Abstract
Testosterone (T) plays a key role in the increase and maintenance of muscle mass and bone density in adult men. Life history theory predicts that environmental stress may prompt a reallocation of such investments to those functions critical to survival. We tested this hypothesis in two studies of rural Bolivian adult men by comparing free T levels and circadian rhythms during late winter, which is especially severe, to those in less arduous seasons. For each pair of salivary TAM/TPM samples (collected in a ~12-hour period), circadian rhythm was considered classic (CCLASSIC) if TAM>110%TPM, reverse (CREVERSE) if TPM>110%TAM, and flat (CFLAT) otherwise. We tested the hypotheses that mean TAM>mean TPM and that mean TLW<mean TOTHER (LW=late winter, OTHER=other seasons). In Study A, of 115 TPM-TAM pairs, 51%=CCLASSIC, 39%=CREVERSE, 10%=CFLAT; in Study B, of 184 TAM-TPM pairs, 55%=CCLASSIC, 33%=CREVERSE, 12%=CFLAT. Based on fitting linear mixed models, in both studies TOTHER-AM>TOTHER-PM (A: p=0.035, B: p=0.0005) and TOTHER-AM>TLW-AM (A: p=0.054, B: p=0.007); TPM did not vary seasonally, and T diurnality was not significant during late winter. T diurnality varied substantially between days within an individual, between individuals and between seasons, but neither T levels nor diurnality varied with age. These patterns may reflect the seasonally varying but unscheduled, life-long, strenuous physical labor that typifies many non-industrialized economies. These results also suggest that single morning samples may substantially underestimate peak circulating T for an individual and, most importantly, that exogenous signals may moderate diurnality and the trajectory of age-related change in the male gonadal axis.
Keywords: andrology, testosterone, seasonality, circadian rhythms, aging, Bolivia
INTRODUCTION
Life history theory argues that mechanisms have evolved for the allocation of finite resources to somatic and reproductive functions such that an individual’s multigenerational inclusive fitness is maximized (Stearns 1992). Reductions in female reproductive investment–including lower levels of gonadal hormones, higher rates of anovulation, decreased probability of conception, and increased risk of early pregnancy loss–are associated with periods of food scarcity, arduous physical activity and/or increased psychosocial stress in several populations (Prior 1987; Ellison et al. 1989b; Panter Brick et al. 1993; Jasienska and Ellison 1998; Nepomnaschy et al. 2004, 2006; Vitzthum et al. 2006, 2008; Vitzthum 2008). Evidence for comparable variation in the principal male gonadal hormone, testosterone (T), is much less clear (Bribiescas 2001).
Most studies of industrialized populations have observed circannual variation in male T (Smals et al. 1976; Andersson et al. 2003, Bellastella et al. 1982; Dabbs 1990; Meriggiola et al. 1996; Nicolau et al. 1984, 1985; Perry et al. 2000; Reinberg et al. 1975, 1988; Valero-Polti and Fuentes-Arderiu 1998; Svartberg et al. 2003; van Anders et al. 2006), although some have not (Baker et al. 1976, Dai et al. 1981, Martikainen et al. 1985, Abbaticchio et al. 1987, Maes et al. 1997; Svaartberg and Barrett-Conner 2004, Brambilla et al. 2007). Collectively, those studies in the northern hemisphere that observed seasonal variation reported peaks in one or more months from March through December. Temperature and daylight duration–either directly or indirectly through their effects on physical activity, weight, and/or sleep patterns–were the most common reasons suggested for T seasonality, but there are few direct tests of these hypotheses. In a sample of amateur wrestlers, T increased with weight gain during the post-competitive season (Strauss et al. 1985). In contrast, T decreased with weight gain in Norwegian men (Svartberg et al. 2003) and male Lese (shifting horticulturalists in the Ituri Forest) (Bentley et al. 1993), and was similar across seasons in Nepalese men despite significant changes in energy balance (Ellison and Panter-Brick 1996).
Findings from studies of circadian rhythms in T are also inconsistent. Circulating T levels in adult men are generally reported to peak upon awakening, declining by as much as half by late evening and rising again during a night’s sleep (Axelsson et al. 2005). However, the significance and universality of such circadian variation remains uncertain, as is the extent to which it reflects circadian control or results from entrainment by behavioral or other factors. In particular, observations are contradictory regarding change in T diurnality with aging and/or age-related modifications of behavioral repertoires Some authors have reported that circadian rhythms are comparable in healthy older and younger men; however, others have concluded that diurnal variation in T is blunted with age, possibly as a consequence of an age-related decline in circulating T (Diver et al. 2003 and references therein).
Most studies find that T levels decline by 30–50% over the course of adulthood (Liu et al. 2005), and the reasons for this change have become a principal focus of research on male aging (e.g., the ensemble model of the male gonadal axis, and the associated biomathematical constructs, developed by Veldhuis and colleagues (Liu et al. 2005, Keenan et al. 2006, Keenan and Veldhuis 2001)). This ensemble model accords well with empirical data and suggests that the precise physiological mechanisms underlying such declines are likely to be multifactorial. However, current understanding of circadian or age-related variation in T is predicated almost entirely on data from industrialized populations. Little is known of circadian variation in T among community-dwelling men in non-industrialized settings, and the scant data on age-related variation suggests significant inter-populational variation for unknown reasons (Ellison et al. 2002).
