponding odds ratio.
Figure 4.
Relationship between cholera outbreaks and air temperature. Y axis refers to percentage of cholera outbreaks during observed air temperatures. X axis refers to the mean air temperatures in °C.
Figure 5.
Odds of cholera outbreaks during above average and below average rainfall and corresponding odds ratio.
Several studies have postulated and quantified the role of coastal water in the epidemiology of cholera.1,5,13–15 However, there is limited understanding of environmental factors responsible for emergence of cholera in noncoastal areas with active riverine systems. Influence of large-scale hydroclimatological processes on cholera prevalence have been reported,11,16,17 indicating cholera outbreaks in the Bengal Delta may be related to asymmetrical spatial and temporal hydroclimatological response to droughts and floods, creating conditions favorable for growth of cholera bacteria. Increased rainfall in the Bengal Delta may result in widespread floods and cholera outbreaks.7,11 Recently, studies have used V. cholerae isolated from patient stool samples to show cholera outbreaks in New Delhi occur during the months of May through September.18 An increase in cholera outbreaks following heavy rainfall is observed in epidemic regions of Africa.19 Similar observations have been reported in Bangladesh,20–22 Haiti,9 and East Africa.23 The role of air temperature has also been highlighted in several recent studies. Incidence of cholera peaked when the air temperature reached 26°C.24 Isolation of cholera bacteria increased significantly when the air temperature was above 25°C in the flatlands of India.25 Analysis of several freshwater sites concluded that an early summer season (April–June) with warm air temperatures was conducive to proliferation V. cholerae in the environment.25
Recent epidemiological observations point out that the northern region of the Indian sub-continent, which has no coastal connection, has endured several cholera epidemics26–29; obviously, environmental reservoirs of cholera bacteria causing outbreaks and prevalent transmission mechanisms in the region, can be concluded to be linked. In the Indus river basin, all locations of reported outbreaks have been within 25 miles of the nearest river: Delhi (Yamuna river), Lahore and Ludhiana (Sutlej river), Sialkot (Chenab river), Rawalpindi and Multan (Jhelum river), Peshawar (Kabul river), Dera Ismail Khan (Indus river), and Sirsa (Sirsa river). Rivers have long been recognized as an ecological corridor and habitat of cholera bacteria and its host, the copepod.1,7,22,30 Thus, warm air temperature, increased evaporation and water temperature, and decreased water levels in rivers, the primary source of water for household use in the region, are linked to cholera outbreaks. When there is a decrease in water level, an increase in salinity will occur.31 Increased salinity creates favorable environmental conditions for growth of cholera bacteria13,32–34 and, together with increased rainfall and poor sanitation, yields a higher likelihood of a cholera epidemic.
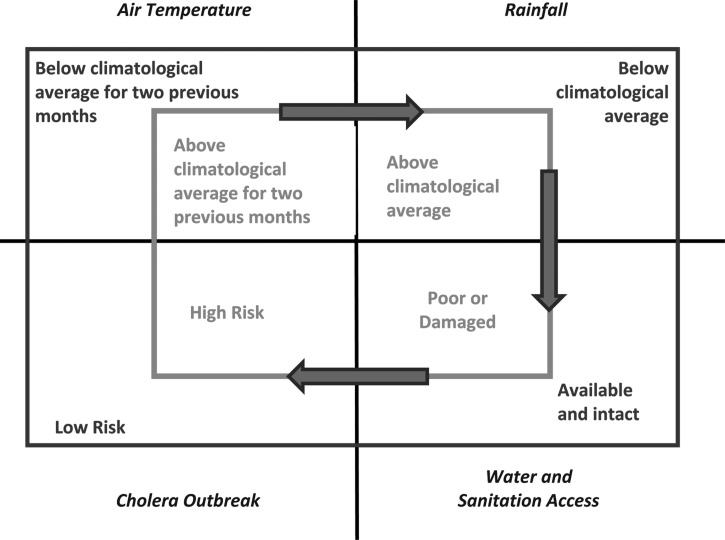
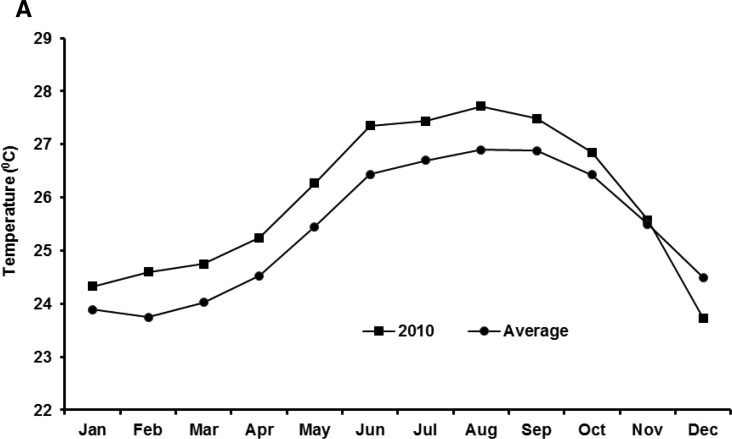
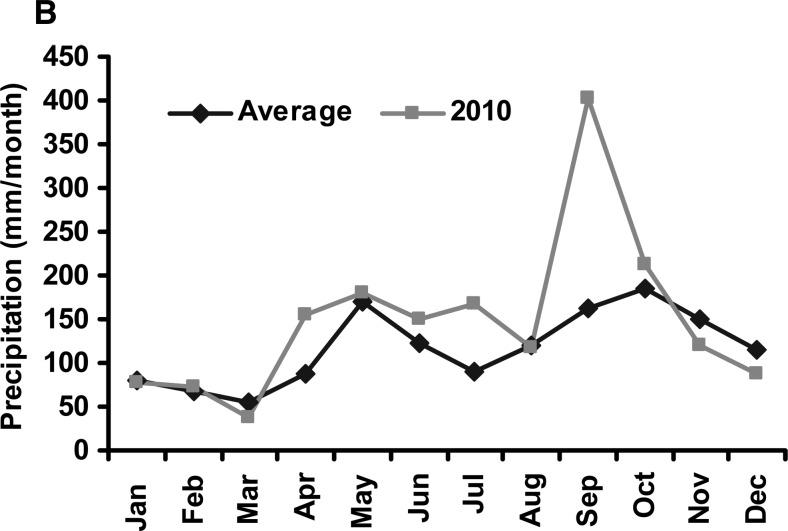
