Abstract
Introduction
To evaluate the efficacy and toxicity of the combination of celecoxib and docetaxel in patients with advanced non-small cell lung cancer (NSCLC) after failure of platinum-based therapy.
Methods
Patients with relapsed NSCLC received celecoxib 400 mg orally twice daily beginning 7 days before the first cycle of docetaxel and the celecoxib was continued with no interruption. Docetaxel 75 mg/m2 was administered intravenously on a 21-day cycle. The primary end point of the study was the 6-month survival rate.
Results
Twenty-four patients were enrolled and twenty patients were treated (median age 60, M:F 16:8). Most patients had a baseline performance status of 1. The objective response rate was 10% (95% CI, 0-25%) and the 6-month survival rate was 59% (95% CI 37 – 80%). Median survival time was 6.9 months (95% CI, 2.8 – 15.2 months) and the 1- and 2-year survival rates were 36% (95% CI, 15 – 57%) and 1% (95% CI, 0 – 10%), respectively. The most frequent grade ≥ 3 adverse events were neutropenia (58%) and neutropenic fever (21%) which resulted in early closure of the trial.
Conclusions
The addition of celecoxib to docetaxel did not appear to improve the response rate and survival compared to docetaxel alone. The combination demonstrated considerable neutropenia and complications from febrile neutropenia that suggests celecoxib may enhance the marrow toxicity of docetaxel.
Keywords: Non small cell lung cancer, COX-2, relapsed, docetaxel, celecoxib
INTRODUCTION
Each year in the United States over 200,000 new cases of lung cancer are diagnosed, the vast majority of which are non-small cell lung carcinoma (NSCLC)1. Approximately 40% of patients present with metastatic disease and will only be candidates for palliative chemotherapy. Standard initial therapy usually consists of a platinum-based drug regimen sometimes with the addition of bevacizumab2,3. However, with a median progression free survival of 3-4 months, many patients are candidates for subsequent therapy.
Docetaxel was the first agent approved for the treatment of advanced NSCLC after the failure of initial platinum-based therapy. Unfortunately, the response rate is only around 7% with a 1-year survival rate of 32-37%4,5. Efforts have been made to utilize doublet therapy for platinum-refractory or relapsed disease; however toxicity has outweighed the clinical benefit in this setting6,7,8,9. More effective and better tolerated therapy is needed for patients who progress after platinum-based treatment.
Cyclooxygenase-2 (COX-2) is an inducible enzyme that facilitates the conversion of arachidonic acid to prostaglandins involved in the regulation of normal growth responses, but has also been implicated in aberrant cellular growth and angiogenesis10,11. Prostaglandins derived from COX-2 may stimulate oncogenesis through the inhibition of immune surveillance and apoptosis in addition to the promotion of angiogenesis and tumor invasion12,13,14,15,16,17. Specifically, COX-2 expression facilitates the formation of prostaglandin-E2(PGE2) which promotes the production and release of vascular endothelial growth factor (VEGF), an angiogenic growth factor18. Overexpression of COX-2 has also been found to increase production of the antiapoptotic proteins Bcl-2 and survivin in lung cancer cell lines19,20. Tumoral COX-2 mRNA expression has been associated with decreased survival and early relapses in patients with resected NSCLC21. Previous studies have demonstrated that approximately 70% of NSCLCs overexpress COX-2 when compared to normal lung tissue and given the involvement of COX-2 in facilitating tumor angiogenesis and inhibiting apoptosis of malignant cells, it is an attractive target for cancer therapy22,23.
Preclinical studies utilizing COX-2 inhibitors have demonstrated a direct anti-tumor effect in NSCLC models24. The addition of a COX-2 inhibitor to taxane chemotherapy might be beneficial as in vitro experiments have demonstrated that taxanes induce COX-2 and subsequent prostaglandin synthesis which may result in reduced effectiveness of the chemotherapy25. Indeed, in human NSCLC cell lines, docetaxel plus the COX-2 inhibitor nimesulide demonstrated improved cytotoxicity compared to single-agent taxane therapy26. With these considerations, we designed a phase II study to evaluate the effectiveness and tolerability of docetaxel plus celecoxib in patients with NSCLC who progressed after platinum-based chemotherapy.
