Magnetic resonance imaging (MRI) is a powerful noninvasive imaging technique that has greatly impacted basic biological research as well clinical diagnosis of cancer and other diseases.[1] Conventional MR contrast agents are T1 (e.g. Gd-DTPA) or T2-based (e.g. iron oxide), which cause significant longitudinal or transverse relaxation of protons, respectively.[2] Despite their success in many biological applications, one potential limitation is the lack of multi-chromatic features that allows for simultaneous detection of multiple signals. Recently, 19F has received significant attention in MR imaging and spectroscopy studies.[3] Compared to 1H-MRI, 19F-MRI has little biological background due to the low levels of endogenous fluorine in the body. Moreover, 19F has 100% natural abundance and its gyromagnetic ratio (40.06 MHz/T) is second only to 1H, which makes it more sensitive for detection over other nuclei.[3f]
In this study, we report on the development of “multi-colored” pH-activatable 19F-MRI nanoprobes with tunable pH transitions. Recently, extensive efforts have been dedicated to the development of stimuli-responsive nanoprobes.[4] Various nanosystems that respond to pH,[5] enzymatic expression,[6] redox reaction,[7] temperature,[8] and light[9] have been reported. Among these stimuli, pH stands out as an important physiological parameter that plays a critical role in both the intracellular (pHi) and extracellular (pHe) milieu.[10] For example, dysregulated pH was described as another hallmark of cancer, where a “reverse” pH gradient across the cell membrane is observed in cancer cells compared to normal cells.[11] A variety of different types of MRI agents have been reported for measuring pH,[12] but all have a rather broad pH response which may limit the accuracy of pH measurement, particularly when the pH perturbation in the pathological tissue is small. Moreover, it is often necessary to administer another pH-insensitive agent to correct for the contribution of agent concentration to obtain pH-sensitive signals, which makes the procedure complicated and difficult to perform.[13]
Herein we report the development of pH-sensitive 19F-MRI nanoprobes with a binary (ON/OFF) response to a specific, narrow pH transition (0.25 pH unit). We theorize that a collection of such nanoprobes where each pH transition is encoded with a specific 19F signature will allow for a simple readout of environmental pH through an “activation barcode”. To demonstrate this proof of concept, we synthesized three 19F-MRI nanoprobes with different pH transitions and 19F-reporters (Scheme 1). Through these nanoprobes, we show in phantom studies the feasibility of using either 19F spectroscopy or imaging to discriminate the pH differences in the microenvironment (i.e. 7.4, 6.5, 5.5 and 4.5).
Scheme 1.
(a) Schematic of pH-activatable ON/OFF 19F-MRI nanoprobes from ionizable diblock copolymers. At pH > pKa, the hydrophobic segments self-assemble into micelle core leading to 19F signal suppression due to restricted polymer chain motion. Upon pH activation (pH < pKa), micelle disassembly leads to dissociated unimers and strong 19F signal. (b) Chemical structures of three representative diblock copolymers containing different pH responsive segments and 19F reporter moieties, where their pKa's and 19F chemical shifts (in ppm, relative to trifluoroacetic acid, or TFA) are shown in parenthesis, respectively.
The initial challenge in designing a set of multi-colored pH-activatable 19F-nanoprobes is two-fold: first is the availability of reporter molecules that can be distinguished by MRS/I. For this purpose, 19F is highly advantageous over 1H probes as many 19F reporter molecules have diverse chemical shifts and narrow peak widths that can be easily differentiated. The second is to devise an activation mechanism in which the signal intensities of these 19F reporter molecules are highly responsive to the pH changes in the environment. In this regard, we adopted a strategy of using changes in spin-spin relaxations between the micelle and unimer states to turn ON/OFF 19F signals in response to pH.[3e, 3i] 19F reporters are introduced to the ionizable block (PR) of amphiphilic copolymers consisting of hydrophilic PEO segment and tertiary amine/ammonium segment (Scheme 1b). We hypothesize that at pH > pKa, hydrophobic micelle assembly results in highly restricted chain motions and short spin-spin relaxation times (T2→0) to effectively broaden and eliminate the 19F signals; at pH < pKa, protonation of ammonium groups will result in micelle disassembly, conformational flexibility in dissociated polymer chains, and reappearance of the previous 19F signal.
