Abstract
Premorbid adjustment varies widely among individuals with schizophrenia, and has been shown to bear significantly on prodrome and onset characteristics, and on cognition, symptoms, and functioning after onset. The current analysis focused on the Premorbid Adjustment Scale (PAS), a retrospective measure assessing social and academic function at several time points from early childhood to illness onset. In an effort to explore discrete developmental subtypes, we applied latent class growth analysis (LCGA) to data from the PAS in our sample of individuals with schizophrenia (N = 208), finding three latent trajectory classes. The first of these classes showed consistently adequate-to-good social and academic functioning prior to onset; the second showed initially good function and deterioration with time until onset; the third showed poor functioning in childhood that deteriorated further during the years up to diagnosis. The classes differed significantly in terms of age of onset, processing speed, and functioning after onset. There were no significant differences in symptomatology. Our findings illustrate a potentially powerful methodological approach to the problem of heterogeneity in schizophrenia research, and add weight to the notion that aspects of premorbid history may be useful for subtyping schizophrenia patients. The potential implications of this subtyping strategy, including those pertaining to potential genetics studies, are discussed.
Introduction
Clinical and biological heterogeneity in schizophrenia are fundamental challenges for understanding illness etiology and developing treatments. Yet, efforts to develop classification schemes that reliably identify subpopulations have been inconclusive and have fostered a long-running debate as to whether schizophrenia is better characterized as a collection of etiologically distinct subtypes or as a continuum of lesser to greater illness severity (Goldberg & Weinberger, 1995; Jablensky, 2006). One enduring strategy in this area of research has been to subdivide the population of people with schizophrenia based on aspects of developmental history prior to the onset of illness. For example, Bleuler (1924) made a distinction between “process” and “reactive” schizophrenia. Individuals with “process” psychosis exhibited slower, earlier, and more insidious onset, greater chronicity, and a more severe illness course (Garmezy, 1970). Individuals experiencing “reactive” psychosis, on the other hand, became symptomatic later and fairly suddenly, often after a traumatic event, and exhibited a less severe illness course. These observations were recapitulated in systematic studies of the impact of premorbid social adjustment on onset and course of illness (Quitkin, Rifkin, & Klein, 1976).
Haas and Sweeney (1992) described three subtypes based mainly on information about social and academic development from the Premorbid Adjustment Scale (PAS; Cannon-Spoor, Potkin, & Wyatt, 1982): one group with adequate-to-good social and academic adjustment, one deteriorating group, and one showing consistently poor adjustment prior to diagnosis. Another variation on these themes was proposed by Corcoran et al. (2003), who separated people with schizophrenia into a “never normal” group and a deteriorating group, based on narratives of premorbid history. Weickert et al. grouped people with schizophrenia according to deterioration in cognitive functioning, approximated as the difference between estimated premorbid IQ and current IQ (2000). They found three groups: one with scores on both measures close to the population average; another with preserved premorbid IQ but lower current IQ, suggesting deterioration from premorbid cognitive performance; and another with poor performance on both measures, indicating consistently poor cognitive function. This consistently poor-performing group also showed language and visual processing deficits relative to the other groups.
The strategy of basing schizophrenia subgroupings on premorbid history draws support from the neurodevelopmental hypothesis of schizophrenia (Marenco & Weinberger, 2000, Weinberger & Levitt, 2011). This hypothesis holds that the illness emerges as a result of abnormal brain development, beginning long before the emergence of psychotic symptoms, and that these neurodevelopmental abnormalities are often reflected in aberrations in behavior and functioning in children and adolescents who go on to develop schizophrenia (Rapoport, Addington, & Frangou, 2005). A number of longitudinal studies have identified social, behavioral, cognitive, and academic antecedents of the disorder (e.g., Amminger, 1999; Cannon et al., 2002). Subtle cognitive deficits on various IQ subtests have been especially widely-documented in children and adolescents who will later be diagnosed with schizophrenia (Cannon et al., 2000; Niendam et al., 2003; Reichenberg et al, 2010; Woodberry, Giuliano, & Seidman, 2008).
