Abstract
Background
Chronic kidney disease is characterised by low estimated glomerular filtration rate (eGFR) and high albuminuria, and is associated with adverse outcomes. Whether these risks are modified by diabetes is unknown.
Methods
We did a meta-analysis of studies selected according to Chronic Kidney Disease Prognosis Consortium criteria. Data transfer and analyses were done between March, 2011, and June, 2012. We used Cox proportional hazards models to estimate the hazard ratios (HR) of mortality and end-stage renal disease (ESRD) associated with eGFR and albuminuria in individuals with and without diabetes.
Findings
We analysed data for 1 024 977 participants (128 505 with diabetes) from 30 general population and high-risk cardiovascular cohorts and 13 chronic kidney disease cohorts. In the combined general population and high-risk cohorts with data for all-cause mortality, 75 306 deaths occurred during a mean follow-up of 8·5 years (SD 5·0). In the 23 studies with data for cardiovascular mortality, 21 237 deaths occurred from cardiovascular disease during a mean follow-up of 9·2 years (SD 4·9). In the general and high-risk cohorts, mortality risks were 1·2–1·9 times higher for participants with diabetes than for those without diabetes across the ranges of eGFR and albumin-to-creatinine ratio (ACR). With fixed eGFR and ACR reference points in the diabetes and no diabetes groups, HR of mortality outcomes according to lower eGFR and higher ACR were much the same in participants with and without diabetes (eg, for all-cause mortality at eGFR 45 mL/min per 1·73 m2 [νs 95 mL/min per 1·73 m2], HR 1·35; 95% CI 1·18–1·55; νs 1·33; 1·19–1·48 and at ACR 30 mg/g [νs 5 mg/g], 1·50; 1·35–1·65 νs 1·52; 1·38–1·67). The overall interactions were not significant. We identified much the same findings for ESRD in the chronic kidney disease cohorts.
Interpretation
Despite higher risks for mortality and ESRD in diabetes, the relative risks of these outcomes by eGFR and ACR are much the same irrespective of the presence or absence of diabetes, emphasising the importance of kidney disease as a predictor of clinical outcomes.
Introduction
Chronic kidney disease is a global public health problem,1,2 affecting 10–16% of the adult population worldwide.3–8 Decreased estimated glomerular filtration rate (eGFR) and increased urinary albumin excretion predict the major health outcomes of this disorder, including end-stage renal disease (ESRD) and death, across a wide range of settings.9–13 Whether these associations are consistent across diseases or are differentially modified by the presence or absence of a particular disease or condition is uncertain.
Diabetes is the leading cause of chronic kidney disease in the developed world,14,15 and people with diabetes and chronic kidney disease have a greatly increased risk of all-cause mortality, cardiovascular mortality, and kidney failure. We did a meta-analysis to assess whether the associations between eGFR, albuminuria, and mortality and kidney outcomes are the same in individuals with and without diabetes.
Methods
Study selection criteria
Sources of data for the Chronic Kidney Disease Prognosis Consortium are described elsewhere.9–12,16 Briefly, we included studies that had at least 1000 participants (not applied to studies that predominantly included patients with chronic kidney disease), baseline information about eGFR and albuminuria, and at least 50 events for each outcome of interest. We restricted our analyses to participants aged at least 18 years. Sample size varies slightly compared with our accompanying article about hypertension17 because of differences in requisite variables and their availability across cohorts. Data transfer and analysis took place between March, 2011, and June, 2012. Our study was approved by the institutional review board at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD, USA).
Procedures
We calculated eGFR with the Chronic Kidney Disease Epidemiology Collaboration equation.16,18 In situations in which creatinine measurement was not previously standardised to isotope dilution mass spectrometry, creatinine concentrations were reduced by 5%, the established calibration factor.19 Although urine albumin-to-creatinine (ACR) ratio is formally recommended, we also included studies in which urine albumin excretion rate, urine protein-to-creatinine ratio (PCR), or quantitative dipstick protein were measured.20 We defined diabetes as fasting glucose of at least 7·0 mmol/L, non-fasting glucose of at least 11·1 mmol/L or glycated haemoglobin (HbA1c) of at least 6·5%, use of glucose-lowering drugs, or self-reported diabetes. We defined hypertension as systolic blood pressure of at least 140 mm Hg, diastolic blood pressure of at least 90 mm Hg, or use of antihypertensive drugs. We defined hypercholesterolaemia as total cholesterol of at least 5·0 mmol/L in participants with previous cardiovascular disease and of at least 6·0 mmol/L in those without previous history of cardiovascular disease. Participants with a history of myocardial infarction, coronary revascularisation, heart failure, or stroke were deemed to have a history of cardiovascular disease. We defined smoking status as present versus former or never.
Primary outcomes were all-cause mortality, cardiovascular mortality, and ESRD, where cardiovascular mortality included deaths due to myocardial infarction, heart failure, sudden cardiac death, or stroke. We defined ESRD as initiation of renal replacement therapy or death from kidney disease other than acute kidney injury.
Statistical analysis
We applied a two-stage analytical approach, whereby each study was analysed separately with a prescribed analysis plan, followed by a random-effects meta-analysis consisting of all study-level results. Participants with missing eGFR, albuminuria, or diabetes data at baseline were excluded; we imputed the mean for missing values of other baseline covariates. Appendix pp 21–24 show the analysis overview and analytical notes for individual studies. We quantified heterogeneity with the I2 statistic and χ2 test.9 In view of the similarity of results in the general and high-risk populations and the large number of mortality outcomes in these populations, we combined them for the meta-analysis of mortality outcomes. Since most ESRD cases were from the chronic kidney disease cohorts, the associations of eGFR and albuminuria with ESRD are shown mainly for these cohorts.
In each study, we fitted eGFR linear splines (knots were placed at each 15 mL/min per 1·73 m2 interval from 30–105 mL/min per 1·73 m2 [90 mL/min per 1·73 m2 in chronic kidney disease cohorts]) and their product terms with diabetes status at baseline. We adjusted Cox models for age, sex, ethnicity (black vs non-black), smoking, systolic blood pressure, total cholesterol, body-mass index, history of cardiovascular disease, and albuminuria (log-transformed ACR and PCR as continuous variables or dipstick as a categorical variable [negative, trace, 1+, 2+, and ≥3+]). This approach provided hazard ratios (HR) for eGFR (with a reference of eGFR 95 mL/min per 1·73 m2 in the general and high risk cohorts and 50 mL/min per 1·73 m2 in the chronic kidney disease cohorts) in both the diabetes and no diabetes groups. We assessed interaction as the ratio of HR in individuals with diabetes versus those without diabetes at each 1 mL/min per 1·73 m2 of eGFR from 15–120 mL/min per 1·73 m2 (60 mL/min per 1·73 m2 in chronic kidney disease cohorts, point-wise interaction). To test the overall interaction across the eGFR range, we pooled the coefficients for product terms between eGFR splines and diabetes status (representing the difference in slopes between the diabetes and no diabetes groups) with an inverse-variance weighted approach. We used much the same methods for the log-ACR risk association analyses (knots at 10 mg/g, 30 mg/g, and 300 mg/g; reference at ACR 5 mg/g in the general and high-risk cohorts and knots at 30 mg/g, 300 mg/g, and 1000 mg/g; reference at ACR 20 mg/g in the chronic kidney disease cohorts). We adjusted eGFR with its spline terms. In analyses stratified by age, sex, or hypertension, we modelled ACR linearly on a logarithmic scale. To appreciate the association of diabetes with mortality and ESRD risk, estimates of both diabetes and no diabetes groups were compared with one reference point of eGFR 95 mL/min per 1·73 m2 (50 mL/min per 1·73 m2 in chronic kidney disease cohorts) and ACR 5 mg/g (20 mg/g in chronic kidney disease cohorts) in the no diabetes group.