We present a theoretical pathway, shown in Figure 6, connecting large-scale hydroclimatological processes with cholera occurrence in epidemic regions. Two rectangles are depicted, with the inner rectangle representing conditions associated with cholera in an epidemic region. If air temperature is above the climatological average for 2 months, and is followed by above climatological average rainfall, in combination with poor or damaged water and sanitation access, it is probable that the region will experience a cholera epidemic. On the other hand, the outer rectangle, shown in Figure 6 , encapsulates conditions under which cholera generally does not occur. If any of the conditions of the inner circle are not met, the likelihood of cholera decreases. Two extreme case studies are offered as validation of this hypothesis. The recent outbreak of cholera in Haiti affected the entire population of the country and it followed a massive earthquake that damaged much of the region's already severely limited water and sanitation infrastructure. Following the earthquake, Haiti experienced above average warm air temperatures, in Figure 7A (representing 50 years of average air temperatures over Haiti taken from the National Oceanic and Atmospheric Administration [NOAA] reanalysis data) showing that air temperature in the year 2010 was one standard deviation higher than the long-term average. Similarly, as shown in Figure 7B Haiti received anomalously high rainfall during the months of September and October, 2010. The combination of these factors may be linked to the outbreak of cholera in Haiti during October 2010 (additional detail is provided in the section Cholera in Haiti).
Figure 6.
Theoretical framework for predicting cholera outbreaks in epidemic regions.
Figure 7.
(A) Air temperature in Haiti in 2010 and average of air temperature data for last 50 years. (B) Monthly rainfall in Haiti in 2010 compared with historical rainfall data.
Although the cholera outbreak in Haiti can be linked to destruction of water and sanitation infrastructure after the earthquake, and continued unhygienic living conditions in refugee camps, a similar disaster in Pakistan in 2005 reportedly did not lead to a massive cholera outbreak. In contrast, following the flooding that occurred in Pakistan during 2010, more than 600,000 people sought treatment of diarrheal disease. A key difference between the Pakistan 2005 and 2010 disasters was that environmental conditions fostering bacterial growth and proliferation in the latter instance were present, i.e., during 2010. The 2005 Pakistan flooding occurred just before winter in a mountainous region, with extremely cold conditions, not conducive to growth and spread of V. cholerae. Conversely, the August 2010 floods occurred in the flatlands of Pakistan with conditions enhancing propagation of cholera bacteria. A major cholera epidemic did not occur after the severe hurricane that battered the Gulf Coast of the United States in 2005 and this raises a question, namely that despite Hurricane Katrina, a cholera epidemic did not occur in Louisiana, a state severely impacted by the event. Over the past 5 years, a total of 44 cases of cholera have been reported in the United States, however none led to an outbreak and it is concluded that this is because robust water and sanitation infrastructure and effective prevention methods to halt the spread of the disease exist in the United States.