It is also clear, however, that premorbid adjustment varies widely among people with schizophrenia, and that this variation is linked to a number of course, symptom, and outcome variables. A recent review by Schmael et al. (2007) synthesized previous findings. Premorbid adjustment has been related to sex, with males showing generally worse premorbid adjustment (e.g., Bailer, Brauer, & Rey, 1996; Rabinowitz, Smedt, Harvey, & Davidson, 2002). Poor premorbid adjustment has also been linked to earlier age of onset (e.g., Krauss, Marwinski, Held, Rietschel, & Freyberger, 1998; Allen, Frantom, Strauss, & van Kammen, 2005). With respect to symptom measures, poor premorbid adjustment predicts both increased negative symptoms (Addington, van Mastrigt, & Addington, 2003; Haim, Rabinowitz, & Bromet, 2006) and overall severity of illness (Bailer et al., 1996; Ucok, Polat, Cakir, & Genc, 2006; Ucok, Polat, Genc, Cakir, & Turan, 2004). Additionally, there is a widely-reported association between poor premorbid adjustment and various indexes of poor outcome in schizophrenia, including Global Assessment of Functioning ratings (GAF; Fleischhaker et al., 2005), relapse during follow-up of first-episode psychosis (Gleeson, Rawlings, Jackson, & McGorry, 2005), and suicide (Stephens, Richard, & McHugh, 1999). Premorbid adjustment has also been shown to predict cognitive function in people with schizophrenia, with studies showing that poor premorbid adjustment predicts, among other things, deficits in verbal memory (Rund et al., 2004) and visual learning (Levitt, O’Donnell, McCarley, Nestor, & Shenton, 1996). Studies examining both premorbid adjustment and age of onset in combination have yielded interesting associations with post-onset cognitive performance. DeQuardo et al. (1994) linked early onset and poor premorbid function to lower IQ and higher ventricle-brain ratio. Earlier onset and poor premorbid function have also been associated with deficits in attention and executive function (Basso, Nasrallah, Olson, & Bornstein, 1997; Silverstein, Mavroleftos, & Close, 2002). The groupings identified by Haas and Sweeney have been associated recently with severity of impairment in a number of cognitive domains, as well as global cognitive impairment (Bechard-Evans, Iyer, Lepage, Joober, & Malla, 2010).
Advances in clustering methodologies have permitted people with schizophrenia to be subtyped using a wide variety of indicator variables with few or no a priori hypotheses. As these methods have evolved, a number of groups have included details of developmental history as part of their subtyping strategies. Farmer, McGuffin, and Spitznagel (1983) used one clustering technique to discern two relatively distinct groups on the basis of premorbid adjustment, family history, and current symptomatology. One group developed symptoms later, functioned well before onset, and showed a predominantly paranoid symptom profile after onset. The other group showed early onset, poor premorbid adjustment and a symptom profile of bizarre behavior and negative symptoms, and was more likely than the first group to have a family history of schizophrenia. Using a different, “minimally-supervised” clustering method, Sham, Castle, Wessely, Farmer, and Murray (1996) identified a possible subtype of schizophrenia similar to this latter group, comprising individuals who showed a longer prodromal phase, pronounced negative symptoms, and poor neuroleptic response.
In the current work, we used a minimally-supervised clustering method, latent class growth analysis (LCGA), to discern social and academic developmental trajectories in our patient sample using information from the Premorbid Adjustment Scale (Cannon-Spoor, Potkin, & Wyatt, 1982). We then examined the developmental clusters we found in relation to illness onset and a number of post-onset cognitive, symptom, and functioning variables.
