Abstract
This exploratory study examines family sleep patterns and quality in a setting of normative napping and co-sleeping. Participants comprised 78 members of 16 families from two locales in Egypt, Cairo and village. Each family member provided a history of sleeping arrangements, one week of continuous activity records, and details of each sleep event. Sleep records documented late-onset and dispersed sleep patterns with extensive co-sleeping. Of recorded sleep events, 69% involved co-sleeping, 24% included more than one co-sleeper, and only 21% were solitary. Mid-late afternoon napping occurred on 31% of days and night sleep onsets averaged after midnight. Age and gender structured sleep arrangements and together with locale, extensively explained sleep behavior (onset, duration, total) and quality. Co-sleepers had fewer night arousals, shorter and less variable night sleep duration, and less total sleep. Increased solitary sleep in adolescents and young adults was associated with increased sleep dysregulation, including exaggerated phase shifts in males and more nighttime arousals in females. Where normative, co-sleeping may provide psychosensory stimuli that moderate arousal and stabilize sleep. Such moderating features may address important self-regulatory developmental needs during adolescence.
Keywords: co-sleeping, bed sharing, sleep quality, adolescence, cultural differences
Sleep and sociality typically have been regarded as distinctive if mutually influential activities. Thus, family dynamics, social pursuits, and the wider context established by work, socioeconomic, neighborhood, and ecologic (climate, housing) conditions all have been considered with respect to how they interfere, support, or compete with sleep quality and quantity (Wolfson, 1996). Comparative ethnographic data suggest an alternate view by indicating that sleep is construed as a form of social behavior in many societies (Worthman & Melby, 2002). In such contexts, co-sleeping is regarded as expectable, protective, comforting, and integral to foundational relationships and emotional patterns of family life (Welles-Nystrom, 2005; Yang & Hahn, 2002). In these settings, formal bedtimes may be absent and napping more prevalent. Added to a more dispersed or distributed sleep pattern is greater latitude for flexibility in sleep schedule, both within and among individuals. One concern about such cross-cultural findings has been that these practices may characterize small, marginalized or historical populations, but do not generalize to contemporary urban society. Others have pointed to evidence that traditional practices such as co-sleeping may not be universally beneficial, including increased sleep disturbance related to co-sleeping in infancy (Hunsley & Thoman, 2001) and childhood (Hayes, Parker, Sallinen, & Davare, 2001; D. Liu, Caldji, Sharma, Plotsky, & Meaney, 2000). Contravening this view, a longitudinal study of American families identified no long-term negative and few positive impacts of early co-sleeping (Okami, Weisner, & Olmstead, 2002).
Against this background of uncertainty about optimal sleep contexts at different stages of the life course, the particular lack of a family systems approach stands out. Accordingly, we report an exploratory family sleep study in Egypt, which has one of the longest histories of urbanized, complex, cosmopolitan society, exhibits the circum-Mediterranean bimodal sleep pattern, offers both very high and moderate density settlement patterns, and maintains a tradition of co-sleeping.
Social Relations, Sleeping Arrangements, and Sleep Quality
Emerging understandings of social relationships and health underscore the importance of their affective quality over mere physical presence (Kiecolt-Glaser & Newton, 2001; Uchino, Cacioppo, & Kiecolt-Glaser, 1996). The same is true of sleep. From infancy onward, the quality of social life influences the quality of sleep (Wolfson, 1996). Poorly attached infants, children living with family stress (marital conflict, maternal depression, family disorganization; Benoit, Zeanah, Boucher, & Minde, 1992; El-Sheikh, Buckhalt, Mize, & Acebo, 2006; Gregory, Ely, O’Connor, Rijsdijk, & Plomin, 2005; Sadeh, Raviv, & Gruber, 2000), evaluation stress in young adults (Sadeh, Keinan, & Daon, 2004), and marital conflict (Kiecolt-Glaser & Newton, 2001), attachment anxiety (Carmichael & Reis, 2005), or loneliness among adults (Cacioppo et al., 2002) all are associated with sleep disturbances. In part, such disturbances may be related to the disruptive effects of social disorganization on daily schedules and sleep habits (Seifer, Sameroff, Dickstein, & Hayden, 1997). But poor sleep may arise from the cognitive-affective impact of disturbed social relationships upon arousal-regulating mechanisms that drive sleep-wake patterns. Hence, Sadeh and colleagues (Sadeh et al., 2004) have suggested that observed effects of stress on sleep reflect two stages in sleep-wake responses to stress, one of fragmented or curtailed sleep related to alarm, hypervigilance, and activation, and a second, of hypersomnia related to disengagement, withdrawal, and shutdown. The influence of cognitive-affective dynamics on regulation of arousal and sleep is further underscored by evidence for substantial placebo effects in pharmacologic treatment for sleep problems. In a small crossover-design study of young adult female university students with reported sleep difficulty, objective and subjective measures of sleep quality were significantly improved on placebo over non-placebo nights (Fratello et al., 2005).
Although positive social relations and social security outside the sleep setting demonstrably enhance sleep quality, the effects of social factors within that setting remain contested or poorly understood. The health and developmental consequences of co-sleeping in infancy and early childhood have been a focus of intense scrutiny and debate, particularly with regard to a contributory role in infant mortality (McKenna & McDade, 2005; Task Force on Sudden Infant Death Syndrome, 2005). In middle childhood, bed-sharing, but not co-rooming, has been associated with increased sleep problems (Blader, Kopleicz, Abikoff, & Foley, 1997). In contrast to these findings, an eighteen-year longitudinal study of American families has shown no negative and few positive effects of bed-sharing in infancy and childhood on psychobehavioral outcomes (Okami et al., 2002).
Relationships as Regulators: The Role of Sleep
Against a background of contestation, one circumstance calls attention to a likely neutral or positive value of co-sleeping for development and sleep regulation. Historical and ethnographic records document habitual co-sleeping from birth as commonplace (McKenna, Thoman, Anders, Sadeh, Schechtman, & Glotzbach, 1993; Worthman & Melby, 2002). Indeed, co-sleeping may qualify as the most intimate behavior that may be shared by partners of all genders and ages. By sharing their sleeping hours, co-sleepers in close body contact share space, air, warmth, and time (one-third of the day) during a critical chronobiological period. Such shared experience creates a space for mutual regulation (McKenna, Mosko, Dungy, & McAnninch, 1991). Work among rodents has demonstrated the organizational developmental roles of expectable environments of rearing and functioning, particularly maternal behavior and early postnatal conditions, on many developing systems, including those regulating arousal and affect (Caldji, Diorio, & Meaney, 2000; Meaney, 2001). Thus, Hofer (Hofer, 1978, 1984, 1994) has suggested that relationships act as regulators that inform development and shape adult function. Different developmental periods thus present particular opportunities and vulnerabilities to contextual cues that drive regulation of affect and arousal (Crawford, 1994; McKenna, 2000).
Adolescence appears to be one such developmental period when cognitive-affective arousal regulation undergoes heightened sensitivity to contextual cues. During adolescence, brain development leads to significant maturation of emotional-cognitive processes that regulate behavior. Grey matter volume, and inferentially number of neuronal cell bodies, peaks and then declines during adolescence at ages that vary by region and individual (Giedd, 2004). Giedd and colleagues (Giedd et al., 1999) therefore have suggested that synaptic production and pruning during adolescence may create a sensitive period for influence from factors shaping experience, namely context and adolescent behavior. This period may extend into early adulthood, as exemplified in the dorsal prefrontal cortex, a region related to impulse control which continues to mature into the twenties. Dahl (2002) has emphasized the potential importance of poor sleep practices for impairing adolescent maturation of cognitive-affective processes crucial to social competence. Added to the evidence for a phase shift in adolescent sleep patterns (Carskadon, Vieira, & Acebo, 1993), these arguments suggest the importance of ecological factors such as family sleep practices for adolescent emotional-cognitive development and expand our attention to the impact of such practices beyond infancy. However, the cross-cultural applicability of such constructs and expectations has not been thoroughly tested. The present study represents a preliminary step in this direction by exploring the social ecology of sleep and sleep practices among Egyptian families.
