Abstract
The chromatin structure at a promoter can define how a gene is regulated. Studies of two yeast genes expressed in the G1 phase of the cell cycle, HO and CLN2, have provided important paradigms for transcriptional regulation. Although the SBF (Swi4/Swi6 Box Factor) transcription factor activates both genes, the chromatin landscapes that regulate SBF binding are different. Specifically, the CLN2 promoter is constitutively available for SBF binding, whereas HO has a complex two-step promoter in which chromatin changes in one region allow SBF to bind at a downstream location. These studies reveal the role of chromatin in defining the regulatory properties of promoters.
Key Terms: cell cycle, chromatin, promoter, gene activation
Transcriptional regulation of genes within the cell cycle
Chromatin structure has long been implicated in gene transcription regulation. Here we examine the regulation of HO and CLN2, two yeast genes expressed in G1 phase of the cell cycle. These two genes are activated by the same transcription factor, SBF, which is composed of two subunits, Swi4 and Swi6. HO encodes an endonuclease that cleaves at the MAT locus to initiate mating type interconversion. HO is tightly repressed, as inappropriate expression of an endonuclease could be toxic. The CLN2 gene encodes a G1 cyclin required for the G1/S cell cycle transition, and it should be expressed every cell cycle.
Although HO and CLN2 are activated by the same transcription factor, the regulatory properties of these two genes is totally different due to the chromatin structures of their promoters. The HO gene is difficult to activate because of its chromatin structure, whereas the chromatin structure at the CLN2 promoter ensures that the gene is reliably activated every cell cycle. We understand the molecular mechanisms which dictate how chromatin defines regulatory properties of these promoters, and these paradigms can provide insights for other regulatory systems.
Regulation of the HO gene
Chromatin has long been known to repress transcription, with transcriptional activators required to overcome this repression [1]. Genetic screens for regulators of HO expression identified important chromatin regulators such as SWI/SNF, Gcn5, and Sin3 [2–4]. Molecular studies have revealed a complex choreography of factors acting on chromatin at the HO promoter as a prelude to transcriptional activation [5, 6].
HO is under complex regulation, and the HO promoter incorporates motifs that produce three forms of transcriptional signals. (1) Binding sites spread throughout the HO promoter are recognized by the a1/α2 heterodimeric repressor, composed of subunits expressed from both the MATa and MATα alleles. This ensures that HO is expressed in haploid but not in diploid cells, as diploids have no need for mating type interconversion [7]. (2) Budding yeast divide asymmetrically, and binding sites for the Ash1 protein ensure that HO is not expressed in daughter cells following mitosis. This asymmetric expression of HO explains why only mother cells switch mating type. (3) HO is cell cycle regulated, being expressed only in late G1/early S phases after the commitment point for the cell cycle (Box 1).
The promoter for HO is large compared to the average yeast gene, with the nearest gene more than 3 kb upstream. Two major promoter regions have been identified: URS1 and URS2 (Fig 1). URS1 extends from 1000 to 1900 bases upstream of the HO transcriptional start site,, and URS2 from 200 to 900 bases upstream. URS1 contains two binding sites for the Swi51 transcription factor, which is required for HO expression. Swi5 is cell cycle regulated; it enters the nucleus and binds DNA after cells progress through anaphase in mitosis. Swi5 binds DNA only briefly, as it is rapidly degraded. Thus Swi5 is not present at the promoter at the time in G1/S when HO is transcribed. This suggests that although this DNA-binding factor may be required for gene activation, it may not be at the promoter at the time of transcription. Interestingly yeast contains another zinc finger protein, Ace2, that has a DNA-binding domain and DNA-binding specificity nearly identical to that of Swi5, and shows similar cell cycle regulation as Swi5 [8, 9], although Ace2 is present only in daughter cells [10]. However, although Ace2 does recognize sites in the HO promoter in vitro, it does not activate HO and does not bind to the HO promoter in vivo [8]. It is believed that HO chromatin is structured to prevent Ace2 from binding while still allowing Swi5 to bind, although the mechanism is unclear.
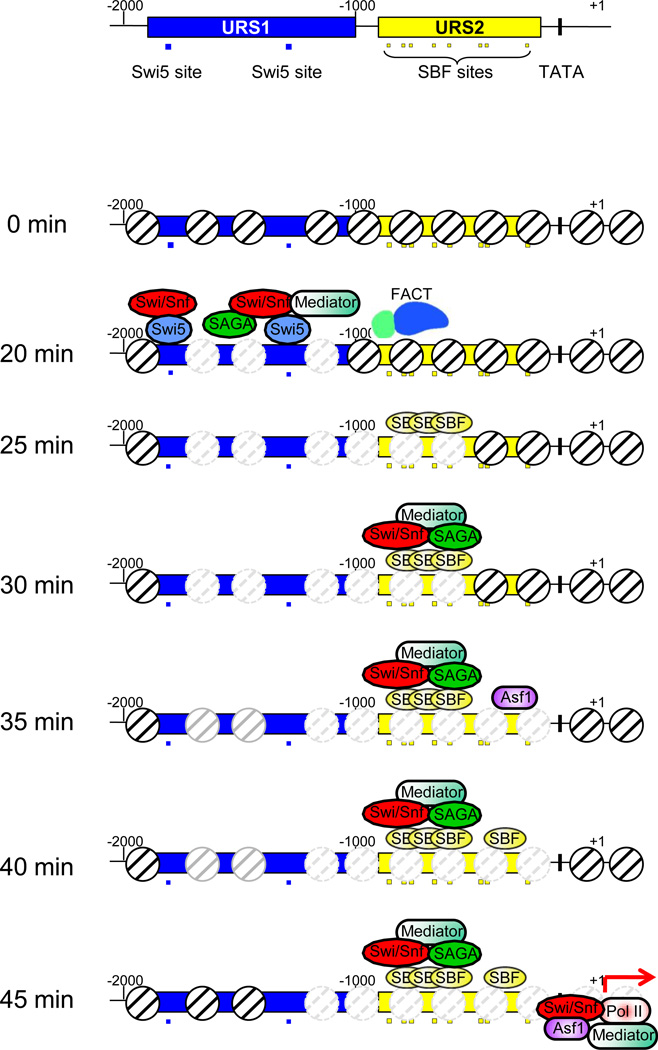
Fig. 1. Sequence of binding events at the HO promoter.
The top panel shows the structure of the HO promoter including the URS1 promoter element (blue), with two Swi5 binding sites (blue-filled squares), and the URS2 promoter element (yellow) with eight SBF binding sites (yellow-filled squares). The lower panels show nucleosome eviction events as well as binding by factors at time points following release from the G2/M arrest. The G2/M arrest is at 0 min, and at 25°C cells pass START, the commitment point for the G1/S transition, at 30–40 min following release. At 20 min Swi5 binds and recruits SWI/SNF, SAGA, and Mediator, leading to nucleosome eviction within URS1 (indicated by grey dashed-lined nucleosomes). FACT recruitment at 20 min facilitates nucleosome loss and SBF binding at 25 min at the upstream part of URS2, and subsequent recruitment of SWI/SNF, SAGA, and Mediator at 30 min. At 35 min Asf1-dependent nucleosome loss at the downstream end of URS2 permits subsequent SBF binding in this region. The partial repopulation of nucleosomes at URS1 is indicated by grey solid-lined nucleosomes. Finally, at 45 min the gene is transcribed.