For initial development, we first synthesized poly-(ethylene oxide)-b-poly[2-(diisopropylamino) ethyl methacrylate-r-trifluoroethyl methacrylate] (PEO-b-P(DPA-r-TFE)) copolymer using atom transfer radical polymerization method.[14] To investigate the optimal composition, we synthesized a series of PEO-b-P(DPA-r-TFE) copolymers with increasing molar ratios (5 to 75 mol%) of TFE component (Table S1-S2, Fig. S1). On one hand, a higher TFE content should lead to stronger 19F signals while, too much TFE may override the pH response from DPA segment and induce micelle aggregation even at low pH. Gel permeation chromatography (GPC) and 1H NMR characterization demonstrated that all copolymers had similar molecular weights (1.5-1.8 × 104 Da) and polydispersity (Table S1, Fig. S1). pH titration of the copolymers showed that the TFE content had a considerable influence on the pKa and pH response of the copolymers. At 5 mol% of TFE, the pKa is 6.3, similar to the PEO-b-PDPA copolymer without TFE.[5c] An increase in TFE content decreased the pKa of the copolymers (Fig. S2a). Based on these pKa values, we chose pH 4.0 (below the pKa's of all the copolymers) to evaluate the effect of TFE content on 19F signal intensity (δF = 2.3 ppm for TFE relative to TFA). The 19F signal intensity as a funciton of TFE content showed a bell-shaped response curve, where it reached a maximum at 40 mol% TFE. At pH 4.0, dynamic light scattering experiments showed that all the copolymers except the PEO-b-P(DPA16-r-TFE44) (73 mol%) were in the unimer state as indicated by their small size (<10 nm in diameter) (Fig. S2c). Instead, PEO-b-P(DPA16-r-TFE44) copolymer formed micelles with a hydrodynamic diameter of 44 nm despite most of the amino groups were protonated at this pH. The decrease of 19F intensity can be explained by the rapid increase of spin-spin relaxation (or decreased T2) at higher molar fraction of TFE (Fig. S2e). Data show T2 is relatively unchanged (>40 ms) when the TFE content is below 20 mol%. Based on these data, we chose 20 mol% (i.e. PEO-b-P(DPA48-r-TFE12) as the optimal 19F-reporter composition in subsequent pH response studies.
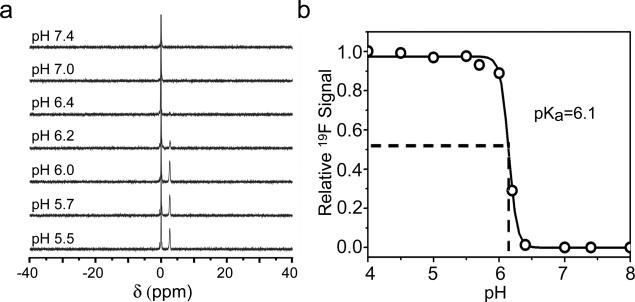
19F-NMR spectra of PEO-b-P(DPA48-r-TFE12) copolymer collected as a function of pH demonstrate ultra-pH responsive behavior (Fig. 1), similar to previously reported fluorescent nanoparticles.[5c, 5d] Below pH 6.0, we observed complete activation of 19F signals; above pH 6.2, the 19F signals largely disappeared. The pH difference (ΔpH10-90%) between 10 to 90% signal difference is 0.25 pH. This ultra-pH response is a unique property of this class of ionizable amphiphilc block copolymers, where hydrophobicity-driven micellization dramatically increased the cooperative deprotonation of the ammonium blocks.[5c, 5d] Transmission electron microscopy (TEM) of PEO-b-P(DPA48-r-TFE12) verified the formation of micelles at pH 7.4 (above its pKa of 6.1) and complete micelle dissociation at pH 5.0 (Fig. S3a). The micelle-unimer transition was further corroborated by 1H NMR (Fig. S3b) and dynamic light scattering (DLS), where hydrodynamic diameters were changed from 40 to 6 nm at pH 7.4 and 5.0, respectively (Fig. S3c).
