BACKGROUND
The majority of potentially preventable early trauma deaths still result from uncontrolled hemorrhage.1–7 Despite studies demonstrating an advantage for early balanced resuscitation of platelets, plasma, and red blood cells [RBCs] in severely injured patients8–12, it remains difficult to readily identify those most likely to benefit.1 This approach is referred to as damage control resuscitation and has been shown in numerous retrospective investigations to have a mortality benefit for patients ultimately requiring massive transfusion [MT].8–13
Time to initiation of balanced resuscitation14–17 is postulated to be a major determinant in improved outcomes. Several authors have attempted to develop physiologic, hemodynamic, laboratory, and mechanism parameters that can reliably predict who will require MT.1,14,18–23 Previously investigated algorithms have relied on retrospective data, vary in the included components, differ in calculation complexity, and have yet to elucidate a widely accepted approach to avoid under-triage of patients who will require MT.1,24 Many of the scores lack real-time application and even the most simplified versions are plagued by significant risk of under-triage. In addition, recent work has demonstrated differential utility of commonly investigated military triggers in a civilian retrospective cohort.1
Utilizing the entire patient cohort from the Prospective Observational Multicenter Major Trauma Transfusion [PROMMTT] study7, the predictive ability of individual transfusion triggers common to previously reported scoring systems are prospectively investigated. The goal is to determine the utility of individual triggers compared to a Massive Transfusion Score [MTS] for expeditious identification of who is likely to benefit from damage control resuscitation.
METHODS
Study Population
PROMMTT was a prospective, multicenter observational cohort study conducted at ten Level 1 trauma centers in the US from July 2009 to October 2010.7 The primary objective of PROMMTT was to investigate in hospital mortality in all patients surviving for at least 30 minutes after ED admission. To be included in the PROMMTT cohort, patients had to be major trauma patients at least 16 years old, requiring trauma team activation, arriving from the scene, and receiving at least 1 unit RBC within 6 hours.7 The original PROMMTT study as well as this secondary analysis was approved by the institutional review boards [IRBs] of each study site and the Data Coordinating Center [DCC]. The US Army Human Research Protections Office also provided second level review and approval of the PROMMTT study.
Trigger Selection
The Individual Transfusion Trigger study (Cincinnati ITT Study, CITT)1 and the Assessment of Blood Consumption (ABC) score15,18 have shown promise in the literature for predictive utility of MT and ease of use in the civilian population. The CITT triggers were adapted from previously published military triggers. The CITT triggers included systolic blood pressure [SBP] <90 mm Hg, hemoglobin [hgb] <11 g/dL, temperature <35.5 °C, international normalized ratio [INR] >1.5, and base deficit [BD] >=6. The ABC score includes SBP<90 mmHg, heart rate [HR] >= 120 beats per minute (bpm), Focused assessment for the sonography of trauma [FAST] exam positive, and mechanism of injury (penetrating). From the CITT and ABC studies, eight unique triggers were identified for study inclusion.
Data Collection
Data collection was conducted under the standard operating procedure manual and site coordinators of the PROMMTT study. Research assistants were available 24/7 in the study sites and responded to all major trauma activations to record real-time collection of relevant data variables. The observers recorded exact times of transfusion products, fluids, interventions, and patient outcomes. Following conclusion of the active resuscitation phase of the observation as defined by PROMMTT7, patients were followed on a daily basis until discharge or death.
The initial ED value for each trigger was recorded and verified in the medical records when possible prior to submission to the DCC. As in previous work, the first laboratory data available on arrival was counted as the ED laboratory result if they were drawn in the ED or performed as a point of care test in the ED.
Data Analysis
The DCC provided the data as a de-identified patient level data set containing all relevant study variables including demographics, injury characteristics, ED vital signs, arrival times, transfusion data, ED interventions, laboratory studies, radiographic studies, operative interventions, and outcomes. Cause of death was determined by the treating attending physician. Initial ED values were utilized to determine if a patient met each trigger cut-off value.
For each trigger, comparison was made between patients receiving a MT versus no MT within specific time intervals. Patients were classified in the massive transfusion at 24 hours [MT24h] group if they received 10 or more units of RBCs within 24 hours of initial ED presentation. Alternatively, a separate analysis was done for MT at 6 hours [MT6h] if patients received 10 or more units of RBCs within 6 hours. To address potential survivor bias, sensitivity analysis was done including early hemorrhagic deaths with each MT variable (MT24h plus hemorrhagic deaths within 24 hours [MT24h+]; MT6h plus hemorrhagic deaths within 6 hours [MT6h+]).
To determine individual predictive utility of a trigger for predicting MT, the Odds Ratio [OR] and 95% confidence interval [CI] of receiving a MT was calculated using logistic regression. Sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV] were calculated for each trigger. The percentage of correct classification (true positives plus true negatives divided by total) was determined. Chi-square, T-test, and Mann-Whitney tests were used to compare relevant groups as applicable. All data analysis was performed using SAS/STAT (version 9.2, Cary, NC) and SPSS (version 18).