Methods
Sample
Individuals included in current analyses (N = 208; 74.5% male; 86.1% Caucasian) were schizophrenia probands evaluated as part of their participation in the CBDB/NIMH Genetic Study of Schizophrenia (Egan et al., 2001). All participants provided informed consent. Participants were excluded from the study if they had an IQ of less than 70, showed evidence of a learning disability, had abused drugs or alcohol within the last 6 months, or had a history of head trauma with a loss of consciousness. Ages in the sample ranged from 20 to 62. All participants in the current analysis were medically screened and were interviewed by 2 psychiatrists to ensure that they met diagnostic criteria for a schizophrenia spectrum disorder.
Measures
The Premorbid Adjustment Scale
The Premorbid Adjustment Scale (PAS; Cannon-Spoor et al., 1982) is a retrospective measure mainly focused on an individual’s social and academic adjustment at a maximum of four points prior to illness onset: childhood (up to age 11), early adolescence (12 to 15), late adolescence (17 to 18), and adulthood (19 and older). The scale includes only time points before illness onset (e.g., if an individual experiences onset at age 20, only childhood, early adolescence, and late adolescence time points will be included). It was administered to the parents of all participants as part of a detailed assessment protocol. The PAS consists of interview questions concerning sociability, peer relationships, academic performance (for time ranges before adulthood), adaptation to school (for time ranges before adulthood), and socio-sexual aspects of development (for time ranges after childhood). Responses for each of these sections were coded into ratings between 0 and 6, and the sum of scores at each time range was divided by the greatest possible sum of points, yielding a score between 0 and 1 for each time range. Higher scores on the test indicate worse premorbid adjustment; a score of 0 indicates development with no difficulties. Some individuals with very early onset of illness are missing data for later PAS time points. In order to focus on more typical schizophrenia cases, enhance generalizability, and simplify data analysis, we included subjects who had PAS scores for all four time points.
Cognitive measures
A 3-hour battery of neuropsychological tests was administered to participants by trained evaluators. IQ was assessed using a four-subtest estimate from the revised Wechsler Adult Intelligence Scale (WAIS-R; Wechsler, 1981). Exploratory and confirmatory factor analyses were performed on a subset these variables (as listed and described in detail in Dickinson, Goldberg, Gold, Elvevåg, & Weinberger, in press), yielding factor-based composites representing six broad cognitive domains: (1) Verbal Memory, (2) Nback, (3) Visual Memory, (4) Processing Speed, (5) Card Sorting, and (6) Span. Additionally, all cognitive measures were standardized and averaged to yield a measure of general cognition (g).
Measures of symptoms and functioning
Symptomatology was assessed by two psychiatrists using the Positive and Negative Syndrome Scale (PANSS; Kay, Opler, & Fizbein, 1986). Each item on the scale is scored from 1 to 7, with higher scores signifying worse symptomatology. Consistent with the factor analytic literature (e.g., Lindenmayer, Grochowski, & Hyman, 1995) and internal confirmatory factor analyses (Fortgang & Wallwork, unpublished), these data are organized into five symptom domains: Positive, Negative, Depressed, Excited, and Disorganized/Concrete. Functioning was assessed using the Global Assessment of Functioning Scale ratings (GAF; American Psychiatric Association [DSM-IV-TR], 2000). The scale assesses psychological, social, and occupational functioning on a score of 0–100, with higher scores indicating better overall functioning.
Statistical Analyses
Latent Class Growth Analysis
All analyses were performed using Mplus (version 5.2). We examined latent premorbid trajectories of adjustment in our sample using latent class growth analysis (LCGA). This technique falls under the heading of finite mixture modeling, which decomposes heterogeneity in a sample by dividing it into a finite number of latent subgroups based on profiles of indicator variables. Relative to other finite mixture modeling techniques (such as latent class analysis), LCGA is well-suited to discerning classes defined by different developmental trajectories, because indicator variables can be measures from successive time points and the model incorporates growth parameters relating the indicator variables to time. LCGA is essentially a special case of growth mixture modeling (GMM) in which the growth parameters are assumed to be invariant within classes (Muthén & Muthén, 2000; Jung & Wickrama, 2007). LCGA readily incorporates covariates into model selection.