Figure 1.
(a) 19F spectra of 2 mg/mL PEO-b-P(DPA48-r-TFE12) micelles in deuterated acetate buffers at different pH. TFA was used as an external reference with its chemical shift set as 0. (b) Normalized 19F signal intensity as a function of pH. Data was obtained from (a).
To investigate the ON/OFF pH-activatable MR imaging capability of the nanoprobes, we prepared a sample with two concentric tubes where both tubes were filled with PEO-P(DPA48-r-TFE12) at 25 mg/mL but the pH of the inner and outer tubes were controlled at 5.0 and 7.4, respectively. Axial 1H MRI images showed two compartments with similar signal intensities (left panel, Fig. 2a). In contrast, the corresponding 19F MRI images showed an intense signal (ON) in the inner tube but no signal (OFF) in the outer tube (right panel, Fig. 2a). We quantified the signal intensity in different regions of interest (ROI) over the background noise (Fig. 2b). At 55 mins, the 19F SNR reached 31-fold for the PEO-b-P(DPA48-r-TFE12) nanoprobes at pH 5.0 (ON state). Then we compared the contrast of 19F images between the ON and OFF states at pH 5.0 and 7.4, respectively. The contrast ratio (SNRpH5.0/SNRpH7.4) is 27 fold based on 19F images, demonstrating that 19F reporter on the polymers are highly responsive to the pH changes in the environment. In comparison, the SNRpH5.0/SNRpH7.4 ratio from the 1H images was only 1.2.
Figure 2.
(a) 1H and 19F MRI images of PEO-b-P(DPA48-r-TFE12) (25 mg/mL) phantom at pH 5.0 (inner tube) and 7.4 (outer tube). (b) SNR of 19F signals for PEO-b-P(DPA48-r-TFE12) as a function of scanning time at pH 5.0 (left panel) and comparison of SNR ratios at pH 5.0 and 7.4 from both 1H and 19F MRI images (right).
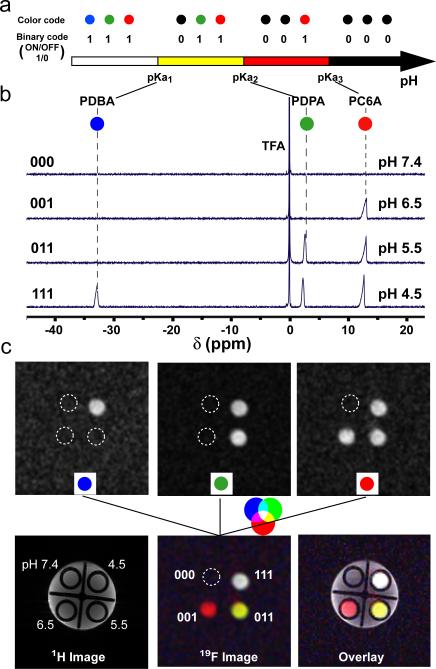
Finally, we investigated the “barcode” concept using a mixture of 19F-MRI nanoprobes with different pH transitions and 19F reporter molecules to distinguish pH in the microenvironment. In addition to TFE (δF = 2.3 ppm), we introduced two additional 19F reporter molecules (Scheme 1b, DFB and BTFB, δF = −33.2 and 13.0 ppm, respectively). These reporter molecules were incorporated into two new copolymers with different pH sensitivities, poly(ethylene oxide)-b-poly[2-(pentamethylene imino) methacrylate-r-2-(methacryloyloxy) ethyl 3,5-bis(trifluoromethyl) benzoate] (PEO-b-P(C6A-r-BTFB)) and poly(ethylene oxide)-b-poly[2-(dibutylamino) methacrylate-r-2-(methacryloyloxy) ethyl 3,5-difluorobenzoate] (PEO-b-P(DBA-r-DFB)) (Table S3). pH titration experiments demonstrated similar ultra-pH responsive properties of the two new copolymers (Fig. S4). The pKa's of the PEO-b-P(C6A-r-BTFB) and PEO-b-P(DBA-r-DFB) copolymers were 7.0 and 5.0, respectively, in addition to PEO-b-P(DPA-r-TFE) (pKa = 6.1). Based on these pKa's, we defined a three-digit barcode where each digit corresponds to one nanoprobe (with pKa from low to high), and has a binary response (1 for ON, 0 for OFF). For better visual demonstration, we also assigned a single color to each nanoprobe for the ON state (black for the OFF state). Such a barcode design allows for the direct readout of microenvionment pH within two adjacent pKa's in which one nanoprobe is ON and the other is OFF (Fig. 3a).