Determination of the FAST trigger
FAST was reported [rFAST] in only 67% of the cohort. Some centers did not perform FAST in certain patients (penetrating trauma) and those in extremis may have gone directly to the operating room without a FAST. Given data were likely not missing at random, a result was substituted for those with missing FASTs using a modified method described in the Prince of Wales Hospital [PWH] transfusion score.20 Diagnostic peritoneal lavage [DPL] was used when no FAST was available. If a patient went to the operating room from the ED with no FAST or DPL [ORFAST], repair of major solid organ or vascular abdominal injury was substituted for a positive FAST as these injuries would frequently be associated with significant hemorrhage on abdominal entry. For those with no immediate abdominal operation but who did have an ED abdominal CT scan, a FAST result was determined from presence or absence of intra-abdominal hemorrhage.20 If none of the above were available, a FAST variable was not reported. The derived FAST [dFAST] represented the reported or surrogate results and was determinable for 96% of the cohort (TABLE 1). Sensitivity analysis was performed with dFAST included and not included in each model.
Table 1.
Derivation of the dFAST variable
| Variable | n (% total cohort) |
% MT | OR MT | |
|---|---|---|---|---|
| FAST or DPL reported (N=874 total) |
26%** | 2.1 (1.5–2.9) | ||
| FAST reported [rFAST] |
838 (67%) |
26%* | ||
| No FAST, but DPL Known [DPL] |
36 (3%) |
36%* | ||
| No FAST/DPL but went to OR [ORFAST] (N=263 total) |
28%** | 2.4 (1.2–4.9) | ||
| No abdominal Operation |
160 (13%) |
28% | ||
| Abdominal Operation |
103 (8%) |
28% | ||
| No FAST/DPL/OR but ED CT scan [CT] |
63 (5%) |
5% | N/A | |
| Derived FAST [dFAST] |
Total | 1199 (96%) |
||
rFAST vs. DPL - p=NS (Mann-Whitney)
FASTorDPL reported vs. ORFAST - p=NS (Mann-Whitney)
Massive Transfusion Score [MTS]
If data were not available or determinable for a trigger in at least 75% of the patients, it was not included as a variable in the MTS. In the subset of patients for whom data were available for all triggers [ALL group], the adjusted ORs for MT of each trigger were determined using a hierarchical mixed effects logistic regression nested for study site. Two analyses were then performed to identify the best predictive MTS. First, each criterion based upon the individual predictive adjusted ORs was assigned a weighted value if the trigger was met. Alternatively, each criterion was equally weighted with 1 point assigned for each trigger met.
Total scores were calculated at the patient-level and comparison was made between those receiving MT and no MT based upon threshold scores. The overall accuracy for prediction of MT were determined for the final MTS models (criterion equally weighted) using area under the receiver operator cure (AUC). Correlation coefficients of predicting MT using MTS were calculated. The OR for requiring MT was determined for a MTS>=2.
RESULTS
Overall
The PROMMTT cohort included 1245 patients from a total screened potentially eligible population of 12,561. Penetrating trauma was the mechanism of injury in 35%. Based upon transfusion data, 297 (24%) received a MT24h (>10 units RBCs in the initial 24 hours). The percentage of patients receiving MT24h was equivalent between those with a rFAST, DPL, and ORFAST [TABLE 1]. For each trigger, data was available for a variable number of patients with temperature being the least frequently known parameter (51%) [TABLE 2]. INR was available in 87% (1081/1245).
Table 2.
Likelihood of massive transfusion and Mean RBC transfusion volume for Individual transfusion triggers.
| Trigger | Data available n=pts |
Data available % pts |
Mean Units RBCs Transfused | Likelihood of MT (OR, 95% CI) |
% MT if trigger exceeded (PPV) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigger, Yes | Trigger, No | p-value | MT 24h | MT 24h+ | MT 6h+ | MT 24h | MT24h+ | MT6h+ | |||
| INR >1.5 | 1081 | 87% | 13.5 +/−1.0 | 6.6 +/−0.3 | p<0.0001 |
3.4 (2.5–4.7) |
4.0 (2.9–5.5) |
5.8 (4.0–8.2) |
43% | 49% | 40% |
| SBP <90 mm Hg |
1213 | 97% | 11.2 +/−0.7 | 6.5 +/−0.3 | p<0.0001 |
2.6 (1.9–3.4) |
2.5 (1.9–3.3) |
2.4 (1.7–3.3) |
36% | 37% | 25% |
| Hgb <11 g/dL | 1198 | 96% | 10.3 +/−0.6 | 6.8 +/−0.3 | p<0.0001 |
2.4 (1.9–3.2) |
2.6 (2.0–3.4) |
3.5 (2.6–4.8) |
34% | 37% | 28% |
| BD >=6 | 960 | 77% | 10.5 +/−0.6 | 5.5 +/−0.3 | p<0.0001 |
2.8 (2.0–3.9) |
3.0 (2.2–4.2) |
4.5 (3.0–6.9) |
32% | 35% | 25% |
| dFAST (+) | 1245 | 100% | 12.0 +/−0.7 | 7.0 +/−0.3 | p<0.0001 |
2.4 (1.8–3.2) |
2.4 (1.8–3.2) |
2.9 (2.1–3.9) |
37% | 40% | 30% |
| HR>=120 bpm |
1218 | 98% | 9.9 +/−0.6 | 7.3 +/−0.3 | p<0.0001 |
1.5 (1.2–2.0) |
1.6 (1.2–2.1) |
1.8 (1.3–2.4) |
29% | 31% | 23% |
| Penetrating | 1242 | 100% | 9.0 +/−0.6 | 7.7 +/−0.3 | p=0.04 | 1.0 (0.8–1.4) |
1.1 (0.8–1.4) |
1.5 (1.1–2.0) |
24% | 26% | 21% |
| Temp <35.5 C |
630 | 51% | 7.7 +/−0.8 | 6.4 +/−0.4 | p=0.11 | 1.5 (0.9–2.4) |
1.6 (1.0–2.5) |
1.5 (0.9–2.6) |
22% | 28% | 28% |
MT 24 h: 10+ units RBCs in 24 hours; MT 24h+ : 10+ units RBCs in 24 hours plus hemorrhagic deaths within 24 hours; MT6h+ : 10+ units RBCs at 6 hours plus hemorrhagic deaths within 6 hours; INR: Internal normalized ratio; SBP:systolic blood pressure; Hgb: hemoglobin; g/dL: grams per deciliter; BD: base deficit; HR: heart rate; bpm: beats per minute; Temp: temperature; OR: odds ratio; CI: confidence interval; PPV: positive predictive value
Utility of Individual Transfusion Triggers
For all triggers except temperature, if the trigger was met, the mean units of transfused RBCs was greater in the group who met that target than the group that did not meet the target [TABLE 2]. The largest difference in RBC utilization was seen for the INR trigger (13.5 units vs. 6.6, p<0.0001).