URS2 contains eight binding sites for the SBF DNA-binding factor, which also activates CLN2. SBF is cell-cycle regulated in several ways. Phosphorylation by the Cdc28 Cyclin Dependent Kinase (CDK) regulates nuclear localization of Swi6, so that SBF can only bind DNA from early G1 through early S phase [11]. Additionally, SBF activity is inhibited during early G1 by two factors, Whi5 and Stb1, until phosphorylation by CDK terminates their inhibition, allowing SBF to then activate transcription [12–15]. There is also a second G1 specific factor, MBF, that does not regulate HO but does contribute to CLN2 activation [16].
Normally HO is expressed exclusively in mother cells because the Ash1 protein prevents HO expression in daughter cells [17]. ASH1 encodes a DNA-binding protein that recruits the Rpd3(L) histone deacetylase complex to promoters to repress transcription [18]. ASH1 is expressed transiently in late M phase, and ASH1 mRNA is transported to the bud tip in daughter cells where it is translated into protein. This mRNA transport results in a much higher concentration of Ash1 in daughters compared to mothers, effectively blocking HO expression. Ash1 is often described as the “daughter-specific” repressor, but this term is not fully accurate as Ash1 is not localized exclusively in daughter cells. Quantitation of Ash1 localization by immunofluorescence microscopy shows that Ash1 is present in both mother and daughter cells, but with substantially more protein present in daughters [19]. Ash1 may affect HO expression in mother cells, as an ash1 mutation results in increased frequency of mating type switching in mother cells [19] and an ash1 mutation allows HO expression in mothers in the absence of the normally required Gcn5 acetyltransferase [20]. Thus, Ash1 acts in both daughter and mother cells, but has a quantitatively more significant role in daughters. The high concentration of Ash1 in daughter cells effectively blocks HO expression, whereas in mother cells Ash1 merely contributes to making chromatin in the HO promoter repressive without precluding the possibility of expression, as described later. The genetic interactions between the Gcn5 acetyltransferase and Ash1, which recruits a deacetylase, suggest control of histone modification is critical in regulating HO expression.
Thus, HO has a complex promoter with binding sites for various factors to ensure appropriate expression at the correct time in the correct cells. However, the sequence-specific DNA-binding factors, alone, are not sufficient to overcome the repressive chromatin barriers, and additional coactivators are needed.
Chromatin factors regulate HO
In addition to the Swi5 and SBF sequence-specific DNA-binding factors, the SWI/SNF chromatin remodeling complex, the Gcn5 histone acetyltransferase, the Mediator complex, and the Rpd3(L) HDAC are also required for HO expression, demonstrating the importance of chromatin in regulation of HO. A landmark paper by Cosma et al [5] used cells synchronized within the cell cycle to show that DNA-binding and chromatin factors are sequentially recruited to distinct regions of the HO promoter. This study pioneered the use of chromatin immunoprecipitation (ChIP) assays to study events at a complex promoter, and developed the paradigm of sequential recruitment of factors that was subsequently observed for many other promoters [21, 22].
Swi5 binds to the promoter first and recruits SWI/SNF, which the Gcn5-containing SAGA (Spt-Ada-Gcn5-Acetyltransferase) complex, whose acetyltransferase activity permits the SBF activator to bind. Although this simple linear pathway of Swi5 → SWI/SNF → SAGA → SBF is attractive, subsequent studies showed that this model is not adequate [6, 20]. The original study used strains with an ash1 mutation, presumably to increase the sensitivity of the ChIP assay, allowing promoter events that occur in both mother and daughter cells to both contribute to the ChIP signal. Although daughter cells contain much more Ash1 than mothers, there is Ash1 protein in mothers, and the data suggest the absence of Ash1 affects the fine balance of chromatin acetylation at HO in mother cells [18, 20]. For example, in an ash1 mutant SWI/SNF recruitment to HO occurs despite a gcn5 mutation, but in an ASH1 strain with the wild type ASH1 gene Gcn5 is required for SWI/SNF recruitment. Thus, instead of the Swi5 → SWI/SNF → SAGA pathway seen in the ash1 mutant, in ASH1 strains SWI/SNF and SAGA are mutually dependent on one another for recruitment. Similarly, the original study placed SBF at the end of the pathway because disruption of the Swi6 subunit of SBF in an ash1 mutant strain did not affect recruitment of SWI/SNF or SAGA to URS2 [5]. By contrast, an experiment conducted in a swi6 ASH1 strain shows that SBF is required for SWI/SNF and SAGA recruitment to URS2 [18]. The presence of Ash1 therefore alters the mechanism of HO activation significantly: in an ash1 mutant SAGA is required to allow SBF to bind, while in the wild type strain SBF recruits SWI/SNF and SAGA to URS2.
Sequential changes at the promoter during the cell cycle
HO promoter activation involves sequential actions, first a set of contemporaneous events at URS1, followed by series of events at URS2. Not only are multiple DNA-binding and chromatin-remodeling factors important for regulation of HO expression in wild-type cells, but the order of recruitment is also crucially regulated (Fig 1; Supplemental Movie). In the G2 phase of the cell cycle nucleosomes are positioned at the promoter, such that the two Swi5 binding sites at −1800 and −1300 within nucleosome depleted regions [23, 24]. The SWI5 gene is transcribed in S phase and G2, but the protein is phosphorylated by the Cdc28 CDK during those phases, masking the NLS of Swi5 and resulting in its retention in the cytoplasm [25]. Entry into anaphase triggers release of the Cdc14 phosphatase which removes the inhibitory phosphorylations from the NLS of Swi5, allowing it to enter the nucleus and bind DNA [26]. Genetic evidence suggests that an interaction between the two Swi5 binding sites in the HO URS1 is required for gene activation [27]. Swi5 interacts directly with three chromatin complexes, SWI/SNF, SAGA, and Mediator, and recruits them to HO URS1 contemporaneously [6]. None of these chromatin complexes are bound at HO in a swi5 mutant, and therefore Swi5 is required for their recruitment. Eviction of nucleosomes from URS1 is also observed at this time when Swi5 binds. Mutant analysis shows that Swi5 and SWI/SNF are absolutely required for nucleosome loss, while gcn5 and gal11 mutations (affecting the SAGA and Mediator complexes, respectively) alter the kinetics of nucleosome eviction. ChIP assays show acetylation of H3 and H4 tails also occurs at this time. The acetylation might mark nucleosomes that are to be evicted soon, or it may reflect marking of nucleosomes neighboring the eviction site, as the resolution of the ChIP experiment was not high enough to distinguish occupying and nearby nucleosomes.