Figure 3.
(a) Schematic illustration of the activation barcode concept for direct readout of pH within adjacent pKa's. See text for details. (b) 19F spectra of a mixture of three PEO-b-P(R-r-F) nanoprobes in acetate buffers of different pH (7.4, 6.5, 5.5, 4.5). TFA was used as an external reference. (c) 19F MR imaging of the same nanoprobe mixture in solutions with different pH. Detection of each 19F reporter was accomplished by selective activation at its chemical shift (upper three panels). A “barcode map” (bottom middle panel) can be obtained by fusion of three 19F reporter images. 19F MR image was overlayed with 1H image to show the spatial registration.
To validate this concept, we performed a double blind experiment, where four solutions at pH 7.4, 6.5, 5.5 and 4.5 were first prepared containing the same mixture of the three nanoprobes. 19F spectroscopy was then obtained for each solution. Figure 3b shows a clearly distinguished barcode pattern of nanoprobe activation. More specifically, the (000) solution corresponds to the solution at pH 7.4, where all the nanoprobes were OFF. Accordingly, the (001), (011) and (111) solutions correspond to solutions with pH values at 6.5, 5.5 and 4.5, respectively. The nanoprobe barcodes successfully distinguished the solution pH. Lastly, addition of fetal bovine serum (5 or 10%) in nanoprobe solutions at pH 4.5 did not affect the signal contrast significantly, demonstrating successful 19F detection in biologically relevant media (Fig. S5).
In addition to 19F spectroscopy, we also used 19F MRI to spatially resolve the nanoprobe activation map. A phantom sample was prepared where 4 smaller tubes (each containing the same nanoprobe mixture in solutions at pH 7.4, 6.5, 5.5, and 4.5) were placed in a bigger tube with water only. T1-weighted 1H MRI images show similar signal intensity from all the tubes and the surrounding water (Fig. 3c). For 19F MR imaging, we selectively activated each 19F reporter at its chemical shift to examine the nanoprobe activation. Based on results from each 19F channel, we were able to obtain the barcode information for the different regions of interest (Fig. 3c). Potentially, by combining the 19F spectroscopy and imaging capabilities, we can generate a pH map where each voxel can be encoded with an activation barcode to indicate its environmental pH with spatial discrimination.