On univariate analysis, the likelihood of requiring MT24h was greatest when the INR target was exceeded (OR 3.4, 95% CI 2.5–4.7), followed by BD (OR 2.8, 2.0–3.9), and SBP (OR 2.6, 1.9–3.4) [TABLE 2]. The INR trigger was positive in 19% of patients and 43% of these patients got a MT24h. If any individual trigger was met, at least 22% (range 22–43%) of the patients went on to receive MT24h [TABLE 2].
In order to account for patients who would have likely received MT24h but died from hemorrhage prior to receiving 10 units RBCs, a sensitivity analysis was performed including these patients in the MT group (MT24h+). This analysis had little effect on the predictability of the individual transfusion triggers with the exception of an increase in the likelihood of receiving MT (OR 4.0) and the number receiving MT (49%) for the INR trigger [TABLE 2].
Sensitivity, Specificity, NPV, and correct classification percentage for each individual trigger was also unaffected by including hemorrhagic deaths within 24 hours [TABLE 3]. NPV for MT24h+ for each trigger exceeded 75% with BD being the highest (85%) followed by temperature (82%), and HGB (82%). For each trigger, using only that single trigger, patients were correctly classified 57–75% of the time with INR having the highest rate.
Table 3.
Utility of individual transfusion triggers
| Trigger | % Correctly classified | Sensitivity | Specificity | NPV | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MT 24h |
MT24h+ | MT6+ | MT 24h |
MT24h+ | MT6h+ | MT 24h |
MT24+ | MT6+ | MT 24h |
MT24h+ | MT6+ | |
| INR >1.5 | 74% | 75% | 80% | 36% | 37% | 48% | 86% | 87% | 86% | 82% | 81% | 90% |
| SBP <90 | 69% | 68% | 70% | 45% | 44% | 45% | 76% | 76% | 75% | 82% | 81% | 88% |
| Hgb <11 | 65% | 66% | 68% | 53% | 54% | 62% | 69% | 70% | 69% | 83% | 82% | 90% |
| BD >=6 | 56% | 57% | 55% | 74% | 75% | 82% | 50% | 51% | 49% | 86% | 85% | 93% |
| dFAST (+) | 68% | 68% | 71% | 43% | 42% | 48% | 76% | 77% | 76% | 80% | 79% | 87% |
| HR>=120 | 64% | 63% | 66% | 38% | 39% | 42% | 71% | 72% | 71% | 79% | 78% | 86% |
| Penetrating | 58% | 58% | 63% | 36% | 36% | 43% | 65% | 65% | 66% | 76% | 75% | 85% |
| Temp <35.5 C | 71% | 70% | 73% | 27% | 26% | 16% | 80% | 82% | 89% | 84% | 82% | 80% |
MT 24h: 10+ RBCs in 24 hours; MT 24h+ : 10+ RBCs in 24 hours plus all hemorrhagic deaths within 24 hours; MT 6h+ : 10+ RBCs in 6 hours plus all hemorrhagic deaths within 6 hours; RBC: red blood cells; INR: Internal normalized ratio; SBP:systolic blood pressure; Hgb: hemoglobin; g/dL: grams per deciliter; HR: heart rate; bpm: beats per minute; Temp: temperature; NPV: negative predictive value
Massive Transfusion at 6 hours
The individual transfusion triggers were also predictive of massive transfusion at 6 hours (MT6h+). INR remained the most predictive, followed by BD, and HGB [TABLE 2]. If any trigger was exceeded, MT was seen within 6 hours in between 21–40% of patients exceeding the relevant trigger. Sensitivity and NPV were increased for each trigger at 6 hours compared with 24 hours [TABLE 3]. Correct classification was also improved with the exception of a slight decrease for the BD trigger.
Massive Transfusion Score [MTS]
Given temperature did not distinguish between RBC utilization [TABLE 2] and data was available in only 51% of the cohort, it was excluded from the MTS. When dFAST was utilized, 66% (822/1245) of the patients had all 7 remaining triggers known (ALL group) including 67% (199/297) of those receiving a MT24h. Using mixed effects hierarchical multiple logistic regression, adjusted ORs for MT were calculated for the ALL group [TABLE 4]. INR remained the most significant predictor in each model.