Cholera in Haiti
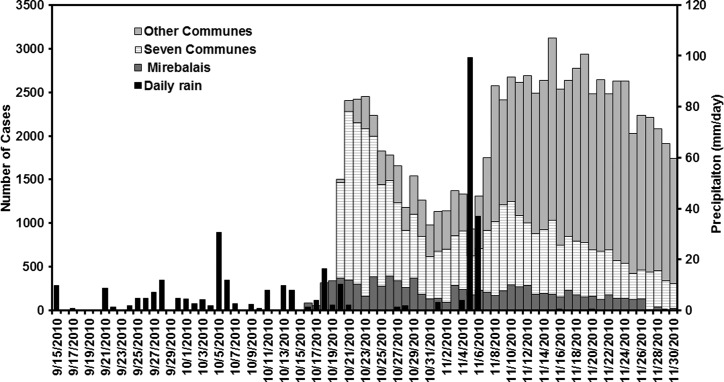
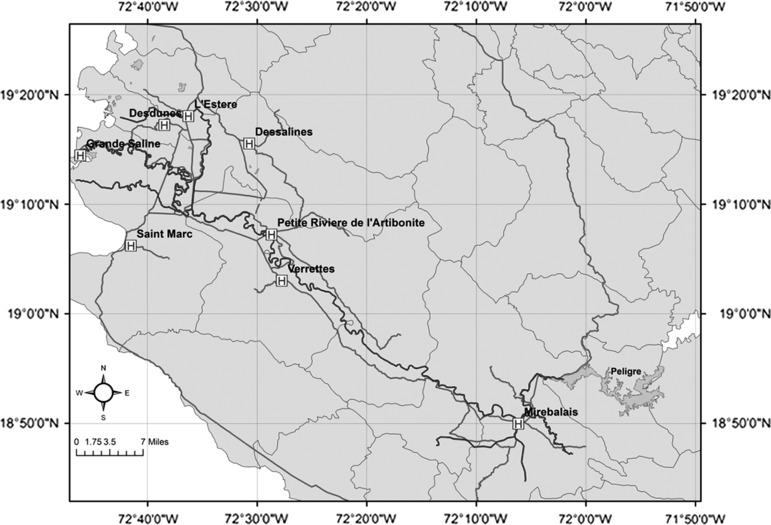
Cholera that broke out in Haiti following the massive earthquake of 2010 has been widely reported as potentially a human-driven outbreak, where United Nations (UN) peacekeepers from Nepal were assumed to be carriers of the V. cholerae 01.35,36 However, the role of regional hydroclimatological processes in the Haitian outbreak has not been addressed. The pathway presented in Figure 6 suggests environmental conditions were near optimal for an outbreak of cholera in Haiti. The air temperature analysis given in Figure 7A, shows high air temperature preceding the cholera outbreak in Haiti. In fact, the 2010 air temperature during the months preceding the cholera outbreak in Haiti was above the long-term climatological average by one standard deviation. The combination of a lack of safe water and sanitation system, elevated air temperatures, and above average rainfall (Figure 7B), produced conditions optimal for cholera by October 2010. For Haiti, we used daily cholera case data reported by Piarroux37 that comprises three time series: cholera cases in Mirebalais, total cases reported from seven communes (St-Marc, Dessalines, Desdunes, Grande Saline, Lestere, Petite-Rivière-de-l'Artibonite, Verrettes), and from other communes (Nord and Sue-Est) of Haiti (Figure 8 ). The first cholera case was reported on October 13, 2010 in the Mirebalais region (Meille) located near Artibonite River (Sack D, personal communication). On October 20, 2010, ∼1000 cases of cholera were recorded in seven communes downstream of the Artibonite River (Figure 9 ).
Figure 8.
Cholera cases in Mirebalais, seven communes (St-Marc, Dessalines, Desdunes, Grande Saline, Lestere, Petite-Rivière-de-l'Artibonite, Verrettes) and other communes (in Nord and Sue-Est) of Haiti (data obtained from Piarroux and others37). Daily rainfall data were obtained from the Tropical Rainfall Measuring Mission (TRMM) satellite (black).
Figure 9.
Location of health centers reporting cholera cases in communes along the Artibonite River on October 20, 2010, Haiti. MINUSTAH, United Nations Stabilization Mission in Haiti (extracted from Piarroux and others37).
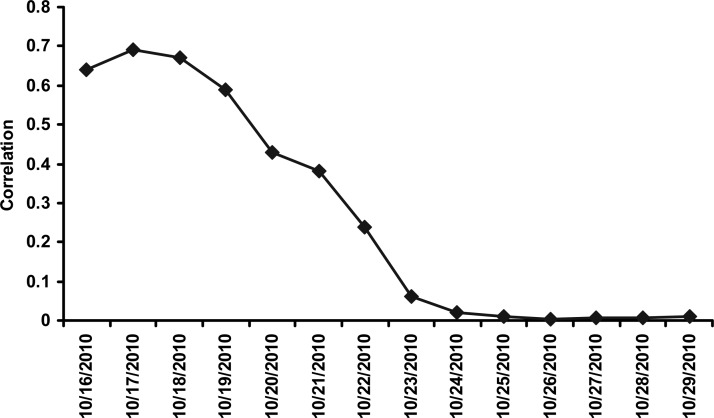
Daily precipitation data were obtained from Tropical Rainfall Measuring Mission (TRMM) satellite sensors over the Haiti region. A 6-day lagged Spearman rank correlation (Figure 10) between rainfall and cholera cases in Mirebalais and seven communes were 0.53 (P < 0.001) and 0.44 (P < 0.001), respectively, the highest statistically significant correlation over the course of 30 days. Given the fact that air temperatures were significantly high in 2010, the strong correlation between rainfall and cholera cases indicates rainfall is an important contributing factor in producing environmental conditions optimal for proliferation of V. cholerae in communities where water and sanitation infrastructure had been destroyed or were non-existent. In fact, Haiti received a total of 209 mm rainfall, with 26 rain days out of the 30 preceding the first reported cholera case (Figure 8). For the other communes, located far from the Artibonite River, correlation was not statistically significant and was expected to be weaker than the Mirebalais and seven downstream communes. Other communes are located on the northern and southern coasts of Haiti—which are farther from the Artibonite River. Hurricane Thomas lashed the region with heavy rainfall along the coast of Haiti on November 5–6, 2010, as shown in Figure 8. The surge in cholera cases in the other communes can be related to widespread inundation caused by the hurricane rainfall in combination with movement of the population over this time period to other regions. In fact, after the hurricane, a spike in cholera cases was observed in the other communes, whereas Mirebalais and the seven communes showed a relatively stable number of cholera cases.
Figure 10.
Thirty-day lagged correlation between daily precipitation and Mirebalais and seven Communes (data from October 16 to November 30, 2010).