PATIENTS AND METHODS
Eligibility
Patients with histologically or cytologically documented NSCLC were entered onto this study between November 2001 and May 2002. Patients were required to have evidence of progressive or relapsed disease during or after treatment with platinum-containing chemotherapy for stage IIIA, IIIB or IV NSCLC. Chemotherapy, radiation therapy and major surgery were not allowed within two weeks of starting celecoxib. In addition, any non-steroidal anti-inflammatory drug (NSAID) therapy must have been discontinued 30 days prior to the initiation of treatment with the exception of ≤ 325 mg/day of aspirin for cardiovascular conditions. Other requirements included measurable or evaluable disease, age ≥ 18, Zubrod performance status (PS) of 0-2, Absolute Neutrophil Count (ANC) ≥ 1500/mm3, platelet count ≥ 100,000/mm3, total bilirubin ≤ the institutional upper limit of normal, SGOT ≤ 2.5× the upper limit of normal and serum creatinine ≤ 1.5 mg/dL (132.6 mol/L). Exclusion criteria also included an allergy to sulfa drugs, prior therapy with docetaxel, body weight below 50 kilograms and symptomatic, uncontrolled brain or leptomeningeal disease. Patients were ineligible if they had peripheral neuropathy of grade ≥ 2, a thromboembolic event within 4 weeks of study entry, a history of gastrointestinal bleeding within 6 months of study entry or peptic ulcer disease of any duration. The trial was approved by the local Institutional Review Boards and written informed consent was obtained from all patients.
Treatment
Patients were treated with celecoxib 400 mg administered orally twice daily beginning 7 days before the first cycle of docetaxel. Patients were asked to take each dose with a meal. Docetaxel was administered at a dose of 75 mg/m2 and was repeated every 21 days. Therapy continued until progression or unacceptable toxicity. Patients could be maintained on celecoxib after discontinuation of docetaxel for reasons other than disease progression. Each cycle was of 21 day duration except the first cycle which lasted for 28 days as this cycle included the 7-day induction of celecoxib prior to the first docetaxel infusion.
Dose adjustment for toxicity
Full dose of docetaxel was delivered if the ANC ≥ 1,500/mm3 and platelets ≥ 100,000/mm3 and non-hematologic toxicity ≤ grade 1; but the dose of docetaxel was reduced by 20% if the nadir ANC was ≤ 500/mm3 and/or the nadir platelet count was ≤ 25,000/mm3. Docetaxel was delayed for ANC < 1500/mm3 and/or platelets ≤ 100,000/mm3, or grade ≥ 3 non-hematologic toxicity for a maximum of 2 weeks. If ≥ grade 3 non-hematologic toxicity was observed at any point, then the dose of subsequent cycles of docetaxel was reduced by 20%. Docetaxel could begin when toxicity resolved to ≤ grade 1. Patients experiencing ≥ grade 3 neurotoxicity resulted in discontinuation of protocol therapy. Grade 2 neurotoxicity resulted in a maximum delay of 2 weeks of docetaxel and a subsequent 20% dose reduction.
Celecoxib was not held or reduced for hematologic toxicity, but was reduced to 300 mg orally twice daily for an increase in serum creatinine between 50-100% of the pre-therapy value and was held for a serum creatinine > 100% of the pre-therapy value. If the creatinine level recovered to < 100% increase from pre-therapy levels within a 2-week period, then the dose was reduced to 300 mg twice daily for all subsequent treatments. Celecoxib was also held for grade ≥ 3 non-hematologic toxicity and full dose therapy could resume within a 2-week period if the toxicity resolved to grade ≤ 1.
Assessment of response and toxicity
Patients were considered evaluable for toxicity assessment if treatment with celecoxib was started and were eligible for response if they received at least one dose of celecoxib and docetaxel. Patients underwent appropriate scans to evaluate for response after every two cycles of treatment. Response to therapy was assessed according to the RECIST criteria27. Celecoxib and docetaxel were discontinued if a patient developed progressive disease or life-threatening/irreversible toxicity that was not manageable with symptomatic care or dose reduction and/or delay. All toxicity was graded according to the NCI Common Toxicity Criteria version 2.0 (http://ctep.cancer.gov/reporting/ctc.html).