The indicator variables from which we derived latent classes were PAS scores at each of the four age ranges. Based on these variables, models fitting between 1 and 8 classes were run successively. Models were fitted multiple times using random seed values to avoid convergence on local maxima. In order to account for demographic differences within our sample, we considered sex, race, age at the time of testing, and family socioeconomic status as potential covariates within the analysis. In preliminary analyses, we tested the effects of all of these covariates on PAS scores at all time points, and none showed any reliable relationship with PAS scores. However, given the well-documented link between premorbid adjustment and sex (Schmael, 2007), we retained sex as a covariate in the analyses, and examined the other variables as outcome variables.
The best-fitting classification model was determined based on the Bayesian Information Criterion (BIC; Schwartz, 1978), a criterion calculated from the log-likelihood of the model and the number of parameters in the model. The lowest BIC suggests the best fit. We also consulted the Vuong-Lo-Mendell-Rubin test (Lo, Mendell & Rubin, 2001) using the TECH11 option in Mplus. These tests assess the discrepancies in likelihood between the model being tested with a model with one fewer class; significant results in these tests indicate that a K-class model fits the data better than a model with (K-1) classes.
Relating Class Groupings to Outcome Variables
After discerning trajectories in premorbid adjustment based solely on our indicator variables and covariates, we sought to detect mean-level differences in variables pertaining to onset, as well as post-onset functioning, cognition, and symptoms. Main analyses tested mean-level differences among classes in variables of interest with the Wald Test of Mean Equality (Asparouhov and Muthén, 2007) using “pseudo-class random draws.” Briefly, pseudo-class random draws are part of a resampling technique in which individual class assignments are permitted to vary across multiple random classifications based on individual posterior probability distributions. Using the pseudo-class random draws, class-specific means and variances can be computed and used in regressions and mean comparisons across classes (Clark and Muthén, 2009; see Asparouhov and Muthén, 2007 or Wang, Brown, & Bandeen-Roche, 2005 for more detail).
We conducted additional exploratory analyses. Although each individual could be assigned to a “most probable class” based on posterior probabilities of class membership, each individual had some probability of membership in each class. Thus, using the full sample for each analysis, we also examined within-class associations with onset, cognition, symptom, and functioning variables. To do so, we logarithmically transformed each individual’s probability (p between 0 and1) of belonging to each class and, for each class, we regressed transformed membership probability on our variables of interest. Clark and Muthén (2009) suggest cautious interpretation of these analyses, as probability estimates for group membership are not observed variables but statistical estimates, and may yield inflated regression parameters.
Results
Table 1 shows the values of log-likelihood, BIC, and number of parameters for all models assessed.
Table 1.
Bayesian Information Criterion values and Vuong-Lo-Mendell-Rubin Likelihood Test p-values for solutions specifying 1–8 classes.
| Number of classes | Bayesian Information Criterion |
Vuong-Lo-Mendell-Rubin Likelihood Test p-value |
|---|---|---|
| 1-class | −408.397 | N/A |
| 2-class | −529.244 | 0.0771 |
| 3-class | −558.059 | 0.0025 |
| 4-class | −551.548 | 0.5283 |
| 5-class | −549.365 | 0.2333 |
| 6-class | −533.019 | 0.3400 |
| 7-class | −521.863 | 0.5360 |
| 8-class | −511.734 | 0.2239 |
A three-class model showed the lowest value on the BIC. The difference in BIC values between the three- and four-class models was modest (see Table 1). The size and profile of the two more extreme classes was similar in the three- and four-class models; the main difference between the two models is that the three-class model contained one large intermediate class, whereas the four-class model separated this large class into two intermediate groups.