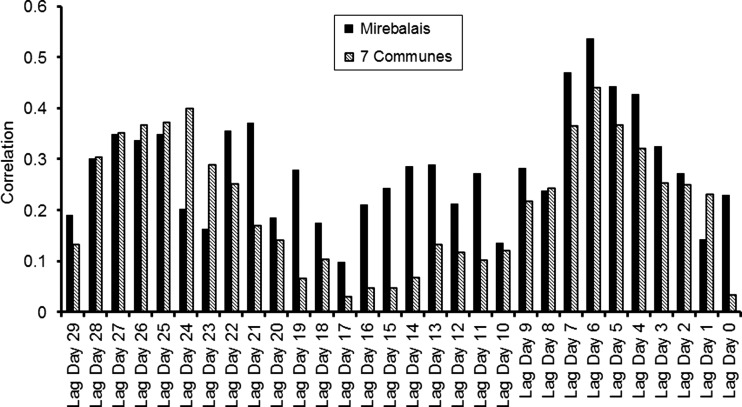
The data presented in Pairroux and others (2011) reported a strong correlation between cholera cases in Mirebalais and seven communes (Table 2 in Reference 37). They noted that cholera was reported in seven communes 5 days after the first cholera case was reported in Mirebalais (Figure 2 in Reference 37). However, counteracting statistical evidence is presented here to show that it is equally uncertain that spread of cholera occurred from Mirebalais to seven communes. Simultaneous Spearman rank correlation between the two time series, obtaining statistically significant values were calculated.37 However, when correlation is computed between Day 1 cholera case in Mirebalais with Day 6 cholera case in the seven communes, Day 1 Spearman correlation, indeed, is very high (0.65, P < 0.01; Figure 11). However, correlation decreases as lag increases, almost statistically insignificant at a lag of 5 days (0.40; P < 0.11). Simultaneous occurrence using time series of cholera cases in the Mirebalais and other communes is assumed to occur by the Artibonite River with the reasonable assumption that the Artibonite River is heavily contaminated with domestic waste, including human pathogens, e.g., V. cholerae 01. This assumption does not take into account the spatial spread of the disease, which is the secondary transmission mechanism.
Figure 11.
Lagged Spearman correlation between Mirebalais and seven communes.
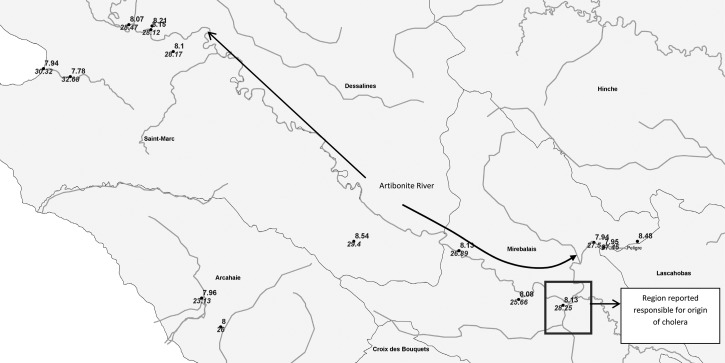
Complementary evidence, however, highlights the role of environmental processes related to cholera in Haiti. Overwhelming evidence in the literature shows that V. cholerae is autochthonous to the aquatic environment in both freshwater and estuarine systems25,38–41; previous studies have shown that the pH value of ∼8.013,42 and warm water temperatures between 19 and 28°C33,43 are related to an increased concentration of cholera bacteria. Hence, it is equally plausible that V. cholerae was already present in the aquatic environment of Haiti and that autochthonous V. cholerae played a role in the epidemic.3 The triggering point of the epidemic may well have been the convergence of optimal environmental conditions of warm temperatures, heavy rainfall followed by flooding (as evidenced from time series analysis of historical cholera in North India), and destruction of an already inadequate water and sanitation infrastructure. If true, we should then observe optimal pH and water temperatures in the Artibonite River during the month of October. In a field survey along the Artibonite River, October 11–28, 2011, the average pH and water temperature were 8.1 and 28.50°C, respectively (Figure 12), values within the optimal range for selective growth of V. cholerae. Previous research in Kolkata and Dhaka have shown that precipitation is associated with an increase in cholera cases most likely caused by the feeding of nutrient-rich runoff into water bodies and flooding of the water supply intended for human consumption with river water harboring V. cholerae.14,21
Figure 12.
In situ pH (ranges between 7 to 9) and water temperature (ranges between 23 to 33) observations collected between October 11 and 28, 2011.