We tested for three-way interactions between the kidney measures, hypertension, and diabetes by obtaining an inverse variance weighted average of coefficients of the three-way product terms (kidney measure*hypertension*diabetes) in each cohort and then pooled across cohorts. This statistic shows the overall interaction of coexistence of hypertension and diabetes as compared with what we would expect from the interaction by hypertension alone (ie, no diabetes) and diabetes alone (ie, no hypertension). We also tested the interaction by doing meta-regression analysis with a random-effects model, which allowed us to assess the chronic kidney disease-diabetes interaction with studies enrolling individuals with only diabetes or only no diabetes.21
We did categorical analyses to compare the risk in 32 categories of eGFR (<15, 15–29, 30–44, 45–59, 60–74, 75–89, 90–104, ≥105 mL/min per 1·73 m2) and albuminuria (ACR <10, 10–29, 30–299, ≥300 mg/g or PCR <15, 15–49, 50–499, ≥500 mg/g; or dipstick test results: negative, trace, 1+, 2+ or more), in general and high-risk cohorts. In chronic kidney disease cohorts the categorical analyses were based on 20 categories of eGFR (<15, 15–29, 30–44, 45–74, ≥75 mL/min per 1·73 m2) and albuminuria (ACR <30, 30–299, 300–999, ≥1000 mg/g; or PCR <50, 50–499, 500–1499, ≥1500 mg/g; or dipstick negative or trace, 1+, 2+, 3+ or more).
We used Stata (version 11.2) for analysis. We judged p values less than 0·05 to be statistically significant.
Role of the funding source
The sponsors had no role in the study design, data collection, analysis, data interpretation, or writing of the report. KM and JC had full access to all analyses and all authors had final responsibility for the decision to submit for publication, informed by discussions with the collaborators.
Results
Table 1 and appendix pp 1–3 show baseline characteristics from individual studies. 1 024 977 participants were included, of whom 128 505 (13%) had diabetes. Participants with diabetes were generally older than those without diabetes and had a higher prevalence of hypertension, hypercholesterolaemia, and cardiovascular disease.
Table 1.
Baseline characteristics of the individual studies by the presence or absence of diabetes
| Individuals with diabetes
|
Individuals without diabetes
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age | Women | Black | HTN | History of CVD | HC | Smoking | eGFR | Albuminuria‡ | N | Age | Women | Black | HTN | History of CVD | HC | Smoking | eGFR | Albuminuria‡ | |
| General population cohorts | ||||||||||||||||||||
| Aichi | 306 | 53 (5·5) | 9% | 0% | 46% | 2% | 26% | 42% | 96 (14·5) | 8% | 4425 | 48 (7·0) | 21% | 0% | 24% | 1% | 20% | 29% | 97 (13·8) | 2% |
| ARIC* | 1901 | 63 (5·6) | 52% | 35% | 66% | 24% | 44% | 13% | 84 (19·0) | 22% | 9489 | 63 (5·7) | 57% | 19% | 44% | 12% | 34% | 15% | 84 (14·8) | 6% |
| AusDiab* | 933 | 63 (12·5) | 47% | 0% | 69% | 23% | 60% | 13% | 78 (17·9) | 22% | 10 105 | 51 (14·1) | 55% | 0% | 29% | 7% | 43% | 16% | 87 (16·4) | 5% |
| Beaver Dam CKD | 496 | 66 (10·0) | 56% | 0% | 69% | 28% | 57% | 15% | 77 (19·8) | 12% | 4361 | 62 (11·2) | 56% | 0% | 48% | 13% | 54% | 20% | 80 (17·8) | 3% |
| Beijing* | 433 | 62 (8·7) | 48% | 0% | 67% | 25% | 35% | 22% | 82 (15·2) | 13% | 1126 | 59 (9·7) | 51% | 0% | 51% | 15% | 26% | 24% | 84 (13·8) | 3% |
| CHS* | 471 | 77 (4·6) | 52% | 24% | 75% | 43% | 39% | 9% | 75 (19·5) | 39% | 2517 | 78 (4·8) | 60% | 15% | 62% | 29% | 38% | 8% | 74 (17·0) | 17% |
| CIRCS | 530 | 57 (7·8) | 43% | 0% | 53% | 2% | 19% | 39% | 88 (14·9) | 8% | 11 341 | 54 (8·8) | 62% | 0% | 35% | 1% | 13% | 26% | 89 (15·0) | 3% |
| COBRA* | 614 | 53 (10·3) | 57% | 0% | 58% | 14% | 43% | 36% | 102 (20·7) | 21% | 2258 | 51 (10·8) | 51% | 0% | 40% | 7% | 33% | 40% | 103 (16·4) | 6% |
| ESTHER | 1850 | 64 (6·4) | 48% | 0% | 68% | 28% | 48% | 16% | 84 (20·7) | 23% | 7790 | 62 (6·6) | 57% | 0% | 57% | 15% | 45% | 16% | 84 (20·2) | 9% |
| Framingham* | 281 | 63 (8·7) | 42% | 0% | 69% | 17% | 17% | 13% | 85 (21·6) | 33% | 2675 | 58 (9·7) | 54% | 0% | 37% | 5% | 23% | 15% | 89 (18·0) | 10% |
| Gubbio* | 89 | 56 (5·7) | 44% | 0% | 58% | 11% | 52% | 35% | 87 (11·1) | 6% | 1592 | 54 (5·8) | 56% | 0% | 38% | 4% | 48% | 31% | 84 (11·6) | 4% |
| HUNT* | 1703 | 66 (13·3) | 51% | 0% | 81% | 28% | N/A | 19% | 82 (19·5) | 25% | 7913 | 61 (14·9) | 56% | 0% | 83% | 21% | N/A | 22% | 86 (19·7) | 10% |
| IPHS | 5164 | 62 (8·8) | 50% | 0% | 66% | 9% | 24% | 27% | 82 (14·4) | 7% | 90 201 | 59 (10·3) | 67% | 0% | 49% | 5% | 18% | 20% | 86 (13·9) | 2% |
| MESA* | 846 | 65 (9·4) | 47% | 39% | 67% | 0% | 37% | 13% | 84 (19·9) | 28% | 5887 | 62 (10·3) | 54% | 26% | 42% | 0% | 27% | 13% | 82 (15·8) | 7% |
| MRC | 957 | 81 (4·5) | 52% | 0% | 79% | 26% | N/A | 10% | 57 (15·8) | 11% | 11 414 | 81 (4·6) | 62% | 0% | 76% | 17% | N/A | 12% | 57 (14·5) | 7% |
| NHANES III* | 1810 | 61 (15·1) | 53% | 30% | 60% | 27% | N/A | 19% | 86 (25·5) | 34% | 13 753 | 45 (19·2) | 53% | 27% | 25% | 8% | N/A | 26% | 102 (23·9) | 9% |
| Ohasama | 150 | 65 (7·5) | 67% | 0% | 47% | 5% | 26% | 13% | 82 (11·8) | 6% | 1323 | 63 (9·5) | 66% | 0% | 41% | 2% | 17% | 14% | 83 (13·2) | 7% |
| PREVEND* | 311 | 61 (9·3) | 42% | 2% | 73% | 16% | 61% | 31% | 83 (16·4) | 36% | 8063 | 49 (12·6) | 50% | 1% | 32% | 5% | 39% | 34% | 89 (15·6) | 10% |
| RanchoBernardo* | 178 | 74 (10·2) | 51% | 0% | 77% | 17% | 40% | 3% | 69 (18·8) | 25% | 1296 | 70 (12·1) | 61% | 0% | 52% | 9% | 27% | 8% | 74 (16·7) | 13% |