Cultural Variation in Regulation of Sleep and Family Processes
Although most available literature concerns western postindustrial populations, recent work has addressed greater ecological and cultural diversity. Reports document normative napping and co-sleeping in populations as diverse as agrarian Brazil (Reimao et al., 2000b; Reimao, Souza, Medeiros, & Almirao, 1998) and urban China or Japan (X. Liu, Liu, Owens, & Kaplan, 2005; Park, Matsumoto, Shinkoda, Nagashima, Kang, & Seo, 2001). Findings contrasting with extant literature on American and European populations concerning relationships of sleep practices to sleep outcomes include reduced rates of insomnia in Brazil (Reimao et al., 2000a), increased rates of sleep problems in China (X. Liu et al., 2005), and a balance of nap and nighttime sleep in Japan (Fukuda & Ishihara, 2002) that each reflect a distinctive facet of social ecology.
One potential factor underlying these diverse patterns is the cognitive-affective impact of co-sleeping on arousal regulation. Consequently, the social meaning of co-sleep merits consideration. Sleeping companions–who sleeps with whom–mirror and cement social relationships and social structure (Caudill & Plath, 1986). Membership in a social unit (e.g., family, couple, age set, platoon) commonly determines sleeping partners. Social regulation of who sleeps with whom reflects the moral order (Shweder, Jensen, & Goldstein, 1995) and core values around belonging, identity, care, and intimacy (Ben-Ari, 1996). Thus, mothers sleep with infants for their protection, nurture, and comfort. Whether and when husbands and wives sleep together depends on culturally defined forms and purposes of marriage, including marital relations, sexual mores, and gender roles related to work and parenting. Hence, sleeping arrangements map social identity and belonging. The effects of social relations on affective-cognitive processes regulating arousal further suggest that the quality of these relations may moderate the impact of co-sleeping on sleep quality. On the one hand, secure or affectionate relations may conduce to positive impact of co-sleeping, while hostile or alienated relations with co-sleepers may exacerbate sleep difficulties. Furthermore, co-sleeping has been associated with parental attempts to address child psychological distress or physical illness (Hayes et al., 2001; X. Liu et al., 2005; X. Liu, Liu, & Wang, 2003; Lozoff, Askew, & Wolf, 1996), which complicates interpretation of causality in associations between sleeping arrangements and outcomes.
The period of puberty and adolescence is marked by altered sleep arrangements. Sexual propriety generally prohibits co-sleeping by post-pubertal brothers and sisters or children and parents. As shown by cross-cultural evidence, adolescents frequently are moved into separate beds or sleeping spaces (Worthman & Melby, 2002). Thus, adolescents are less likely to co-sleep. In a representative example, bed sharing among urban Chinese decreased from 56% in 7 year-olds to 7% in 11–13 year-olds (X. Liu et al., 2003). Such comparative evidence suggests that adolescents may be more at risk for social isolation in sleep at a developmental stage when social context plays a particularly powerful role in the regulation of arousal and sleep-wake states.
Here, we report data from an exploratory study of sleep and activity patterns in two focal samples of Egyptian families. We evaluate several hypotheses. 1. Contemporary Egyptian families exhibit flexible, distributed sleep schedules in a context of co-sleeping; 2. Co-sleeping is associated with better sleep as measured by sleep duration and arousals; 3. The impact of sleep conditions is more pronounced among adolescents compared to family members of other ages.
Method
Study Sites and Participants
A convenience sample of 16 families was recruited from two locales, one urban (Cairo) and one village (Mahallat Marhum, Tanta District, Lower Egypt). Participating families were recruited through fliers and direct solicitation in the city and by word of mouth in the village. Exclusion criteria included presence of infant under age two years, chronic illness, and use of sleep medication. The two subsamples comprised 10 urban and 6 village households. Urban household size averaged 4.7 persons, range 3 – 8, n = 46 persons; rural averaged 5.0, range 3 – 7, n = 32 persons. Gender composition of the sample was balanced, with 51% male. Cairo is a densely populated city (171 persons per square mile) estimated in 2001 as having 17.3 million inhabitants. Although not strictly rural (population around 60,000), Mahallat Marhum is officially considered a village and includes a high proportion of families that pursue farming on a part- or full-time basis and retains much of its village character. Families in Cairo resided in apartments (n = 8) or semi-detached dwellings (n = 2), while those in Mahallat Marhum resided in individual non-detached dwellings. SES of the relatively affluent and educated participating families approximated equivalence at each site, comprising trades (printer, truck driver, shopkeeper) to professions (teacher, bureaucrat, nurse, doctor) in the city, and landholding farmers to professionals (accountant, teachers) in the village.
As for most societies, sleep practices in Egypt are poorly documented, although substantial earlier (village: Ammar, 1966; Fakhouri, 1972; Cairo: Kader, 1987; Shoshan, 1993) and contemporary (village: Abu Lughod, 1983; Morsy, 1993; Hopkins, 1987, 1998; Cairo: Early, 1993; Hoodfar, 1997; Wikan, 1980) ethnographic literatures on domestic village and urban life are available. A well-recognized feature of Egyptian sleep practice common among circum-Mediterranean peoples is biphasic sleep distributed in afternoon and late night bouts. Similar to other groups living in climates with high mid-day heat, Egyptians eat the main meal of the day around mid-afternoon, then nap in the late afternoon. In the evening, activity picks up again, including shopping/trading, socializing, and some work. The night bout of sleep begins sometime after midnight and ends after dawn. Urban and rural settings provide a series of contrasts. Intense heat entrains this pattern in summer: during the study period, Cairo maximum temperature averaged 91–97°F, and minima averaged 63–70°F. Cairo has extremely high population densities: crowding and cramped living conditions are endured by all but the most affluent. Large families live in small apartments or even single rooms, sleeping together on sofas or platform beds around the walls and on mats in the center of the room. Co-sleeping is ubiquitous and maintenance of sleep must occur despite the bustle of family life, particularly for afternoon napping. External and internal noise levels can be substantial. The city is thoroughly electrified and offers a huge range of nighttime attractions for trade, entertainment, or social activity. Class markedly modulates levels of crowding, noise, pollution, and amenities such as electric fans.
Our village site is located in the densely settled Nile delta of Lower Egypt. Affluent Nile delta farming villages have relatively large population sizes and high densities. Nucleated families live on floors of extended family compounds. Families often sleep in one room, with parents and children on the platform bed, and adolescents on floor mats or divans. The animals’ stable is directly adjacent. Such villages have fitful electrical supply, little or no sanitation or air conditioning, and a lower density of TV and other diversions. Sleep is bimodal also, again due to heat, but the fall of darkness ushers longer nights and more extended night sleep than seen in Cairo. Under such conditions, we expected multiple and multi-age sleeping partners, frequent presence of animals, integration of sleep in ongoing social activity, and fluid bed- and wake times.
Procedures
Focal families were each studied for one week during the interval of July 3 – August 4, 2000, beginning just after school year’s end. The period was selected to represent sleep when family schedules are not dictated by school, and when heat is strong yet not at its most severe. Measures included questionnaire, structured and non-structured interview, physiologic recording, and activity records to characterize sleep habits, beliefs, architecture, and conditions of average Egyptian families. Data were collected by combined teams of American and Egyptian investigators: the former were the anthropologist authors of this report and the latter were physicians in the Field Epidemiology Training Program in the Ministry of Health. On average, four visits were made to each family and conducted primarily in Arabic. The initial visit was to obtain consent, collect background information, and explain the activity record; collection of activity records by the family started on the following day. The second visit occurred two days after the first: at that time, most questionnaires were completed, the first day’s activity record was reviewed and feedback given to participants, including the family member acting as activity data recorder. The third visit occurred three days after the second: it focused on review of activity records and documention of sleep events. The fourth and final visit occurred three days later, or the day after collection of activity records was complete. Families were compensated for their participation time with gifts in value to $50. Such compensation was set by experienced field epidemiologists in the Egyptian Ministry of Health to be appropriate yet not coercive.
Measures
Individual Sleep History
Each household member provided a detailed history of sleeping arrangements, from infancy to the present. Interviewers asked about duration of breastfeeding and co-sleeping with parent or parents in infancy, rooming and sleeping arrangements thereafter, age at marriage and sleeping arrangements after marriage, arrangements after the arrival of each successive child, and adjustments to arrangements with changes in housing or the maturation of children.