Discussion and Summary
Cholera occurrence is characteristically different in epidemic and endemic regions. Epidemic cholera regions are located away from but within 25 miles of a major river system. The proposed hypotheses for cholera in epidemic regions are likely to be initiated during episodes of prevailing warm air temperature with low river flows, creating favorable environmental conditions for growth of cholera bacteria. Heavy rainfall, followed by inundation and destruction of sanitation infrastructure, accelerates interaction between contaminated water and human activities, resulting in an epidemic. Analysis of large-scale hydroclimatological factors related to outbreaks of cholera in epidemic regions globally was accomplished using 26 years of historical data for North India, enabling demonstration that a combination of warm air temperature, followed by heavy rainfall, and appropriate transmission mechanisms, are linked to, and very likely a trigger for cholera epidemics. The question remains, however, can epidemics of cholera in Haiti or other epidemic regions be predicted? Our theoretical framework (Figure 6) and analysis of historical cholera from the Indus Basin as well as Haiti and also inherent autochthonous nature of the cholera bacteria to aquatic environment, suggests that the cholera outbreak in Haiti bore strong environmental signatures. Because cholera has not been officially reported in that country for the past 50 years, an indigenous hypothesis is not out of the question because acute diarrheal patients in Haiti had not been tested for V. cholerae. Because individuals from other countries traveled to Haiti to provide aid and serve on relief missions at the time of earthquake, imported initiation was concluded.35 Analysis of strains from the recent Haiti outbreak showed similarity to strains from South Asia,35 but more recent strain data analysis indicates greater complexity in defining a definitive origin of the strain.3 Using a highly sensitive and discriminating microbiological approach, high prevalence of V. cholerae non-O1/O139 that may have contributed to the reported cholera cases in the first 3 weeks of the Haitian cholera epidemic were isolated from clinical cases of cholera. Using whole genome sequencing and subsequent genomic analysis, two distinct Vibrio populations, V. cholerae O1 and V. cholerae non-O1/O139, were identified and concluded to have contributed to the cholera epidemic in Haiti. Results of comprehensive genomic analysis and high resolution phylogenetic analysis showed that V. cholerae O1 populations were clonal, resembling epidemic isolates from South Asia and Africa, an observation in agreement with a possible single source of the V. cholerae 01. Vibrio cholerae non-O1/O139 populations, on the other hand, were not clonal, but resembled toxigenic V. cholerae O1 circulating in the Western hemisphere, indicating indigenous origin. Moreover, all of the clinical non-O1/O139 strains were phylogenetically placed into two closely related clusters within a monophyletic clade, data consistent with an “epidemic genotype” and indicating a role for the indigenous V. cholerae non-O1/O139 population, either alone or in concert with V. cholerae O1 in this epidemic. A similar role for V. cholerae non-O1/non-O139 strains was identified in a large-scale molecular analysis of strains isolated during a cholera outbreak in Kolkata, India.44 Interestingly, before the Haiti outbreak of cholera, the public health risk of human pathogens in surface waters of Haiti had not been evaluated and no reportable waterborne diseases were listed.45 Our recent collaboration with the United States CDC reported V. cholerae non-O1/O139, Vibrio parahaemolyticus, human pathogenic viruses, and Cryptosporidium spp. in samples collected from a river, canal, lake, and marine water in Haiti.46 Within this context, a single introduction of V. cholerae O1 may not explain the epidemic of cholera in Haiti in its entirety, because environmental conditions conducive to rapid growth and transmission of cholera bacteria, and the presence of an indigenous population of V. cholerae, may have played a role in the cholera epidemic in Haiti. Introduction alone cannot proceed to epidemic proportions without supporting conditions and transmission pathways. Ambient environmental conditions in the fall (September–October–November) season of 2010 and lack of sufficient safe water and sanitation infrastructure in Haiti after the earthquake provided a convergence in October, 2010, enabling the outbreak of cholera to become a lethal epidemic.
There is increasing evidence that proven cholera intervention strategies would greatly benefit from a predictive surveillance capability that would be able to identify vulnerable population groups at risk of imminent cholera outbreaks at regional scales.47–50 For example, if the strong likelihood of a new cholera epidemic in Port-Au-Prince is known two months in advance, such a warning will enable health authorities in Haiti to prepare for and minimize the impact of the elevated incidence of cholera in potentially vulnerable localities by implementing carefully planned prevention approaches. A reliable and robust cholera prediction model coupled with a spatio-temporal population vulnerability or risk map of potential cholera outbreaks will allow: Vaccination of vulnerable demographic groups (children and elders) in advance, mobilization of expert resources (physicians, nurses, and public health workers), allocation of material (water purification equipment and oral hydration therapy, implementation of water, sanitation, and hygiene (WASH) regulations and practices in regions at risk of outbreaks, and education and awareness campaigns for epidemic warnings and WASH compliance.23,30,47,49,51
The disease burden can be significantly reduced if the previous established prevention measures are implemented, proactively, ahead of impending outbreaks.30 Water purification improved sanitation,52 and filtration techniques53 have shown promise in reducing cholera infection at the point-of-use level, but scaling up such programs to national levels is not possible without sufficient lead time. Recent evidence indeed suggests that a planned implementation of WASH infrastructures in vulnerable localities can significantly reduce cholera prevalence; it has been effective in Haiti recently in bringing the overall case-fatality ratio down to < 2% from as high as 12% seen during the initial weeks.54 A tropical epidemic region, like Haiti, suffering a disaster devastating an already overburdened water and sanitation infrastructure at a time of the year when both climate and environmental conditions favor proliferation of V. cholerae, will be prone to an epidemic of disease. The challenge that remains is to develop a cholera prediction model for epidemic regions, identifying optimizing environmental conditions and incorporating enabling transmission mechanisms. When either a major natural disaster strikes a coastal town or significant civil disorder seriously damages living conditions of a population center in the tropical belt, a cholera tracking mechanism should be operational that monitors the climate and environment surrounding the vulnerable population by satellite remote sensing.1 With the knowledge of monitoring water and sanitation infrastructure, displaced populations and their convergence with environmental conditions and transmission pathways, a prediction capability of high probability can provide warning of potential cholera epidemics and allow mobilization of public health measures.
ACKNOWLEDGMENTS
Dr. Jutla acknowledges start-up from Statler College of Engineering and Mineral Resources, West Virginia University and NASA-EpSCOR programs. Dr. Jutla also thanks Juli Trtanj, Ocean and Human Health Innitiative-NOAA, for her in-kind assistance and support for this work. This work is also supported through grants from National Institutes of Health (2RO1A1039129-11A2) and National Oceanic and Atmospheric Admistration (NOAA-SO660009) to Dr. Colwell/Dr Huq. Further, ICDDR,B acknowledges its donors for providing unrestricted support to the Centre's research efforts.