| REGARDS* | 5671 | 66 (8·7) | 52% | 56% | 79% | 46% | N/A | 14% | 83 (24·4) | 30% | 21 498 | 65 (9·5) | 55% | 36% | 54% | 29% | N/A | 14% | 86 (18·6) | 11% |
| Severance | 4517 | 54 (10·0) | 38% | 0% | 47% | 1% | 60% | 31% | 86 (15·0) | 17% | 71 684 | 45 (11·7) | 50% | 0% | 24% | 2% | 36% | 29% | 90 (14·8) | 4% |
| Taiwan | 25515 | 56 (11·5) | 47% | 0% | 49% | 12% | 31% | 25% | 81 (18·7) | 12% | 488 333 | 41 (13·7) | 50% | 0% | 15% | 4% | 12% | 24% | 93 (17·4) | 1% |
| ULSAM* | 207 | 71 (0·8) | 0% | 0% | 85% | 42% | 57% | 16% | 77 (11·0) | 29% | 896 | 71 (0·6) | 0% | 0% | 72% | 35% | 58% | 21% | 75 (10·7) | 13% |
| High-risk cohorts | ||||||||||||||||||||
| ADVANCE* | 10595 | 66 (6·4) | 42% | 0% | 83% | 25% | 57% | 15% | 78 (17·2) | 31% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| CARE | 580 | 61 (8·3) | 19% | 7% | 91% | 100% | 74% | 11% | 74 (17·0) | 25% | 3518 | 58 (9·4) | 13% | 3% | 84% | 100% | 80% | 17% | 76 (15·8) | 12% |
| KEEP | 23053 | 59 (13·4) | 66% | 30% | 79% | 21% | N/A | 10% | 81 (23·6) | 20% | 54 847 | 52 (15·7) | 69% | 32% | 60% | 10% | N/A | 12% | 88 (22·6) | 9% |
| KP Hawaii† | 19183 | 59 (13·9) | 48% | 0% | N/A | 20% | N/A | 14% | 80 (23·8) | 39% | 20 701 | 59 (15·1) | 52% | 0% | N/A | 14% | N/A | 13% | 78 (22·5) | 28% |
| MRFIT | 597 | 48 (5·8) | 0% | 9% | 73% | 0% | 58% | 54% | 87 (14·2) | 6% | 12 257 | 46 (6·0) | 0% | 7% | 66% | 0% | 50% | 59% | 87 (13·1) | 4% |
| Pima* | 1361 | 44 (14·3) | 57% | 0% | 40% | 0% | 12% | 24% | 110 (24·6) | 48% | 3705 | 28 (11·4) | 56% | 0% | 9% | 0% | 4% | 29% | 124 (14·3) | 10% |
| ZODIAC* | 1095 | 68 (11·4) | 57% | 0% | 87% | 35% | 57% | 19% | 71 (17·8) | 39% | 0 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Chronic kidney disease cohorts | ||||||||||||||||||||
| AASK† | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 1094 | 55 (10·7) | 39% | 100% | 100% | 52% | 44% | 29% | 46 (14·6) | 62% |
| BC CKD | 6659 | 68 (11·5) | 42% | 0% | 93% | 44% | 18% | 7% | 36 (17·8) | 79% | 10767 | 69 (15·2) | 47% | 0% | 73% | 21% | 17% | 5% | 37 (19·9) | 73% |
| CRIB* | 53 | 64 (13·3) | 38% | 17% | 94% | 62% | 60% | 11% | 20 (11·4) | 89% | 255 | 61 (14·4) | 33% | 4% | 95% | 42% | 57% | 14% | 23 (11·1) | 86% |
| Geisinger ACR* | 3227 | 70 (9·8) | 53% | 2% | 89% | 33% | 66% | 10% | 51 (8·3) | 44% | 134 | 70 (11·2) | 57% | 2% | 75% | 19% | 53% | 11% | 49 (11·0) | 38% |
| Geisinger Dip | 1231 | 71 (11·2) | 58% | 1% | 81% | 43% | 50% | 8% | 48 (11·3) | 35% | 3278 | 73 (11·1) | 63% | 1% | 74% | 26% | 42% | 7% | 50 (10·2) | 21% |
| GLOMMS-1 ACR* | 499 | 73 (10·4) | 51% | 0% | 66% | 44% | N/A | 11% | 33 (7·6) | 51% | 38 | 76 (9·1) | 47% | 0% | 42% | 24% | N/A | 16% | 31 (8·2) | 42% |
| GLOMMS-1 PCR† | 105 | 68 (13·5) | 48% | 0% | 68% | 50% | N/A | 14% | 29 (9·3) | 94% | 365 | 70 (15·6) | 48% | 0% | 59% | 30% | N/A | 13% | 29 (9·6) | 95% |
| KPNW | 632 | 70 (9·8) | 55% | 4% | 94% | 57% | 25% | 10% | 46 (11·7) | 43% | 995 | 73 (9·6) | 56% | 3% | 91% | 37% | 21% | 14% | 47 (11·1) | 24% |
| MASTERPLAN* | 220 | 65 (11·2) | 27% | 5% | 98% | 32% | 81% | 20% | 34 (13·4) | 85% | 416 | 58 (12·6) | 33% | 2% | 94% | 20% | 70% | 21% | 37 (14·8) | 85% |
| MDRD† | 107 | 59 (8·7) | 35% | 23% | N/A | 30% | N/A | 13% | 40 (20·4) | 89% | 1623 | 50 (12·8) | 40% | 12% | N/A | 12% | N/A | 12% | 41 (21·1) | 82% |
| MMKD† | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 202 | 47 (12·1) | 34% | 0% | 89% | 12% | 39% | 21% | 47 (30·0) | 95% |
| NephroTest* | 242 | 65 (11·2) | 28% | 8% | 98% | 28% | 74% | 12% | 41 (17·8) | 75% | 686 | 58 (15·4) | 32% | 10% | 91% | 13% | 55% | 11% | 43 (22·0) | 59% |
| RENAAL* | 1513 | 60 (7·4) | 37% | 15% | 97% | 29% | 63% | 18% | 41 (13·1) | 100% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| STENO* | 886 | 44 (11·1) | 43% | 0% | 64% | 7% | 85% | 57% | 84 (26·2) | 49% | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Sunnybrook* | 1734 | 71 (12·4) | 40% | 0% | 90% | 48% | N/A | 4% | 37 (15·0) | 83% | 1651 | 70 (15·6) | 48% | 0% | 80% | 33% | N/A | 5% | 38 (16·1) | 85% |
Data are % or mean (SD) unless otherwise specified. Appendix pp 19–20 show study acronyms and abbreviations. HTN=hypertension. CVD=cardiovascular disease. C=hypercholesterolaemia. eGFR=estimated glomerular filtration rate. ACR=albumin-to-creatinine ratio. PCR=protein-to-creatinine ratio.
Studies with ACR
Studies with PCR.
Proportion of participants with ACR ≥30 mg/g or PCR ≥50 mg/g or dipstick protein ≥1+.
In the 30 combined general population and high-risk cohorts with data for all-cause mortality, 75 306 deaths occurred during a mean follow-up of 8·5 years (SD 5·0). In the 23 studies with data for cardiovascular mortality, 21237 deaths occurred from cardiovascular disease during a mean follow-up of 9·2 years (SD 4·9). When we set one reference point in the no diabetes group, HR for all-cause mortality and cardiovascular mortality at a given eGFR or ACR were 1·2–1·9 times higher in participants with diabetes than in those without diabetes across the entire range of eGFR and ACR (figures 1A, 1C, 2A, 2C). When we set separate references points in the diabetes and no diabetes groups to assess an interaction with diabetes specifically, HR for low eGFR and high ACR were much the same in participants with and without diabetes, showing no point-wise interaction (figures 1B, 1D, 2B, 2D).
Figure 1. Hazard ratios for all-cause and cardiovascular mortality in the combined general and high-risk populations according to eGFR in individuals with and without diabetes.