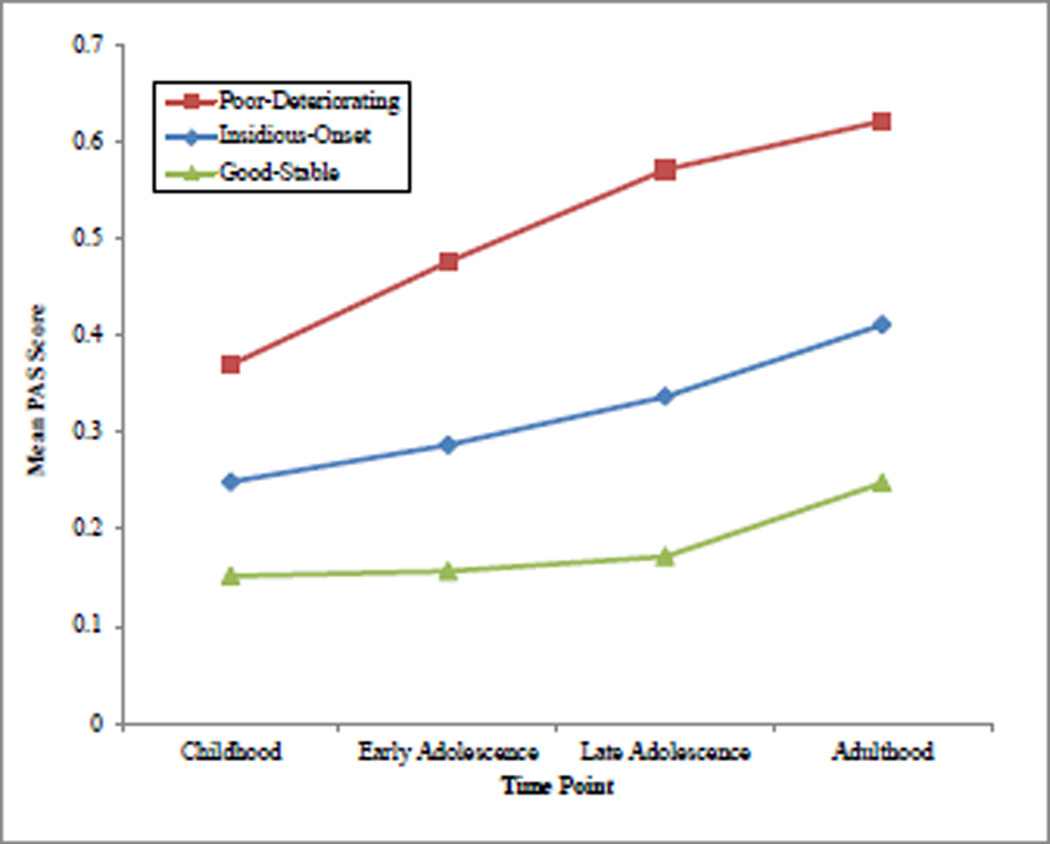
PAS values for our preferred three-class model are displayed graphically in Figure 1. The first group, the good-stable group (31.6% of sample), showed low scores on the PAS Childhood measure (indicating good social and academic adjustment at that time point), with very little increase in scores at time points leading up to onset. The second and largest group, the insidious onset group 55.4% of sample), showed low PAS scores in childhood, but these scores increased steadily to the onset of illness, indicating gradually deteriorating premorbid function. The third group, poor-deteriorating (13.0% of sample), showed high childhood PAS scores, with increases throughout the premorbid years, indicating poor initial adjustment and further steady deterioration. The effect of sex was not significant on any group, or on the intercept, slope, or quadratic parameters for any of the three groups.
Figure 1.
Mean PAS score for all four groups at all four time points.
Wald Tests of Mean Equality were used to test differences between the clusters in demographics, onset, cognition, functioning, and symptomatology. The results of these tests, as well as the means for each of these variables listed by trajectory group, are shown in Table 2. Groups did not differ reliably in demographic variables, nor in age of onset of acute symptomatology, but showed different patterns of prodromal characteristics leading up to onset. The poor-deteriorating group showed the earliest prodromal signs, while prodromal symptoms were markedly later in the good-stable group. The groups differed in years of education, and there was a strong difference between the classes in functional status (GAF) post-onset. Between-groups differences in cognition were apparent only in the processing speed domain, with the poor-deteriorating group showing decreased performance. No between-groups differences in symptomatology were observed.