Statistical Analyses
The primary objective of this study was to assess the 6-month survival rate in patients treated with the combination of docetaxel and celecoxib. To minimize the number of patients required for this study, a two-stage Minimax Simon’s design was used28. This drug combination would be considered not interesting if the 6-month survival rate is < 35%, and it would be of definite clinical interest if the 6-month survival rate is > 55%. With 21 patients in stage I and 39 total patients, the 2-stage design used had a 5% type I error and 80% power in testing the hypothesis. The trial was to be terminated at stage I if ≤ 8 patients survived 6 months. A total of 39 evaluable patients were to be accrued unless undue toxicity warranted early termination of accrual.
To be evaluable for efficacy, the patient had to receive at least one dose of celecoxib and one dose of docetaxel. Other endpoints of this study were treatment toxicity, overall survival duration, and time to treatment failure. Overall survival (OS) was defined as the time from the initiation of celecoxib treatment to death from any cause. Time to treatment failure (TTF) was defined as the time from the initiation of celecoxib treatment to the documentation of disease progression, death due to any cause, or early discontinuation of therapy.
Descriptive analyses for baseline characteristics were performed. Six-month survival rate and median survival were conservatively estimated using Kaplan-Meier method with linear interpolation due to the small sample size29. Time to event endpoints (OS and TTF) were plotted using Kaplan-Meier curves30. The SAS System (Cary, NC) was used for all analyses.
RESULTS
Twenty-four patients were enrolled onto the study between November 2001 and December 2002. Accrual was stopped after 24 patients since a high rate of neutropenic fever was observed. Data were collected until May 12, 2004 when the last patient on study expired. Patient characteristics are summarized in Table 1. The median age at study entry was 60 years (range, 41-76), 67% were males. All patients had received prior platinum-based chemotherapy and half of the patients were deemed platinum refractory (progression within 3 months of treatment). Eighteen patients had received prior therapy with paclitaxel and 10 patients had received two prior regimens. The majority of patients had stage IV disease (79%) and the rest had stage III disease. Sixty-seven percent of the patients had a Zubrod PS of 0 or 1.
Table 1. Patient Characteristics.
| Characteristic | No. | Percentage |
|---|---|---|
| Sex | ||
| Male | 16 | 67 |
| Female | 8 | 33 |
| Age, years | ||
| Median | 60 | |
| Range | 41-76 | |
| Race | ||
| Caucasian | 15 | 63 |
| African American | 7 | 29 |
| Other | 2 | 8 |
| Performance Status | ||
| 0 | 4 | 17 |
| 1 | 12 | 50 |
| 2 | 8 | 33 |
| Stage | ||
| IIIA | 1 | 4 |
| IIIB | 4 | 17 |
| IV | 19 | 79 |
| Number of previous chemo regimens | ||
| 1 | 14 | 58 |
| 2 | 10 | 42 |
| Platinum refractory | 12 | 50 |
| Received prior paclitaxel | 18 | 75 |
Toxicity
A median of 2 cycles were administered (range, 1-14). Four patients (17%) began celecoxib but never received docetaxel. One patient developed pneumonia, was hospitalized and died on day 26. Two patients had a severe decline in performance status due to progressive disease and were removed from study. One patient received the celecoxib for 2 days and then requested to be removed from study. Per protocol design, these 4 patients were not evaluable for treatment response, but were included in the toxicity assessment.
Toxicity data are listed in Table 2. The most common grade 3-4 toxicities were neutropenia (58%) and neutropenic fever (21%) and most of these patients required hospitalization. When the 4 patients that had not received docetaxel were excluded, the rates were 70% and 25% respectively. None developed grade 3-4 thrombocytopenia and only 2 had grade 3 anemia. Six patients (25%) required at least one dose reduction of docetaxel, 5 due to toxicity occurring during the first cycle. Two other patients (8%) had a delay of treatment by 1 week. No patient developed non-hematologic toxicity requiring dose reduction of celecoxib. Twelve patients (50%) required hospitalization during study treatment: 5 for neutropenic fever/sepsis, 4 for progressive dyspnea likely from malignancy, 2 for new onset brain metastases and 1 for non-neutropenic pneumonia.