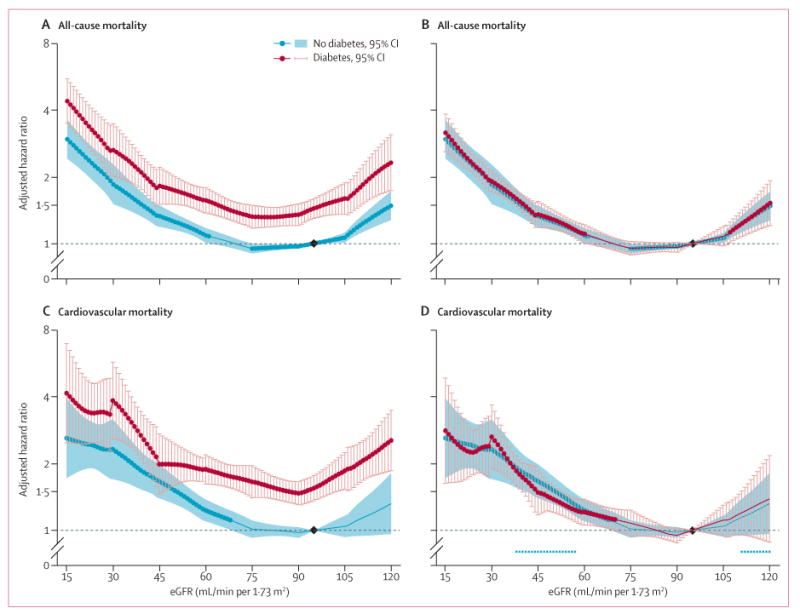
(A, B) All-cause mortality. (C, D) Cardiovascular mortality. Panels A and C use one reference point (diamond, eGFR of 95 mL/min per 1·73 m2 in the no diabetes group) for both individuals with and without diabetes to show the main effect of diabetes on risk. Panels B and D use separate references (diamonds) in the diabetes and no diabetes groups to assess interaction with diabetes specifically. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative, trace, 1+, ≥2+]). Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and eGFR is shown by x signs. eGFR=estimated glomerular filtration rate.
Figure 2. Hazard ratios for all-cause and cardiovascular mortality in the combined general and high-risk populations according to ACR in participants with and without diabetes.
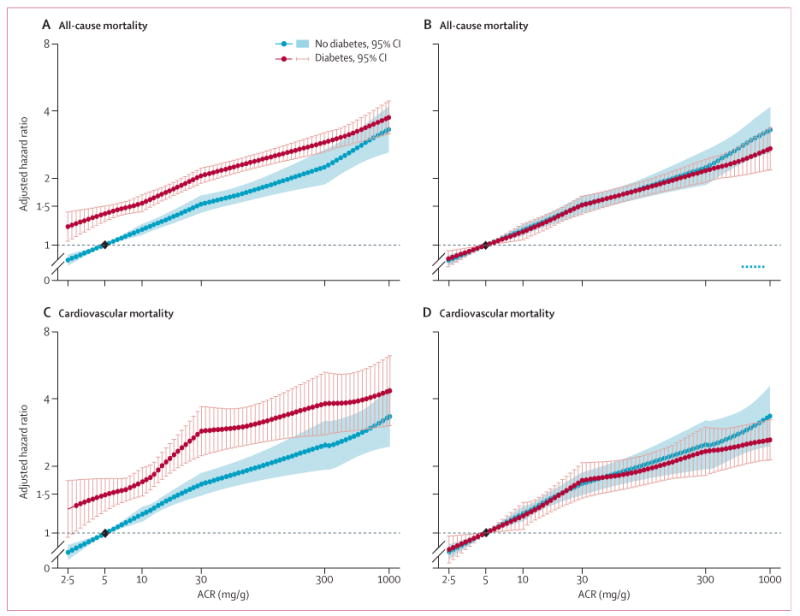
(A, B) All-cause mortality. (C, D) Cardiovascular mortality. Panels A and C use one reference point (diamond, ACR of 5 mg/g in the no diabetes group), for both individuals with and without hypertension to show the main effect of diabetes on risk. Panels B and D use separate references (diamonds) in the diabetes and no diabetes groups to assess interaction with diabetes specifically. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and estimated glomerular filtration rate. Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and ACR is shown by x signs. ACR=albumin-to-creatinine ratio.
The overall interaction of diabetes, computed as relative HR between individuals with diabetes and those without diabetes averaged across the entire range of eGFR for a 15 mL/min per 1·73 m2 reduction was not significant for either outcome (relative HR 1·00; 95% CI 0·97–1·04; p=0·92 for all-cause mortality, 0·97; 0·93–1·02; p=0·20 for cardiovascular mortality). We recorded much the same results for a ten-times increase in ACR (relative HR 0·99; 95% CI 0·91–1·08; p=0·86 for all-cause mortality and relative HR 0·97; 0·86–1·10; p=0·62 for cardiovascular mortality). Heterogeneity of the interaction across studies for all-cause mortality was moderate for both eGFR and ACR; for cardiovascular mortality it was low for eGFR and absent for ACR (appendix p 4). We recorded much the same results with a meta-regression analysis when we included cohorts that enrolled only individuals with diabetes or without diabetes (appendix p 5) or when we analysed the chronic kidney disease cohorts (appendix p 6).
Table 2 shows pooled HR for all-cause mortality and cardiovascular mortality for 32 categories of eGFR and albuminuria in participants with and without diabetes. All-cause mortality and cardiovascular mortality generally increased with lower eGFR and higher albuminuria categories in both the diabetes and no diabetes groups. Moreover, HR were much the same across these groups within a given category. Overall, we did not identify significant interactions with diabetes at any eGFR or albuminuria categories.
Table 2.
Hazard ratios for all-cause and cardiovascular mortality in the combined general and high-risk populations according to eGFR and albuminuria clinical categories in individuals with and without diabetes
| Individuals with diabetes
|
Individuals without diabetes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACR <10, PCR <15, or dipstick negative | ACR 10–29, PCR 15–49, or dipstick trace | ACR 30–299, PCR 50–499, or dipstick 1+ | ACR ≥300, PCR ≥500, or dipstick ≥2+ | Overall | ACR <10, PCR <15, or dipstick negative | ACR 10–29, PCR 15–49, or dipstick trace | ACR 30–299, PCR 50–499, or dipstick 1+ | ACR ≥300, PCR ≥500, or dipstick ≥2+ | Overall | |
| All-cause mortality | ||||||||||
| eGFR ≥105 | 1·27*; 1·07–1·51 | 1·58*; 1·29–1·94 | 2·43*; 1·90–3·11 | 4·38*; 2·97–6·46 | 1·21*; 1·06–1·39 | 1·27*; 1·14–1·41 | 1·62*; 1·35–1·96 | 2·39*; 2·03–2·82 | 5·40*; 3·33–8·76 | 1·20*; 1·12–1·30 |
| eGFR 90–104 | Reference | 1·41*; 1·24–1·59 | 1·73*; 1·45–2·05 | 2·95*; 2·22–3·91 | Reference | Reference | 1·47*; 1·32–1·64 | 1·82*; 1·64–2·03 | 3·23*; 2·39–4·37 | Reference |
| eGFR 75–89 | 0·94; 0·87–1·01 | 1·33*; 1·16–1·54 | 1·59*; 1·35–1·87 | 2·42*; 1·89–3·11 | 0·95; 0·90–1·01 | 0·94; 0·89–1·00 | 1·30*; 1·18–1·44 | 1·60*; 1·40–1·84 | 2·57*; 1·98–3·34 | 0·94*; 0·90–0·98 |
| eGFR 60–74 | 0·99; 0·92–1·07 | 1·32*†; 1·16–1·49 | 1·86*; 1·60–2·16 | 2·98*; 2·36–3·76 | 1·04; 0·97–1·12 | 1·01; 0·95–1·09 | 1·38*†; 1·20–1·59 | 1·86*; 1·64–2·12 | 2·41*; 1·88–3·10 | 1·01; 0·95–1·07 |
| eGFR 45-59 | 1·15*; 1·01–1·30 | 1·82*; 1·60–2·07 | 1·97*; 1·65–2·35 | 3·23*; 2·51–4·15 | 1·18*†; 1·07–1·30 | 1·22*; 1·09–1·35 | 1·70*; 1·49–1·93 | 2·10*; 1·75–2·53 | 3·15*; 2·44–4·06 | 1·19*†; 1·10–1·29 |
| eGFR 30–44 | 1·81*; 1·35–2·44 | 2·25*; 1·87–2·70 | 3·13*; 2·57–3·80 | 4·61*; 3·64–5·84 | 1·65*; 1·48–1·83 | 1·71*; 1·44–2·02 | 2·54*; 2·26–2·86 | 2·89*; 2·31–3·61 | 4·00*; 2·92–5·48 | 1·53*; 1·36–1·73 |
| eGFR 15–29 | 2·69*; 1·78–4·06 | 3·30*; 2·43–4·46 | 4·96*; 3·19–7·72 | 6·80*; 4·76–9·70 | 2·28*; 1·91–2·72 | 3·16*; 2·25–4·45 | 4·01*; 2·86–5·62 | 3·90*; 2·93–5·20 | 6·69*; 4·94–9·08 | 2·27*; 1·86–2·77 |
| eGFR<15 | 12·0*; 3·02–47·6 | 5·88*; 2·43–14·2 | 9·55*; 4·53–20·1 | 14·5*; 8·84–23·8 | 4·46*; 3·26–6·10 | 6·55*; 3·53–12·1 | 8·56*; 5·72–12·8 | 6·91*; 4·67–10·2 | 12·0*; 8·84–16·4 | 4·06*; 3·33–4·95 |