Table 2.
Means and standard deviations for demographic, onset, cognitive, functioning, and symptomatology variables for each group, as well as results of Wald Tests of Mean Equality between groups.
| Good-stable | Insidious-onset | Poor- deteriorating |
Wald Test of Mean Equality |
|||||
|---|---|---|---|---|---|---|---|---|
| Mean | S.E. | Mean | S.E. | Mean | S.E. | Chi Square |
Sig. | |
| Prodrome and Onset | ||||||||
| Age when tested | 36.84 | 1.237 | 38.3 | 0.919 | 36.51 | 1.573 | 1.014 | 0.602 |
| Percent caucasian | 0.82 | 0.051 | 0.89 | 0.032 | 0.87 | 0.073 | 0.826 | 0.662 |
| Family SES | 50.92 | 2.009 | 51.75 | 1.271 | 51.00 | 2.707 | 0.091 | 0.956 |
| Prodrome and Onset | ||||||||
| Age of acute onset | 25.10 | 0.824 | 24.08 | 0.536 | 23.08 | 1.100 | 2.247 | 0.325 |
| Age of prodrome onset | 23.24 | 0.960 | 20.74 | 0.666 | 18.44 | 1.604 | 6.834 | 0.033 |
| Cognition | ||||||||
| Cognitive Factors | ||||||||
| Verbal Memory | 3.53 | 0.123 | 3.55 | 0.101 | 3.61 | 0.238 | 0.098 | 0.952 |
| Nback | 15.11 | 1.511 | 12.83 | 1.143 | 16.95 | 3.460 | 1.658 | 0.436 |
| Visual Memory | 33.26 | 2.322 | 32.48 | 1.929 | 33.21 | 4.092 | 0.046 | 0.977 |
| Processing Speed | 2.67 | 0.107 | 2.50 | 0.077 | 2.21 | 0.136 | 7.490 | 0.024 |
| Card Sorting | 16.91 | 1.364 | 15.06 | 0.959 | 16.47 | 2.040 | 0.626 | 0.731 |
| Span | 3.24 | 0.125 | 3.10 | 0.088 | 3.07 | 0.173 | 0.778 | 0.678 |
| Global Cognitive Ability (g) | −1.26 | 0.105 | −1.42 | 0.077 | −1.41 | 0.181 | 1.013 | 0.603 |
| WAIS Estimated Full-Scale IQ | 94.79 | 1.463 | 93.46 | 1.172 | 91.77 | 2.510 | 1.204 | 0.548 |
| WRAT Reading Standard Score | 104.44 | 1.520 | 102.83 | 1.127 | 103.61 | 2.468 | 0.334 | 0.846 |
| Functioning | ||||||||
| Years of education completed | 15.06 | 0.313 | 14.48 | 0.209 | 13.61 | 0.433 | 7.704 | 0.021 |
| Global Assessment of Functioning | 51.66 | 2.101 | 45.33 | 1.361 | 39.44 | 2.24 | 15.766 | <0.001 |
| Symptomatology | ||||||||
| Positive symptoms | 2.15 | 0.212 | 2.68 | 0.184 | 2.33 | 0.354 | 2.191 | 0.334 |
| Negative symptoms | 2.57 | 0.211 | 3.16 | 0.186 | 3.10 | 0.491 | 2.562 | 0.278 |
| Depression | 1.44 | 0.095 | 1.57 | 0.086 | 1.54 | 0.163 | 0.643 | 0.725 |
| Excitement | 1.10 | 0.075 | 1.18 | 0.058 | 1.50 | 0.205 | 3.493 | 0.174 |
| Disorganization | 2.65 | 0.219 | 3.10 | 0.210 | 3.23 | 0.437 | 1.846 | 0.397 |
In addition to between-cluster differences, we explored the relationship between the likelihood of belonging to each individual class and outcome variables. After logarithmic transformation of each individual’s probability (p between 0 and1) of belonging to each class, and using the whole sample for separate analyses class by class, we regressed transformed membership probability on our outcome variables.