Family Activity Record
We collected continuous 24-hour activity records to maximize the likelihood of capturing all sleep bouts, concurrently for all household members during a 7-day period. Participants reported all activities that occurred, using 10 reporting options (working, chatting/visiting, praying, not at home, eating, drink coffee/tea, lying down, sitting/resting, awake, asleep) with icon modifiers (e.g., cross denoting end of activity, box indicating media use), for each 15-minute time block over the 7-day period. Records were made using specifically designed, culturally appropriate inked stamps of icons for each activity, which obviated a demand for literacy, minimized linguistic ambiguity, and enhanced the ease of recording. At the outset, household members agreed upon a designated recorder who was mandated to ensure complete, continuous collection of activity by each household member. Adults and adolescents in the study filled out the schedules largely on their own. The facilitator was mandated by family members to remind them of the need to maintain records and to keep track of recording materials. In the case of younger children, the facilitator (in four cases, the mother) also was mandated to perform or assist with data entry by the child. Most designated recorders (mean age 19.31 years, range 13 – 31) were adolescents or young adults who assiduously performed this task. All recorders and most participants were literate.
Interviewers initially trained participants and the designated recorder in the use of the stamps and time grid, the meaning of the activity categories, and the procedures for data entry. An explanatory sheet of icons and examples, in Arabic, was provided for ongoing consultation. Such study material was clipped to a board on which the tray for stamps and inkpad was mounted. Participants were then asked to update their schedules at least three times per day (prayer hours were tagged as reminders). The frequency of data entry reported by recorders varied considerably, from nearly continuous entry for each observable household member by some, to the few who missed certain days entirely for specific family members and reconstructed them later. Such variation was linked to accessibility to the person for whom the record was made: parents working outside the home were debriefed mornings and evenings. Nevertheless, participants reported no difficulty recalling relevant information at the time of data entry, largely because the activity categories were rather general. Additionally, participants were allowed to confer with each other regarding their own schedules, and cooperate as needed to update the schedules for all household members.
Periodic Schedule Debriefing
On the second, fifth, and eighth day after activity recording commenced, interviewers visited and reviewed aloud every 15-minute block of data directly with participants or with recorders, consulting participants as needed. We also elicited details for each identified sleep event, including location, companions, and arousals. Interviewers were trained to probe for common errors and resolve them in the debriefing. Such debriefing permitted correction by participants of most data recording errors, whether technical (e.g., confusion among activity categories) or substantive (e.g., garbled recall of a portion of the day during initial data entry). Corrections were elicited when the interviewer detected internal discrepancies within a participant’s record, discrepancies among reports of disparate family members, or gaps in the schedule. Corrections also occurred when participants, upon oral review of their record, realized that the interviewer had drawn erroneous conclusions about what actually happened. Periodic schedule debriefing furthermore permitted collection of detailed ethnographic information, by soliciting specifics and reasons for schedule patterns and sleeping arrangements.
Data analysis
Data for this report are drawn from sleep event records and reports for the daily activity schedules. Daily activity is punctuated by five prayer times, dawn (~4:30 AM), midday (~1 PM), afternoon (~4:30 PM), sunset (~6:30 PM), and evening (9:30 PM). Therefore, the sleep day was defined as the 24-hour interval from 4:00 AM to 4:00 AM on adjacent days. Days where the record for this interval was incomplete were excluded from analysis (60 sleep events excluded), to yield 469 complete sleep days that comprised 622 total sleep events. Gender representation in the final dataset was balanced (52.6% of events from females). For calculations of total sleep per day, events were assigned to the sleep day on which the event began. Nighttime sleep bouts were defined as sleep events that commenced between 8:00 PM and 5:00 AM. Thus, a given sleep day possibly could be coded with no or two bouts of nighttime sleep if sleep patterns were extremely shifted. Afternoon naps were defined as sleep events with onset between 12:00 PM and 8:00 PM.
All analyses were performed in STATA 9.1 and used frequency weights for unbalanced repeated individual measures wherever appropriate. Clock time is reported in 24-hour time.
Results
Summary data for our sample of Egyptian families regarding sleep patterns and budgets by gender, age, sleeping arrangement, and site are provided in Table 1. We present results organized with regard to study hypotheses.
Table 1.
Sleep Patterns in Egyptian Families (Means and Standard Deviations) and by Gender, Age, and Sample Site (n=622)
| Night
|
Day
|
Total Sleep
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| onset | offset | duration | arousals | onset | offset | duration | per day | |||||||||
|
|
|
|
||||||||||||||
| mean time | SD, hours | mean time | SD, hours | mean hours | SD | mean n | SD | mean time | SD, hours | mean time | SD, hours | mean hours | SD | mean hours | SD | |
|
|
|
|
||||||||||||||
| All | 0:45 | 1.8 | 8:32 | 2.6 | 7.6 | 2.2 | 0.7 | 1.1 | 15:45 | 1.5 | 17:29 | 1.8 | 1.8 | 1.0 | 8.4 | 2.6 |
| Gender | ||||||||||||||||
| Female | 0:24 | 1.7 | 8:32 | 2.5 | 7.9 | 2.2 | 0.8 | 1.1 | 15:48 | 1.7 | 17:24 | 1.6 | 1.8 | 1.0 | 8.8 | 3.1 |
| Male | 1:02 | 1.8 | 8:36 | 3.0 | 7.4 | 2.2 | 0.6 | 1.2 | 15:38 | 1.4 | 17:29 | 2.1 | 1.8 | 1.1 | 8.0 | 2.5 |
| Age, years | ||||||||||||||||
| 2–10 | 23:20 | 1.4 | 8:56 | 1.2 | 9.8 | 1.9 | 0.9 | 1.6 | 15:11 | 1.3 | 17:19 | 1.7 | 2.1 | 0.8 | 11.0 | 3.6 |
| 10–20 | 1:03 | 1.8 | 9:31 | 2.2 | 8.3 | 1.5 | 0.6 | 1.1 | 15:41 | 2.2 | 17:28 | 2.4 | 1.9 | 1.4 | 8.8 | 2.2 |
| 20–30 | 1:50 | 1.6 | 9:06 | 3.3 | 7.0 | 2.7 | 0.7 | 1.0 | 16:00 | 1.7 | 18:30 | 1.5 | 2.3 | 1.0 | 7.8 | 3.1 |
| 30–40 | 0:59 | 1.8 | 8:36 | 3.0 | 7.2 | 2.2 | 1.0 | 1.3 | 15:34 | 0.9 | 17:04 | 1.1 | 1.6 | 0.9 | 8.1 | 2.6 |
| 40–60 | 0:21 | 1.5 | 7.38 | 2.9 | 6.5 | 1.8 | 0.5 | 0.9 | 16:00 | 1.4 | 17:16 | 1.9 | 1.4 | 1.0 | 7.5 | 2.1 |
| Co-sleep | ||||||||||||||||
| Yes | 0:19 | 1.6 | 7:49 | 1.9 | 7.5 | 2.0 | 0.6 | 1.0 | 15:46 | 1.5 | 17:25 | 1.8 | 1.7 | 0.9 | 8.2 | 2.6 |