| Overall | Reference | 1·35*; 1·27–1·44 | 1·73*; 1·61–1·86 | 2·67*; 2·31–3·08 | ·· | Reference | 1·31*; 1·23–1·41 | 1·67*; 1·54–1·82 | 2·38*; 2·07–2·75 | ·· |
| Cardiovascular mortality | ||||||||||
| eGFR ≥105 | 1·19; 0·76–1·89 | 1·93*; 1·12–3·33 | 3·00*; 1·49–6·03 | 5·07*; 1·86–13·8 | 1·16; 0·84–1·62 | 1·22; 0·98–1·52 | 1·82*; 1·14–2·91 | 4·00*; 2·82–5·69 | 7·04*; 2·83–17·5 | 1·19; 0·98–1·44 |
| eGFR 90–104 | Reference | 1·28; 0·95–1·74 | 1·74*; 1·28–2·36 | 3·03*; 1·90–4·84 | Reference | Reference | 1·62*; 1·31–2·01 | 1·79*; 1·43–2·24 | 3·39*; 2·12–5·40 | Reference |
| eGFR 75–89 | 1·04; 0·88–1·21 | 1·70*; 1·29–2·23 | 1·79*; 1·41–2·28 | 2·69*; 1·91–3·78 | 1·10; 0·97–1·23 | 1·01; 0·91–1·12 | 1·46*; 1·21–1·77 | 1·80*; 1·51–2·15 | 2·53*; 2·03–3·16 | 1·00; 0·95–1·06 |
| eGFR 60–74 | 1·25*; 1·06–1·47 | 1·56*; 1·21–2·00 | 2·53*; 2·00–3·20 | 3·21*; 2·42–4·27 | 1·27*; 1·12–1·44 | 1·14; 1·00–1·29 | 1·49*; 1·17–1·88 | 2·17*; 1·88–2·50 | 2·38*; 1·78–3·17 | 1·13*; 1·02–1·24 |
| eGFR 45–59 | 1·33*; 1·05–1·68 | 1·75*; 1·31–2·35 | 2·27*; 1·70–3·01 | 3·24*; 2·41–4·35 | 1·32*†; 1·14–1·53 | 1·52*; 1·30–1·77 | 2·19*; 1·86–2·56 | 2·57*; 1·93–3·43 | 3·74*; 2·73–5·12 | 1·48*†; 1·31–1·68 |
| eGFR 30–44 | 2·12*; 1·55–2·89 | 2·49*; 1·62–3·85 | 3·62*; 2·50–5·24 | 5·57*; 4·08–7·59 | 2·08*; 1·73–2·50 | 2·51*; 2·05–3·08 | 2·99*; 2·07–4·33 | 3·52*; 2·76–4·48 | 5·21*; 3·28–8·28 | 2·10*; 1·83–2·40 |
| eGFR 15–29 | 4·10*; 1·75–9·61 | 3·39*; 1·56–7·34 | 5·64*; 2·64–12·1 | 7·96*; 4·89–13·0 | 2·57*; 1·94–3·40 | 5·44*; 3·11–9·51 | 7·12*; 3·12–16·2 | 3·35*; 2·34–4·79 | 8·91*; 4·31–18·4 | 2·70*; 2·06–3·54 |
| eGFR <15 | 19·9*; 1·79–220 | NA | NA | 21·6*; 4·65–99·9 | 6·38*; 1·87–21·8 | 9·63*; 2·29–40·5 | 15·3*; 7·56–31·0 | 8·46*; 5·04–14·2 | 11·9*; 7·62–18·4 | 5·25*; 3·14–8·80 |
| Overall | Reference | 1·43*; 1·25–1·64 | 1·81*; 1·62–2·01 | 2·44*; 1·99–2·98 | ·· | Reference | 1·38*; 1·26–1·52 | 1·72*; 1·51–1·96 | 2·33*; 1·92–2·83 | ·· |
Data are hazard ratio; 95% CI. eGFR is mL/min per 1·73 m2. ACR and PCR are mg/g. Reference categories were eGFR 90–104 mL/min per 1·73 m2 and ACR <10 mg/g, PCR <15 mg/g, or a negative dipstick test for albuminuria. eGFR=estimated glomerular filtration rate. ACR=albumin-to-creatinine ratio. PCR=protein-to-creatinine ratio. NA=not applicable because no data.
p<0·05 relative to the reference category.
p<0·05 for interaction with diabetic status for the corresponding category.
In the 13 chronic kidney disease cohorts, 5960 ESRD events occurred during mean follow-up of 3·8 years (SD 3·2). When we set one reference in the no diabetes group, HR for incident ESRD were higher in participants with diabetes than in those without diabetes across the entire range of eGFR (figure 3A). When we set separate references in the diabetes and no diabetes groups to assess interaction with diabetes specifically, HR for low eGFR were much the same in participants with and without diabetes, showing no point-wise interaction (figure 3C). The overall interaction of diabetes across the entire range of eGFR was not significant for ESRD (relative HR 0·79; 95% CI 0·56–1·13; p=0·19). Variability of the interaction across studies was high (appendix p 7).
Figure 3. Hazard ratios for end-stage renal disease in the chronic kidney disease populations according to eGFR and ACR in participants with and without diabetes.

(A, B) eGFR. (C, D) ACR. Panels A and C use eGFR of 50 mL/min per 1·73 m2 (A) and ACR of 20 mg/g (C) in individuals without diabetes as the reference point (diamond) for both individuals with and without diabetes. Panels B and D use eGFR of 50 mL/min per 1·73 m2 (B) and ACR of 20 mg/g (D) as the reference points (diamond) in diabetic and non-diabetic groups. Blue and red circles denote p<0·05 as compared with the reference (diamond). Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative/ trace, 1+, 2+, ≥3+]) or eGFR. eGFR=estimated glomerular filtration rate. ACR=albumin-to-creatinine ratio.
Conversely, when we set one reference in the no diabetes group, HR for incident ESRD were generally higher in participants with diabetes than in those without diabetes for ACR values greater than 100 mg/g, but were generally lower in those with diabetes for ACR values less than 100 mg/g (figure 3C). Furthermore, when we set separate references in the diabetes and no diabetes groups to assess the interaction with diabetes specifically, the risk of ESRD increased slightly more steeply along with ACR in the diabetes group than in the no diabetes group, but we did not identify a significant point-wise interaction (figure 3D). The overall interaction of diabetes, averaged across the entire range of ACR, was not significant for ESRD (relative HR 1·08; 0·95–1·23; p=0·22). Moreover, heterogeneity of the interaction across studies was absent (appendix p 7). We recorded much the same results when we included cohorts that enrolled only individuals with diabetes or no diabetes (appendix p 8). When we analysed the general population and high-risk cohorts for ESRD, we recorded a steeper risk gradient, particularly for eGFR, in the no diabetes group than in the diabetes group (appendix p 9).
Table 3 shows pooled HR for ESRD for 20 categories of eGFR and albuminuria in participants with and without diabetes. Overall, we identified a significant interaction in only two of the 20 categories.
Table 3.