Both evolutionary and medical sciences have much to gain from comprehensive studies of men in non-industrialized populations. Especially in rural economies unencumbered by fixed schedules, the frequently strenuous activities of adult men may vary daily and/or seasonally yet change little throughout a lifetime (Kaplan 1994). The influence on T levels of less structured albeit demanding quotidian routines, relatively decoupled from age per se, bears directly on whether classic diurnality and/or an age-related decline in T are inherent features of the male gonadal axis or are entrained by extrinsic factors. This question bears, in turn, on the generalizability of current models of the male gonadal axis.
Based on data collected from Bolivian men living in rural agropastoral communities south of La Paz, we tested the hypotheses that T will be lower during late winter, an especially severe season in this environment, and that T diurnality and variation with age are comparable in this population to that typically observed in industrialized populations.
MATERIALS AND METHODS
Study Participants
Protocols in Studies A and B were approved by the Institutional Review Boards at Case Western Reserve University and the University of California (Riverside), respectively. All participants, recruited from dispersed agropastoral communities in Provincías Murillo and Aroma, south of La Paz, Bolivia, were nominally healthy and gave informed consent. In Study A, subjects in a larger investigation of genetic variation in respiratory functioning (Beall et al. 1999) were invited to participate in this study of T; 115 men, aged 21–59 years (mean=38.9, standard deviation=10.8), volunteered and self-collected samples on a single night and following morning at some time during late May through late August (approximately late autumn through late winter), 1994. Study B was conducted within the framework of Project REPA (Reproduction and Ecology in Provincía Aroma) (Vitzthum et al. 2004); 65 men, aged 21–59 years (mean=33.2, standard deviation=7.43), volunteered and self-collected 6 samples during a single week at some time from late May through late October (approximately late autumn through spring), 1996.
Saliva Sample Collection
In Study A, each man self-collected a 5-ml saliva sample shortly before retiring (about 8:00–9:00 pm) and then upon awakening (about 6:00–7:00 am). In Study B, on each of 3 days (Monday, Wednesday, Friday), an assistant visited a participant’s home at about 8:00 am and again at 8:00 pm, and waited while the man self-collected a 5-ml saliva sample according to an established protocol that included rinsing the mouth with cool clean water and waiting at least 20 minutes before collecting saliva, procedures that mitigated contamination from food, drink, blood and coca-chewing (Vitzthum et al. 1993). In both studies, samples were maintained at ambient temperature until shipped to Emory University where they were frozen at −26°C until assayed.
Hormone Assays
Saliva samples were assayed for T using an 125I radioimmunoassay method detailed and comparatively assessed in previous reports (Beall et al. 1992, Dabbs et al. 1995). Assay precision for the low (mean 0.44 ng/dL (0.015 nmol/L)) and high (5.11 ng/dL (0.177 nmol/L)) controls was intra-assay 12.3% and 9.1% and inter-assay 11.0% and 6.6 %, respectively.
Statistical Analyses
Statistical analyses were performed with SPSS version 16.0 (Chicago, IL). For all analyses, T observations were log2 transformed and men’s ages were centered at 30 years (i.e., centered age = subject’s age–30). Centering on a value near the mean or median of an age (or other term’s distribution) is widely recommended for both simple and more complex regressions because it reduces the correlations between the different powers (linear, quadratic, cubic) of the term in fitted models (for additional technical advantages of centering, see West et al., 2008). Correlations of TAM/TPM pairs and the correlations among the multiple TAM and TPM observations in Study B were determined.
To evaluate patterns in circadian rhythms, each TAM/TPM pair was plotted (Figure 1). The circadian rhythm of each pair was defined in this study as classic (CCLASSIC) if TAM>110%TPM, reverse (CREVERSE) if TPM>110%TAM, and flat (CFLAT) otherwise. Any cut-off is necessarily arbitrary; the chosen threshold was purposively conservative to favor the null hypothesis. T diurnality for each man was defined in Study A as (log2TPM)-(log2TAM) and in Study B (which had three days of observations for each man) as [(log2TPM-MON + log2TPM-WED + log2TPM-FRI)/3] – [(log2TAM-MON + log2TAM-WED + log2TAM-FRI)/3)]. Late winter–when temperatures are severely cold, days are short, food scarcity is common, and physical activity is typically less arduous than the preceding harvest and upcoming planting seasons–was defined as July 15 through August 31.
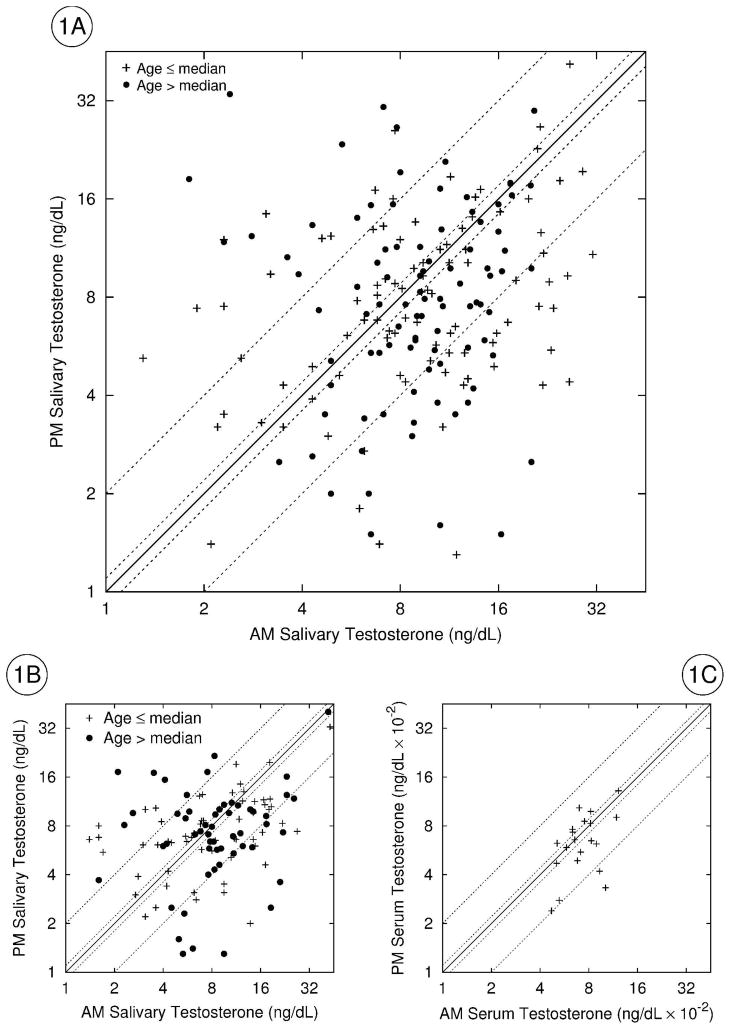
Figure 1.