| No | 1:32 | 1.8 | 9:57 | 3.2 | 8.1 | 2.2 | 0.9 | 1.4 | 15:43 | 1.6 | 17:32 | 2.0 | 1.9 | 1.1 | 8.7 | 3.1 |
| Site | ||||||||||||||||
| Urban | 1:35 | 1.8 | 9:10 | 3.0 | 7.6 | 2.4 | 1.1 | 1.5 | 16:03 | 1.6 | 17:46 | 2.1 | 1.8 | 1.2 | 8.3 | 2.7 |
| Village | 0:05 | 1.6 | 7:47 | 2.1 | 7.7 | 1.2 | 0.4 | 0.6 | 15:22 | 1.4 | 17:07 | 1.5 | 1.8 | 0.7 | 8.6 | 3.0 |
Sleep Patterns in Egyptian Families
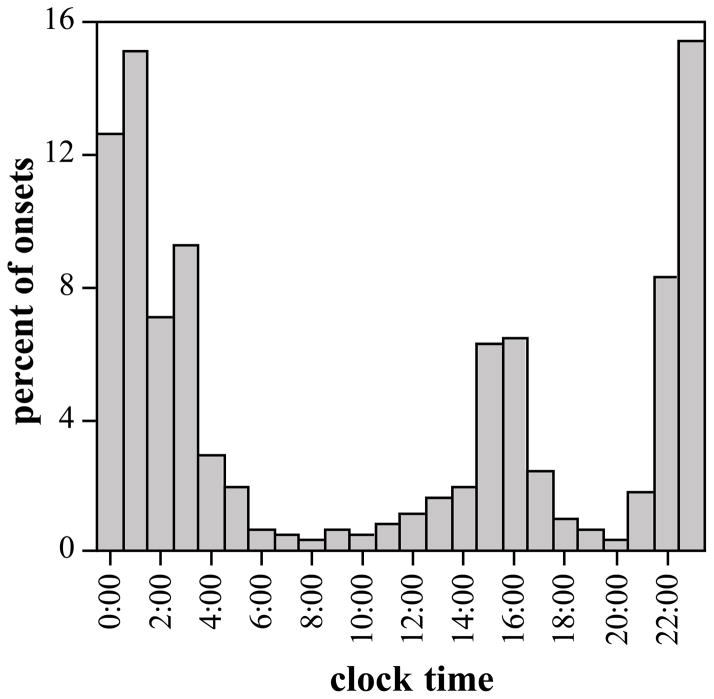
Hypothesis 1 states that sleep in Egyptian families will be diurnal and distributed across the day. Sleep onsets, though dispersed throughout the 24-hour period, exhibited the expected bimodal distribution with a dominant late night peak and a lesser yet substantial peak of onsets in late afternoon (Figure 1). The two types of events, afternoon nap and nighttime sleep, differed in length. Nap duration averaged M = 1.81, SD = 1.05 hours, whereas night sleep duration was M = 7.63, SD = 2.19 hours, t(523) = 29.40, p < .001 (see Table 1). Napping was not universal: 31% of observation days had one or more napping episodes. Schedules of urban households ran later than rural households, such that urban families commenced naps and nighttime sleep respectively 51 and 90 minutes later than rural families, site difference in onset of naps t(130) = 2.60, p = .005; of nighttime sleep t(446) = 8.08, p < .001. Nevertheless, likelihood of afternoon napping did not differ, t(403) = 1.11, p = .13, and the durations of afternoon, nighttime, and total sleep were equivalent in both settings, duration by site for afternoon t(130) = 0.35, p = .36; for nighttime t(391) = 0.68, p = .25; for total sleep t(391) = 1.28, p = .10. Total hours of sleep was marginally greater on days with than on those without napping, with nap: M = 8.27, SD = 3.61; without nap: M = 7.90, SD = 2.10, t(429) = 1.32, p .09. The modest difference reflects decreased duration of nighttime sleep on nap versus non-nap days, with nap: M = 6.30, SD = 3.04; without nap: M = 7.73, SD = 2.06, t(429) = 5.80, p <.001.
Figure 1.
Distribution of onset of sleep events in Egyptian families across the 24-hour day, from midnight (00:00) to subsequent midnight.
Sleep Practices and Sleep Quality
Co-sleeping and Sleeping Arrangements
Co-sleeping was normative in this sample: 69.1% of all recorded sleep events transpired in a bed shared with one to four co-sleepers (Table 2). As reported in lifetime sleep histories, all participants had co-slept with parents from birth and through infancy. Sleep events that did not involve direct co-sleeping were nonetheless socially situated. Of sleep events that did not involve bed sharing, 45% occurred in a room shared with one to three others. Only 21.6% of sleep events were entirely solitary, while 24.2% involved co-sleeping with more than 1 other person. Males were no more likely than females to share a bed, Pearson χ2 1.41, p = 0.23, but they were more likely to share a room if they did not bed-share, Pearson χ2 3.70, p = .05.
Table 2.
Co-Sleeping and Room Sharing in Egyptian Families in Terms of Number of Persons Present, Percent of All Sleep Events Involving Bed or Room-Sharing, and Habitual Arrangement (Proportion of Sleep Events with Co-Sleeping or -Rooming, per Individual) (n=614)
| Variable | Number of Persons
|
Percentage of Sleep Events
|
Habitual Arrangement, by Individual
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Co-Sleep
|
Co-Room
|
Share Bed
|
Share Room
|
Co-Sleeper
|
Co-Roomer
|
|||||
| mean | SD | mean | SD | mean | SD | mean | SD | |||
| All | 0.9 | 0.8 | 1.1 | 0.9 | 69.1 | 77.9 | 0.64 | 0.35 | 0.77 | 0.37 |
| Gender | ||||||||||
| Female | 1.0 | 0.8 | 1.1 | 0.9 | 71.3 | 77.6 | 0.67 | 0.33 | 0.78 | 0.42 |
| Male | 0.9 | 0.8 | 1.1 | 0.8 | 66.8 | 78.1 | 0.61 | 0.38 | 0.78 | 0.41 |
| Age, years | ||||||||||
| 2–10 | 1.5 | 1.0 | 1.6 | 1.0 | 77.4 | 84.0 | 0.74 | 0.27 | 0.84 | 0.29 |
| 10–20 | 0.7 | 0.6 | 0.9 | 0.6 | 60.1 | 77.6 | 0.53 | 0.45 | 0.72 | 0.43 |
| 20–30 | 0.8 | 1.0 | 1.0 | 1.1 | 47.1 | 59.8 | 0.43 | 0.41 | 0.61 | 0.46 |
| 30–40 | 1.3 | 0.9 | 1.5 | 1.0 | 86.9 | 88.5 | 0.85 | 0.16 | 0.90 | 0.19 |
| 40–60 | 0.9 | 0.6 | 1.0 | 0.6 | 79.2 | 83.3 | 0.73 | 0.21 | 0.83 | 0.29 |
| Site | ||||||||||
| Urban | 0.7 | 0.6 | 0.9 | 0.7 | 63.1 | 75.3 | 0.54 | 0.36 | 0.74 | 0.43 |
| Village | 1.2 | 1.0 | 1.3 | 0.9 | 75.2 | 80.7 | 0.74 | 0.31 | 0.80 | 0.27 |
Sleep location also differed by type of sleep event. Nearly all nighttime sleep (93.7%) occurred in the sleeper’s own bedroom, whereas more than a quarter (29.4%) of daytime napping occurred elsewhere, primarily the living room, or another bedroom, Pearson χ2 43.77, p < .001. Daytime napping in these other spaces was more closely associated with solitary sleep (82.3%) than was napping in one’s habitual bedroom, 43.8%; Pearson χ2 14.73, p < .001.
Sleeping Arrangements and Sleep Patterns
Hypothesis 2 states that co-sleeping will be associated with increased sleep quality, as assessed by decreased arousals and longer duration. Whether and with whom participants co-slept depended on the type of sleep event. Participants co-slept during most nighttime sleep events (77.7%), contrasted with only 45.2% of daytime naps, Pearson χ2 47.27, p <.001. Reciprocally, the social context of sleep was related to the timing and length of the sleep bout. Onset of nighttime co-sleeping events was both earlier and less variable than non-cosleeping events, co-sleep: M = 00:19, SD = 1.55 hours; non-cosleep: M = 1:32, SD = 1.81, t(387) = 6.1, p <.001; Levene test of homogeneity f 1.32, p = .04. Nap duration was marginally longer for co-sleepers and co-roomers than solitary sleepers, alone: M = 2.08, SD = 1.48, co-sleep: M = 2.54, SD = 2.27 hours, t(149) = 1.50, p = .07; co-room t(81) = 1.57, p = .06. Conversely, duration of night sleep bouts was somewhat longer when the sleeper was alone than when s/he was co-sleeping, alone: M = 8.15, SD = 2.25 hours; co-sleep: M = 7.52, SD = 2.01 hours, t(350) 2.40, p = .009, but not when the room was shared, alone: M = 8.00, SD = 2.47 hours; co-room: M =8.44, SD = 1.74 hours, t(79) = 0.82, p = .21. Meanwhile, duration of nighttime sleeping bouts was more variable for habitually solitary than co-sleepers. Individual scores for habitual co-sleep or co-room were derived for each participant as the proportion of sleep events with a co-sleeper or –roomer present. A median split by habitual co-sleep revealed that more consistent co-sleepers not only had shorter night sleep durations, more co-sleep: M = 7.48, SD 2.07; less co-sleep: M = 7.87, SD = 2.39, t(389) = 1.70, p = .04, but they also had less variance in sleep duration than less consistent co-sleepers, f 1.58, p < .001. Overall, total amount slept per day declined as the proportion of sleep events that involved co-sleeping increased, t(1, 550) = 2.83, p = .005. Proportion of events involving co-rooming was unrelated to total sleep per day, t(1, 569) = 1.17, p = .24. Thus, co-sleepers reported shorter but more consistent bouts of sleep, providing mixed evidence for our second hypothesis.