Hazard ratios for end-stage renal disease in chronic kidney disease populations according to eGFR and albuminuria clinical categories by the presence or absence of diabetes
| Individuals with diabetes
|
Individuals without diabetes
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ACR <30, PCR <50, or dipstick negative or trace | ACR 30–299, PCR 50–499, or dipstick 1+ | ACR 300–999, PCR 500–1499, or dipstick 2+ | ACR ≥1000, PCR ≥1500, or dipstick ≥3+ | Overall | ACR <30, PCR <50, or dipstick negative or trace | ACR 30–299, PCR 50–499, or dipstick 1+ | ACR 300–999, PCR 500–1499, or dipstick 2+ | ACR ≥1000, PCR ≥1500, or dipstick ≥3+ | Overall | |
| eGFR ≥75 | 1·47; 0·63–3·41 | 2·47*†; 1·29–4·73 | 3·43*†; 1·58–7·47 | 4·42*; 2·20–8·86 | 0·81†; 0·21–3·17 | 0·54; 0·07–4·05 | 0·68†; 0·38–1·20 | 0·74†; 0·33–1·63 | 1·59; 0·54–4·68 | 0·46*†; 0·28–0·75 |
| eGFR 45–74 | Reference | 1·76*; 1·05–2·95 | 2·84*; 1·11–7·27 | 8·01*; 3·62–17·7 | Reference | Reference | 1·69*; 1·23–2·32 | 2·85*; 1·22–6·66 | 3·93*; 2·78–5·55 | Reference |
| eGFR 30–44 | 2·11*†; 1·26–3·51 | 3·35*; 2·07–5·41 | 5·71*; 3·57–9·13 | 8·56*; 5·27–13·9 | 1·86*; 1·58–2·19 | 1·42†; 0·85–2·37 | 3·01*; 2·23–4·06 | 4·20*; 3·04–5·81 | 6·76*; 4·90–9·31 | 1·87*; 1·48–2·38 |
| eGFR 15–29 | 2·98*; 1·68–5·28 | 8·25*; 5·19–13·1 | 23·7*; 8·09–69·3 | 33·7*; 13·8–82·4 | 5·23*; 3·81–7·17 | 6·15*; 3·17–12·0 | 7·94*; 5·93–10·6 | 11·9*; 7·17–19·8 | 28·9*; 10·5–79·6 | 7·64*; 4·99–11·7 |
| eGFR <15 | 1·74; 0·23–13·1 | 34·7*; 4·21–285 | 122*; 4·64–3229 | 35·7*; 21·5–59·4 | 9·46*; 5·52–16·2 | 3·97*; 1·58–10·0 | 16·0*; 11·5–22·4 | 22·7*; 16·1–32·0 | 31·8*; 18·9–53·4 | 18·3*; 10·9–30·6 |
| Overall | Reference | 1·60; 0·85–3·02 | 3·55*; 2·89–4·36 | 6·79*; 4·36–10·6 | ·· | Reference | 1·86*; 1·32–2·62 | 2·70*; 1·78–4·08 | 5·56*; 3·44–9·00 | ·· |
Data are hazard ratio; 95% CI. eGFR is mL/min per 1·73 m2. ACR and PCR are mg/g. Reference categories were eGFR 45–74 mL/min per 1·73 m2 and ACR <30 mg/g, PCR <50 mg/g, or a dipstick test for albuminuria ≤trace.
p<0·05 relative to the reference category.
p<0·05 for interaction with hypertensive status for the corresponding category.
When stratified by hypertension, we identified significant interactions with eGFR, but not ACR, in individuals without hypertension for all-cause mortality (average relative HR for 15 mL/min per 1·73 m2 lower eGFR between diabetes and no diabetes 0·94; 95% CI 0·91–0·97; overall p for interaction=0·0003) and cardiovascular mortality (0·92; 0·86–0·99, overall p for interaction=0·038), showing that lower eGFR was more strongly associated with these outcomes in individuals without diabetes or hypertension. Figure 4 shows results for eGFR and all-cause mortality (appendix pp 10–14 show other outcomes and ACR results). When we tested the three-way interactions of diabetes*hypertension*kidney measures (eGFR or ACR), we did not identify significant interactions except for eGFR and all-cause mortality (p for three-way interaction=0·025). Analyses stratified by age (≥65 years vs <65 years) or sex showed much the same results across strata, with higher incidence rates for the three health outcomes in those with diabetes, but with no significant point-wise or overall interactions for each of the health outcomes within strata of age or sex (appendix pp 15–18). One exception was a higher HR of mortality for ACR recorded in individuals without diabetes than in those with diabetes in participants aged 65 years or older (overall relative HR for 10-fold higher ACR among individuals with diabetes vs those without diabetes was 0·90; 95% CI 0·86–0·96; p=0·0004, appendix p 16).
Figure 4. Hazard ratios for all-cause mortality according to eGFR in participants with and without diabetes in individuals with and without hypertension from the general population and high-risk cohorts.

(A, B) Individuals with hypertension. (C, D) Individuals without hypertension. Blue and red circles denote p<0·05 as compared with the reference (diamond). Significant interaction between diabetes and eGFR is shown by x signs. Hazard ratios were adjusted for age, sex, race, smoking, history of cardiovascular disease, serum total cholesterol concentration, body-mass index, and albuminuria (log albumin-to-creatinine ratio, log protein-to-creatinine, or categorical dipstick proteinuria [negative, trace, 1+, ≥2+])
Discussion
Individuals with diabetes have a higher risk of all-cause and cardiovascular mortality than do those without diabetes across the range of eGFR and ACR. However, relative risks of these health outcomes according to measures of kidney disease are much the same in individuals with and without diabetes. The use of clinically relevant cutoff points showed the importance of both eGFR and ACR with respect to each of these outcomes. When we did similar analyses for ESRD in chronic kidney disease cohorts, results were essentially the same. We did identify a slight eGFR-diabetes interaction with all-cause mortality in individuals without hypertension, suggesting that those with lower kidney function had a slightly higher relative risk of mortality in the absence of diabetes or hypertension. Taken together, these findings show the value of eGFR and albuminuria as predictors of these health outcomes and emphasise their importance as predictors in the presence or absence of diabetes. The accompanying article shows the relevance of these measures of chronic kidney disease in the presence or absence of hypertension.17
In the general population, eGFR and ACR are important correlates of all-cause and cardiovascular mortality outcomes and kidney outcomes.9,11,22–24 These findings were subsequently extended to individuals in a large, provincial registry13,25 and to individuals with kidney disease.12 In all of these studies, individuals with and without diabetes were assessed together.
Kidney disease is a well recognised and important complication of diabetes and diabetic kidney disease is one of the most common causes of chronic kidney disease, with nearly 25% of patients with chronic kidney disease affected by diabetes. Moreover, diabetic kidney disease has increased from 2·2% to 3·3% of the US population from 1998 to 2008, probably because of a concomitant increase in diabetes.26 In individuals with diabetes, both eGFR and albuminuria are important predictors of cardiovascular and kidney outcomes.27 Results of a study of 2420 Pima Indians with diabetes showed the added value of incorporation of albuminuria into prediction models of ESRD and death that included baseline eGFR; this is one of the few previous studies to jointly assess eGFR and ACR in a population with diabetes (panel).28
Associations between eGFR, albuminuria, and outcomes in the absence of diabetes are less well explored, despite the fact that non-diabetic kidney disease is nearly three-quarters of all chronic kidney disease.15 Although non-diabetic kidney disease can be classified as glomerular, vascular, tubulointerstitial, and cystic diseases, our diverse population probably also includes individuals with chronic kidney disease in association with hypertension, obesity, or smoking, in whom the disease is not well characterised.29 Data for progression and consequences of non-diabetic kidney disease are sparse, but were examined in 1666 patients without diabetes from the Modification of Diet in Renal Disease study with predominantly stage 2–4 chronic kidney disease; kidney failure was recorded frequently with long-term follow-up.30 Importantly, the competing risk of cardiovascular outcomes seemed to be lower than in studies that included individuals with diabetic kidney disease. In a sample of 2692 patients with chronic kidney disease from Japan, those with hypertensive and other nephropathies showed an increased risk of cardiovascular events and death compared with individuals with primary kidney disease.31 In 1094 patients with hypertensive nephropathy from the African American Study of Kidney Disease and Hypertension, baseline urinary PCR, but not baseline eGFR, was associated with cardiovascular outcomes.32 Thus, our study adds to existing work by explicitly showing the importance of abnormalities in eGFR and ACR even in the absence of diabetes. Importantly, the relations between eGFR and ACR clinical cutoff points and adverse outcomes were preserved when assessing individuals with and without diabetes separately.