TPM plotted against TAM in 3 studies. Figure 1A=rural Bolivian men from Provincía Aroma (Study B); Figure 1B=rural Bolivian men from Provincía Murillo (Study A); Figure 1C=U.S. men from Spratt et al. (1988). Both axes are log2 scale (nmol/L = 0.0347 ng/dL). Below the solid diagonal line, TPM<TAM (classic circadian rhythm); above the line, TPM>TAM (reverse circadian rhythm). The first pair of dotted lines either side of solid diagonal delineate TAM/TPM pairs defined as “flat” (TAM=TPM±10%). The second pair of dotted lines delineate TAM=2TPM (below the solid diagonal) and TPM=2TAM (above the solid diagonal). +=younger men, ●=older men (defined as above and below the median of each sample in Studies A and B; all men in Figure 1C are ≤ 37 years). In Figure 1A, 3 points outside the plotted area are not included to conserve space and increase clarity.
Linear mixed models (LMM) were fitted to evaluate circadian, seasonal, between day, and age-associated variation in T variables. The protocols for fitting and evaluating models followed West et al. (2007); individuals were treated as random effects in all final models. Age variation was parameterized by various combinations of linear, quadratic, and cubic powers of centered age. Reflecting the a priori expectation that TAM>TPM, TOTHER>TLW, and T decreases with increasing age, significance tests for these hypotheses were one-sided. Tests for significant differences in T variables among days of week were two-sided.
RESULTS
Descriptive statistics for T variables are in Table 1. Plots of each TAM/TPM pair on a log2 scale are in Figure 1. Histograms of T diurnality are in Figure 2.
Table 1.
Descriptive Statistics of Testosterone (ng/dL) Variables
| STUDY A (n=115) | STUDY B (n=65) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Late Winter | Other Seasons | Late Winter | Other Seasons | |||||||||
| AM | PM | Diurnality | AM1 | PM | Diurnality | AM | PM | Diurnality | AM2 | PM | Diurnality | |
|
|
||||||||||||
| Geometric Mean of T | 7.03 | 6.99 | 8.65 | 7.26 | 7.63 | 7.37 | 10.11 | 7.59 | ||||
| Arithmetic Mean of log2T | 2.814 | 2.806 | −0.008 | 3.112 | 2.86 | −0.252 | 2.931 | 2.881 | −0.052 | 3.338 | 2.925 | −0.42 |
| Standard Error of log2T | 0.142 | 0.142 | 0.181 | 0.118 | 0.118 | 0.128 | 0.113 | 0.126 | 0.109 | 0.114 | 0.128 | 0.158 |
log2TOTHER-AM>log2TOTHER-PM (p=0.035), log2TOTHER-AM>log2TLW-AM (p=0.054)
log2TOTHER-AM>log2TOTHER-PM (p=0.0005), log2TOTHER-AM>log2TLW-AM (p=0.007)
Figure 2.
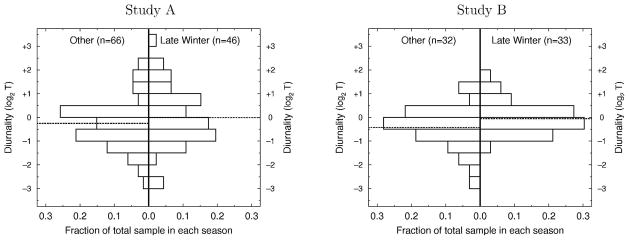
Distribution of individual circadian rhythm [defined as (log2TPM)–(log2TAM) in Study A and as [(log2TPM-MON + log2TPM-WED + log2TPM-FRI)/3] – [(log2TAM-MON + log2TAM-WED + log2TAM-FRI)/3)] in Study B] during late winter and during other seasons in Study A (left) and B (right). Diurnality <0 has higher TAM than TPM. Mean in each sample is indicated by a dashed line. There is substantial individual variation in diurnality in each sample and season. The seasonal difference in diurnality arises from a larger proportion of the sample having TPM>TAM in late winter than in other seasons (see text for additional discussion).