In stronger support of the hypothesis that co-sleeping contributes to better sleep with reduced disturbance, co-sleepers reported fewer arousals during nighttime sleep than did solitary sleepers, solitary: M = 0.87, SD = 1.37, co-sleep: M = 0.61, SD = 1.05, t(355) = 1.72, p = .04 (see Table 1). Sharing a room was not associated with the number of arousals during nighttime sleep, co-room: M = 0.76, SD = 0.97, t(66) = 0.36, p = .36. Napping differed in having few reported arousals, even accounting for shorter duration, likely because an arousal often prompted the end of the nap. In general, nighttime arousal rates declined as the proportion of sleep events that involved co-sleeping increased, t(1, 360) = 3.07, p = .002. This pattern was due solely to strong buffering effects of co-sleeping in females, t(1, 189) = 4.06, p < .001, not males, t(1, 170) = 0.73, p = .46. Proportion of events involving co-rooming was unrelated to total sleep per day, t(1, 360) = 1.17, p = .24.
Age and Sleep Arrangements
Hypothesis 3 states that the impact of sleep conditions is most pronounced among adolescents compared to family members of other ages. Across our sample, total daily sleep declined with age, F (1, 422) = −17.43, p < .001, R2 = .11. Amount of co-sleeping varied by age (see Table 3), with nighttime co-sleeping reaching a nadir in adolescence and early adulthood. Children rarely slept alone at night (3.92% of bouts), sleeping instead with siblings or parents. Solitary sleep (absence of co-sleeper or co-roomer) was largely limited to adolescents and young adults, particularly young adult males. Adolescents were less likely to co-sleep and more likely to sleep alone that any other age group, co-sleep adolescents: M = 0.53, SD = 0.45, other ages: M = 0.69, SD = 0.31, t[608] = 5.01, p = <.001; sleep alone adolescents: M = 0.28, SD = 0.43, other ages: M = 0.21, SD = 0.23, t[608] = 2.16, p = .02. Moreover, the period of adolescence through early adulthood was distinguished by distinctive, highly gendered sleeping arrangements. From childhood until marriage, girls were much more likely than boys to sleep with a parent, Pearson χ2 6.01, p .05, but were twice as likely as boys to have their own bed during adolescence, Pearson χ2 3.54, p .04. At marriage, they co-slept with their husband, along with children as they arrive. By contrast, adolescent boys co-slept with brothers or male cousins throughout childhood and early adolescence, and unmarried young men slept alone until marriage. Therefore, across all observed ages, frequency (women: M = 0.67, SD = 0.33, men: M = 0.61, SD = 0.38, t[620] = 1.79, p = .04) and consistency of co-sleeping among females exceeded that in males, Levene test of homogeneity f 1.32, p = .009.
Table 3.
Age-Stratified Patterns of Co-Sleeping in Egyptian Families by Percentage of All, Night, and Nap Events With Type of Co-Sleeper (n=428)
| Age, years | Co-sleepers, all sleep events | Males’ co-sleepers, nighttime | Females’ Co-sleepers, nighttime | Daytime Naps | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| alone | sib or kin | parent-child | spouse | alone | sib or kin | parent-child | spouse | alone | sib or kin | parent-child | spouse | alone | co-sleep | |
| 2–10 | 11 | 55 | 34 | 0 | 0 | 79 | 21 | 0 | 7 | 43 | 51 | 0 | 30 | 70 |
| 10–20 | 24 | 69 | 7 | 0 | 12 | 88 | 0 | 0 | 26 | 58 | 16 | 0 | 50 | 50 |
| 20–30 | 43 | 16 | 13 | 28 | 89 | 8 | 0 | 0 | 18 | 26 | 11 | 44 | 42 | 58 |
| 30–40 | 11 | 0 | 8 | 80 | 13 | 0 | 9 | 77 | 0 | 0 | 0 | 100 | 42 | 58 |
| 40–60 | 17 | 1 | 6 | 76 | 1 | 1 | 1 | 96 | 6 | 0 | 3 | 91 | 45 | 55 |
The findings presented above document age stratified sleep practices, in which adolescents experience less co-sleeping and more solitary sleep, compared to more customary co-sleeping at other ages. Given the third hypothesis, the question therefore becomes whether the distinctive socioecological arrangements of their sleep environment are related to distinctive profiles of sleep quality among adolescents. Thus, we tested whether the lower rates of co-sleeping during adolescence were associated with differences in sleep quality, by assessing the relationship of solitary sleep to timing and duration of sleep behavior. The aforementioned overall relationship of delayed sleep onset to solitary sleep pertained among adolescents: solitary sleepers ages 10–20 years stayed up much longer than did co-sleepers, alone: M = 01:58, SD = 1.47 hours; co-sleep: 00:34, SD = 1.52 hours, t(129) = 6.1, p < .001. To probe whether the effect was related to habitual sleeping arrangements, we tested the difference between habitual and non-habitual co-sleepers, with similar results, non-habitual: M = 01:40, SD = 1.84 hours; habitual co-sleepers: M = 00:50, SD = 1.54 hours, t(129) = 3.48, p < .001. The link between solitary status and nighttime sleep delay was much more pronounced in males than females, solitary males: M = 02:11, SD = 1.49 hours; solitary females: M = 00:46, SD = 1.07 hours, t(59) = 3.5, p < .001.
We have noted that night sleep duration was shorter for co-sleep than non-cosleep events in the entire sample. The pattern holds among adults, t(196) = 1.63, p = .05, but this difference was smaller and non-significant for adolescents, t(103) = 1.27, p = .09. Room-sharing exclusive of co-sleeping was not related to duration of night sleep in the entire sample, or among adolescents, all: t(79) = 0.82, p = .21; adolescents: t(36) = 0.99, p = .16. However, sleeping alone had a marked effect on sleep disturbance in adolescents and young adults: compared to co-sleepers and –roomers, solitary adolescent sleepers reported increased rates of nighttime arousals, solitary: M = 1.62, SD = 1.96; non-solitary: M = 0.23, SD = 0.48, t(99) = 5.81, p < .001. The same was true of young adults, solitary: M = 0.91, SD = 1.07; non-solitary: M = 0.20, SD = 0.52, t(51) = 2.76, p = .004. These effects were confined to females. Women reported more frequent arousals than males, particularly adolescents, females: M = 0.80, SD = 1.42; males: M = 0.23, SD = 0.5, t(100) = 2.60, p = .005, and young adults, females: M = 0.88, SD = 1.08; males: M = 0.33, SD = 0.66, t(52) = 2.07, p = .02. They also reported much higher rates of arousals in solitary compared to co-sleeping events, solitary: M = 1.30, SD = 1.86; co-sleep: M = 0.70, SD = 0.94, t(186) = 2.60, p = .005, compared to males, solitary vs. co-sleep t(167) = 0.30, p = 0.38. Rates of reported nighttime sleep arousals were higher in children than adolescents, children: M = 0.92, SD = 1.59; adolescents: M = 0.47, SD = 1.06, t(152) = 1.83, p = .04, and no different for adolescents than adults ages 40–60 years, t(206) = 0.09, p = .44. Frequency of customary solitary sleep among children and older adults was too low to assess impact on rates of arousals.
Age, Sleeping Arrangements, and Sleep Behavior
Using ANOVA with weights for sampling frequency, we then tested relationships of our independent variables (co-sleep habits, age, gender, site) to our measures of sleep behavior and quality (onset and duration of night sleep, night sleep arousals, total sleep per day, see Table 4). Age and degree of habitual co-sleeping emerged as significant in all of the models, while site did not. Strong interactions among independent variables (age and gender or co-sleeping and site) in three of the models manifest the impact of cultural practices and ecological circumstances that regulate sleep behavior in each of these dimensions.