Interestingly, mutations in any one of the three coactivator complexes reduces binding of the other two complexes, indicating that coactivator binding is interdependent. Coactivator recruitment by Gcn4 and Adr1 has also been shown to be interdependent at the ARG1 and ADH2 loci [28, 29]. Finally, although Mediator is recruited to the HO promoter as both an early and a late event in promoter activation [30], and Mediator mutations reduce HO expression, Mediator’s exact mechanistic role is not clear.
At the same time as these events at URS1, the FACT (Facilitates Chromatin Transcription) histone chaperone is recruited to the upstream end of URS2, some 500 bp away [6]. FACT is able to modify nucleosome structure, but unlike classical remodelers, FACT changes nucleosomes in the absence of ATP [31]. FACT binding is very transient, and subsequently nucleosomes are depleted at the upstream end of URS2. Importantly, mutations in the FACT complex reduce HO expression and nucleosome eviction at URS2, suggesting that this nucleosome eviction at URS2 is necessary for HO expression. Slightly later in the cell cycle the Asf1 histone chaperone binds to the downstream end of URS2, and at about this time nucleosomes are evicted from this region, implicating that Asf1 evicts nucleosomes at the downstream end of URS2. An asf1 gene disruption results in decreased HO expression and in decreased nucleosome loss at the downstream end of URS2; nucleosome depletion at the upstream end of URS2 is not affected in the asf1 mutant, suggesting that nucleosome eviction events at the upstream and downstream ends of URS2 are independent. The use of two distinct histone chaperones at different times and different sites suggests a sequential modification of nucleosomes with distinct properties that require different methods to overcome each type of repression.
There are eight SBF binding sites within URS2, but SBF does not bind equally to all of these sites in vivo [32]. SBF first binds to the sites at the upstream end of URS2, and only later does it bind, much more weakly, to the downstream end. It appears that nucleosomes inhibit SBF binding, as mutations that reduce nucleosome eviction result in decreased SBF binding. In vitro DNA-binding experiments show SBF binds equally well to sites from the upstream and downstream ends of URS2, suggesting chromatin differences limit binding. This idea is supported by ChIP experiments showing much lower SBF occupancy at HO compared to other promoters. The sequential binding of SBF first to the upstream and then to the downstream sites in URS2 is because SBF nucleosomes are sequentially evicted from each region. The SWI/SNF, SAGA, and Mediator coactivators are recruited to URS2 by SBF, as disruption of the SWI6 gene encoding a SBF subunit eliminates coactivator recruitment. Importantly, the swi6 mutation does not affect coactivator recruitment to the distal URS1 region, and thus events at URS1 are independent of those at URS2, once again supporting a sequential, step-wise removal of chromatin barriers. One might think that the chromatin coactivators recruited by SBF facilitate nucleosome eviction from upstream URS2; however the eviction occurs before the coactivators are recruited (Fig 1). Genetic experiments suggest that the chromatin changes at URS1 facilitate FACT binding to upstream URS2, producing the nucleosome eviction; the underlying mechanisms here remain a major unanswered question.
Many studies have demonstrated a role for FACT in transcriptional elongation, but at HO URS2 FACT functions to promote nucleosome eviction as a prelude to transcription initiation. FACT does not contain a sequence-specific DNA-binding domain, so a major question is how FACT is recruited specifically to the upstream end of HO URS2. Genetic analysis shows that chromatin reorganization at URS1 is required for FACT to bind at upstream end of URS2, as swi5 and swi2 mutations affecting URS1-specific transcription factor and SWI/SNF, respectively, eliminate FACT recruitment [6]. Although SWI/SNF is recruited to upstream end of URS2, this occurs subsequent to FACT binding, and thus FACT recruitment requires SWI/SNF actions in URS1. FACT binding is delayed in strains with gal11 or gcn5 mutations affecting Mediator or SAGA, suggesting that these factors contribute to FACT recruitment. Biochemical experiments show that FACT interacts directly with the Swi6 subunit of SBF, providing a DNA-binding protein that can recruit FACT. However, a swi6 mutation results in only a modest decrease in FACT binding, suggesting that other factors or events at HO contribute to FACT recruitment. Thus, FACT recruitment depends upon both SBF binding as well as SWI/SNF-dependent changes at URS1.
A recent report using microcopy to examine HO expression in single cells demonstrated that mutations affecting the SWI/SNF chromatin remodeler and the Gcn5 histone acetyltransferase do not affect the firing amplitude of the HO promoter [33]. Instead, in these mutants there are two populations of cells; most of the cells do not express HO, and a small fraction express HO at wild type levels. Additionally, certain mutants exhibit exhibits short-term epigenetic memory of HO expression that persists through mitosis.
In summary, HO has an exceedingly complex promoter, with waves of nucleosome eviction occurring along the promoter as the cell cycle progresses. Nucleosomes are lost from four distinct regions of the promoter, and distinct factors and events are required at these regions. SWI/SNF might contribute to all of these nucleosome eviction events, as a swi2 mutation blocks the subsequent binding of FACT and Ash1 and also transcriptional activation. HO functions as a two step promoter: chromatin events at URS1 are required for the subsequent events at URS2, and then transcription is activated by SBF bound at URS2. Finally, HO expression is dependent on SAGA. Interestingly, HO expression is also reduced in taf (TBP-associated factor) mutants, which is unusual as most genes can be classified as either TAF-dependent or SAGA-dependent, in terms of their regulatory properties, once again underlining the stringent nature of the regulation of HO [34].
HO Expression is regulated by TBP binding
Several observations suggest that HO activation is limited by the TATA-binding protein (TBP) binding weakly to the promoter. The TBP site identified at HO (TCTAAATG) has a mismatch from a generous consensus that allows degenerate nucleotides at three of the eight positions [35], and its location within a nucleosome may make it difficult for TBP to bind. The SAGA complex proteins Spt3 and Spt8 interact directly with TBP [36], and Spt3 has been shown to activate transcription at some promoters by stimulating TBP binding [37, 38]. In contrast, Spt3 functions as a negative regulator at HO, as an spt3 mutation allows HO expression despite mutations in SWI/SNF remodeler components or the Gcn5 histone acetyltransferase, which would normally block HO expression [20, 39]. This suggests that SWI/SNF and Gcn5 function at HO, at least in part, to overcome the repression by Spt3. Gcn5 and Spt3 are both part of SAGA, and thus two proteins in the same regulatory complex can have opposing roles in transcriptional regulation. HO expression and TBP binding are both very brief during the cell cycle, but an spt3 mutation results in prolongation of both HO expression and TBP binding [39]. The importance of TBP binding is also supported by experiments with specific alleles of TBP and Spt3 that show allele-specific suppression [39]. HO expression was reduced in a gcn5 mutant, but the gcn5 defect in HO expression was largely suppressed by a TBP point mutation that reduces physical interaction with the Spt3 subunit of SAGA [40]. Importantly, an additional mutation in Spt3 that restores binding to TBP also eliminates HO expression in the Gcn5 mutant [39]. These results, along with ChIP data, support the hypothesis that Spt3 inhibits TBP binding to HO. Additionally, HO is not expressed in a swi2 SWI/SNF mutant, but this defect can be partially suppressed by a V71E substitution in TBP [20]. In summary, the HO promoter is regulated by having a suboptimal TATA site coupled with several mechanisms for discouraging stable binding of the TFIID complex containing TBP and Tafs; all of these must be overcome to achieve transcription.