Table 2. Worst Toxicity (≥Grade 3) Experienced per Patient (n=24) Grade.
| Toxicity | 3 | 4 | 5 |
|---|---|---|---|
| Anemia | 2 | 0 | 0 |
| Neutropenia | 6 | 8 | 0 |
| Neutropenic fever | 3 | 2 | 0 |
| Nausea/Vomiting | 3 | 0 | 0 |
| Neuropathy | 1 | 0 | 0 |
| Dyspnea | 0 | 1 | 1 |
| Pneumonia | 0 | 0 | 1 |
| Mucositis | 1 | 0 | 0 |
| Fatigue/Decline in PS | 2 | 2 | 0 |
Response and Survival
Two of the 20 evaluable patients had a partial response to treatment (10%, 95% CI, 0-25%). Twelve patients had a partial response or stable disease through 2 cycles of therapy for a tumor control rate of 60% (95% CI 40-80%). The 6-month survival rate was 59% (95% CI 37 – 80%).
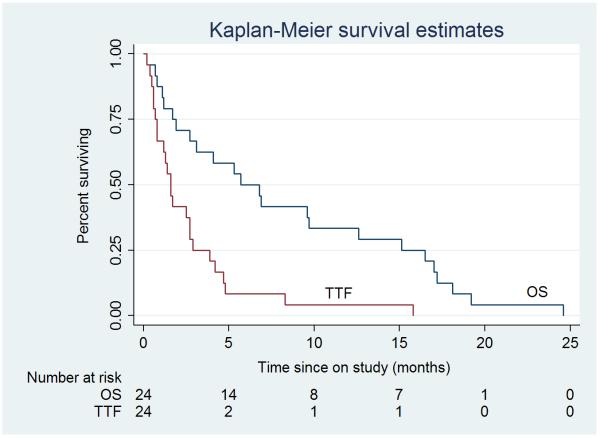
The median time to treatment failure was 1.67 months (95% CI, 1.3 – 2.9). Median overall survival was 6.9 months (95% CI, 2.8 – 15.2 months) and the 1- and 2-year survival rates were 36% (95% CI, 15 – 57%) and 1% (95% CI, 0 – 10%), respectively. The intent to treat (ITT) Kaplan-Meier estimates of TTF and OS for the 24 patients are presented in Figure 1.
Figure 1.
Intent To Treat Kaplan-Meier estimates for overall and time to treatment failure survival for all patients N = 24 (median overall survival, 5.7 months; median time to treatment failure, 1.61 months).
Discussion
Our study achieved the primary endpoint of greater than 6-month survival in 59% of the patients enrolled in stage I, although it is underpowered with poor precision due to the early termination. Unacceptable hematologic toxicity resulted in early closure of the trial which included grade 3-4 neutropenia in 70% and neutropenic fever in 25% of patients who received both celecoxib and docetaxel. Although patient selection may have contributed to this (half of the patients were platinum-refractory, one-third had a PS of 2), the response and survival results are, nevertheless, comparable with two other trials utilizing docetaxel plus celecoxib in advanced NSCLC. Csiki et al utilized the same dose and schedule of docetaxel and celecoxib in 56 patients and reported a response rate of 11%, a median survival of 6 months, and a 1-year survival of 23%31. Nugent et al demonstrated in 39 patients a response rate of 10%, median survival of 11.3 months and 1-year survival of 48%32. However, these results are not much different than those observed with single agent docetaxel, suggesting the addition of celecoxib did not improve overall survival4,5.
Early clinical data utilizing celecoxib as an anti-neoplastic agent seemed promising. A study by Altorki et al demonstrated elevated intratumoral levels of COX-2 and PGE2 after treatment with neoadjuvant carboplatin and paclitaxel, suggesting chemotherapy induced upregulation of COX-233. Neoadjuvant treatment with celecoxib 400 mg twice daily plus chemotherapy substantially reduced intratumoral PGE2 levels evaluated in the post surgical specimens, however, given the small sample size, these results could not be correlated with survival.
Another study by Altorki et al confirmed tolerability of the combination of celecoxib, paclitaxel and carboplatin as neoadjuvant therapy for patients with resectable NSCLC34. A dramatic reduction in tumoral PGE2 levels was noted compared to control patients and results indicated celecoxib may enhance the response of paclitaxel and carboplatin in patients with NSCLC. Csiki et al also reported 5 subjects who underwent assessment of intratumoral PGE2 after administration of celecoxib 400 mg twice daily, 4 of whom had significant decreases in PGE2 post-therapy. This trial also evaluated urinary PGE-M levels (the primary urinary metabolite of PGE2) both pre-and post-administration of celecoxib. A dramatic reduction in previously elevated urinary PGE-M levels correlated with improvement in survival in patients with advanced NSCLC.