Table 4.
Model of Direct and Interactive Effects of Gender, Age Group, and Habitual Co-Sleeping On Aspects of Sleep Pattern (n=424)
| Variable | df | F | eta2 | p | error/SS | effect/MS |
|---|---|---|---|---|---|---|
| Onset, nighttime sleep (adjusted R2 0.68) | ||||||
| Co-Sleep Habits | 15 | 15.12 | 0.16 | <.0001 | 218.53 | 14.56 |
| Age | 41 | 10.80 | 0.32 | <.0001 | 426.67 | 10.41 |
| Gender | 1 | 6.41 | 0.00 | 0.01 | 6.17 | 6.17 |
| Age*Gender | 9 | 6.19 | 0.04 | <.0001 | 53.72 | 5.97 |
| Duration, nighttime sleep (adjusted R2 0.48) | ||||||
| Co-Sleep Habits | 15 | 3.16 | 0.06 | <.0001 | 114.23 | 7.62 |
| Age | 41 | 7.17 | 0.38 | <.0001 | 708.47 | 17.28 |
| Co-sleep*Site | 5 | 4.18 | 0.03 | 0.001 | 50.39 | 10.08 |
| Arousals, nighttime sleep (adjusted R2 0.61) | ||||||
| Co-Sleep Habits | 14 | 22.80 | 0.33 | <.0001 | 149.01 | 10.64 |
| Age | 41 | 10.68 | 0.46 | <.0001 | 204.45 | 4.99 |
| Age*Gender | 5 | 18.60 | 0.10 | <.0001 | 43.42 | 8.68 |
| Total sleep per day (adjusted R2 0.22) | ||||||
| Co-Sleep Habits | 15 | 2.25 | 0.06 | 0.005 | 181.47 | 12.10 |
| Age | 41 | 3.23 | 0.25 | <.0001 | 733.24 | 17.88 |
| Gender | 1 | 4.87 | 0.01 | 0.03 | 26.22 | 26.22 |
In the first model, direct effects of bed-sharing habits, age, gender, and the interaction of age and gender explained much of the variation in night sleep onsets, F (66, 443) = 15.27, p < .001, R2 = .68. The interaction reflects a gender difference in trajectory of bedtime by age, with much later bedtimes for men in early-mid adulthood, ages 20–40 years: men: M = 01:58, SD = 1.63; women: M = 00:41, SD = 1.25, t(108) = 6.33, p = <.001, and no gender difference in bedtimes at adolescence and advanced midlife, ages 10–20 years: t(136) = 0.90, p = .18; aged >40 years: t(138) = 0.03, p = .49. A second model comprised of co-sleep habits, age, and the interaction of co-sleeping habits and site predicted nearly half the variation in duration of nighttime sleep, F (61, 404) = 7.14, p < .001, R2 = .48. Among less habitual co-sleepers, villagers slept longer than urbanites, village: M = 7.69, SD = 2.61; urban: M = 6.84, SD = 2.85, t(219) = 2.17, p = .02, but habitual co-sleepers showed no such village-urban difference, village: M = 7.30, SD = 1.99, urban: M = 7.38, SD = 2.50, t(208) = 0.25, p = .40. Length of night sleep was not related to habitual co-sleeping among villagers, >median co-sleep rate: M = 7.30, SD = 1.99; <median: M = 7.69, SD = 2.61, t(193) = 1.18, p = .12, but tended to do so among Cairenes, >median co-sleep rate: M = 7.30, SD = 1.99; <median: M = 7.69, SD = 2.61, t(234) = 1.48, p = .07.
In a third model, number of nighttime arousals was substantially accounted for by co-sleep habits, age, and the interaction of age with gender, F (60, 377) = 10.70, p < .001, R2 = .61. The bases of the interaction are that women report more nighttime arousals than men at most ages (10–20, 20–30, 40–60 years), women: M = 0.84, SD = 1.28; men: M = 0.32, SD = 0.67, t(368) = 4.77, p = <.001, except in childhood when boys report much more, girls: M = 0.55, SD = 0.74; boys: M = 1.39, SD = 2.19, t(50) = 1.94, p = .03, and in the thirties when there is no difference, women: M = 0.90, SD = 1.41; men: M = 01.15, SD = 1.18, t(38) = 0.61, p = .27. A final model that included age, co-sleeping habits, and gender predicted the total amount of sleep per day, F (57, 429) = 3.13, p < .001, R2 = .22, related to findings presented above, that women slept longer, sleep duration decreased with age, and co-sleeping related to shorter and less variable sleep duration.
Discussion
Sleep records of the Egyptian families in our study confirmed the expected pattern of prevalent daytime napping and pervasive co-sleeping. Egyptian families exhibited the anticipated pattern of bimodal sleep, with the major sleep event at night and a nap in the afternoon. Late onsets for night sleep of relatively short duration (7.6 hours) were counterbalanced by later morning offsets and a mid-late afternoon nap of nearly two hours. This pattern was maintained both in urban Cairo and in a large more rural village, supporting ethnographic evidence that napping and later night sleep figure importantly in the sleep regimen. Indeed, days without naps tended to have less total sleep than those with naps, although naps occurred only on about a third of reported days. Nevertheless, quantities of sleep reported appear to lie within reported ranges for sufficient sleep by age (Sadeh et al., 2000; Wolfson & Carskadon, 1998). Sleep patterns show expected age-related changes of declining total sleep by age and delayed sleep onsets among adolescents, and contribute evidence to the growing body of comparative literature on culture and sleep behavior (X. Liu et al., 2005; Park et al., 2001; Reimao et al., 2000a).
Sleep also was embedded in family relationships. Over three-quarters of nighttime sleep events and nearly half of afternoon naps involved co-sleeping. Only a fifth of all sleep events were solitary, without sharing the bed or room. Hence, sleep involved companions. Findings provide preliminary support for the notion that such sleeping arrangements are related to sleep pattern, quantity, and quality. Overall, sharing a bed had mixed relationships with sleep patterns and quality. Co-sleeping was associated with earlier and less variable onsets of nighttime sleep, shorter and less variable nighttime sleep duration, less sleep disturbance represented by arousals, and less total sleep per day. Hence, co-sleeping appeared to be related to more regular, compact, and undisturbed sleep than co-rooming or solitary arrangements. Sheer physical presence was not sufficient for this set of effects, which were not associated with co-rooming. Such findings contrast with reports linking sleep difficulty to co-sleeping in children (Blader et al., 1997; Hayes et al., 2001). Our data do not allow us to test the possibility that shorter (albeit still substantial) night sleep reflects greater sleep efficiency and consequently reduced need in co-sleepers. Nevertheless, the evidence generally support the view that co-sleeping is related to increased sleep quality in a context where habitual co-sleeping is normative.
Furthermore, sleeping arrangements reported by Egyptian families were culturally patterned, such that that the age, gender, probability of co-sleeping, and relationship to co-sleeper varied with age and gender of the sleeper. Consequently, these factors substantially accounted for observed patterns of night sleep onsets and duration, total sleep, and sleep quality estimated by arousals. Overall, customary practices provided co-sleepers for persons of all ages, although this was notably less true for adolescents and unmarried young adults. When deprived of the accustomed social context of sleep, both genders experienced sleep difficulties associated with youth (Carskadon et al., 1993; Wolfson & Carskadon, 1998). Increased rates of solitary nighttime sleep among youth were associated with more sleep disruption in young women and an increased phase shift that was especially pronounced in young men. These findings agree with existing reports of gender and age effects on sleep patterns and macroarchitecture among western populations (Reyner & Horne, 1995; Robert et al., 2006). But in general, gender differences in interactions of cognitive-affective development with external circumstances in relation to capacity for self-regulation and sleep maintenance during puberty merit greater attention.