Sequential recruitment of the Rpd3(L) histone deacetylase to the promoter
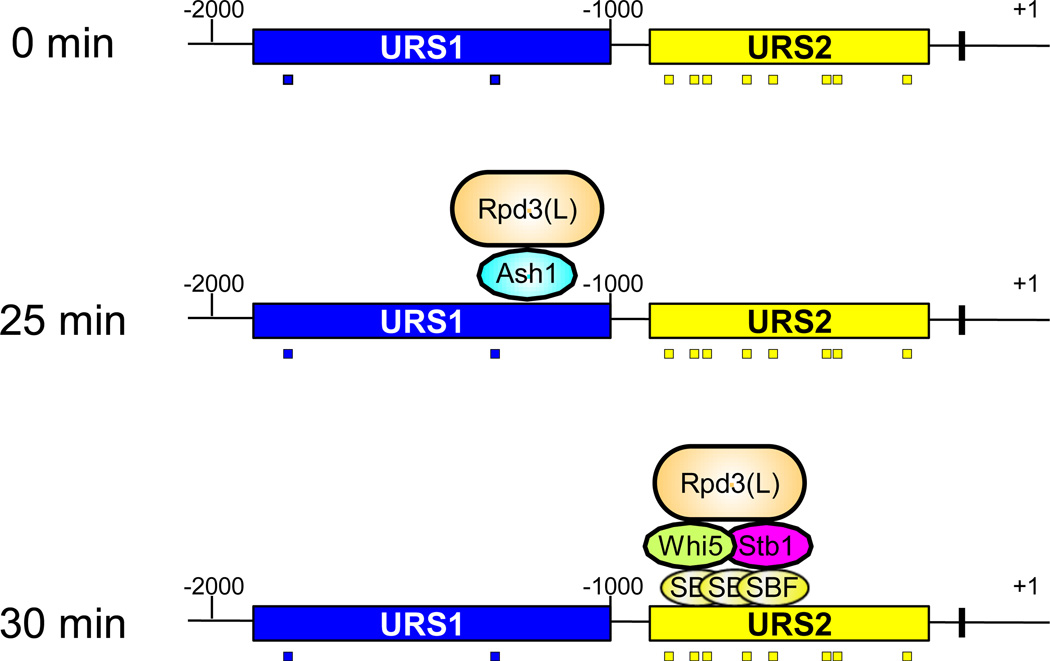
The Rpd3(L) histone deacetylase represses HO transcription, as an rpd3 mutation allows HO to be expressed in the absence of either the Swi5 DNA-binding protein or the Gcn5 histone acetyltransferase. The Rpd3(L) complex is recruited to the HO promoter twice during the cell cycle: first to URS1 by the Ash1 DNA-binding protein, and subsequently to URS2 by SBF [18] (Fig 2). SBF interacts with Rpd3(L) via two intermediary proteins, Whi5 and Stb1 [18, 32, 41, 42]. Rpd3(L) is recruited independently to these two promoter regions [18]. Rpd3(L) functions to repress transcription, and its recruitment to two promoter regions by different factors emphasizes the strong repression at HO.
Fig. 2. Rpd3(L) is recruited twice to the HO promoter during the cell cycle.
The Rpd3(L) histone deacetylase is first recruited to HO URS1 by Ash1, at about 25 min after release from G2/M arrest. Later in the cell cycle, Rpd3(L) is recruited to the URS2 region of the HO promoter by the SBF DNA-binding factors and the intermediary proteins Whi5 and Stb1. There is no evidence that Rpd3(L) shifts its position along the promoter or is handed from one factor to another. It is more likely that Rpd3(L) binding at URS1 ends when Ash1 is degraded, and that a new molecule of Rpd3(L) is recruited by SBF/Whi5/Stb1. The HO promoter is labeled as described in Fig 1.
Ash1 binds only at URS1 and an ash1 mutation results in increased binding of SWI/SNF and Mediator to URS1. An ash1 mutation also suppresses the defect in SWI/SNF binding caused by a gcn5 mutation.
An ash1 mutation also has marked effects on events at URS2 and the TATA box, which are distant from the Ash1 binding site at −1250. There is increased binding of SBF to URS2 in the ash1 mutant, as well as increased recruitment of SWI/SNF and Mediator. A swi2 mutation results in a defect in SBF binding to URS2, but this defect is suppressed by an ash1 mutation. This suggests that chromatin inhibits SBF from binding at URS2, and that SBF binding is regulated in an opposing fashion by SWI/SNF and Ash1, despite the fact that the Ash1 and SBF binding sites are separated by 500 bp. Finally, it is worth noting that Rpd3(L) can also act globally, without being targeted by specific DNA-binding proteins [43].