Table 4.
Adjusted Odds Ratios for each trigger for predicting MT
| MT 24 h | MT 24 hrs + | MT 6 h + | ||||
|---|---|---|---|---|---|---|
| Adjusted ORs (95% CI) | Adjusted ORs (95% CI) | Adjusted ORs (95% CI) | ||||
| Trigger | dFAST | No FAST | dFAST | No FAST | dFAST | No FAST |
| INR >1.5 | 2.2 (1.4–3.3) | 2.1 (1.4–3.1) | 2.5 (1.7–3.7) | 2.4 (1.6–3.5) | 3.9 (2.5–6.0) | 3.6 (2.3–5.5) |
| SBP <90 mmHg | 1.9 (1.3–2.7) | 1.9 (1.4–2.7) | 1.7 (1.2–2.5) | 1.8 (1.3–2.6) | 1.5 (1.0–2.3) | 1.6 (1.1–2.5) |
| Hgb <11 g/dL | 1.8 (1.2–2.5) | 1.8 (1.3–2.5) | 1.8 (1.3–2.6) | 1.8 (1.3–2.6) | 2.0 (1.3–3.0) | 2.0 (1.3–3.0) |
| BD >=6 | 1.8 (1.2–2.6) | 2.0 (1.4–2.9) | 2.0 (1.4–2.9) | 2.1 (1.5–3.0) | 3.0 (1.8–4.9) | 3.2 (2.0–5.2) |
| HR>=120bpm | 1.1 (0.8–1.7) | 1.2 (0.8–1.6) | 1.2 (0.8–1.7) | 1.2 (0.8–1.7) | 1.2 (0.8–1.8) | 1.1 (0.7–1.7) |
| Penetrating | 1.0 (0.7–1.4) | 0.9 (0.6–1.3) | 0.9 (0.6–1.3) | 0.8 (0.6–1.2) | 1.2 (0.8–1.9) | 1.0 (0.7–1.6) |
| dFAST (+) | 1.9 (1.3–2.7) | 1.8 (1.2–2.5) | 1.9 (1.3–2.9) | |||
MT: massive transfusion; MT 24h: 10+ RBCs in 24 hours; MT 24h+ : 10+ RBCs in 24 hours plus all hemorrhagic deaths within 24 hours; MT 6h+ : 10+ RBCs in 6 hours plus all hemorrhagic deaths within 6 hours; INR: Internal normalized ratio; SBP:systolic blood pressure; Hgb: hemoglobin; g/dL: grams per deciliter; dFAST: derived FAST; HR: heart rate; bpm: beats per minute; Temp: temperature
Using the adjusted ORs to determine a relative point value for each trigger, a weighted score was calculated for each patient in the ALL group. The accuracy of predicting MT was then determined (data not shown) for various cutoff values (AUC 0.60–0.69). Alternatively, the criterion were assigned an equal weight of 1 point for each trigger met and the total summed (MTS). This equally weighted score provided the best overall accuracy of receiving a MT (AUCs 0.70) at both 24 hours and 6 hours.
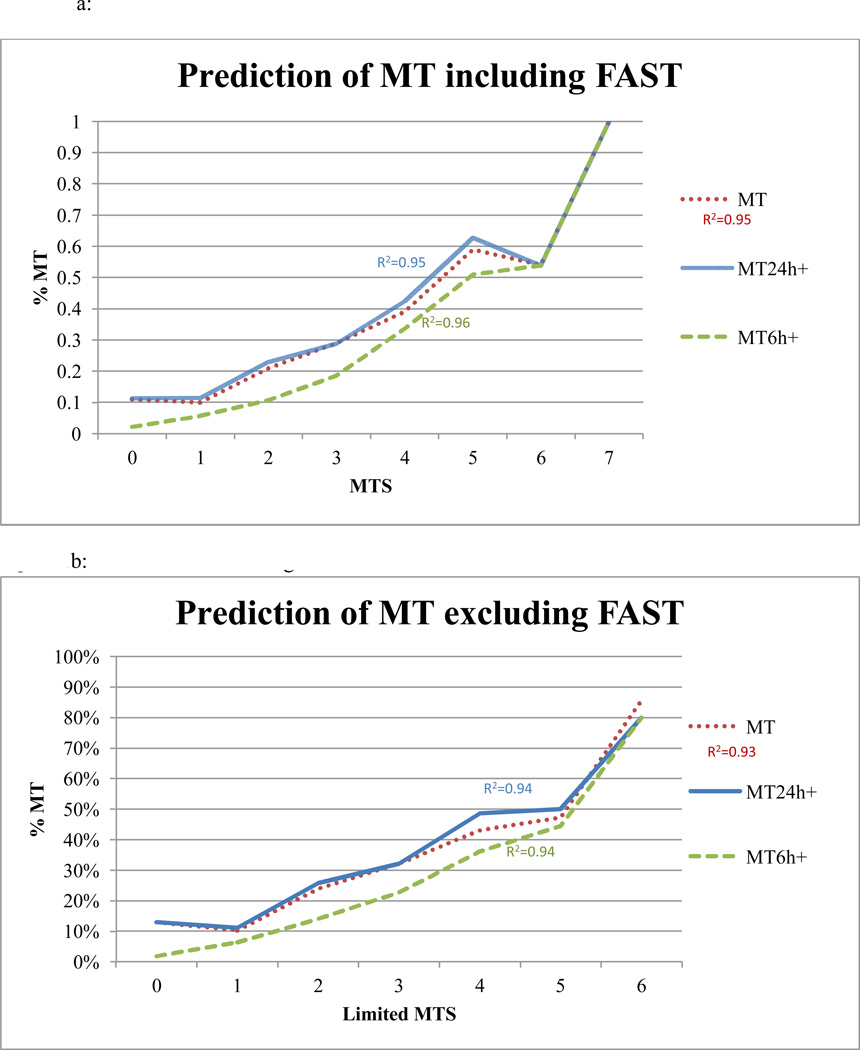
Using the equal weighted model, as the MTS increased it was highly predictive of who received MT24h+ [FIGURE 1]. Patients with a MTS<2 were unlikely to receive MT24+ (NPV 89% ). If any 2 triggers were met, the sensitivity for predicting MT24h+ was 85% and PPV was 33% [TABLE 4]. MT24+ was present in 33% of patients with a MTS >=2 compared with 11% of those with MTS <2 (OR 3.9, CI 2.6–5.8, p<0.0005). The MTS also demonstrated even higher sensitivity (90%), NPV (95%), and correlation for MT prediction within 6 hours [TABLE 5].