Despite these compelling data that suggest COX-2 is a valid in vivo target, COX-2 inhibition has not consistently demonstrated enhancement of the anti-tumor activity of cytotoxics in clinical trials. Gridelli et al utilized gemcitabine and cisplatin with or without the COX-2 inhibitor rofecoxib in patients with untreated advanced NSCLC and no survival benefit was identified35. A recent trial by Lilenbaum et al evaluated docetaxel plus irinotecan or gemcitabine plus irinotecan with or without the addition of celecoxib for second-line therapy in patients with advanced NSCLC36. Survival was actually worse in patients who received chemotherapy plus celecoxib (median survival 6.3 months) compared to the patients who received the chemotherapy alone (median survival 9 months). It was postulated that celecoxib may reduce the level of prostaglandin I2 which has anti-tumor properties, leading to promotion of tumor growth rather than inhibition37.
Unfortunately, efficacy and survival do not seem to have improved with the addition of celecoxib to docetaxel when administered to an unselected population and the marrow toxicity appears to have been enhanced compared to docetaxel alone. The Lilenbaum study reported that 35% of patients who received docetaxel/irinotecan/celecoxib demonstrated grade 3-4 neutropenia compared to 20% who received docetaxel/irinotecan without celecoxib. Similarly, Csiki et al and Nugent et al both reported grade 3-4 neutropenia (57% and 26%, respectively) and febrile neutropenia (15% and 9%, respectively) as two of the most common toxicities of docetaxel plus celecoxib. This is consistent with our study which suggests a synergistic effect of docetaxel plus celecoxib in the development of neutropenia compared to docetaxel alone.
It is possible that COX-2 is required for marrow recovery after cytotoxic chemotherapy38. Preclinical data suggest that chemotherapy-induced bone marrow necrosis requires an inflammatory response to remove dead cells and debris in order to maintain a proper hematopoetic milieu. Mice deficient in the COX-2 gene demonstrated a slow marrow recovery following administration of 5-fluorouracil compared to wild-type mice given the same agent. Interestingly, when hemolysis was induced in the COX-2 deficient mice, erythropoesis was unhindered compared to the wild-type mice suggesting COX-2 was required for repair of marrow damage, but was not necessary for normal marrow hematopoesis.
Recently, results were presented from the Cancer and Leukemia Group B trial 30203 that evaluated celecoxib and/or zileuton (5-LOX inhibitor) plus standard chemotherapy in advanced NSCLC39. Patients received carboplatin and gemcitabine with celecoxib, zileuton, or both and although failure free survival and overall survival did not differ among the three arms (or compared to historic controls), a preplanned analysis of COX-2 expression as a prognostic and predictive marker demonstrated intriguing results. Patients who did not receive celecoxib and demonstrated high intratumoral expression of COX-2 by immunohistochemistry had a worse outcome compared to patients with low expression. This confirmed retrospective studies that suggested overexpression of COX-2 in NSCLC is a negative prognostic factor40. Also, patients treated with celecoxib who demonstrated moderate or high COX-2 expression had an improvement in overall survival compared to those with moderate or high COX-2 expression who did not receive celecoxib. Interestingly, patients with low COX-2 expression treated with celecoxib seemed to have a worse overall survival compared to patients who overexpressed COX-2 and received celecoxib. In our trial, patients were not selected based on COX-2 expression and, as Edelman et al has suggested, a negative effect of celecoxib in patients with low COX-2 expression may have diluted the benefit attained in the patients with moderate to high COX-2 expression.
Non-smokers appear to have less COX-2 activity compared to active and former smokers, suggesting different dose levels would be required for adequate inhibition based on the smoking status of the patient. With a “one size fits all” dose of celecoxib, previous studies have shown that PGE2 production was inhibited to a greater degree in non-smokers31,41. The preselection of NSCLC patients based on tumor expression of COX-2 may be important to observe an antitumor effect of celecoxib. Immunohistochemical analysis of the tumor or surrogate markers of COX-2 expression such as urinary PGE-M levels could be used prospectively to enhance a clinical trial population most likely to respond to celecoxib.