Footnotes
Authors' addresses: Antarpreet Jutla, Department of Civil and Environmental Engineering, West Virginia University, Morgantown, WV, E-mail: asjutla@mail.wvu.edu. Elizabeth Whitcombe, Nur Hasan, Bradd Haley, Anwar Huq, and Rita Colwell, University of Maryland, Maryland Pathogen Research Institute, College Park, MD, E-mails: ewhitcombe@xtra.co.nz, nur.hasan@cosmosid.net, bradd.haley@gmail.com, huqanwar@gmail.com, and rcolwell@umiacs.umd.edu. Ali Akanda, University of Rhode Island, Civil and Environmental Engineering, Kingston, RI, E-mail: akanda@egr.uri.edu. Munir Alam, International Centre for Diarrheal Disease Research, Bangladesh – Microbiology, Dhaka, Bangladesh, E-mail: munirul@icddrb.org. R. Bradley Sack, Johns Hopkins School of Public Health, Department of International Health, Baltimore, MD, E-mail: rsack@jhsph.edu.
References
- 1.Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- 2.Stine OC, Alam M, Tang L, Nair GB, Siddique AK, Faruque SM, Huq A, Colwell R, Sack RB, Morris JG., Jr Seasonal cholera from multiple small outbreaks in rural Bangladesh. Emerg Infect Dis. 2008;14:831–833. doi: 10.3201/eid1405.071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasan NA, Choi S, Eppinger M, Clark PW, Chen A, Alam M, Haley BJ, Taviani E, Hine H, Su Q, Tallon LJ, Prosper JB, Furth K, Hoq MM, Li H, Fraser-Liggett C, Cravioto A, Huq A, Ravel J, Cebula T, Colwell RR. Genomic diversity of 2010 Haitian cholera outbreak strains. Proc Natl Acad Sci USA. 2012;109:E2010–E2017. doi: 10.1073/pnas.1207359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huq A, Colwell RR. Vibrios in the marine and estuarine environment: tracking Vibrio cholerae. Ecosyst Health. 1996;2:198–214. [Google Scholar]
- 5.Lobitz BM, Beck LR, Huq A, Wood B, Fuchs G, Faruque ASG, Colwell RR. Climate and infectious disease: use of remote sensing for detection. Proc Natl Acad Sci USA. 2000;97:1438–1443. doi: 10.1073/pnas.97.4.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffith DC, Kelly-Hope LA, Miller MA. Review of reported cholera outbreaks worldwide, 1995–2005. Am J Trop Med Hyg. 2006;75:973–977. [PubMed] [Google Scholar]
- 7.Akanda AS, Jutla AS, Islam S. Dual peak cholera transmission in Bengal Delta: a hydroclimatological explanation. Geophys Res Lett. 2009;36:L19401. [Google Scholar]
- 8.Jutla AS, Akanda AS, Griffiths J, Islam S, Colwell RR. Warming oceans, phytoplankton, and river discharge: implications for cholera outbreaks. Am J Trop Med Hyg. 2011;85:303–308. doi: 10.4269/ajtmh.2011.11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enserink M. Infectious diseases: Haiti's outbreak is latest in cholera's new global assault. Science. 2010;330:738–739. doi: 10.1126/science.330.6005.738. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control, Lesson 1: Introduction to Epidemiology. http://www.cdc.gov/osels/scientific_edu/ss1978/lesson1/section11.htm Available at. Accessed March 16, 2013.
- 11.Akanda AS, Jutla AS, de Magny GC, Alam M, Siddique AK, Sack RB, Huq A, Colwell RR, Islam S. Hydroclimatic influences on seasonal and spatial cholera transmission cycles: implications for public health intervention in the Bengal Delta. Water Resour Res. 2011a;47:W00H07. [Google Scholar]
- 12.Rinaldo A, Bertuzzo E, Mari L, Righetto L, Blokesch M, Gatto M, Rodriguez-Iturbe I. Reassessment of the 2010–2011 Haiti cholera outbreak and rainfall-driven multiseason projections. Proc Natl Acad Sci USA. 2012;109:6602–6607. doi: 10.1073/pnas.1203333109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huq A, Sack RB, Nizam A, Longini IM, Nair GB, Ali A, Morris JG, Khan MNH, Siddique AK, Yunus M, Albert MJ, Sack DA, Colwell RR. Critical factors influencing the occurrence of Vibrio cholerae in the environment of Bangladesh. Appl Environ Microbiol. 2005;71:4645–4654. doi: 10.1128/AEM.71.8.4645-4654.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constantin de Magny G, Murtugudde R, Sapianob M, Nizam A, Brown C, Busalacchi A, Yunus M, Nair G, Gil A, Calkins J, Manna B, Rajendran K, Bhattacharya M, Huq A, Sack R, Colwell RR. Environmental signatures associated with cholera epidemics. Proc Natl Acad Sci USA. 2008;105:17676–17681. doi: 10.1073/pnas.0809654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jutla AS, Akanda AS, Islam S. Satellite space-time variability of chlorophyll in Bay of Bengal: connections to cholera outbreaks. Remote Sens Environ. 2012;123:196–206. doi: 10.1016/j.rse.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertuzzo E, Casagrandi R, Gatto M, Rodriguez-Iturbe I, Rinaldo A. On spatially explicit models of cholera epidemics. J R Soc. Interface. 2009;7:321–333. doi: 10.1098/rsif.2009.0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertuzzo E, Azaele S, Maritan A, Gatto M, Rodriguez-Iturbe I, Rinaldo A. On the space-time evolution of a cholera epidemic. Water Resour Res. 2008;44:W01424. [Google Scholar]