FIGURE 1. Prediction of MT based upon the Massive Transfusion Score (MTS).
a: Prediction of MT using the MTS including FAST
b: Prediction of MT using the Limited MTS
MT: massive transfusion; MTS: massive transfusion score; MT 24 h: 10+ units RBCs in 24 hours; MT 24h+ : 10+ units RBCs in 24 hours plus hemorrhagic deaths within 24 hours; MT6h+ : 10+ units RBCs at 6 hours plus hemorrhagic deaths within 6 hours;
Table 5.
Performance of the model for prediction of MT with a MTS>=2
| MT at 24 hrs | MT at 24 hrs + hemorrhagic deaths within 24 hrs |
MT at 6 hrs + hemorrhagic deaths within 6 hrs |
||||
|---|---|---|---|---|---|---|
| MTS>=2 | dFAST | No FAST | dFAST | No FAST | dFAST | No FAST |
| Sensitivity | 85% | 81% | 85% | 81% | 90% | 87% |
| Specificity | 41% | 47% | 41% | 47% | 39% | 45% |
| PPV | 31% | 32% | 33% | 34% | 22% | 23% |
| NPV | 89% | 89% | 89% | 88% | 95% | 95% |
| R2 | 0.95 | 0.93 | 0.95 | 0.94 | 0.96 | 0.94 |
| OR MT (95% CI) |
3.9 (2.5–5.9) | 3.7 (2.5–5.5) | 3.9 (2.6–5.8) | 3.8 (2.6–5.6) | 6.0 (3.3–10.9) | 5.7 (3.4–9.7) |
MT: massive transfusion; PPV: positive predictive value; NPV: negative predictive value; R2: correlation coefficient; OR: odds ratio; CI: confidence interval
Inclusion of the dFAST variable in the MTS improved the sensitivity of the models while not biasing the contribution of each individual trigger. For example, the ORs of all the triggers for prediction of MT24+ and the overall model ORs for MT remained nearly identical with and without inclusion of the dFAST [TABLE 4&5]. Also, penetrating trauma and HR were not independent predictors of MT; however, inclusion of the variables in the MTS improved the sensitivity (MT24h+ 75% to 85%).
In order to explore the utility of the MTS if not all 7 triggers were known for a given patient, the model was applied in the remaining 34% of the cohort [NOT ALL group]. The model remained predictive of receiving a MT (R2 = 0.94) as the number of triggers met increased. Sensitivity and NPV for predicting MT24+ remained high at 82% and 89%, respectively. Overall in the NOT ALL group, 35% of patients required MT24+ if the MTS>=2 compared with 11% for those with MTS<2 (OR 4.4, 2.5–7.5, p<0.0005).
DISCUSSION
Previous studies have shown a survival advantage to early balanced resuscitation for patients ultimately requiring MT.8–12 Despite these advances, early identification of patients with a high likelihood of needing significant transfusion remains a challenge in the trauma bay.
A number of predictive scores have been developed from retrospective data, however, the scores have variable accuracy and sensitivity.8–12,24,25 The most promising score remains the TASH weighted score which requires complex calculations and thus limits its ease of use.19,24,25 Additionally, all but 1 civilian score excluded INR24 and it has been recently shown to be a significant individual predictor in a retrospective cohort of patients who required early operative intervention (CITT).1
The present study is the first to prospectively examine the predictive ability of individual triggers to expeditiously identify those who are likely to receive MT. This cohort represents a diverse population more closely reflective of the patient spectrum encountered in civilian trauma centers compared with previous studies.7 Although this is the best currently available prospective cohort for testing transfusion triggers, it is important to acknowledge that patients needed to receive at least 1 unit of RBCs for enrollment.
All triggers from the CITT except temperature remained significant individual predictors of MT. Although the correct classification rate and MT rate with a positive INR trigger were slightly lower in the present study compared with CITT1, the results remain consistent with INR as the best individual trigger followed by SBP, BD, and Hgb. Importantly, all the individual triggers remained significant negative predictors (NPV >80%) of MT. Given the clinical utility of the laboratory parameters hypothesized in the CITT study and validated in the present prospective cohort, particular effort should be undertaken to obtain these parameters as rapidly as possible on patient arrival.