Perhaps cultural changes in sleeping arrangements contribute to these patterns in Egyptian adolescents by promoting individualism manifested in separate bedrooms for study, or individual beds for personal space (Dwairy & Menshar, 2006). Conversely, solitary sleeping may be reactive, such that adolescents with skewed schedules or sleep difficulties are more likely to sleep alone. Our cross-sectional data cannot address direction of causality distinguishing these alternatives. Egyptian parents and youth in our sample identified TV watching (especially late night movies) and for younger males, video games and net surfing, as important reasons for sleep delay; young women also attributed sleep disruption to worry and anxiety. Our empirical results agree with previous findings of higher rates of reported sleep disturbance among females than males in a large sample of 12–18 year old urban and rural Egyptian students (Galal, Hamad, & Hassan, 2001). As elsewhere, insomnia is closely linked to mood disorders among Egyptian youth and adults (Okasha, 2004). Presence of a sleeping partner may buffer adolescents from disruptive effects of stress or dysregulated arousal on sleep, by passive provision of security and soothing, and active provision of social support or counterbalancing feedback. Insofar as relationships act as regulators (Hofer, 1984), lack of an accustomed sleeping partner removes a behavioral, psychological, and physiological entrainer that can be particularly important for adolescents. Absence of such psychosensory inputs may be especially challenging because in adolescence, potential for sleep dysregulation related to stimulus seeking and distraction and/or social vigilance and introspection is very high (Dahl, 2002). In this sense, sleep hours spent with others can be companionable, antagonizing withdrawal and reducing social isolation (Cacioppo et al., 2002), buffering stress and arousal to levels compatible with sleep (Sadeh et al., 2004), and contributing to sleep quality that promotes mental and physical health (Bursztyn & Stessman, 2005). Solitary sleep adds to number of hours alone, which stresses capacity for emotional and physiological self-regulation at a developmental period for reorganization and vulnerability in this capacity. Our findings and other evidence suggest that such benefits accrue at any age, but Dahl has proposed that the important developmental task of emotional-cognitive maturation at adolescence is highly sensitive to sleep quality and sufficiency (Dahl, 2002). We would emphasize that such maturation also is sensitive to social context, including that of sleep.
Limitations and Future Directions
Our exploratory study has distinct limitations. First, sample size is small and representativeness is limited by the sample’s middle class, urban character and by our use of convenience sampling with a cross-sectional design. Restriction to families with children over two years of age eliminates both those with small infants, and elders without children. Second, the narrow sampling period—comprising a single month in early summer just at the end of the school year and the beginning of summer school break—gives a profile of sleep patterns under specific conditions and cannot represent seasonal and calendrical variation in patterns across the year (BaHamman, 2003). Although this approach yields a picture of sleep patterns during a normative transitional period that challenges family function, it represents neither sleep patterns under more stable and school-restricted schedules, nor families without schooled children. Moreover, lack of longitudinal data precludes definitive assessment of regulatory and developmental effects of sleeping arrangements such as habitual co-sleeping. Third, activity records have methodological limitations including reliance on participant self-reports. Use of a designated record-keeper to prompt or elicit individual records facilitated more timely and complete diary recording by all family members, but is subject to variable effectiveness of record-keepers and reliability of self-report. Self report diaries have been found to correspond closely to activity monitoring in American high school students of ages similar to our designated record-keepers, but in a quite different cultural context (Carskadon, Acebo, Wolfson, Tzischinsky, & Darley, 1997). Cognitive load is also a consideration for participants unfamiliar with tracking time or activity. Daily recording can be more burdensome for large and very busy families. Moreover, the need for participant training and multiple follow-up visits contributes to substantial investigator and participant burden. Fourth, our measure of sleep quality is limited, and we did not assess affective outcomes (e.g., mood) or subjective factors such as sleep quality and difficulty, attitudes to co-sleep partners, and family relations. Finally, use of multivariable analysis with multiple measures applied to a rather small sample gives grounds for caution in interpretation of results.
Who sleeps with whom reflects the social order and informs the emotional-regulatory content of relationships. This exploratory study of habitually co-sleeping Egyptian families has provided preliminary documentation of family sleep patterns in a setting where co-sleeping is customary and habitual. Our findings suggest that the timing and amount of sleep are largely determined by direct and interactive effects of gender, age, and age-stratified sleeping arrangements. Moreover, they support the possibility that habitual co-sleeping can be beneficial, and that a day’s sleep can be obtained routinely through less consolidated means that include institutionalized napping. Further, some findings indicate that solitary sleep for adolescents is linked to sleep dysregulation related to extreme phase shifts in sleep for males and sleep disruption for females. For societies such as the U.S., where values of autonomy and privacy place a premium on solitary rooming and sleep, our findings also raise the question of whether such practices contribute to risk for difficulties in sleep, affect, and behavior regulation among adolescents.
Acknowledgments
Funding was provided by the University Research Committee, the International Collaboration and Exchange Directorate of Emory University, and National Institutes of Mental Health MH57761. We gratefully acknowledge the contributions of study participants in Cairo and Mahallat Marhum, Egypt. Research was performed with permission from the Ministry of Health of Egypt, through sponsorship from the Field Epidemiology Training Unit (FETP) under administrative direction from Drs. Abdel-Nasser and Moharram Khalifa. We thank Drs. Eman Helmy, Waleed Ibrahim, and Amgad El Kholy of the FETP, and Tayseer Salem and Ammar Divan for their roles in data collection.
References
- BaHamman A. Sleep pattern, daytime sleepiness, and eating habits during the month of Ramadan. Sleep and Hypnosis. 2003;5:165–174. [Google Scholar]
- Ben-Ari E. From mothering to othering: Organization, culture, and nap time in a Japanese day-care center. Ethos. 1996;24:136–164. [Google Scholar]
- Benoit D, Zeanah CH, Boucher C, Minde KK. Sleep disorders in early-childhood–association with insecure maternal attachment. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31:86–93. doi: 10.1097/00004583-199201000-00013. [DOI] [PubMed] [Google Scholar]
- Blader JC, Kopleicz HS, Abikoff H, Foley C. Sleep problems of elementary school children. A community survey. Archives of Pediatrics and Adolescent Medicine. 1997;151:473–480. doi: 10.1001/archpedi.1997.02170420043007. [DOI] [PubMed] [Google Scholar]
- Bursztyn M, Stessman J. The siesta and mortality: Twelve years of prospective observations in 70-year-olds. Sleep. 2005;28:345–347. [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, et al. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science. 2002;13:384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biological Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carmichael CL, Reis HT. Attachment, sleep quality, and depressed affect. Health Psychology. 2005;24:526–531. doi: 10.1037/0278-6133.24.5.526. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Wolfson A, Tzischinsky O, Darley C. REM sleep on MTLTS in high school students is related to circadian phase. Sleep Research. 1997;26:705. [Google Scholar]
- Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16:258–262. doi: 10.1093/sleep/16.3.258. [DOI] [PubMed] [Google Scholar]
- Caudill W, Plath DW. Who sleeps by whom? Parent-child involvement in urban Japanese families. In: Lebra TS, Lebra WP, editors. Japanese culture and behavior: Selected readings. Honolulu: University of Hawaii Press; 1986. pp. 247–279. [Google Scholar]
- Crawford CJ. Parenting practices in the Basque country: Implications of infant and childhood sleeping location for personality development. Ethos. 1994;22:42–82. [Google Scholar]