- 18.Das S, Gupta S. Diversity of Vibrio cholerae strains isolated in Delhi, India, during 1992–2000. J Health Popul Nutr. 2005;23:44–51. [PubMed] [Google Scholar]
- 19.Bompangue Nkoko D, Giraudoux P, Plisnier PD, Tinda AM, Piarroux M, Sudre B, Horion S, Tamfum JJ, Ilunga BK, Piarroux R. Dynamics of cholera outbreaks in great lakes region of Africa, 1978–2008. Emerg Infect Dis. 2011;17:2026–2034. doi: 10.3201/eid1711.110170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashizume M, Armstrong B, Hajat S, Wagatsuma Y, Faruque AS, Hayashi T, Sack DA. The effect of rainfall on the incidence of cholera in Bangladesh. Epidemiology. 2008;19:103–110. doi: 10.1097/EDE.0b013e31815c09ea. [DOI] [PubMed] [Google Scholar]
- 21.Hashizume M, Faruque AS, Terao T, Yunus M, Streatfield K, Yamamoto T, Moji K. The Indian Ocean dipole and cholera incidence in Bangladesh: a time-series analysis. Environ Health Perspect. 2011;119:239–244. doi: 10.1289/ehp.1002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cash BA, Rodó X, Kinter JL., III Links between tropical pacific SST and cholera incidence in Bangladesh: role of the western tropical and central extratropical pacific. J Clim. 2009;22:1641–1660. [Google Scholar]
- 23.Reyburn R, Kim DR, Emch M, Khatib A, Von Seidlein L, Ali M. Climate variability and the outbreaks of cholera in Zanzibar, east Africa: a time series analysis. Am J Trop Med Hyg. 2011;84:862–869. doi: 10.4269/ajtmh.2011.10-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gurbanov S, Akhmadov R, Shamkhalova G, Akhmadova S, Haley BJ, Colwell RR, Huq A. Occurrence of Vibrio cholerae in municipal and natural waters and incidence of cholera in Azerbaijan. EcoHealth. 2012;8:468–477. doi: 10.1007/s10393-012-0756-8. [DOI] [PubMed] [Google Scholar]
- 25.Mishra A, Taneja N, Sharma RK, Kumar R, Sharma NC, Sharma M. Amplified fragment length polymorphism of clinical and environmental Vibrio cholerae from a freshwater environment in a cholera-endemic area, India. BMC Infect Dis. 2011;11:249. doi: 10.1186/1471-2334-11-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taneja N, Biswal M, Tarai B, Sharma M. Emergence of Vibrio cholerae O1 biotype ElTor serotype Inaba in North India. Jpn J Infect Dis. 2005;58:238–240. [PubMed] [Google Scholar]
- 27.Taneja N, Kaur J, Sharma K, Singh M, Kalra JK, Sharma NM, Sharma M. A recent outbreak of cholera due to Vibrio cholerae O1 Ogawa in and around Chandigarh, North India. Indian J Med Res. 2003;117:243–246. [PubMed] [Google Scholar]
- 28.Taneja N, Mishra A, Sangar G, Singh G, Sharma M. Cholera outbreaks in north India due to new variants of Vibrio cholerae O1 El Tor. Emerg Infect Dis. 2009;15:352. doi: 10.3201/eid1502.080943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taneja N, Samanta P, Mishra A, Sharma M. Emergence of tetracycline resistance in Vibrio cholerae O1 biotype ElTor serotype Ogawa from North India. Indian J Pathol Microbiol. 2010;53:865–866. doi: 10.4103/0377-4929.72014. [DOI] [PubMed] [Google Scholar]
- 30.Akanda AS, Jutla AS, Gute DM, Evans T, Islam S. Reinforcing cholera intervention with prediction aided prevention. Bull World Health Organ. 2012;90:243–244. doi: 10.2471/BLT.11.092189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson D, Dettinger M, Cayan D, Dileo J, Isaacs C, Smith R. River salinity variations in response to discharge: Examples from Western United Stated during the early 1900s. In: Isaacs C, Tharp V, editors. Proceedings of the Twelfth Annual Pacific Workshop Interagency Ecological Program. Technical Report 46. California Department of Water Resources; 1996. pp. 145–153. [Google Scholar]
- 32.Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Louis VR, Russek-Cohen E, Choopun N, Rivera IN, Gangle B, Jiang SC, Rubin A, Patz J, Huq A, Colwell RR. Predictability of Vibrio cholerae in Chesapeake Bay. Appl Environ Microbiol. 2003;69:2773–2785. doi: 10.1128/AEM.69.5.2773-2785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Islam MS, Mahmuda S, Morshed MG, Bakht HB, Khan MN, Sack RB, Sack DA. Role of cyanobacteria in the persistence of Vibrio cholerae O139 in saline microcosms. Can J Microbiol. 2004;50:127–131. doi: 10.1139/w03-114. [DOI] [PubMed] [Google Scholar]
- 35.Chin C, Sorenson J, Harris JB, Robins WP, Charles RC, Jean-Charles RR, Bullard J, Webster DR, Kasarskis A, Peluso P, Paxinos EE, Yamaichi Y, Calderwood SB, Mekalanos JJ, Schadt EE, Waldor MK. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enserink M. Haiti's cholera outbreak. Cholera linked to UN Forces, but questions remain. Science. 2011;332:776–777. doi: 10.1126/science.332.6031.776. [DOI] [PubMed] [Google Scholar]