The parameters of FAST, HR>=120 bpm and penetrating mechanism also had significant ability to discriminate between those requiring and not requiring significant RBC volumes. The inclusion of the dFAST variable, which uses surrogate data to derive a FAST parameter when the data were missing, provided an improvement in sensitivity of the models without biasing the outcomes of interest. As an example, the ORs of prediction of MT at 24 hours and 6 hours remained essentially statistically unchanged.
In this study, an equally weighted MTS had no trade-off in accuracy compared with a weighted score. Additionally, the MTS that resulted in a screening test with the best sensitivity and reliability given the consequences of delayed initiation of balanced resuscitation was desired. The sensitivity or ability of a test to identify correctly those who have the condition of interest should be high when the consequences for missing a positive case are significant1. Driving a model to a higher sensitivity trades off a degree of accuracy which was tolerable in this clinical scenario given that consequences for over-triage are minimal compared to the substantially increased mortality with under-triage.
The MTS was both sensitive and reliable in predicting MT at 24 hours or 6 hours as the number of positive triggers increased. Additionally, the NPV was improved with combining the triggers into the MTS compared to using individual triggers alone. In fact, the ability to exclude (NPV 95%) the likelihood of MT using the score was excellent when considering early significant hemorrhage (MT6h+). Thus, if patients had less than 2 positive triggers (MTS<2), they were highly unlikely to receive a MT by 6 hours. Importantly, the score was also useful for prediction of MT even if data was not available for every trigger.
Previously published transfusion scores with equal weighting and thus, ease of use, have focused on high accuracy over sensitivity due to concern for over-triage. This has resulted in poorer model sensitivity (53–75%)24 compared with the current MTS model (85% MT24h+, 90% MT6h+). The sensitivity of prior scores has been especially poor when validation studies have been attempted.24 Reliance on the MTS alone would result in initiating balanced resuscitation in a proportion of patients who would ultimately not require MT. Although inappropriate use and risk of RBCs and plasma transfusion are of concern, there are data to suggest that balanced resuscitation may still benefit severely injured trauma patients who do not ultimately require MT volumes.1,25,26 Although the utility of individual triggers are validated in this study, the combined MTS should be further studied in future prospective studies.
Prior work has been unable to account for survivor bias in determining predictive MT algorithms given the retrospective nature of the data collection. The PROMMTT study included cause of death variables allowing determination of those who died from early exsanguination. By performing a sensitivity analysis assuming these patients would have required MT if they had survived long enough to receive 10 units of RBCs, the model performance remained consistent.
CONCLUSION
Parameters that can be obtained early in the initial ED evaluation are valid predictors for determining likelihood of MT. The overall sensitivity for predicting significant blood volume needs was improved by combining the triggers into the MTS. The score can be applied with ease in an expeditious manner early in the patient course as a guide to avoid under-triage of patients most likely to benefit from balanced resuscitation of platelet, plasma, and RBCs.
Acknowledgments
Source of Funding – PROMMTT was funded by the U.S. Army Medical Research and Material Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center (DCC) was supported by CTSA funds from NIH grant UL1 RR024148. Data for this study was provided by the DCC on behalf of the PROMMTT Study group and this particular study received no additional funding for completion.
The sponsors did not have any role in the design and conduct of the study, collection, management, analysis and interpretation of the data; nor in preparation, review or approval of the manuscript, or the decision to submit this manuscript for publication.
Appendix
Prospective Observational Multicenter Major Trauma Transfusion Study Group:
Data Coordinating Center, University of Texas Health Science Center at Houston: Mohammad H. Rahbar, PhD (principal investigator); John B. Holcomb, MD (co-investigator); Erin E. Fox, PhD (co-investigator and study coordinator); Deborah J. del Junco, PhD (co-investigator); Bryan A. Cotton, MD, MPH (co-investigator); Charles E. Wade, PhD (co-investigator); Jiajie Zhang, PhD (co-investigator); Nena Matijevic, PhD (co-investigator); Yu Bai, MD, PhD (co-investigator); Weiwei Wang, PhD (co-investigator); Jeanette Podbielski, RN (study coordinator); Sarah J. Duran, MSCIS (data manager); Ruby Benjamin-Garner, PhD (data manager); Robert J. Reynolds, MPH (data manager).
PROMMTT Clinical Sites:
Brooke Army Medical Center: Christopher E. White, MD (principal investigator); Kimberly L. Franzen, MD (co-investigator); Elsa C. Coates, MS, RN (study coordinator).
Medical College of Wisconsin: Karen J. Brasel, MD, MPH (principal investigator); Pamela Walsh (study coordinator).
Oregon Health and Sciences University: Martin A. Schreiber, MD (principal investigator); Samantha J. Underwood, MS (study coordinator); Jodie Curren, RN, BSN (study coordinator).
University of California, San Francisco: Mitchell J. Cohen, MD (principal investigator); M. Margaret Knudson, MD (co-investigator); Mary Nelson, RN, MPA (study coordinator); Mariah S. Call, BS (study coordinator).