In Study A, the Pearson correlation between log2TPM and next morning log2TAM was 0.301 (p<0.001). In Study B, the correlations between same-day log2TAM and log2TPM (each day evaluated separately) were <0.20 and not significant. Most correlations among the AM observations were significant but modest: log2TAM-Mon v. log2TAM-Wed: r = 0.489 (p<0.001); log2TAM-Mon v. log2TAM-Fri: r = 0.417 (p<0.001); log2TAM-Wed v. log2TAM-Fri: r = 0.197 (p=0.122). All of the associations among the PM observations were <0.24 and non-significant. The circadian pattern on one day did not predict that of another day. Day of week was not a significant covariate in any fitted LMM.
There was striking heterogeneity in circadian rhythms, both between men and between days within an individual (Figure 1, plots A and B; Figure 2). In Study A, of 115 TAM/TPM pairs, 51%=CCLASSIC, 39%=CREVERSE, and 10%=CFLAT. In Study B, of 184 TAM/TPM pairs, 55%=CCLASSIC, 33%=CREVERSE, and 12%=CFLAT. In both studies, similar proportions of each diurnal pattern were observed in younger and older men and, in Study B, on each of 3 weekdays. Variation in T variables was not associated with age in either study.
In both studies, mean diurnality in late winter was substantially less than in other seasons (A: −0.008 v. −0.252; B: −0.052 v. −0.420). These differences in mean circadian rhythms reflected a shift in the distribution of diurnality (Figure 2), such that during late winter a smaller proportion of men exhibited classic circadian variation (i.e., either a rise in T during the night or a drop during the day). Even so, there was still marked heterogeneity in circadian patterns throughout the time spans of both studies.
Morning T levels (Table 1) were significantly lower during late winter than during other seasons (log2TLW-AM < log2TOTHER-AM in Study A: 2.814 v. 3.112 (p=0.054); in Study B: 2.931 v. 3.338 (p=0.007)) but evening levels did not vary seasonally (log2TLW-PM v. log2TOTHER-PM in Study A: 2.806 v. 2.860 (p=0.38; in Study B: 2.881 v. 2.925 (p=0.40). In other words, in both studies: TAM-OTHER > TPM-OTHER ≈ TAM-LW ≈ TPM-LW. These patterns underlie the absence of circadian variation in late winter (log2TLW-AM v. log2TLW-PM in Study A: 2.814 v. 2.806 (p=0.48); in Study B: 2.931 v. 2.881 (p=0.34) and significant diurnality in other seasons (log2TOTHER-AM > log2TOTHER-PM in Study A: 3.112 v. 2.860 (p=0.035); in Study B: 3.338 v. 2.925 (p=0.0005)).
DISCUSSION
Together these two studies provide clear evidence of seasonality in both morning T level and T diurnality in a non-industrialized population. In these rural Bolivian men, the mean levels of T across seasons and at morning and evening within each season suggest that during late winter, T fails to rise during the night to the level observed during other seasons and that over the course of a late winter day, mean T levels are about the same, in contrast to the decline in mean T observed during other seasons. The demonstration of parallel seasonality in two independent studies, one in 1994 and another in 1996, conducted in two separate sets of communities using different protocols to collect comparable data substantially mitigates any possibility that the observed seasonality is anomalous.
The reduction in mean TAM during late winter in these altiplano communities (altitude ~4000m) is most likely multifactorial. During this season, the days are short, mean low temperatures are typically several degrees below freezing, and wind/dust storms are not uncommon. People may sleep longer, but perhaps less comfortably as fuels are too precious for most to use for heating homes, apart from the heat generated by cooking. Food stores from the fall harvest are declining, and many families are unable to purchase more foodstuffs. Documented responses to seasonal nutritional stress in the Andes include reduced activity levels and fewer meals to reduce post-prandial energy loss (Leonard and Thomas 1989). All these environmental and behavioral factors can be expected to modify T levels. Experimentally controlled short-term fasting in both primates and humans (reviewed in Cameron 1996) and daylight fasting during Ramadan (Bogdan et al. 2001, Mesbahzadeh et al. 2005) are associated with reductions in T and shifts in T diurnality. Both sleep and activity patterns are also known to influence T levels and circadian rhythms (Kern et al. 1995, Luboshitzky et al. 2001, Axelsson et al. 2005). Additional research is needed to test these specific hypotheses in rural Bolivian men. The pattern of seasonality in these Bolivians is distinct from that observed in Lese men (Bentley et al. 1993), the only other report of T seasonality in a non-industrialized population. In the Lese, TAM levels were comparable in both seasons, but TPM levels were lower in August than in June. Because energetic conditions had improved and the men had gained an average of 2 kg during the observation period, the reason for the seasonal decline in evening T was unclear.
In the present studies of Bolivians, all indicators of T levels and T diurnality were comparable for younger and older members. Most studies of industrialized populations (Axelsson et al. 2005, Diver et al. 2003, Liu et al. 2005, Keenan et al. 2006) have observed a 1%–2% T decline/year at older ages (40–90 years). Findings from the few reports for non-industrialized populations are mixed (Ellison et al. 1989, Beall et al. 1992, Bentley et al. 1993, Bribiescas 1996, Campbell et al. 2003, Ellison et al. 2002), some observing an age-related T decline, others not, even within the same population. This inconsistency may be the result of some studies having relatively fewer men at much older ages, but 51 of the 115 participants in Study A were ≥40 years of age. However, all men were younger than 60 years of age in both of the present studies. Although it may be that T declines in rural Bolivian men at ages >60 years, no significant decline at younger ages was detected in these studies.