Binding of the Ash1 repressor occurs after URS1 activation events
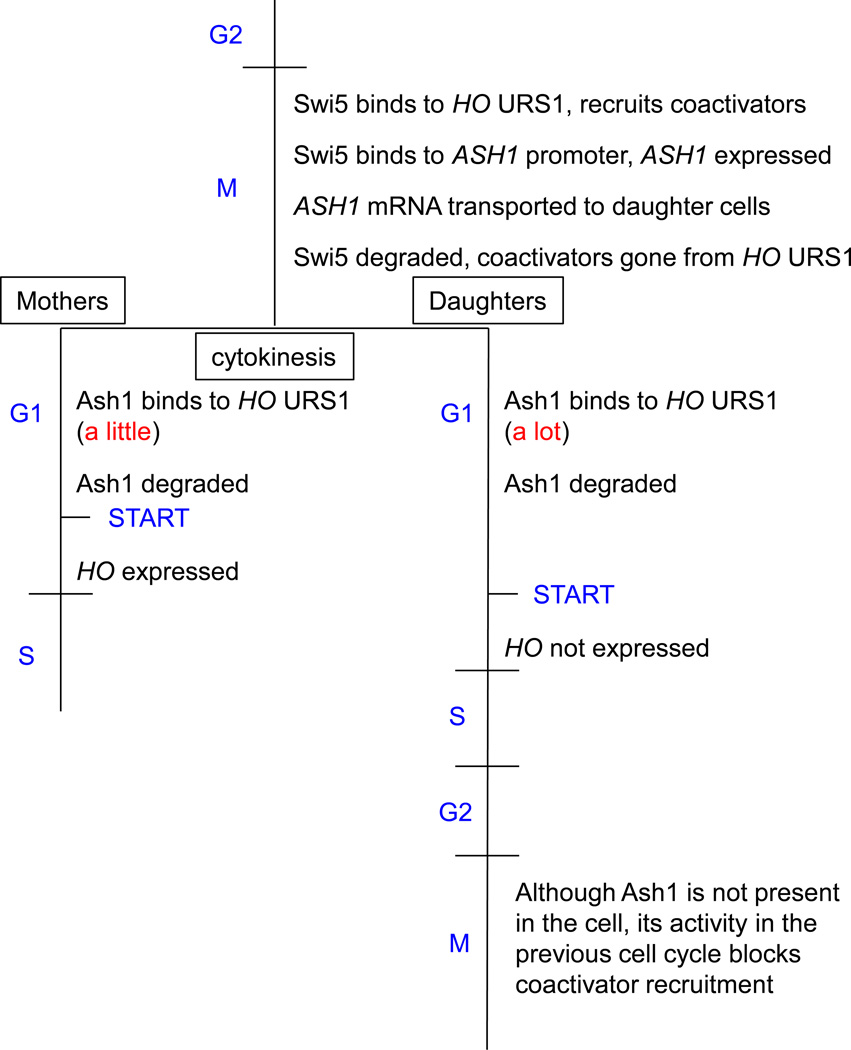
Ash1 is cell cycle regulated, and the timing of its expression raises major conceptual issues regarding how it has such a profound effect on HO expression. ASH1 is activated by the Swi5 transcription factor, which also initiates the events leading to HO transcription. Swi5 enters the nucleus in late M/early G1 and binds to both the ASH1 and HO promoters. SWI/SNF, SAGA, and Mediator are recruited to HO URS1 by Swi5 at this time. Swi5 also promotes ASH1 expression, and the newly transcribed ASH1 mRNA is transported mostly to daughter cells and translated: Ash1 protein then enters the (primarily daughter cell) nucleus [17]. Ash1 inhibits SWI/SNF and Mediator binding to URS1, yet ChIP experiments show that Ash1 binds to HO URS1 only after Swi5 and the coactivators have already left the scene [18]. Ash1 is an unstable protein and is present only briefly during the cell cycle [18, 44]. Ash1 inhibits SWI/SNF and Mediator binding to URS1, but Ash1 is present in the nucleus only after these coactivators have left HO URS1. Thus, Ash1 must have had its effect during the previous cell cycle. Ash1 most likely affects subsequent gene activation by recruiting the Rpd3(L) histone deacetylase, and these modifications likely persist through the next cell cycle to affect binding of SWI/SNF and Mediator. This provides an interesting model to study epigenetic control of transcription.
Regulation of CLN2
The CLN2 G1 cyclin gene is important for cell cycle progression and has been studied extensively. Although the mechanism controlling CLN2 expression was at one time controversial [45, 46][47, 48], work by the Cross lab was able to establish conclusively that a positive feedback loop regulates CLN2 expression [49]. This single cell microscopic method has been recently used to characterize how CLN2 expression is activated by the SBF DNA-binding transcription factor [50]. This is the same SBF factor that functions at HO, but the regulation of these two genes is very different. The CLN2 promoter is free of nucleosomes to ensure that the gene is efficiently expressed in every cell cycle, while the chromatin at HO makes the gene very difficult to activate.
Chromatin determines the probability of CLN2 activation
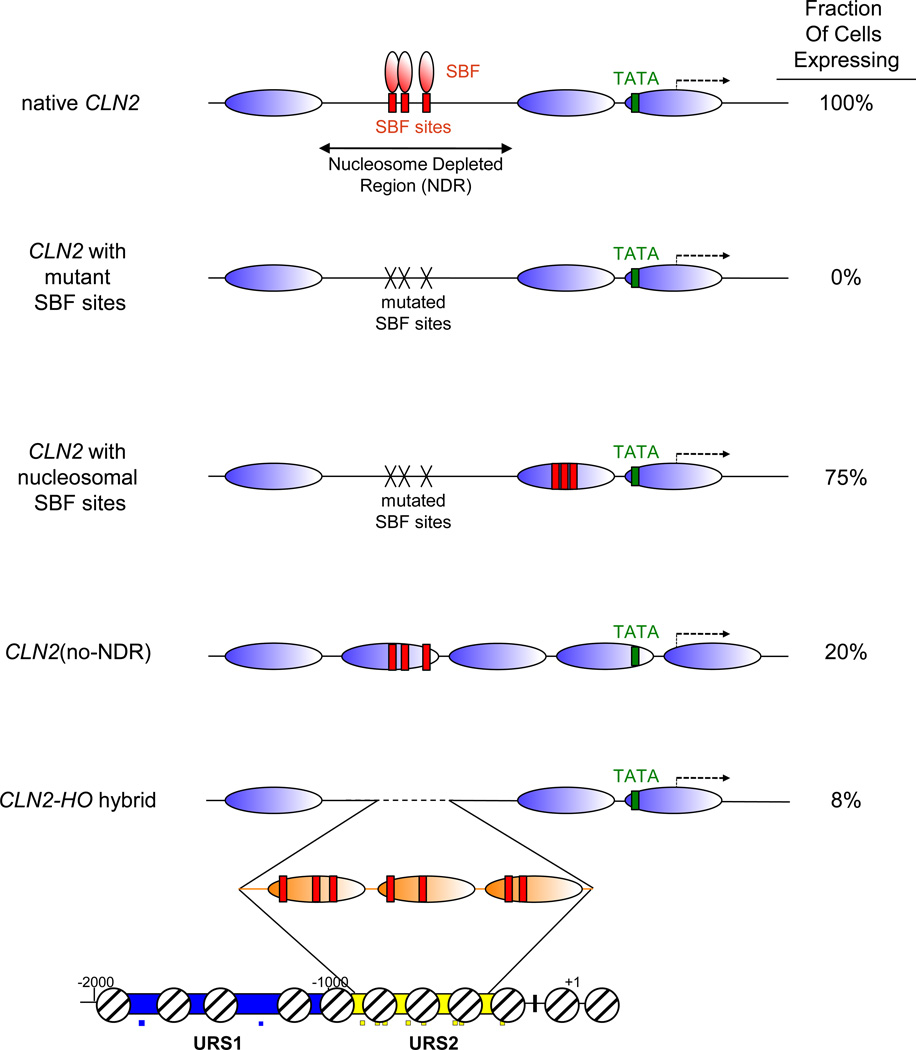
Using a destabilized GFP reporter under the control of the CLN2 promoter to determine when CLN2 is expressed, Bai et al. [50] found that CLN2-GFP was reliably expressed every cell cycle. The three SBF sites in the CLN2 promoter lie within a 300 bp nucleosome depleted region (NDR), and as expected CLN2 expression is eliminated by mutations in these SBF sites (Fig 3). When the authors reintroduced new SBF sites so that they were present under a nucleosome, they found that the resulting promoter had markedly different properties. The promoter was activated in only 75% of cell cycles, while the native promoter is expressed in 100% of cell cycles. Importantly, the chromatin environment of the biding site did not affect the level of expression, and thus the chromatin environment of the binding site affects the probability that the gene would be activated in any individual cell cycle. These results suggest that nucleosomes can limit the accessibility of a transcription factor to bind and thus the likelihood of gene activation.