In conclusion, our study demonstrates that celecoxib added to docetaxel may enhance marrow toxicity and, in unselected patients, there is no clear improvement in survival. Evaluation of celecoxib with or without chemotherapy in appropriately selected patients may be beneficial and warrants further investigation.
Acknowledgments
Aventis (currently Sanofi-Aventis) provided funding. Celecoxib was provided by Pharmacia (currently Pfizer). Supported in part by Cancer Center Support Grant CA-22453 from the National Cancer Institute.
Footnotes
Presented in part at the Thirty-Ninth Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June, 2003.
Written informed consent was obtained from all patients prior to initiation of therapy.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 3.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 4.Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000;18:2095–2103. doi: 10.1200/JCO.2000.18.10.2095. [DOI] [PubMed] [Google Scholar]
- 5.Fossella FV, DeVore R, Kerr RN, et al. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol. 2000;18:2354–2362. doi: 10.1200/JCO.2000.18.12.2354. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Shih J, Lee C, et al. Phase II study of docetaxel and ifosfamide combination chemotherapy in non-small cell lung cancer patients failing previous chemotherapy with or without paclitaxel. Lung Cancer. 2003;39:209–214. doi: 10.1016/s0169-5002(02)00445-2. [DOI] [PubMed] [Google Scholar]
- 7.Munoz A, Rubio I, Mane J, et al. Phase II study of docetaxel/vinorelbine in patients with non-small cell lung cancer previously treated with platinum-based chemotherapy. Clin Lung Cancer. 2002;4:168–173. doi: 10.3816/clc.2002.n.024. [DOI] [PubMed] [Google Scholar]
- 8.Kosmas C, Tsavaris N, Vadiaka M, et al. Gemcitabine and docetaxel as second-line chemotherapy for patients with non-small cell lung carcinoma who fail prior paclitaxel plus platinum-based regimens. Cancer. 2001;92:2902–2910. doi: 10.1002/1097-0142(20011201)92:11<2902::aid-cncr10103>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 9.Spiridonidis CH, Laufman LR, Carman L, et al. Second-line chemotherapy for non-small cell lung cancer with monthly docetaxel and weekly gemcitabine: a phase II trial. Ann Oncol. 2001;12:89–94. doi: 10.1023/a:1008306616994. [DOI] [PubMed] [Google Scholar]
- 10.Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10:4266s–4269s. doi: 10.1158/1078-0432.CCR-040014. [DOI] [PubMed] [Google Scholar]
- 11.Leahy KM, Ornberg RL, Wang Y, et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–631. [PubMed] [Google Scholar]
- 12.Plescia OJ, Smith AH, Grinwich K. Subversion of immune system by tumor cells and role of prostaglandins. Proc Natl Acad Sci USA. 1975;72:1848–1851. doi: 10.1073/pnas.72.5.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng H, Shao J, Morrow JD, et al. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362–366. [PubMed] [Google Scholar]
- 14.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 15.Williams CS, Tsujii M, Reese J, et al. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105:1589–1594. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dohadwala M, Luo J, Zhu L, et al. Non-small cell lung cancer cyclooxygenase-2-dependent invasion is mediated by CD44. J Biol Chem. 2001;276:20809–20812. doi: 10.1074/jbc.C100140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolina M, Sharma S, Lin Y, et al. Specific inhibition of cyclooxygenase-2 restores antitumor reactivity by altering the balance of IL-10 and IL-12 synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 19.Liu CH, Chang SH, Narko K, et al. Overexpression of cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J Biol Chem. 2001;276:18563–18569. doi: 10.1074/jbc.M010787200. [DOI] [PubMed] [Google Scholar]
- 20.Krysan K, Merchant FH, Zhu L, et al. [accessed October 25,2007];COX-2-dependent stabilization of survivin in non-small cell lung cancer. FASEB J. 2003 doi: 10.1096/fj.03-0369fje. published online November 3. Available at: http://www.fasebj.org. [DOI] [PubMed] [Google Scholar]