Compared to reports of up to two-fold differences between TPM and TAM levels (Axelsson et al. 2005), diurnal variation appears blunted in rural Bolivian men, especially in late winter. However, the sample means mask considerable heterogeneity in circadian rhythms. In both studies, about half of the TAM/TPM pairs did not conform to the expected circadian rhythm (Figure 1). Because the criterion for testing the null hypothesis was conservative (i.e., a TAM/TPM pair was considered classic if TAM>110%PM), this estimate of the proportion of non-conforming pairs can be considered a minimum. Absence of classic diurnality is not equivalent, however, to an absence of circadian variation per se. In both studies, only 10–12% of the pairs had TAM values within TPM±10%; that is, only a small proportion of TAM/TPM pairs were consistent with the blunting of diurnality suggested by the TAM and TPM sample means. In the remaining pairs (39% in Study A, 33% in Study B), circadian variation was as pronounced as that observed among the classic diurnal pairs, but the timing of the observed peak and nadir was reversed. In Study B, the modest correlations among the post-waking TAM levels across 3 days and the absence of significant correlations among the TPM values indicate substantial intra-individual variation in both daily absolute T levels and T circadian rhythms.
Notably, this marked within and between individual variation in diurnality is not fully explained by the severe conditions during late winter. In Figure 2, it is clear that the significant seasonal difference in mean circadian rhythm arises from a modest shift in the distribution of diurnality such that there are fewer TAM/−TPM pairs exhibiting the classic pattern in late winter. Nonetheless, both classic and reverse diurnality are still commonly exhibited in all seasons.
These observations suggest that some factor(s) underlies an apparent modulation of T temporal patterning such that peak T does not always occur in these Bolivian men shortly after waking. As already noted, dietary, sleep and activity patterns are known to modify T levels and circadian rhythms. As sleeping styles typical in most industrialized countries are not, in fact, common to most of the world’s population (Worthman and Melby 2002), circadian rhythms may also vary accordingly. Likewise, most men outside industrialized countries, and some within, engage daily in often strenuous physical labor that may stimulate T production during the day and hence blunt and/or shift the timing of diurnal variation. Such physiological responses have been documented among subsistence hunters; the typical drop in T from morning to evening observed in !Kung San men of Botswana was cut by half on days spent hunting for food (Worthman and Konner 1987).
Few published studies include individual data that would permit an assessment of within-sample variation in circadian rhythms. A handful of agricultural and forager populations exhibit differences between mean TAM and mean TPM substantially less than two-fold (Ellison et al. 1989, Bentley et al. 1993, Bribiescas 1996, Campbell et al. 2003). However, as only summary statistics were reported, it is unclear if the apparent blunting of diurnal variation in these samples holds for all study participants or if there is significant heterogeneity in circadian rhythms within each sample. Such variation is evident in the range (0.2 to 5.9) among individuals for the ratio of AM/PM T included in one report (Beall et al. 1992), and Ellison et al. (1989) noted that 5 of 29 study participants had higher mean TPM than mean TAM.
The limited published data available from U.S. samples suggest that circadian rhythms may, in fact, be more heterogeneous in industrialized populations than widely appreciated. Figure 1C plots serum TAM versus TPM reported for 20 U.S. males aged 18–37 years (Spratt et al. 1988). Although participants were observed in the laboratory for 24 hours and slept between 2300 and 0700 hours, they nonetheless exhibited substantial variation in T diurnality. Using the criteria defined here, 45%= CCLASSIC, 35%=CREVERSE, and 20%=CFLAT. The authors, noting two other studies in which some individuals did not exhibit classic T diurnality, also concluded that T levels need not necessarily reach a nadir during the evening in all men (Spratt et al. 1988 and references therein).
Both the reasons for heterogeneity in T diurnality and the consequences of such variation for aging, health and reproductive functioning merit further study. Likewise, as has been previously emphasized (Ellison et al. 2002), the apparent absence in some populations of an age-related T decline deserves greater attention. In particular, larger studies with frequent sampling and careful measurement of hypothesized covariates, including the timing and duration of behaviors relative to sampling, are called for. Until more is learned, we should not assume that any potential determinant has a constant impact on T levels over a 24-hour period. For example, the effect of a missed evening meal may differ from that of a meal missed at midday.
The present studies of Bolivian men collected only 2 samples per individual for each 24-hour observation period. Therefore, the greater of these observations may not have represented that man’s peak on that day nor would the lower value necessarily have been the nadir. Clearly a single sample, even one taken shortly after awakening, cannot be assumed to represent a given individual’s peak T and cannot be relied upon, alone, to characterize variation in T levels within or between populations. A significant clinical implication is that single morning samples may substantially underestimate peak circulating T for a patient.
Research predicated on an ensemble model of the male gonadal axis (Liu et al. 2005; Keenan et al. 2006; Keenan and Veldhuis 2001) has yielded valuable insight into the mechanisms behind disrupted regulation during aging, but circadian linkages have yet to be fully investigated (Keenan and Veldhuis 2001). This new evidence of varying T diurnality within and between individuals suggests the need for a more thorough examination of the relationship of circadian systems to regulation of testicular activity.
Likewise, life history theory has generated promising avenues of research into the proximate and evolutionary determinants of variation in T levels, and the consequences of these for male investment in somatic maintenance and reproductive effort (Wingfield et al. 1990, Ellison and Panter-Brick 1996, Bribiescas 2001, Campbell et al. 2003, Gray 2003, Muehlenbein and Bribiescas 2005, Muller et al. 2009). At the same time, findings from studies testing specific hypotheses, particularly in human males, are inconsistent. Accounting for seasonal and individual variation in circadian rhythms could help to resolve these apparent contradictions.