- 37.Piarroux R, Barrais R, Faucher B, Haus R, Piarroux M, Gaudart J, Magloire R, Raoult D. Understanding the cholera epidemic, Haiti. Emerg Infect Dis. 2011;17:1161–1167. doi: 10.3201/eid1707.110059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haley BJ, Chen A, Grim CJ, Clark P, Diaz CM, Taviani E, Hasan N, Sancomb E, Elnemr W, Islam M, Huq A, Colwell R, Benediktsdóttir E. Vibrio cholerae in a historically cholera-free country. Environmental Microbiology Reports. 2012;4:381–389. doi: 10.1111/j.1758-2229.2012.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grim CJ, Zo Y, Hasan NA, Ali A, Chowdhury WB, Islam A, Rashid MH, Alam M, Morris JG, Jr, Huq A, Colwell RR. RNA colony blot hybridization method for enumeration of culturable Vibrio cholerae and Vibrio mimicus bacteria. Appl Environ Microbiol. 2009;75:5439–5444. doi: 10.1128/AEM.02007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro RL, Otieno MR, Adcock PM, Phillips-Howard PA, Hawley WA, Kumar L, Waiyaki P, Nahlen BL, Slutsker L. Transmission of epidemic Vibrio cholerae O1 in rural western Kenya associated with drinking water from Lake Victoria: an environmental reservoir for cholera? Am J Trop Med Hyg. 1999;60:271–276. doi: 10.4269/ajtmh.1999.60.271. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh AR, Koley H, De D, Garg S, Bhattacharya MK, Bhattacharya SK, Manna B, Nair G, Shimada T, Takeda T. Incidence and toxigenicity of Vibrio cholerae in a freshwater lake during the epidemic of cholera caused by serogroup O139 Bengal in Calcutta, India. FEMS Microbiol Ecol. 1994;14:285–292. [Google Scholar]
- 42.Huq A, West PA, Small EB, Huq MI, Colwell RR. Influence of water temperature, salinity, and ph on survival and growth of toxigenic Vibrio cholerae Serovar O1 associated with live copepods in laboratory microcosms. Appl Environ Microbiol. 1984;48:420–424. doi: 10.1128/aem.48.2.420-424.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hood MA, Winter PA. Attachment of Vibrio cholerae under various environmental conditions and to selected substrates. FEMS Microbiol Ecol. 1997;22:215–223. [Google Scholar]
- 44.Chatterjee S, Ghosh K, Raychoudhuri A, Chowdhury G, Bhattacharya MK, Mukhopadhyay AK, Ramamurthy T, Bhattacharya SK, Klose KE, Nandy RK. Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata. Indian Journal of Clinical Microbiology. 2009;47:1087–1095. doi: 10.1128/JCM.02026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control Launching a National Surveillance System after an Earthquake. 2013. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5930a1.htm Available at. Accessed April 5, 2013.
- 46.Kahler AM, Haley BJ, Mull BJ, Narayanan J, Chen A, Purcell NC, Colwell RR, Huq A, Hill VR. 112th General Meeting of the American Society for Microbiology; June 16–19, 2012; San Francisco, CA. 2012. p. 2493. (abstr) [Google Scholar]
- 47.Ivers LC, Farmer P, Almazor C, Léandre F. Five complementary interventions to slow cholera: Haiti. Lancet. 2010;376:2048–2051. doi: 10.1016/S0140-6736(10)62243-X. [DOI] [PubMed] [Google Scholar]
- 48.Sack DA, Sack RB, Chaignat C-L. Getting serious about cholera. N Engl J Med. 2006;355:649–651. doi: 10.1056/NEJMp068144. [DOI] [PubMed] [Google Scholar]
- 49.Antarpreet S, Jutla, Ali S, Akanda, Shafiqul Islam. A framework for predicting endemic cholera using satellite derived environmental determinants. Environmental Modelling & Software. 2013;47:148–158. [Google Scholar]
- 50.Jutla AS, Akanda AS, Huq A, Faruque A, Colwell R, Islam S. A water marker monitored by satellites to predict endemic cholera. Remote Sensing Letters. 2013;4:822–831. doi: 10.1080/2150704X.2013.802097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barreto ML, Genser B, Strina A, Teixeira MG, Assis AM, Rego RF, Teles CA, Prado MS, Matos SM, Santos DN, dos Santos LA, Cairncross S. Effect of city-wide sanitation programme on reduction in rate of childhood diarrhoea in northeast Brazil: assessment by two cohort studies. Lancet. 2007;370:1622–1628. doi: 10.1016/S0140-6736(07)61638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bartram J. Flowing away: water and health opportunities. Bull World Health Organ. 2008;86:2. doi: 10.2471/BLT.07.049619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colwell R. Cholera outbreaks and ocean climate social research. An International Quarterly. 2006;73:753–760. [Google Scholar]
- 54.USAID (United States Agency for International Development) Haiti–Cholera. Fact Sheet 22 2011 [Google Scholar]