- 21.Yuan A, Yu CJ, Shun CT, et al. Total cyclooxygenase-2 mRNA levels correlate with vascular endothelial growth factor mRNA levels, tumor angiogenesis and prognosis in non-small cell lung cancer patients. Int J Cancer. 2005;115:545–555. doi: 10.1002/ijc.20898. [DOI] [PubMed] [Google Scholar]
- 22.Koki A, Kahn NK, Woerner BM, et al. Cyclooxygenase-2 in human pathological disease. Adv Exp Med Biol. 2002;507:177–184. doi: 10.1007/978-1-4615-0193-0_28. [DOI] [PubMed] [Google Scholar]
- 23.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase 2 occurs frequently in human lung cancers, specifically in adenocarcinoma. Cancer Res. 1998;58:3761–3764. [PubMed] [Google Scholar]
- 24.Hida T, Kozaki K, Ito H, et al. Significant growth inhibition of human lung cancer cells both in vitro and in vivo by the combined use of a selective cyclooxygenase-2 inhibitor, JTE-522, and conventional anticancer agents. Clin Cancer Res. 2002;8:2443–2447. [PubMed] [Google Scholar]
- 25.Subbaramaiah K, Hart JC, Norton L, et al. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2: Evidence for involvement of ERK1/2 and p38 mitogen-activated protein kinase pathways. J Biol Chem. 2000;275:14838–14845. doi: 10.1074/jbc.275.20.14838. [DOI] [PubMed] [Google Scholar]
- 26.Hida T, Kozaki K, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res. 2000;6:2006–2011. [PubMed] [Google Scholar]
- 27.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 28.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 29.Lee E. Statistical Methods for Survival Data Analysis. (ed 3rd) Wiley & Sons, Inc.; 2003. pp. 76–91. [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 31.Csiki I, Morrow J, Sandler A, et al. Targeting cyclooxygenase-2 in recurrent non-small cell lung cancer: a phase II trial of celecoxib and docetaxel. Clin Cancer Res. 2005;11:6634–6640. doi: 10.1158/1078-0432.CCR-05-0436. [DOI] [PubMed] [Google Scholar]
- 32.Nugent F, Mertens W, Graziano S, et al. Docetaxel and cyclooxygenase-2 inhibition with celecoxib for advanced non-small cell lung cancer progressing after platinum-based chemotherapy: a multicenter phase II trial. Lung Cancer. 2005;48:267–273. doi: 10.1016/j.lungcan.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Altorki N, Port J, Zhang F, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res. 2005;11:4191–4197. doi: 10.1158/1078-0432.CCR-05-0108. [DOI] [PubMed] [Google Scholar]
- 34.Altorki NK, Keresztes RS, Port DM, et al. Celecoxib, a selective cyclo-oxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol. 2003;21:2645–2650. doi: 10.1200/JCO.2003.07.127. [DOI] [PubMed] [Google Scholar]
- 35.Gridelli C, Gallo C, Ceribelli A, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8:500–512. doi: 10.1016/S1470-2045(07)70146-8. [DOI] [PubMed] [Google Scholar]
- 36.Lilenbaum R, Socinski MA, Altorki NK, et al. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non-small-cell lung cancer. J Clin Oncol. 2006;24:4825–4832. doi: 10.1200/JCO.2006.07.4773. [DOI] [PubMed] [Google Scholar]
- 37.Csiki I, Johnson DH. Did targeted therapy fail cyclooxygenase too? J Clin Oncol. 2006;24:2798–4800. doi: 10.1200/JCO.2006.08.0622. [DOI] [PubMed] [Google Scholar]
- 38.Lorenz M, Slaughter HS, Wescott DM, et al. Cyclooxygenase-2 is essential for normal recovery from 5-fluorouracil-induced myelotoxicity in mice. Exp Hematol. 1999;27:1494–1502. doi: 10.1016/s0301-472x(99)00087-9. [DOI] [PubMed] [Google Scholar]
- 39.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy − Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 40.Khuri FR, Wu H, Lee JJ, et al. Cyclooxygenase-2 overexpression is a marker of poor prognosis in stage I non-small cell lung cancer. Clin Cancer Res. 2001;7:861–867. [PubMed] [Google Scholar]
- 41.Gross ND, Boyle JO, Morrow JD, et al. Levels of prostaglandin E metabolite, the major urinary metabolite of prostaglandin E2, are increased in smokers. Clin Cancer Res. 2005;11:6087–6093. doi: 10.1158/1078-0432.CCR-05-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]