Most importantly, although formal evolutionary and clinical models provide powerful tools for analyzing complex systems, all physiological mechanisms are, in fact, integrated components of an organism functioning within a signal-generating milieu. The evidence of seasonal variation and the absence of an age-related T decline in these rural Bolivians implies that the dynamic responsiveness of the male gonadal axis to behavioral, psychosocial, and/or environmental contexts may, at least to some extent, moderate the trajectory of age-related change in the axis. Should further research confirm this hypothesis, the implications for clinical practice and future studies of male evolutionary endocrinology would be substantial.
Acknowledgments
Grant Support: National Institutes of Mental Health: MH57761 (to CMW)
National Science Foundation: SBR 9221724 (to CMB), SBR 9506107 (to VJV)
University of California Regents (to VJV)
We thank the participants in these studies for their patience, the many research assistants for their help in collecting samples, Roger Mundry (MPI-Evolutionary Anthropology, Leipzig) for statistical advice, and a reviewer for thoughtful commentary. This work was supported by grants from the U.S. National Science Foundation (SBR 9221724 to CMB, SBR 9506107 to VJV), the U.S. National Institutes of Mental Health (MH57761 to CMW), and the University of California Regents (to VJV).
Footnotes
A contribution to the special issue on “Evolutionary Endocrinology: Integrating Proximate Mechanisms, Ontogeny, and Evolved Function,” Plenary Session at 2008 HBA Meetings
References
- Abbaticchio G, de Fini M, Giagulli VA, Santoro G, Vendola G, Giorgino R. Circannual rhythms in reproductive functions of human males, correlations among hormones and hormone-dependent parameters. Andrologia. 1987;19:353–361. doi: 10.1111/j.1439-0272.1987.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Andersson AM, Carlsen E, Petersen JH, Skakkebaek NE. Variation in levels of serum inhibin B, testosterone, estradiol, luteinizing hormone, follicle-stimulating hormone, and sex hormone-binding globulin in monthly samples from healthy men during a 17-month period: possible effects of seasons. J Clin Endocrinol Metab. 2003;88:932–937. doi: 10.1210/jc.2002-020838. [DOI] [PubMed] [Google Scholar]
- Axelsson J, Ingre M, Akerstedt T, Holmback U. Effects of acutely displaced sleep on testosterone. J Clin Endocrinol Metab. 2005;90:4530–4535. doi: 10.1210/jc.2005-0520. [DOI] [PubMed] [Google Scholar]
- Baker HW, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary testicular system with age. Clin Endocrinol. 1976;5:349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Beall CM, Worthman CM, Stallings J, Strohl KP, Brittenham GM, Barragan M. Salivary testosterone concentration of Aymara men native to 3600 m. Ann Hum Biol. 1992;19:67–78. doi: 10.1080/03014469200001932. [DOI] [PubMed] [Google Scholar]
- Beall CM, Almasy LA, Blangero J, Williams-Blangero S, Brittenham GM, Strohl KP, Decker MJ, Vargas E, Villena M, Soria R, Alarcon AM, Gonzales C. Percent of oxygen saturation of arterial hemoglobin among Bolivian Aymara at 3900–4000 m. Am J Phys Anthropol. 1999;108:41–52. doi: 10.1002/(SICI)1096-8644(199901)108:1<41::AID-AJPA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bellastella A, Esposito V, Mango A, D’Alessandro B. Temporal relationship between circannual levels of luteinizing hormone and testosterone in prepubertal boys with constitutional short stature. Chronobiologia. 1982;9:123–125. [PubMed] [Google Scholar]
- Bentley GR, Harrigan AM, Campbell B, Ellison PT. Seasonal effects on salivary testosterone levels among Lese males of the Ituri Forest, Zaire. Am J Hum Biol. 1993;5:711–717. doi: 10.1002/ajhb.1310050614. [DOI] [PubMed] [Google Scholar]
- Bogdan A, Bouchareb B, Touitou Y. Ramadan fasting alters endocrine and neuroendocrine circadian patterns. Meal–time as a synchronizer in humans? Life Sciences. 2001;68:1607–1615. doi: 10.1016/s0024-3205(01)00966-3. [DOI] [PubMed] [Google Scholar]
- Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Lack of seasonal variation in serum sex hormone levels in middle-aged to older men in the Boston area. J Clin Endocrinol Metab. 2007;92:4224–4229. doi: 10.1210/jc.2007-1303. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. Testosterone levels among Ache hunter-gatherer men. Hum Nature. 1996;7:163–188. doi: 10.1007/BF02692109. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. Reproductive ecology and life history of the human male. Am J Phys Anthropol (Suppl 33) 2001:148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- Campbell B, O’Rourke MT, Lipson SF. Salivary testosterone and body composition among Ariaal males. Am J Hum Biol. 2003;15:697–708. doi: 10.1002/ajhb.10203. [DOI] [PubMed] [Google Scholar]
- Dabbs JM., Jr Age and seasonal variation in serum testosterone concentration among men. Chronobiol Int. 1990;7:245–249. doi: 10.3109/07420529009056982. [DOI] [PubMed] [Google Scholar]