Fig. 3. Ash1 repression at URS1 persists from the previous cell cycle.
During G2 phase Swi5 binds to HO URS1, recruiting coactivators that alter URS1 chromatin. Concurrently, Swi5 binds to the ASH1 promoter, and the expressed ASH1 mRNA is transported to daughter cells and translated. Swi5 is quickly degraded ending the coactivator presence at URS1. Ash1 binds to URS1 after cytokinesis, and is quickly degraded. Thus, the effects of Ash1 revealed through genetic analysis, occur in the second M phase, whereas Ash1 was bound to the promoter much earlier in the cell cycle. START, in late G1, refers to the commitment point for the G1/S transition
Recent work addresses the question of what generates the NDR at CLN2 [51]. In addition to SBF sites, the CLN2 NDR contains evolutionarily conserved binding sites for other factors, including sites for Reb1, Mcm1, and Rsc3. Mutating all of these binding sites eliminated the NDR, leading to the expected decrease in SBF occupancy. Unlike the native CLN2 promoter which is expressed every cell cycle, this CLN2(no-NDR) promoter is unreliably expressed, with transcriptional activation occurring in about 20% of cell cycles (Fig 3). It is not clear why CLN2 with nucleosomal SBF sites (Fig 3, line 3) is expressed more efficiently than the CLN2(no-NDR) derivative (Fig 3, line 4). It could because one promoter has the SBF sites closer to the TATA, the different placement of the TATA, or the stability of these nucleosomes in vivo.
Comparing HO and CLN2
The CLN2 and HO promoters both contain SBF sites, but the SBF sites in CLN2 are in an NDR while those at HO are covered by nucleosomes. ChIP experiments show much more SBF binding to the CLN2 promoter than to the HO promoter, suggesting that SBF has difficulty binding to nucleosome-occupied sites [18]. A hybrid CLN2-HO promoter was constructed in which a 534 bp region of HO URS2, including 8 SBF binding sites, was inserted into a CLN2 promoter lacking its native SBF sites [50]. The SBF sites in this hybrid promoter are covered by nucleosomes, and this CLN2-HO hybrid promoter drove expression in only 8% of cell cycles. This was much less than was seen for the CLN2(no-NDR) promoter, suggesting that the nucleosomes at HO URS2 create a stronger barrier for SBF binding than those at the CLN2(no-NDR) promoter (Fig 3). The Swi4 subunit is the limiting factor in SBF, and increasing expression of Swi4 resulted in an increased fraction of cells expressing the CLN2-HO hybrid promoter. This result is consistent with the model that repressive nucleosomes covering HO URS2 make it difficult for SBF to bind and induce gene activation.
The CLN2 and HO promoters show regulatory differences, presumably because of the differences in nucleosome occupancy of the SBF binding sites, which affects the amount of SBF that binds. Although SBF recruits SWI/SNF and Gcn5/SAGA to both the HO and CLN2 promoters, the two genes have different coactivator requirements for activation. HO expression requires the SWI/SNF chromatin remodeler and the Gcn5 histone acetyltransferase, while CLN2 expression is largely unaffected by swi2 or gcn5 mutations. The difference is that CLN2 has a simple promoter, where SBF binds within an NDR, whereas for the HO promoter the SBF binding sites are embedded within extremely repressive chromatin. HO is a two step promoter, where chromatin events at URS1 are required to evict nucleosomes at URS2 to permit SBF to bind; the chromatin changes at URS1 require SWI/SNF and SAGA.
Concluding Remarks
Although the studies of HO and CLN2 have provided important insights into gene regulation, there remain important questions about how chromatin determines regulation of these genes. It is not understood how factors binding at CLN2 create an NDR, and why HO nucleosomes are much more repressive than nucleosomes from the CLN2(no-NDR) mutant promoter. HO activation requires two distinct histone chaperones at different times and different promoter positions, suggesting these nucleosomes have different properties. Further work is needed to explain why unlocking these nucleosomes to permit eviction requires different machinery. HO clearly functions as a multistep promoter, but we do not understand how chromatin events at URS1 cause subsequent changes at the distal URS2 promoter element, including recruitment of FACT. Similarly, it is not clear how promoter binding by the Ash1 repressor can affect SBF binding to sites 500 bp away. The genetics suggest that histone acetylation plays a critical role in the regulation of HO expression, but this is not understood at the mechanistic level. Although both are expressed at the same time in the cell cycle, Cln2 and Ho have different cellular roles: Cln2 is a G1 cyclin that promotes cell cycle progression, whereas Ho is an endonuclease whose expression is tightly restricted. The CLN2 promoter ensures that the promoter fires each cell cycle while HO has multiple layers of chromatin with different properties, making accidental activation highly improbable and allowing better control over intentional activation. Based on previous work in the transcription field, it seems likely that regulatory paradigms such as these, found in yeast, will also be found to contribute significantly to the regulation of gene expression in metazoans. The same transcriptional machinery is used in all eukaryotes, and there are the same types of chromatin modifications and positioning. Thus far, only in yeast has it been possible to dissect out the molecular mechanisms at complex promoters to understand how chromatin can make one promoter activated efficiently every cell cycle while for another activation requires specific circumstances. It seems probable that NDRs and repressive chromatin will be identified as important regulatory features in promoters in metazoans.
Supplementary Material
Fig. 4. Nucleosomes from the HO promoter are very repressive when placed at CLN2.
The native CLN2 promoter has SBF binding sites within a nucleosome depleted region, and the gene is expressed in 100% of cell cycles (top line). Mutating the SBF sites eliminates expression (line 2), while replacing the SBF sites, but within a nucleosomal context, reduces the fraction of cell cycles in which the CLN2 promoter fires (lines 3 and 4). Inserting a region of the HO promoter with SBF binding sites (indicated by orange nucleosomes) into CLN2 promoter lacking its SBF sites results in a hybrid promoter that expresses in very few cell cycles (line 4). The region of the HO promoter where the DNA with these three nucleosomes originates is indicated.
Highlights.
The yeast HO and CLN2 genes are both activated by the SBF factor and expressed in G1 phase of the cell cycle.
Activation of HO requires multiple activators to sequentially evict nucleosomes from distinct promoter regions in a multi-step promoter.
The CLN2 promoter has SBF binding sites in a nucleosome depleted region, ensuring reliable expression in each cell cycle.
The transcriptional regulation of these two genes illuminate how chromatin structure can define regulatory properties of promoters.
ACKNOWLEDGMENTS
This work was supported by NIH Grant GM039067.