Probability of membership in the good-stable class was associated robustly with later prodromal onset and higher global functioning, β = .214, t(183) = 2.969, p = .003 and β = .244, t(188) = 3.443, p = .001, respectively. Higher performance on processing speed was associated with likelihood of membership in this group, β = .182, t(202) = 2.634, p = .009, as was less severe symptomatology in terms of decreased conceptual disorganization, β = −.168, t(158) = −2.143, p = .034. Membership in the insidious onset class was associated with increased positive and negative symptoms after the onset of illness, β = .157, t(154) = 1.976, p = .050 and β = .176, t(156) = 2.232, p = .027 respectively. Probability of belonging to the poor-deteriorating class had a range of implications. Membership in this class was associated with lower educational attainment, β = −.174, t(205) = −2.528, p = .012, earlier onset of prodromal symptoms, β = −.209, t(183) = −2.885, p = .004, and impaired global functioning, β = −.192, t(188) = −2.682, p = .008. Additionally, membership probability was associated with lower processing speed scores and higher scores on the PANSS Excitement factor, β = −.192, t(202) = −2.784, p = .006 and β = .360, t(154) = 4.785, p < .001 respectively.
Discussion
In the current study we used information about academic and social history prior to illness onset to separate people with schizophrenia into three clusters of individuals: a “good-stable” group, an “insidious-onset” group, and a “poor-deteriorating” group. The largest group was the insidious-onset group, which comprised individuals who started with good adjustment in childhood that deteriorated until onset. The remainder of our sample fell into the two more extreme developmental trajectory groupings: the good-stable and poor-deteriorating groups. The good-stable group was identified with a developmental history relatively free of problems in adjustment as measured by the PAS. By contrast, individuals in the poor-deteriorating group started with poor adjustment in childhood that became increasingly problematic until the onset of schizophrenia.
Having identified these groupings based solely on information about social and academic adjustment before illness, we examined whether these clusters showed different patterns in terms of prodrome and onset, and in terms of cognition, symptoms, and functioning after onset. We found no differences between groups in key demographic variables, including age, race, and family SES. For other variables, there were pronounced differences. Members of the good-stable group were rated as higher functioning than the other classes on the GAF and showed the highest degree of educational attainment of the groups. Furthermore, their scores on the Processing Speed cognitive factor were higher on average than those attained by the other groups. In general, their post-onset cognitive performance appeared to be relatively good, and their symptoms relatively mild. In contrast, members of the poor-deteriorating group showed an aggressive course of illness onset and much poorer outcome after onset. Strikingly, poor-deteriorating class members developed subtle prodromal symptoms approximately 4.8 years earlier than good-stable class members. They achieved a lower level of education than the other groups -- approximately 1.5 years less education than those in the good-stable class. Cognitively, these individuals showed the largest impairments in post-diagnosis processing speed, a core dimension of cognitive impairment leading up to and after the onset of schizophrenia (Dickinson et al., 2007; Niendam et al., 2003; Mesholam-Gately et al., 2009). People classified in this group also were judged to have markedly impaired overall functioning relative to the rest of the sample: the difference between the average GAF rating of the poor-deteriorating group and the good-stable group was over ten points, a particularly notable difference given the relative restriction of range in GAF ratings among individuals with schizophrenia. With respect to symptomatology, no significant mean differences were observed compared to other classes; however, higher probability of poor-deteriorating class membership was strongly associated with higher levels of PANSS Excitement, possibly suggesting a special role for symptoms of impulsivity, hostility and agitation within this class.