In summary, we report the feasibility of a series of multichromatic pH-activatable 19F nanoprobes encoded with different 19F reporters at specific pH transitions. Compared to small molecular pH sensors (typically 2 pH unit for 10 fold signal change across pKa), the pH response of these nanoprobes is extremely sharp (ΔpHON/OFF~0.25 pH) and can be used as binary indicators for a specific pH transition. The current three nanoprobe collection provides the proof of concept and allows for a qualitative measurement of environmental pH. This nanoplatform can potentially overcome the instrument complexity and short T1 limitation of the 13C-based hyperpolarization probes.[15] Moreover, compared to chemical exchange saturation transfer (CEST) or 1H agents where small pH-dependent chemical shifts are quantified,[12c, 16] the chemical shifts of 19F reporters are widely separated and easily differentiated for binary readout and data processing. Development of additional nanoprobes with more refined pH transitions will be useful to narrow the pH transitions and improve the precision of pH measurement. In addition, use of hybrid nanoparticles to include all 19F-encoded polymers in one system could further unify pharmacokinetics and biodistribution during in vivo study. Through a barcode map from 19F-imaging spectroscopy, it is conceivable to generate a pH map in three dimensions. Along with these exciting potentials, one main challenge in subsequent preclinical translation of these nanoprobes is the relatively low detection sensitivity of 19F-MRS/I. Optimization of MR scan time, pulse sequence or coil design should further improve the current detection limit (0.16 mg/mL 19F). Image resolution can also be compromised to achieve higher detection sensitivity. Upon successful demonstration, the 19F nanoprobes will add to the existing arsenal of pH sensors to measure tissue pH, an important physiological parameter in many pathological indications (e.g. cancer, inflammation, and osteoporosis).
Supplementary Material
Footnotes
This work is supported by the NIH (R01CA129011, R01EB013149). We acknowledge the assistance of the Southwestern Small Animal Imaging Resource, which is supported in part by NCI U24 CA126608, the Simmons Cancer Center through an NCI Cancer Center Support Grant (P30 CA142543).
Supporting information for this article is available on the WWW under http://www.angewandte.org.
Contributor Information
Xiaonan Huang, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Gang Huang, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Shanrong Zhang, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Koji Sagiyama, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Osamu Togao, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Xinpeng Ma, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Yiguang Wang, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Yang Li, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Todd C. Soesbe, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA)
Baran D. Sumer, Department of Otolaryngology, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA)
Masaya Takahashi, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
A. Dean Sherry, Advance Imaging Research Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
Jinming Gao, Department of Pharmacology, Harold C. Simmons Comprehensive Cancer Center, UT Southwestern Medical Center at Dallas, 5323 Harry Hines Blvds, Dallas.Texas 75390 (USA).
References