GLOSSARY
- Swi5
A zinc finger protein that functions as a sequence-specific DNA-binding protein. Swi5 is present in the nucleus briefly in late G1 and early G1. It binds to sites within HO URS1, but HO expression occurs only later in the cell cycle
- SBF
A sequence-specific DNA-binding factor composed of two subunits, Swi4 and Swi6. The Swi6 subunit is present in the nucleus only bind DNA from early G1 through early S phase. SBF is inactive as a transcriptional activator during much of this time, due to interactions with the Whi5 and Stb1 proteins that recruit the Rpd3(L) histone deacetylase. The Cyclin Dependent Kinase relieves this inhibition as cells progress past START, the commitment point for the G1/S transition
- MBF
A transcription factor related to SBF. MBF contains two subunits, Mbp1 and Swi6. Mbp1 is inhibited by the Nrm1 factor, instead of by Whi5 and Stb1. MBF and SBF both activate CLN2 expression, but MBF does not activate HO
- SWI/SNF
A multiprotein complex that contains an ATP-dependent chromatin remodeler that repositions nucleosomes in vivo. Mutations in SWI/SNF subunits reduce HO expression and prevent nucleosome eviction at the HO promoter. Swi5 interacts directly with SWI/SNF and recruits it to URS1. SBF recruits SWI/SNF to HO URS2, but it has not been determined whether this is a direct interaction
- SAGA
A multiprotein complex that contains an acetylation module with the Gcn5 histone acetyltransferase. Mutations in GCN5 reduce HO expression. SAGA contains other modules including the Spt3/Spt8 module that interacts with TBP, a histone deubiquitinase module, and an architectural module that includes proteins that interact with activation domains. Swi5 interacts directly with SAGA and recruits it to URS1. SBF recruits SAGA to HO URS2, but it has not been determined whether this is a direct interaction
- Mediator
A multiprotein complex that interacts with both transcriptional activators and the basal transcription machinery. The major function of Mediator is to help recruit RNA pol II to promoters. A mutation affecting the Gal11 subunit of Mediator reduces HO expression. Swi5 interacts directly with Mediator and recruits it to URS1. SBF recruits Mediator to HO URS2, but it has not been determined whether this is a direct interaction
- TAFs
TAFS are TBP Associated Factors, subunit of the TFIID multiprotein complex that contains TBP (TATA Binding Protein). Activation of some promoters requires TBP, alone, while others require TBP in the context of the larger TFIID complex. The fact that HO expression is reduced in a TAF mutant demonstrates that HO activation requires TFIID
- FACT
FACT is chromatin reorganizer, meaning it can modify nucleosome structure; unlike classical remodelers, FACT changes nucleosomes in the absence of ATP. FACT is composed of two subunits, Spt16 and Pob3, and its activity is supported by the small HMG box protein Nhp6. FACT is also described as a histone chaperone, a protein that shields histones from inappropriate interactions and facilitates nucleosome assembly
- Asf1
Asf1 is a histone chaperone that specifically interacts with H3-H4 dimers
- Rpd3(L)
The Rpd3(L) complex contains the Rpd3(L) histone deacetylase, the Sin3 structural component, as well as other subunits that allow it to interact specifically with sequence-specific DNA-binding proteins
- Whi5 and Stb1
Whi5 and Stb1 both function as intermediaries between SBF and Rpd3(L), allowing SBF to recruit Rpd3(L) to promoters. Phosphorylation of Whi5 and Stb1 by CDK ends this interaction and allows transcriptional activation by SBF
- Ash1
Ash1 is a sequence-specific DNA-binding protein that transiently interacts with Rpd3(L) and recruits it to promoters
- Spt3
Spt3 is a subunit of the SAGA complex that, with Spt8, interacts directly with TBP. Although Spt3/8 stimulates TBP binding at many promoters, at HO Spt3/8 inhibit TBP binding and thus limit the duration of promoter activation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
In yeast, the wild type gene is upper case and italicized, i.e. SWI5. A mutant gene is lower case and italicized, i.e. swi5. A protein is not italicized, with only the first character in upper case, i.e. Swi5.
REFERENCES
- 1.Li B, et al. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Peterson CL, Herskowitz I. Characterization of the yeast SWI1, SWI2 and SWI3 genes, which encode a global transcriptional activator of transcription. Cell. 1992;68:573–583. doi: 10.1016/0092-8674(92)90192-f. [DOI] [PubMed] [Google Scholar]
- 3.Breeden L, Nasmyth K. Cell cycle control of the yeast HO gene: cis-and trans-acting regulators. Cell. 1987;48:389–397. doi: 10.1016/0092-8674(87)90190-5. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, et al. The Saccharomyces cerevisiae SIN3 gene, a negative regulator of HO, contains four paired amphipathic helix motifs. Mol. Cell. Biol. 1990;10:5927–5936. doi: 10.1128/mcb.10.11.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cosma MP, et al. Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell. 1999;97:299–311. doi: 10.1016/s0092-8674(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 6.Takahata S, et al. FACT and Asf1 regulate nucleosome dynamics and coactivator binding at the HO promoter. Mol. Cell. 2009;34:405–415. doi: 10.1016/j.molcel.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathias JR, et al. Repression of the yeast HO gene by the MATalpha2 and MATa1 homeodomain proteins. Nucleic Acids Res. 2004;32:6469–6478. doi: 10.1093/nar/gkh985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Voth WP, et al. Forkhead proteins control the outcome of transcription factor binding by antiactivation. EMBO J. 2007;26:4324–4334. doi: 10.1038/sj.emboj.7601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sbia M, et al. Regulation of the yeast Ace2 transcription factor during the cell cycle. J. Biol. Chem. 2008;283:11135–11145. doi: 10.1074/jbc.M800196200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazanka E, et al. The NDR/LATS family kinase Cbk1 directly controls transcriptional asymmetry. PLoS biology. 2008;6:e203. doi: 10.1371/journal.pbio.0060203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sidorova JM, et al. Cell cycle-regulated phosphorylation of Swi6 controls its nuclear localization. Mol. Biol. Cell. 1995;6:1641–1658. doi: 10.1091/mbc.6.12.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costanzo M, et al. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell. 2004;117:899–913. doi: 10.1016/j.cell.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 13.de Bruin RA, et al. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell. 2004;117:887–898. doi: 10.1016/j.cell.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Costanzo M, et al. G1 transcription factors are differentially regulated in Saccharomyces cerevisiae by the Swi6-binding protein Stb1. Mol. Cell. Biol. 2003;23:5064–5077. doi: 10.1128/MCB.23.14.5064-5077.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Bruin RA, et al. Stb1 collaborates with other regulators to modulate the G1-specific transcriptional circuit. Mol. Cell. Biol. 2008;28:6919–6928. doi: 10.1128/MCB.00211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris MR, et al. Binding specificity of the G1/S transcriptional regulators in budding yeast. PloS one. 2013;8:e61059. doi: 10.1371/journal.pone.0061059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cosma MP. Daughter-specific repression of Saccharomyces cerevisiae HO: Ash1 is the commander. EMBO Rep. 2004;5:953–957. doi: 10.1038/sj.embor.7400251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahata S, et al. Repressive Chromatin Affects Factor Binding at Yeast HO (Homothallic Switching) Promoter. J. Biol. Chem. 2011;286:34809–34819. doi: 10.1074/jbc.M111.281626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sil A, Herskowitz I. Identification of an asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell. 1996;84:711–722. doi: 10.1016/s0092-8674(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 20.Mitra D, et al. SWI/SNF binding to the HO promoter requires histone acetylation and stimulates TATA-binding protein recruitment. Mol. Cell. Biol. 2006;26:4095–4110. doi: 10.1128/MCB.01849-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cosma MP. Ordered recruitment: gene-specific mechanism of transcription activation. Mol. Cell. 2002;10:227–236. doi: 10.1016/s1097-2765(02)00604-4. [DOI] [PubMed] [Google Scholar]