University of Cincinnati: Peter Muskat, MD (principal investigator); Jay A. Johannigman, MD (co-investigator); Bryce RH Robinson, MD (co-investigator); Richard Branson (co-investigator); Dina Gomaa, BS, RRT (study coordinator); Cendi Dahl (study coordinator).
University of Pittsburgh Medical Center: Louis H. Alarcon, MD (principal investigator); Andrew B. Peitzman, MD (co-investigator); Stacy D. Stull, MS, CCRC (study coordinator); Mitch Kampmeyer, MPAS, CCRC, PA-C (study coordinator); Barbara J. Early, RN, BSN, CCRC (study coordinator); Helen L. Shnol, BS, CRC (study coordinator); Samuel J. Zolin, BS (research associate); Sarah B. Sears, BS (research associate).
University of Texas Health Science Center at Houston: John B. Holcomb, MD (co-principal investigator); Bryan A. Cotton, MD, MPH (co-principal investigator); Marily Elopre, RN (study coordinator); Quinton M. Hatch, MD (research associate); Michelle Scerbo (research associate); Zerremi Caga-Anan, MD (research associate).
University of Texas Health Science Center at San Antonio: John G. Myers, MD (co-principal investigator); Ronald M. Stewart, MD (co-principal investigator); Rick L. Sambucini, RN, BS (study coordinator); Marianne Gildea, RN, BSN, MS (study coordinator); Mark DeRosa CRT (study coordinator); Rachelle Jonas, RN, BSN (study coordinator); Janet McCarthy, RN (study coordinator).
University of Texas Southwestern Medical Center: Herbert A. Phelan, MD (principal investigator); Joseph P. Minei, MD (co-investigator); Elizabeth Carroll, MD (study coordinator).
University of Washington: Eileen M. Bulger, MD (principal investigator); Patricia Klotz, RN (study coordinator); Keir J. Warner, BS (research coordinator).
Footnotes
- Dr. Holcomb reports serving on the board for Tenaxis, Winkenwerder Company, the Regional Advisory Council for Trauma, and the National Trauma Institute; providing expert testimony for the Department of Justice; grants funded by Haemonetics Corporation, and KCI USA, Inc. and patent royalities paid through his institution.
- Dr. Wade reported serving on the Science Board for Resuscitation Products, Inc and the Advisory Board for Astrazeneca.
- No other disclosures by other authors are reported.
Presented at: The American Association for the Surgery of Trauma (AAST) Annual Meeting, September 2012, Kauai, Hawaii
Disclaimer – The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
IRB approval – The original PROMMTT study as well as this secondary analysis was approved at each study site and the Data Coordinating Center by the local institutional review boards. The US Army Human Research Protections Office also provided second level review and approval for PROMMTT.
Level of Evidence: I - Prospective, observational, multi-center trial testing previously developed trigger criteria; diagnostic test
Major Author Contributions
Literature search – Callcut, Cotton, Robinson
Study design – Callcut, Cotton, Fox, Wade, Holcomb, Muskat, Robinson
Data Collection - Cotton, Muskat, Fox, Wade, Holcomb, Schreiber, Rahbar, Cohen, Brasel, Bulger, Robinson, del Junco, Myers, Knudson, Alarcon
Data Analysis – Callcut, Cotton, Fox
Data Interpretation – Callcut, Cotton, Muskat, Robinson
Manuscript Writing – Callcut, Cotton, Robinson
Critical Revision –Cotton, Muskat, Fox, Wade, Holcomb, Schreiber, Rahbar, Cohen, Brasel, Bulger, Robinson, del Junco, Myers, Knudson, Alarcon
Contributor Information
Rachael A Callcut, Email: callcutr@sfghsurg.ucsf.edu.
Bryan A Cotton, Email: Bryan.A.Cotton@uth.tmc.edu.
Peter Muskat, Email: muskatp@ucmail.uc.edu.
Erin E Fox, Email: Erin.E.Fox@uth.tmc.edu.
Charles E Wade, Email: Charles.E.Wade@uth.tmc.edu.
John B Holcomb, Email: John.Holcomb@uth.tmc.edu.
Martin A Schreiber, Email: schreibm@ohsu.edu.
Mohammad H. Rahbar, Email: Mohammad.H.Rahbar@uth.tmc.edu.
Mitchell J Cohen, Email: mcohen@sfghsurg.ucsf.edu.
M. Margaret Knudson, Email: pknudsen@sfghsurg.ucsf.edu.
Karen J Brasel, Email: kbrasel@mcw.edu.
Eileen M Bulger, Email: ebulger@u.washington.edu.
Deborah J del Junco, Email: Deborah.J.DelJunco@uth.tmc.edu.
John G. Myers, Email: MyersJG@uthscsa.edu.
Louis H Alarcon, Email: alarconl@upmc.edu.
Bryce RH Robinson, Email: robinsbc@ucmail.uc.edu.