- 1.a Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]; b Laurent S, Forge D, Port M, Roch A, Robic C, Vander Elst L, Muller RN. Chem. Rev. 2008;108:2064–2110. doi: 10.1021/cr068445e. [DOI] [PubMed] [Google Scholar]; c Orel SG, Schnall MD. Radiology. 2001;220:13–30. doi: 10.1148/radiology.220.1.r01jl3113. [DOI] [PubMed] [Google Scholar]
- 2.a Jun YW, Lee JH, Cheon J. Angew. Chem. Int. Ed. Engl. 2008;47:5122–5135. doi: 10.1002/anie.200701674. [DOI] [PubMed] [Google Scholar]; b Khemtong C, Kessinger CW, Gao J. Chem Commun (Camb) 2009:3497–3510. doi: 10.1039/b821865j. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Sun C, Lee JS, Zhang M. Adv. Drug Delivery Rev. 2008;60:1252–1265. doi: 10.1016/j.addr.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a Janjic JM, Srinivas M, Kadayakkara DK, Ahrens ET. J. Am. Chem. Soc. 2008;130:2832–2841. doi: 10.1021/ja077388j. [DOI] [PubMed] [Google Scholar]; b Jiang ZX, Liu X, Jeong EK, Yu YB. Angew. Chem. Int. Ed. Engl. 2009;48:4755–4758. doi: 10.1002/anie.200901005. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Mizukami S, Takikawa R, Sugihara F, Hori Y, Tochio H, Walchli M, Shirakawa M, Kikuchi K. J. Am. Chem. Soc. 2008;130:794–795. doi: 10.1021/ja077058z. [DOI] [PubMed] [Google Scholar]; d Mizukami S, Takikawa R, Sugihara F, Shirakawa M, Kikuchi K. Angew. Chem. Int. Ed. Engl. 2009;48:3641–3643. doi: 10.1002/anie.200806328. [DOI] [PubMed] [Google Scholar]; e Oishi M, Sumitani S, Nagasaki Y. Bioconjugate Chem. 2007;18:1379–1382. doi: 10.1021/bc7002154. [DOI] [PubMed] [Google Scholar]; f Ruiz-Cabello J, Barnett BP, Bottomley PA, Bulte JW. NMR Biomed. 2011;24:114–129. doi: 10.1002/nbm.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Tanaka K, Kitamura N, Naka K, Chujo Y. Chem Commun (Camb) 2008:6176–6178. doi: 10.1039/b815022b. [DOI] [PubMed] [Google Scholar]; h Yamaguchi K, Ueki R, Nonaka H, Sugihara F, Matsuda T, Sando S. J. Am. Chem. Soc. 2011;133:14208–14211. doi: 10.1021/ja2057506. [DOI] [PubMed] [Google Scholar]; i Takaoka Y, Sakamoto T, Tsukiji S, Narazaki M, Matsuda T, Tochio H, Shirakawa M, Hamachi I. Nat. Chem. 2009;1:557–561. doi: 10.1038/nchem.365. [DOI] [PubMed] [Google Scholar]; j Yu JX, Hallac RR, Chiguru S, Mason RP. Prog. Nucl. Magn. Reson. Spectrosc. 2013 doi: 10.1016/j.pnmrs.2012.10.001. http://dx.doi.org/10.1016/j.pnmrs.2012.1010.1001. [DOI] [PMC free article] [PubMed]; k Yu JX, Kodibagkar VD, Cui W, Mason RP. Curr. Med. Chem. 2005;12:819–848. doi: 10.2174/0929867053507342. [DOI] [PubMed] [Google Scholar]
- 4.a Lee ES, Gao Z, Bae YH. J. Controlled Release. 2008;132:164–170. doi: 10.1016/j.jconrel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Torchilin V. Eur. J. Pharm. Biopharm. 2009;71:431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a Bae Y, Fukushima S, Harada A, Kataoka K. Angew. Chem. Int. Ed. Engl. 2003;42:4640–4643. doi: 10.1002/anie.200250653. [DOI] [PubMed] [Google Scholar]; b Lynn DM, Amiji MM, Langer R. Angew. Chem. Int. Ed. Engl. 2001;40:1707–1710. [PubMed] [Google Scholar]; c Zhou K, Liu H, Zhang S, Huang X, Wang Y, Huang G, Sumer BD, Gao J. J. Am. Chem. Soc. 2012;134:7803–7811. doi: 10.1021/ja300176w. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhou K, Wang Y, Huang X, Luby-Phelps K, Sumer BD, Gao J. Angew. Chem. Int. Ed. Engl. 2011;50:6109–6114. doi: 10.1002/anie.201100884. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Lee ES, Na K, Bae YH. J. Controlled Release. 2003;91:103–113. doi: 10.1016/s0168-3659(03)00239-6. [DOI] [PubMed] [Google Scholar]
- 6.a Bernardos A, Aznar E, Marcos MD, Martinez-Manez R, Sancenon F, Soto J, Barat JM, Amoros P. Angew. Chem. Int. Ed. Engl. 2009;48:5884–5887. doi: 10.1002/anie.200900880. [DOI] [PubMed] [Google Scholar]; b Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wang C, Chen Q, Wang Z, Zhang X. Angew. Chem. Int. Ed. Engl. 2010;49:8612–8615. doi: 10.1002/anie.201004253. [DOI] [PubMed] [Google Scholar]