- 22.Biddick R, Young ET. The disorderly study of ordered recruitment. Yeast. 2009;26:205–220. doi: 10.1002/yea.1660. [DOI] [PubMed] [Google Scholar]
- 23.Jiang C, Pugh BF. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome biology. 2009;10:R109. doi: 10.1186/gb-2009-10-10-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brogaard K, et al. A map of nucleosome positions in yeast at base-pair resolution. Nature. 2012;486:496–501. doi: 10.1038/nature11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moll T, et al. The role of phosphorylation and the CDC28 protein kinase in cell cycle-regulated nuclear import of the S. cerevisiae transcription factor SWI5. Cell. 1991;66:743–758. doi: 10.1016/0092-8674(91)90118-i. [DOI] [PubMed] [Google Scholar]
- 26.Visintin R, et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk- dependent phosphorylation. Mol. Cell. 1998;2:709–718. doi: 10.1016/s1097-2765(00)80286-5. [DOI] [PubMed] [Google Scholar]
- 27.McBride HJ, et al. Long-range interactions at the HO promoter. Mol. Cell. Biol. 1997;17:2669–2678. doi: 10.1128/mcb.17.5.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biddick RK, et al. The transcriptional coactivators SAGA, SWI/SNF, and mediator make distinct contributions to activation of glucose-repressed genes. J. Biol. Chem. 2008;283:33101–33109. doi: 10.1074/jbc.M805258200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Govind CK, et al. Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol. Cell. Biol. 2005;25:5626–5638. doi: 10.1128/MCB.25.13.5626-5638.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhoite LT, et al. The Swi5 activator recruits the Mediator complex to the HO promoter without RNA polymerase II. Genes Dev. 2001;15:2457–2469. doi: 10.1101/gad.921601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Formosa T. The role of FACT in making and breaking nucleosomes. Biochim. Biophys. Acta. 2012;1819:247–255. doi: 10.1016/j.bbagrm.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahata S, et al. The E2F functional analogue SBF recruits the Rpd3(L) HDAC, via Whi5 and Stb1, and the FACT chromatin reorganizer, to yeast G1 cyclin promoters. EMBO J. 2009;28:3378–3389. doi: 10.1038/emboj.2009.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Q, et al. Stochastic expression and epigenetic memory at the yeast HO promoter. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1306113110. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huisinga KL, Pugh BF. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell. 2004;13:573–585. doi: 10.1016/s1097-2765(04)00087-5. [DOI] [PubMed] [Google Scholar]
- 35.Rhee HS, Pugh BF. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature. 2012;483:295–301. doi: 10.1038/nature10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohibullah N, Hahn S. Site-specific cross-linking of TBP in vivo and in vitro reveals a direct functional interaction with the SAGA subunit Spt3. Genes Dev. 2008;22:2994–3006. doi: 10.1101/gad.1724408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dudley AM, et al. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 1999;13:2940–2945. doi: 10.1101/gad.13.22.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhaumik SR, Green MR. Differential Requirement of SAGA Components for Recruitment of TATA-Box- Binding Protein to Promoters In Vivo. Mol. Cell. Biol. 2002;22:7365–7371. doi: 10.1128/MCB.22.21.7365-7371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, et al. Regulation of TATA-binding protein binding by the SAGA complex and the Nhp6 high-mobility group protein. Mol. Cell. Biol. 2003;23:1910–1921. doi: 10.1128/MCB.23.6.1910-1921.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laprade L, et al. Characterization of new Spt3 and TATA-binding protein mutants of Saccharomyces cerevisiae: Spt3 TBP allele-specific interactions and bypass of Spt8. Genetics. 2007;177:2007–2017. doi: 10.1534/genetics.107.081976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, et al. Recruitment of Cln3 cyclin to promoters controls cell cycle entry via histone deacetylase and other targets. PLoS biology. 2009;7:e1000189. doi: 10.1371/journal.pbio.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang D, et al. Dual regulation by pairs of cyclin-dependent protein kinases and histone deacetylases controls G1 transcription in budding yeast. PLoS biology. 2009;7:e1000188. doi: 10.1371/journal.pbio.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogelauer M, et al. Global histone acetylation and deacetylation in yeast. Nature. 2000;408:495–498. doi: 10.1038/35044127. [DOI] [PubMed] [Google Scholar]
- 44.Liu Q, et al. SCFCdc4 enables mating type switching in yeast by cyclin-dependent kinase-mediated elimination of the Ash1 transcriptional repressor. Mol. Cell. Biol. 2011;31:584–598. doi: 10.1128/MCB.00845-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cross FR, Tinkelenberg AH. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell. 1991;65:875–883. doi: 10.1016/0092-8674(91)90394-e. [DOI] [PubMed] [Google Scholar]
- 46.Dirick L, Nasmyth K. Positive feedback in the activation of G1 cyclins in yeast. Nature. 1991;351:754–757. doi: 10.1038/351754a0. [DOI] [PubMed] [Google Scholar]
- 47.Dirick L, et al. Roles and regulation of Cln-Cdc28 kinases at the start of the cell cycle of Saccharomyces cerevisiae. The EMBO journal. 1995;14:4803–4813. doi: 10.1002/j.1460-2075.1995.tb00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuart D, Wittenberg C. CLN3, not positive feedback, determines the timing of CLN2 transcription in cycling cells. Genes Dev. 1995;9:2780–2794. doi: 10.1101/gad.9.22.2780. [DOI] [PubMed] [Google Scholar]
- 49.Skotheim JM, et al. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature. 2008;454:291–296. doi: 10.1038/nature07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bai L, et al. Nucleosome-depleted regions in cell-cycle-regulated promoters ensure reliable gene expression in every cell cycle. Dev Cell. 2010;18:544–555. doi: 10.1016/j.devcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bai L, et al. Multiple Sequence-Specific Factors Generate the Nucleosome-Depleted Region on CLN2 Promoter. Mol. Cell. 2011;42:465–476. doi: 10.1016/j.molcel.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.