REFERENCES
- 1.Callcut RA, Johannigman JA, Kadon KS, Hanseman DJ, Robinson BR. All massive transfusion criteria are not created equal: defining the predictive value of individual transfusion triggers to better determine who benefits from blood. J Trauma. 2011 Apr;70(4):794–801. doi: 10.1097/TA.0b013e3182127e40. [DOI] [PubMed] [Google Scholar]
- 2.Moore FA, Nelson T, McKinley BA, et al. Is there a role for aggressive use of fresh frozen plasma in massive transfusion of civilian trauma patients? Am J Surg. 2008 Dec;196(6):948–958. doi: 10.1016/j.amjsurg.2008.07.043. discussion 958–960. [DOI] [PubMed] [Google Scholar]
- 3.Kashuk JL, Moore EE, Johnson JL, et al. Postinjury life threatening coagulopathy: is 1:1 fresh frozen plasma:packed red blood cells the answer? J Trauma. 2008 Aug;65(2):261–270. doi: 10.1097/TA.0b013e31817de3e1. discussion 270–261. [DOI] [PubMed] [Google Scholar]
- 4.Krug EG, Sharma GK, Lozano R. The global burden of injuries. Am J Public Health. 2000 Apr;90(4):523–526. doi: 10.2105/ajph.90.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niles SE, McLaughlin DF, Perkins JG, et al. Increased mortality associated with the early coagulopathy of trauma in combat casualties. J Trauma. 2008 Jun;64(6):1459–1463. doi: 10.1097/TA.0b013e318174e8bc. discussion 1463–1455. [DOI] [PubMed] [Google Scholar]
- 6.Demetriades D, Murray J, Charalambides K, et al. Trauma fatalities: time and location of hospital deaths. J Am Coll Surg. 2004 Jan;198(1):20–26. doi: 10.1016/j.jamcollsurg.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Rahbar MH, Fox EE, del Junco DJ, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study. Resuscitation. 2012 Apr;83(4):459–464. doi: 10.1016/j.resuscitation.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holcomb JB, Wade CE, Michalek JE, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008 Sep;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 9.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007 Oct;63(4):805–813. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 10.Zink KA, Sambasivan CN, Holcomb JB, Chisholm G, Schreiber MA. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am J Surg. 2009 May;197(5):565–570. doi: 10.1016/j.amjsurg.2008.12.014. discussion 570. [DOI] [PubMed] [Google Scholar]
- 11.Brown LM, Aro SO, Cohen MJ, et al. A high fresh frozen plasma: packed red blood cell transfusion ratio decreases mortality in all massively transfused trauma patients regardless of admission international normalized ratio. J Trauma. 2011 Aug;71(2 Suppl 3):S358–S363. doi: 10.1097/TA.0b013e318227f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. J Am Coll Surg. 2007 Oct;205(4):541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J Trauma. 2007 Feb;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 14.McLaughlin DF, Niles SE, Salinas J, et al. A predictive model for massive transfusion in combat casualty patients. J Trauma. 2008 Feb;64(2 Suppl):S57–S63. doi: 10.1097/TA.0b013e318160a566. discussion S63. [DOI] [PubMed] [Google Scholar]
- 15.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? J Trauma. 2009 Feb;66(2):346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 16.Cotton BA, Dossett LA, Au BK, Nunez TC, Robertson AM, Young PP. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. J Trauma. 2009 Nov;67(5):1004–1012. doi: 10.1097/TA.0b013e3181bcb2a8. [DOI] [PubMed] [Google Scholar]
- 17.Dente CJ, Shaz BH, Nicholas JM, et al. Early predictors of massive transfusion in patients sustaining torso gunshot wounds in a civilian level I trauma center. J Trauma. 2010 Feb;68(2):298–304. doi: 10.1097/TA.0b013e3181cf7f2a. [DOI] [PubMed] [Google Scholar]
- 18.Cotton BA, Dossett LA, Haut ER, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. J Trauma. 2010 Jul;(69 Suppl 1):S33–S39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 19.Yucel N, Lefering R, Maegele M, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. J Trauma. 2006 Jun;60(6):1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. discussion 1236–1227. [DOI] [PubMed] [Google Scholar]
- 20.Rainer TH, Ho AM, Yeung JH, et al. Early risk stratification of patients with major trauma requiring massive blood transfusion. Resuscitation. 2011 Jun;82(6):724–729. doi: 10.1016/j.resuscitation.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Vandromme MJ, Griffin RL, McGwin G, Jr., Weinberg JA, Rue LW, 3rd, Kerby JD. Prospective identification of patients at risk for massive transfusion: an imprecise endeavor. Am Surg. 2011 Feb;77(2):155–161. [PubMed] [Google Scholar]
- 22.Ruchholtz S, Pehle B, Lewan U, et al. The emergency room transfusion score (ETS): prediction of blood transfusion requirement in initial resuscitation after severe trauma. Transfus Med. 2006 Feb;16(1):49–56. doi: 10.1111/j.1365-3148.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- 23.Larson CR, White CE, Spinella PC, et al. Association of shock, coagulopathy, and initial vital signs with massive transfusion in combat casualties. J Trauma. 2010 Jul;(69 Suppl 1):S26–S32. doi: 10.1097/TA.0b013e3181e423f4. [DOI] [PubMed] [Google Scholar]
- 24.Maegele M, Brockamp T, Nienaber U, et al. Predictive Models and Algorithms for the Need of Transfusion Including Massive Transfusion in Severely Injured Patients. Transfus Med Hemother. 2012 Apr;39(2):85–97. doi: 10.1159/000337243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maegele M, Lefering R, Wafaisade A, et al. Revalidation and update of the TASH-Score: a scoring system to predict the probability for massive transfusion as a surrogate for life-threatening haemorrhage after severe injury. Vox Sang. 2011 Feb;100(2):231–238. doi: 10.1111/j.1423-0410.2010.01387.x. [DOI] [PubMed] [Google Scholar]