- 7.a Li YL, Zhu L, Liu Z, Cheng R, Meng F, Cui JH, Ji SJ, Zhong Z. Angew. Chem. Int. Ed. Engl. 2009;48:9914–9918. doi: 10.1002/anie.200904260. [DOI] [PubMed] [Google Scholar]; b Saito G, Swanson JA, Lee KD. Adv. Drug Delivery Rev. 2003;55:199–215. doi: 10.1016/s0169-409x(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 8.a Choi SW, Zhang Y, Xia Y. Angew. Chem. Int. Ed. Engl. 2010;49:7904–7908. doi: 10.1002/anie.201004057. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jeong B, Bae YH, Kim SW. J. Controlled Release. 2000;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- 9.a Skirtach AG, Munoz Javier A, Kreft O, Kohler K, Piera Alberola A, Mohwald H, Parak WJ, Sukhorukov GB. Angew. Chem. Int. Ed. Engl. 2006;45:4612–4617. doi: 10.1002/anie.200504599. [DOI] [PubMed] [Google Scholar]; b Volodkin DV, Skirtach AG, Mohwald H. Angew. Chem. Int. Ed. Engl. 2009;48:1807–1809. doi: 10.1002/anie.200805572. [DOI] [PubMed] [Google Scholar]; c Febvay S, Marini DM, Belcher AM, Clapham DE. Nano Lett. 2010;10:2211–2219. doi: 10.1021/nl101157z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5th ed. Garland Science; New York: 2008. [Google Scholar]
- 11.Webb BA, Chimenti M, Jacobson MP, Barber DL. Nat. Rev. Cancer. 2011;11:671–677. doi: 10.1038/nrc3110. [DOI] [PubMed] [Google Scholar]
- 12.a Aime S, Delli Castelli D, Terreno E. Angew. Chem. Int. Ed. Engl. 2002;41:4334–4336. doi: 10.1002/1521-3773(20021115)41:22<4334::AID-ANIE4334>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]; b Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. J. Magn. Reson. Imaging. 2002;16:430–450. doi: 10.1002/jmri.10181. [DOI] [PubMed] [Google Scholar]; c Sheth VR, Li Y, Chen LQ, Howison CM, Flask CA, Pagel MD. Magn. Reson. Med. 2012;67:760–768. doi: 10.1002/mrm.23038. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ward KM, Balaban RS. Magn. Reson. Med. 2000;44:799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]; e Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Nat. Med. 2003;9:1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]; f Zhang S, Wu K, Sherry AD. Angew. Chem. Int. Ed. Engl. 1999;38:3192–3194. [PubMed] [Google Scholar]; g Wu Y, Soesbe TC, Kiefer GE, Zhao P, Sherry AD. J. Am. Chem. Soc. 2010;132:14002–14003. doi: 10.1021/ja106018n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ. Magn. Reson. Med. 2006;55:309–315. doi: 10.1002/mrm.20773. [DOI] [PubMed] [Google Scholar]; b Raghunand N, Howison C, Sherry AD, Zhang S, Gillies RJ. Magn. Reson. Med. 2003;49:249–257. doi: 10.1002/mrm.10347. [DOI] [PubMed] [Google Scholar]
- 14.a Tsarevsky NV, Matyjaszewski K. Chem. Rev. 2007;107:2270–2299. doi: 10.1021/cr050947p. [DOI] [PubMed] [Google Scholar]; b Ma YH, Tang YQ, Billingham NC, Armes SP, Lewis AL, Lloyd AW, Salvage JP. Macromolecules. 2003;36:3475–3484. [Google Scholar]
- 15.Gallagher FA, Kettunen MI, Day SE, Hu DE, Ardenkjaer-Larsen JH, Zandt R, Jensen PR, Karlsson M, Golman K, Lerche MH, Brindle KM. Nature. 2008;453:940–943. doi: 10.1038/nature07017. [DOI] [PubMed] [Google Scholar]
- 16.a Provent P, Benito M, Hiba B, Farion R, Lopez-Larrubia P, Ballesteros P, Remy C, Segebarth C, Cerdan S, Coles JA, Garcia-Martin ML. Cancer Res. 2007;67:7638–7645. doi: 10.1158/0008-5472.CAN-06-3459. [DOI] [PubMed] [Google Scholar]; b McMahon MT, Gilad AA, DeLiso MA, Berman SM, Bulte JW, van Zijl PC. Magn. Reson. Med. 2008;60:803–812. doi: 10.1002/mrm.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.