Our findings suggest that several major health outcomes, including ESRD and death, are increased in individuals with chronic kidney disease, irrespective of the cause of their impaired kidney function. Although the absolute risks of ESRD, all-cause mortality, and cardiovascular mortality are higher in patients with chronic kidney disease and diabetes than in those without diabetes throughout the ranges of eGFR and ACR, the relative risks of these outcomes are much the same, and similar clinical thresholds for diagnosis and classification of chronic kidney disease can be used in individuals with and without diabetes. These findings show the importance of identification of abnormalities in kidney measures in patients even in the absence of diabetes.
Our data suggest that cardiovascular and all-cause mortality outcomes are an important source of mortality in patients, irrespective of whether or not they have diabetes, underscoring the importance of identifying and treating risk factors for cardiovascular disease in all patients with chronic kidney disease. Glycaemic control is an important component in the management of patients with diabetic kidney disease,33 although the HbA1c goal can be tailored based on individual patient needs. Control of hypertension and dyslipidaemia are crucial management issues in diabetic kidney disease, but are also important in those without diabetes. Additionally, vigilant monitoring of risk factors in patients with chronic kidney disease who do not have diabetes is an important implication of our work, because of the finding of increased cardiovascular mortality risk in individuals with chronic kidney disease who did not have either diabetes or hypertension.
Strengths of our study include a sample size of more than a million participants derived from 40 countries. We did a study-level meta-analysis with a harmonised analysis plan. Additionally, we used a new eGFR equation that improves risk prediction.16 In view of the breadth and depth of our study sample, our findings can be generalised to a wide range of clinical settings. Nevertheless, some limitations warrant mention. Black participants in our study were from the USA; Africa remains unrepresented. We did not use a centralised laboratory and assays, because this approach is not feasible for a study of this scope and magnitude. Serum creatinine was not standardised in all studies; however, we recorded much the same results when we limited our analysis to studies with serum creatinine measurements standardised to isotope dilution mass spectrometry (data not shown). We relied on single, spot urine samples instead of 24 h urine data. However, single spot urine samples are more predictive of outcomes than are 24 h collections in people with diabetic kidney disease.34 We defined diabetes with standard population-based definitions and were not able to differentiate type 1 from type 2 diabetes, although worldwide most diabetes is type 2. Information about diet and exercise were not included, and we did not consider detailed information on medication use. Our results are drawn primarily from observational studies; therefore we cannot infer causality or render specific treatment recommendations.
Supplementary Material
Panel.
Research in context
Systematic review
Sources of data for the Chronic Kidney Disease Prognosis Consortium are provided elsewhere.9–12,16 We searched PubMed with the terms “diabetes”, “non-diabetes”, “chronic kidney disease”, “mortality”, end-stage renal disease”, and “cardiovascular disease” and identified only a few studies focused on outcomes related to chronic kidney disease in individuals with diabetes26–28 and only three studies focused on chronic kidney disease in those without diabetes,30–32 even though individuals without diabetes make up nearly three-quarters of those with chronic kidney disease. However, the strength of the association in individuals with diabetes as compared with those without diabetes had not yet been assessed.
Interpretation
Our findings emphasise the importance of kidney disease as a predictor of clinical outcomes, even in the absence of diabetes.
Acknowledgments
The Chronic Kidney Disease Prognosis Consortium Data Coordinating Center is underpinned by a programme grant from the US National Kidney Foundation (NKF funding sources include Abbott and Amgen). Various sources (appendix pp 25–27) have supported enrolment and data collection including laboratory measurements, and follow-up in the collaborating cohorts of the Chronic Kidney Disease Prognosis Consortium.
Funding
US National Kidney Foundation.
Footnotes
Contributors
KM, MW, JC, PEdJ, and C-PW conceived the study concept and design. KM, JC, and the Chronic Kidney Disease Prognosis Consortium investigators and collaborators acquired the data. KM, MW, JC, and the Data Coordinating Center members analysed the data. All authors took part in the interpretation of the data. CSF, KM, MW, JC, and RGN, drafted the report, and all authors provided critical revisions for important intellectual content. All collaborators shared data and were given the opportunity to comment on the report. KM and JC obtained funding for Chronic Kidney Disease Prognosis Consortium and individual cohort and collaborator support is listed in appendix pp 25–27.
Chronic Kidney Disease Prognosis Consortium investigators and collaborators
Appendix pp 19–20 show study acronyms and abbreviations.AASK J Wright, L Appel, T Greene, B C Astor.ADVANCE J Chalmers, S MacMahon, M Woodward, H Arima. Aichi H Yatsuya, K Yamashita, H Toyoshima, K Tamakoshi. ARIC J Coresh, B C Astor, K Matsushita, Y Sang. AusDiab R C Atkins, K R Polkinghorne, S Chadban. Beaver Dam CKD A Shankar, R Klein, B E K Klein, K E Lee. Beijing Cohort H Wang, F Wang, L Zhang, L Zuo. British Columbia CKD A Levin, O Djurdjev. CARE M Tonelli, F M Sacks, G C Curhan. CHS M Shlipak, C Peralta, R Katz, L Fried. CIRCS H Iso, A Kitamura, T Ohira, K Yamagishi. COBRA T H Jafar, M Islam, J Hatcher, N Poulter, N Chaturvedi. CRIB M J Landray, J R Emberson, J N Townend, D C Wheeler. ESTHER D Rothenbacher, H Brenner, H Müller, B Schöttker. Framingham C S Fox, S-J Hwang, J B Meigs. Geisinger R M Perkins. GLOMMS-1 Study N Fluck, L E Clark, G J Prescott, A Marks, C Black. Gubbio M Cirillo. HUNT S Hallan, K Aasarød, C M Øien, M Radtke. IPHS F Irie, H Iso, T Sairenchi, K Yamagishi. Kaiser Permanente NW D H Smith, J W Weiss, E S Johnson, M L Thorp; KEEP A J Collins, J A Vassalotti, S Li, S-C Chen. KP Hawaii B J Lee. MASTERPLAN J F Wetzels, P J Blankestijn, A D van Zuilen; MDRD M Sarnak, A S Levey, V Menon. MESA M Shlipak, M Sarnak, C Peralta, R Katz, H J Kramer, I H de Boer. MMKD F Kronenberg, B Kollerits, E Ritz. MRC Older People P Roderick, D Nitsch, A Fletcher, C Bulpitt. MRFIT A Ishani, J D Neaton. NephroTest M Froissart, B Stengel, M Metzger, J-P Haymann, P Houillier, M Flamant. NHANES III B C Astor, J Coresh, K Matsushita. Ohasama T Ohkubo, H Metoki, M Nakayama, M Kikuya, Y Imai. Pima Indian R G Nelson, W C Knowler. PREVEND R T Gansevoort, P E de Jong, B K Mahmoodi, S J L Bakker. Rancho Bernardo S K Jassal, E Barrett-Connor, J Bergstrom. RENAAL H J Lambers Heerspink, B E Brenner, D de Zeeuw. Renal REGARDS D G Warnock, P Muntner, S Judd, W McClellan. Severance S H Jee, H Kimm, J Jo, Y Mok, J E Lim. STENO P Rossing, H-H Parving. Sunnybrook N Tangri, D Naimark. Taiwan GP C-P Wen, S-F Wen, C-K Tsao, M-K Tsai. ULSAM J Ärnlöv, L Lannfelt, A Larsson. ZODIAC H J Bilo, H Joosten, N Kleefstra, K H Groenier, I Drion. Steering Committee: B C Astor, J Coresh (Chair), R T Gansevoort, B Hemmelgarn, P E de Jong, A S Levey, A Levin, K Matsushita, C-P Wen, M Woodward. Data Coordinating Center: S H Ballew (Coordinator), J Coresh (Principal investigator), M Grams, B K Mahmoodi, K Matsushita (Director), Y Sang (Lead programmer), M Woodward (Senior statistician); administrative support: L Camarata, X Hui, J Seltzer, H Winegrad.