- Dabbs JM, Jr, Campbell BC, Gladue BA, Midgley AR, Navarro MA, Read GF, Susman EJ, Swinkels LM, Worthman CM. Reliability of salivary testosterone measurements: a multicenter evaluation. Clin Chem. 1995;41:1581–1584. [PubMed] [Google Scholar]
- Dai WS, Kuller LH, LaPorte RE, Gutai JP, Falvo-Gerard L, Caggiula A. The epidemiology of plasma testosterone levels in middle-aged men. Am J Epidemiol. 1981;114:804–816. doi: 10.1093/oxfordjournals.aje.a113251. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol. 2003;58:710–717. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- El-Migdadi F, Nusier M, Bashir N. Seasonal pattern of leutinizing, follicle-stimulating hormone, testosterone and progesterone in adult population of both sexes in the Jordan Valley. Endocr Res. 2000;26:41–48. doi: 10.1080/07435800009040144. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Hum Repro. 2002;17:3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Lipson SF, Meredith MD. Salivary testosterone levels in males from the Ituri Forest of Zaire. Am J Hum Biol. 1989a;1:21–24. doi: 10.1002/ajhb.1310010106. [DOI] [PubMed] [Google Scholar]
- Ellison PT, Panter-Brick C. Salivary testosterone levels among Tamang and Kami males of central Nepal. Hum Biol. 1996;68:955–965. [PubMed] [Google Scholar]
- Ellison PT, Peacock NR, Lager C. Ecology and ovarian function among Lese women of the Ituri Forest, Zaire. Am J Phys Anthropol. 1989b;78:519–526. doi: 10.1002/ajpa.1330780407. [DOI] [PubMed] [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am J Phys Anthropol. 2003;122:279–286. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Jasienska G, Ellison PT. Physical work causes suppression of ovarian function in women. Proc Biol Sci. 1998;265(1408):1747–1751. doi: 10.1098/rspb.1998.0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan H. Evolutionary and wealth flows theories of fertility: empirical tests and new models. Pop Develop Rev. 1994;20:753–791. [Google Scholar]
- Keenan DM, Veldhuis JD. Hypothesis testing of the aging male gonadal axis via a biomathematical construct. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1755–R1771. doi: 10.1152/ajpregu.2001.280.6.R1755. [DOI] [PubMed] [Google Scholar]
- Keenan DM, Takahashi PY, Liu PY, Roebuck PD, Nehra AX, Iranmanesh A, Veldhuis JD. An ensemble model of the male gonadal axis: illustrative application in aging men. Endocrinology. 2006;147:2817–2828. doi: 10.1210/en.2005-1356. [DOI] [PubMed] [Google Scholar]
- Kern W, Perras B, Wodick R, Fehm HL, Born J. Hormonal secretion during nighttime sleep indicating stress of daytime exercise. J Appl Physiol. 1995;79:1461–1468. doi: 10.1152/jappl.1995.79.5.1461. [DOI] [PubMed] [Google Scholar]
- Leonard WR, Thomas RB. Biosocial responses to seasonal food stress in highland Peru. Hum Biol. 1989;61:65–85. [PubMed] [Google Scholar]
- Liu PY, Iranmanesh A, Nehra AX, Keenan DM, Veldhuis JD. Mechanisms of hypoandrogenemia in healthy aging men. Endocrinol Metab Clin N Am. 2005;34:935–955. doi: 10.1016/j.ecl.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Luboshitzky R, Zabari, Shen-Orr, Herer P, Lavie P. Disruption of the nocturnal testosterone rhythm by sleep fragmentation in normal men. J Clin Endocrinol Metab. 2001;86:1134–1139. doi: 10.1210/jcem.86.3.7296. [DOI] [PubMed] [Google Scholar]
- Martikainen H, Tapanainen J, Vakkuri O, Leppäluoto J, Huhtaniemi I. Circannual concentrations of melatonin, gonadotrophins, prolactin and gonadal steroids in males in a geographical area with a large annual variation in daylight. Acta Endocrinol. 1985;109:446–450. doi: 10.1530/acta.0.1090446. [DOI] [PubMed] [Google Scholar]
- Maes M, Mommen K, Hendrickx D, Peeters D, D’Hondt P, Ranjan R, DeMeyer F, Scharpe S. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol. 1997;46:587–598. doi: 10.1046/j.1365-2265.1997.1881002.x. [DOI] [PubMed] [Google Scholar]
- Meriggiola MC, Noonan EA, Paulsen CA, Bremner WJ. Annual patterns of luteinizing hormone, follicle stimulating hormone, testosterone and inhibin in normal men. Hum Reprod. 1996;11:248–252. doi: 10.1093/humrep/11.2.248. [DOI] [PubMed] [Google Scholar]
- Mesbahzadeh B, Ghiravani Z, Mehrjoofard H. Effect of Ramadan fasting on secretion of sex hormones in healthy single males. East Mediterr Health J. 2005;11:1120–1123. [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. Am J Hum Biol. 2005;17:527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proc Roy Soc (B) 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch K, McConnell D, Strassmann BI, England BG. Stress and female reproductive functioning: a study of daily variations in cortisol, gonadotrophins, and gonadal steroids in a rural Mayan population. Am J Hum Biol. 2004;16:523–532. doi: 10.1002/ajhb.20057. [DOI] [PubMed] [Google Scholar]
- Nepomnaschy PA, Welch KB, McConnell DS, Low BS, Strassmann BI, England BG. Cortisol levels and very early pregnancy loss in humans. Proc Natl Acad Sci (USA) 2006;103:3938–3942. doi: 10.1073/pnas.0511183103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau GY, Haus E, Lakatua DJ, Bogdan C, Sackett-Lundeen L, Popescu M, Berg H, Petrescu E, Robu E. Circadian and circannual variations of FSH, LH, testosterone, dehydroepiandrosterone-sulfate (DHEA-S) and 17-hydroxy progesterone (17 OH-Prog) in elderly men and women. Endocrinologie. 1985;23:223–246. [PubMed] [Google Scholar]