- Dahl RE. The regulation of sleep-arousal, affect, and attention in adolescence: some questions and speculations. In: Carskadon MA, editor. Adolescent sleep patterns: Biological, social, and psychological influences. New York: Cambridge University Press; 2002. pp. 269–284. [Google Scholar]
- Dwairy M, Menshar KE. Parenting style, individuation, and mental health of Egyptian adolescents. Journal of Adolescence. 2006;29:103–117. doi: 10.1016/j.adolescence.2005.03.002. [DOI] [PubMed] [Google Scholar]
- El-Sheikh M, Buckhalt J, Mize J, Acebo C. Marital conflict and disruption of children’s sleep. Child Development. 2006;7:31–43. doi: 10.1111/j.1467-8624.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- Fratello F, Curcio G, Ferrara M, Marzano C, Couyoumdjian A, Petrillo G, et al. Can an inert sleeping pill affect sleep? Effects on polysomnographic, behavioral and subjective measures. Psychopharmacology. 2005;181:761–770. doi: 10.1007/s00213-005-0035-2. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Ishihara K. Routine evening naps and night-time sleep patterns in junior high and high school students. Psychiatry and Clinical Neurosciences. 2002;56:229–230. doi: 10.1046/j.1440-1819.2002.00986.x. [DOI] [PubMed] [Google Scholar]
- Galal SB, Hamad S, Hassan M. Self-reported adolescents’ health and gender: An Egyptian study. Eastern Mediterranean Health Journal. 2001;7:625–634. [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijedenbos A, et al. Brain development during childhood and adolescence: A longitudinal fMRI study. Nature Neuroscience. 1999;10:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Ely TC, O’Connor TG, Rijsdijk FV, Plomin R. Family influences on the association between sleep problems and anxiety in a large sample of pre-school aged twins. Personality and Individual Differences. 2005;39:1337–1348. [Google Scholar]
- Hayes MJ, Parker KG, Sallinen B, Davare AA. Bedsharing, temperament, and sleep disturbance in early childhood. Sleep. 2001;24:657–662. doi: 10.1093/sleep/24.6.657. [DOI] [PubMed] [Google Scholar]
- Hofer M. Hidden regulatory processes in early social relationships. In: Bateson PP, Klopfer PH, editors. Perspectives in ethology: II. Social behavior. Oxford: Plenum; 1978. pp. 135–166. [Google Scholar]
- Hofer MA. Relationships as regulators: A psychobiologic perspective on bereavement. Psychosomatic Medicine. 1984;46:183–197. doi: 10.1097/00006842-198405000-00001. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Hidden regulators in attachment, separation, and loss. Monographs of the Society for Research in Child Development. 1994;59(2–3):250–283. [PubMed] [Google Scholar]
- Hunsley M, Thoman EB. The sleep of co-sleeping infants when they are not co-sleeping: Evidence that co-sleeping is stressful. Developmental Psychobiology. 2001;40:14–22. doi: 10.1002/dev.10009. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. Journal of Neuroendocrinology. 2000;12(1):5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- Liu X, Liu L, Wang R. Bed sharing, sleep habits, and sleep problems among Chinese school-aged children. Sleep. 2003;26:839–844. doi: 10.1093/sleep/26.7.839. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Askew G, Wolf AW. Cosleeping and early childhood sleep problems: Effects of ethnicity and socioeconomic status. Journal of Developmental & Behavioral Pediatrics. 1996;17:9–15. doi: 10.1097/00004703-199602000-00002. [DOI] [PubMed] [Google Scholar]
- McKenna JJ. Cultural influences on infant and childhood sleep biology, and the science that studies it: Toward a more inclusive paradigm. In: Loughlin GM, Caroll JL, Marcus CL, editors. Sleep and breathing in children: A developmental approach. New York: Marcel Dekker; 2000. [Google Scholar]
- McKenna JJ, McDade TW. Why babies should never sleep alone: A review of the co-sleeping controversy in relation to SIDS, bedsharing and breast feeding. Paediatric Respiratory Reviews. 2005;6:134–152. doi: 10.1016/j.prrv.2005.03.006. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Mosko S, Dungy C, McAnninch J. Sleep and arousal patterns among co-sleeping mother-infant pairs: Implications for SIDS. American Journal of Physical Anthropology. 1991;83:331–347. doi: 10.1002/ajpa.1330830307. [DOI] [PubMed] [Google Scholar]
- McKenna JJ, Thoman EB, Anders T, Sadeh A, Schechtman V, Glotzbach S. Infant-parent co-sleeping in an evolutionary perspective: Implications for understanding infant sleep development and the Sudden Infant Death Syndrome (SIDS) Sleep. 1993;16:263–282. doi: 10.1093/sleep/16.3.263. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Okami P, Weisner TS, Olmstead R. Outcome correlates of parent-child bedsharing: An eighteen-year longitudinal study. Developmental and Behavioral Pediatrics. 2002;23:244–253. doi: 10.1097/00004703-200208000-00009. [DOI] [PubMed] [Google Scholar]
- Okasha A. Focus on psychiatry in Egypt. British Journal of Psychiatry. 2004;185:266–272. doi: 10.1192/bjp.185.3.266. [DOI] [PubMed] [Google Scholar]
- Park YM, Matsumoto K, Shinkoda H, Nagashima H, Kang MJ, Seo YJ. Age and gender difference in habitual sleep-wake rhythm. Psychiatry and Clinical Neurosciences. 2001;55:201–202. doi: 10.1046/j.1440-1819.2001.00825.x. [DOI] [PubMed] [Google Scholar]
- Reimao R, Souza JC, Gaudioso CE, Guerra HdC, Alves AdC, Oliveira JCF, et al. Nocturnal sleep pattern in native Brazilian Terena adults. Arquivos de Neuro-Psiquiatria. 2000a;58:233–238. doi: 10.1590/s0004-282x2000000200005. [DOI] [PubMed] [Google Scholar]
- Reimao R, Souza JC, Gaudioso CE, Guerra HdC, Alves AdC, Oliveira JCF, et al. Siestas among Brazilian native Terena adults: A study of daytime napping. Arquivos de Neuro-Psiquiatria. 2000b;58:39–44. doi: 10.1590/s0004-282x2000000100006. [DOI] [PubMed] [Google Scholar]
- Reimao R, Souza JC, Medeiros MM, Almirao RI. Sleep habits in native Brazilian Terena children in the state of Mato Grosso do Sul, Brazil. Arquivos de Neuro-Psiquiatria. 1998;56:703–707. doi: 10.1590/s0004-282x1998000500001. [DOI] [PubMed] [Google Scholar]
- Reyner A, Horne JA. Gender- and age-related differences in sleep determined by home-recorded sleep logs and actimetry from 400 adults. Sleep. 1995;18:127–134. [PubMed] [Google Scholar]
- Robert JJ, Hoffmann RF, Emslie GJ, Hucghes C, Rintelmann J, Moore J, et al. Sex and age differences in sleep macroarchitecture in childhood and adolescent depression. Sleep. 2006;29:351–358. doi: 10.1093/sleep/29.3.351. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Keinan G, Daon K. Effects of stress on sleep: The moderating role of coping style. Health Psychology. 2004;23:542–545. doi: 10.1037/0278-6133.23.5.542. [DOI] [PubMed] [Google Scholar]
- Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Developmental Psychology. 2000;36:291–301. doi: 10.1037//0012-1649.36.3.291. [DOI] [PubMed] [Google Scholar]
- Seifer R, Sameroff A, Dickstein S, Hayden L. Parental psychopathology and sleep variation in children. Child and Adolescent Psychiatric Clinics of North America. 1997;5:715–727. [Google Scholar]
- Shweder RA, Jensen LA, Goldstein WM. Who sleeps by whom revisited: A method for extracting the moral goods implicit in practice. In: Goodnow JJ, Miller PJ, Kessel F, editors. Cultural practices as contexts for development. San Francisco, CA: Jossy-Bass; 1995. pp. 21–39. [DOI] [PubMed] [Google Scholar]
- Task Force on Sudden Infant Death Syndrome. The changing concept of Sudden Infant Death Syndrome: Diagnostic coding shifts, controversies regarding the sleeping environment, and new variables to consider in reducing risk. Pediatrics. 2005;116:1245–1255. doi: 10.1542/peds.2005-1499. [DOI] [PubMed] [Google Scholar]
- Uchino BM, Cacioppo JT, Kiecolt-Glaser JK. The relationship between social support and physiological process: A review with emphasis on underlying mechanisms and implications for health. Psychological Bulletin. 1996;119:488–531. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Welles-Nystrom B. Co-sleeping as a window into Swedish culture: Considerations of gender and health care. Scandinavian Journal of Caring Sciences. 2005;19:354–360. doi: 10.1111/j.1471-6712.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Wolfson AR. Sleeping patterns of children and adolescents: Developmental trends, disruptions, and adaptations. Child and Adolescent Psychiatric Clinics of North America. 1996;5:549–568. [Google Scholar]
- Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69:875–887. [PubMed] [Google Scholar]
- Worthman CM, Melby M. Toward a comparative developmental ecology of human sleep. In: Carskadon MA, editor. Adolescent sleep patterns: Biological, social, and psychological influences. New York: Cambridge University Press; 2002. pp. 69–117. [Google Scholar]
- Yang CK, Hahn HM. Cosleeping in young Korean children. Journal of Developmental & Behavioral Pediatrics. 2002;23:151–157. doi: 10.1097/00004703-200206000-00004. [DOI] [PubMed] [Google Scholar]