Conflicts of interest
KM’s institution has received grants from the US National Kidney Foundation, Amgen, and US National Institutes of Health. MW’s institution has received a grant from Servier; he has received consultancy fees from Roche and speaker’s fees from Sanofi and Servier. JCh’s institution has received grants from Servier; and he has received speaker’s fees from them. HJLH has served as a consultant for Abbott, Reata, Johnson & Johnson, and Vitae; and he has received speaker’s fees from Abbott. BJL has received travel expenses from Kidney Disease: Improving Global Outcomes. RMP has received grants from the US National Kidney Foundation. PR’s institution has received consultancy fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, and Novo Nordisk, and has received grants from Abbott, Novartis, Pfizer, and speaker’s fees from Abbott, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Lilly, and Novo Nordisk; he holds stock in Novo Nordisk. JAV has received consultancy fees from CTI Clinical Trial Services and Litholink Chronic Kidney Disease Advisory Board; his institution has received grants from the US National Institutes of Health; and he has received speaker’s fees from Gore Creative Technologies and Elsevier. JCo’s institution has received grants from the US National Kidney Foundation, US National Institutes of Health, and Amgen, and has received consultancy fees from Amgen and Merck. PEdJ’s institution has received a grant from the Dutch Kidney Foundation. CSF, HJGB, TS, MT, KY, C-PW and RGN declare that they have no conflicts of interest.
See Online for appendix
Contributor Information
Caroline S Fox, National Heart, Lung, and Blood Institute’s Framingham Heart Study and the Center for Population Studies Framingham, MA, USA; Division of Endocrinology, Brigham and Women’s Hospital and Harvard Medical School, Boston MA, USA.
Kunihiro Matsushita, John Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Mark Woodward, John Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; The George Institute for Glocal Health, University of Sydney, Sydney, NSW, Australia.
Henk JG Bilo, Department of Internal Medicine, Isala Clinics, Zwolle, Netherlands.
John Chalmers, The George Institute for Glocal Health, University of Sydney, Sydney, NSW, Australia.
Hiddo J Lambers Heerspink, Department of Clinical Pharmacology, University Medical Centre Groningen, University of Groningen, Groningen, Netherlands.
Brian J Lee, Kaiser Permanente Hawaii Region, Moanalua Medical Center, Honolulu, HI, USA.
Robert M Perkins, Nephrology Department, Geisinger Medical Center, Danville, PA, USA.
Peter Rossing, Steno Diabetes Center, Gentofte, Denmark; HEALTH, University of Aarhus, Aarhus, Denmark.
Toshimi Sairenchi, Department of Public Health, Dokkyo Medical University School of Medicine, Shimotugagun-Mibu, Japan; Ibaraki Health Plaza, Ibaraki Health Service Association, Mito, Japan.
Marcello Tonelli, Department of Medicine, University of Alberta, Edmonton, AB, Canada.
Joseph A Vassalotti, US National Kidney Foundation, New York, NY, USA.
Kazumasa Yamagishi, Ibaraki Health Plaza, Ibaraki Health Service Association, Mito, Japan; Department of Public Health Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan; Osaka Medical Centre for Health Science and Promotion, Osaka, Japan.
Josef Coresh, John Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Paul E de Jong, Department of Nephrology, University Medical Centre Groningen, University of Groningen, Groningen, Netherlands.
Chi-Pang Wen, China Medical University Hospital, Taichung, Taiwan; Institute of Population Health Science, National Health Research Institutes, Zhunan, Taiwan.
Robert G Nelson, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Phoenix AZ, USA.
References
- 1.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med. 2006;354:997–99. doi: 10.1056/NEJMp058318. [DOI] [PubMed] [Google Scholar]
- 2.Meguid El Nahas A, Bello AK. Chronic kidney disease: the global challenge. Lancet. 2005;365:331–40. doi: 10.1016/S0140-6736(05)17789-7. [DOI] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, et al. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 4.Hallan SI, Coresh J, Astor BC, et al. International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. J Am Soc Nephrol. 2006;17:2275–84. doi: 10.1681/ASN.2005121273. [DOI] [PubMed] [Google Scholar]
- 5.Chadban SJ, Briganti EM, Kerr PG, et al. Prevalence of kidney damage in Australian adults: the AusDiab kidney study. J Am Soc Nephrol. 2003;14(suppl 2):S131–38. doi: 10.1097/01.asn.0000070152.11927.4a. [DOI] [PubMed] [Google Scholar]
- 6.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371:2173–82. doi: 10.1016/S0140-6736(08)60952-6. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;(2 suppl 1):39. S1–266. [PubMed] [Google Scholar]
- 9.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79:1341–52. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 11.Gansevoort RT, Matsushita K, van der Velde M, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes in both general and high-risk populations. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. 2011;80:93–104. doi: 10.1038/ki.2010.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–40. doi: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tonelli M, Muntner P, Lloyd A, et al. Using proteinuria and estimated glomerular filtration rate to classify risk in patients with chronic kidney disease: a cohort study. Ann Intern Med. 2011;154:12–21. doi: 10.7326/0003-4819-154-1-201101040-00003. [DOI] [PubMed] [Google Scholar]
- 14.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291:844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 15.US Renal Data System. USRDS 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 16.Matsushita K, Mahmoodi BK, Woodward M, et al. Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 2012. 307:1941–51. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoodi BK, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012 doi: 10.1016/S0140-6736(12)61272-0. published online Sept 24. http://dx.doi.org/10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed]
- 18.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Coresh J, Greene T, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–72. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 20.Miller WG, Bruns DE, Hortin GL, et al. Current issues in measurement and reporting of urinary albumin excretion. Clin Chem. 2009;55:24–38. doi: 10.1373/clinchem.2008.106567. [DOI] [PubMed] [Google Scholar]
- 21.Thompson S, Kaptoge S, White I, Wood A, Perry P, Danesh J. Statistical methods for the time-to-event analysis of individual participant data from multiple epidemiological studies. Int J Epidemiol. 2010;39:1345–59. doi: 10.1093/ije/dyq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halbesma N, Kuiken DS, Brantsma AH, et al. Macroalbuminuria is a better risk marker than low estimated GFR to identify individuals at risk for accelerated GFR loss in population screening. J Am Soc Nephrol. 2006;17:2582–90. doi: 10.1681/ASN.2005121352. [DOI] [PubMed] [Google Scholar]
- 23.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol. 2009;20:1069–77. doi: 10.1681/ASN.2008070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishani A, Grandits GA, Grimm RH, et al. Association of single measurements of dipstick proteinuria, estimated glomerular filtration rate, and hematocrit with 25-year incidence of end-stage renal disease in the multiple risk factor intervention trial. J Am Soc Nephrol. 2006;17:1444–52. doi: 10.1681/ASN.2005091012. [DOI] [PubMed] [Google Scholar]
- 25.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423–29. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 26.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–39. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20:1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berhane AM, Weil EJ, Knowler WC, Nelson RG, Hanson RL. Albuminuria and estimated glomerular filtration rate as predictors of diabetic end-stage renal disease and death. Clin J Am Soc Nephrol. 2011;6:2444–51. doi: 10.2215/CJN.00580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levey AS. Clinical practice. Nondiabetic kidney disease. N Engl J Med. 2002;347:1505–11. doi: 10.1056/NEJMcp013462. [DOI] [PubMed] [Google Scholar]
- 30.Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. 2008;73:1310–15. doi: 10.1038/ki.2008.67. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama M, Sato T, Miyazaki M, et al. Increased risk of cardiovascular events and mortality among non-diabetic chronic kidney disease patients with hypertensive nephropathy: the Gonryo study. Hypertens Res. 2011;34:1106–10. doi: 10.1038/hr.2011.96. [DOI] [PubMed] [Google Scholar]
- 32.Norris K, Bourgoigne J, Gassman J, et al. Cardiovascular outcomes in the African American Study of Kidney Disease and Hypertension (AASK) Trial. Am J Kidney Dis. 2006;48:739–51. doi: 10.1053/j.ajkd.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 33.Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambers Heerspink HJ, Gansevoort RT, Brenner BM, et al. Comparison of different measures of urinary protein excretion for prediction of renal events. J Am Soc Nephrol. 2010;21:1355–60. doi: 10.1681/ASN.2010010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