- Nicolau GY, Lakatua D, Sackett-Lundeen L, Haus E. Circadian and circannual rhythms of hormonal variables in elderly men and women. Chronobiol Int. 1984;1:301–319. doi: 10.3109/07420528409063911. [DOI] [PubMed] [Google Scholar]
- Panter-Brick C, Lotstein DS, Ellison PT. Seasonality of reproductive function and weight loss in rural Nepali women. Hum Reprod. 1993;8:684–690. doi: 10.1093/oxfordjournals.humrep.a138120. [DOI] [PubMed] [Google Scholar]
- Perry HM, 3rd, Miller DK, Patrick P, Morley JE. Testosterone and leptin in older African-American men: relationship to age, strength, function and season. Metabolism. 2000;49:1085–1091. doi: 10.1053/meta.2000.7710. [DOI] [PubMed] [Google Scholar]
- Prior JC. Physical exercise and the neuroendocrine control of reproduction. Baillieres Clin Endocrinol Metab. 1987;1:299–317. doi: 10.1016/s0950-351x(87)80065-4. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Lagoguey M, Chauffournier JM, Cesselin F. Circannual and circadian rhythms in plasma testosterone in five healthy young Parisian males. Acta Endocrinol. 1975;80:732–734. doi: 10.1530/acta.0.0800732. [DOI] [PubMed] [Google Scholar]
- Reinberg A, Smolensky MH, Hallek M, Smith KD, Steinberger E. Annual variation in semen characteristics and plasma hormone levels in men undergoing vasectomy. Fertil Steril. 1988;49:309–315. doi: 10.1016/s0015-0282(16)59721-0. [DOI] [PubMed] [Google Scholar]
- Smals AG, Kloppenborg PW, Benraad TJ. Circannual cycle in plasma testosterone levels in man. J Clin Endocrinol Metab. 1976;42:979–982. doi: 10.1210/jcem-42-5-979. [DOI] [PubMed] [Google Scholar]
- Spratt DI, O’Dea LSL, Schoenfeld D, Butler J, Rao PN, Crowley WF. Neuroendocrine-gonadal axis in men: frequent sampling of LH, FSH, and testosterone. Am J Physiol. 1988;254(5 Pt1):E658–E666. doi: 10.1152/ajpendo.1988.254.5.E658. [DOI] [PubMed] [Google Scholar]
- Strauss RH, Lanese RR, Malarkey WB. Weight loss in amateur wrestlers and its effect on serum testosterone levels. J Am Med Assoc. 1985;254:3337–3338. [PubMed] [Google Scholar]
- Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- Svaartberg J, Barrett-Conner E. Could seasonal variation in testosterone levels in men be related to sleep? The Aging Male. 2004;7:205–210. doi: 10.1080/13685530412331284696. [DOI] [PubMed] [Google Scholar]
- Svartberg J, Jorde R, Sundsfjord J, Bonaa KH, Barrett-Connor E. Seasonal variation of testosterone and waist to hip ratio in men: the Tromso Study. J Clin Endocrinol Metab. 2003;88:3099–3104. doi: 10.1210/jc.2002-021878. [DOI] [PubMed] [Google Scholar]
- Valero-Polti J, Fuentes-Arderiu X. Annual rhythmic variations of follitropin, lutropin, testosterone and sex-hormone-binding globulin in men. Clin Chim Acta. 1998;271:57–71. doi: 10.1016/s0009-8981(97)00239-8. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hampson E, Watson NV. Seasonality, waist-to-hip ratio, and salivary testosterone. Psychoneuroendocrinology. 2006;31:895–899. doi: 10.1016/j.psyneuen.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ. Evolutionary models of women’s reproductive functioning. Annu Rev Anthropol. 2008;37:53–73. [Google Scholar]
- Vitzthum VJ, von Dornum M, Ellison PT. Effect of coca-leaf chewing on salivary progesterone assays. Am J Phys Anthropol. 1993;92:539–544. doi: 10.1002/ajpa.1330920410. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ, Spielvogel H, Thornburg J. Interpopulational differences in progesterone levels during conception and implantation in humans. Proc Natl Acad Sci USA. 2004;101:1443–1448. doi: 10.1073/pnas.0302640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitzthum VJ, Spielvogel H, Thornburg J, Brady W. A prospective study of early pregnancy loss in humans. Fertil Steril. 2006;86:373–379. doi: 10.1016/j.fertnstert.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Vitzthum VJ, Spielvogel H, Thornburg J. Seasonality of early pregnancy loss in humans: risk is higher during periods of greater physical exertion. 2008 Under review. [Google Scholar]
- West BT, Welch KB, Galecki AT. Linear Mixed Models: A Practical Guide Using Statistical Software. Chapman & Hall/CRC; Boca Raton: 2007. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The “Challenge Hypothesis”: theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Natur. 1990;136:829–846. [Google Scholar]
- Worthman CM, Melby MK. Toward a comparative developmental ecology of human sleep. In: Carskadon MA, editor. Adolescent Sleep Patterns: Biological, Social, and Psychological Influences. Cambridge University Press; Cambridge, UK: 2002. pp. 69–117. [Google Scholar]
- Worthman CM, Konner MJ. Testosterone levels change with subsistence hunting effort in !Kung San men. Psychoneuroendocrinology. 1987;12:449–458. doi: 10.1016/0306-4530(87)90079-5. [DOI] [PubMed] [Google Scholar]