In relating distinct premorbid courses to different patterns of onset, and of cognition, symptomatology and functioning after onset, our findings support the long-held idea that people with schizophrenia can be subgrouped on the basis of premorbid history. Our good-stable group, in showing a shorter prodrome, and less severe overall post-onset course, may approximate Bleuler’s “reactive” schizophrenia (1924) or Haas and Sweeney’s best group (1992) – individuals who maintain relatively good adjustment until a sudden, precipitous onset of illness. Meanwhile, our poor-deteriorating group started with poor early childhood adjustment that worsened steadily, similar to Bleuler’s “process” schizophrenia or similar groupings ascertained by other groups (Farmer et al., 1983; Haas & Sweeney, 1992; Sham et al., 1996). Similar to findings with earlier classification schemes (Weickert et al., 2000), and to findings addressing poor premorbid adjustment in general (Schmael et al., 2007), our poor-deteriorating group showed impairments in cognition and functioning after diagnosis. Our findings with regard to symptomatology were more equivocal, but exploratory within-class analyses showed varying associations with probabilities of class membership for the different classes.
The two extreme subgroups in current analyses came most clearly into focus. They accounted for 44.6% of the overall sample. The remaining 55.4% were classified within the “insidious onset” class, whose profile is less clear. Refinement of the current classification scheme might increase the size of the extreme classes somewhat, but it appears that, in a scheme based on social and academic adjustment before the onset of schizophrenia, a sizable proportion of the typical sample will fall into a fuzzier middle ground, between the extremes. A further point is that average PAS scores and the values of many of the onset, cognition, symptomatology, and functioning variables followed a roughly linear pattern across classes.
These findings again frame the question whether the current scheme and similar schemes are better understood as continua of premorbid adjustment or as sets of discrete classes (Goldberg & Weinberger, 1995). Current and recent research suggest several reasons to sidestep the debate about discrete classes versus continua of severity. As Jablensky (2006) argues, whether a given classification scheme is characterized as discrete, continuous, or hybrid matters less than whether it has predictive power and advances “mechanistic explanation of disease phenomena” (p. 828). Whether we have simply discerned three classes of increasing illness severity (from the good-stable class, to the insidious-onset class, to the poor-deteriorating class), as opposed to three qualitatively different classes, is difficult to determine. However, even where classification is based on more continuous data, methodological advances may enhance the utility of the schemes for discriminating discontinuous classes. Our exploratory regressions, in which probability of membership in each individual class was used to predict variables of interest, confirmed and extended the information from the mean comparisons across classes. For example, these analyses confirmed that probability of class membership for both the good-stable and the poor-deteriorating classes is robustly associated with age of prodromal onset, global functioning post-onset, and processing speed performance post-onset. However, the analyses also revealed more qualitative differences among the classes in relation to symptomatology. Probability of membership in the poor-deteriorating class was more strongly associated with the PANSS Excitement factor than with any other variable, but was not significantly related to positive, negative or disorganized symptoms. Class membership probability for the insidious-onset class showed a significant association to classic negative and positive symptoms, but not excitement or other symptom dimensions. Probability of membership in the good-stable class related significantly only to disorganized symptoms. While these results are exploratory (Clark and Muthén, 2009), they provide interesting, tentative evidence that the groups discerned in this analysis may be qualitatively different from one another in regard to symptoms, even as they differ more continuously in terms of prodromal symptoms, functioning and cognition. Thus, current analyses show that even a fairly narrow classification scheme – focused only on patterns of premorbid social and academic adjustment – predicts prodromal patterns and patterns of and cognition, symptoms and functioning after onset that are distinct to some degree from class to class. A multi-dimensional approach to classification, which incorporates a more diverse mixture of variables across dimensions of assessment (e.g., developmental history, premorbid adjustment, cognition, symptoms) may yield groups that are more qualitatively different from one another (Sham et al., 1996).
One limitation of the current study is the use of a retrospective informant-report measure as a basis for information about functioning in periods before illness onset. However, Brill, Reichenberg, Weiser, and Rabinowitz (2008) compared the PAS with contemporary measures of adjustment, using Draft Board scores of Israeli conscripts who later developed schizophrenia; the correlations were robust. Thus, while future studies might help to compare the validity of the PAS with measures of functioning in adulthood, there are encouraging results about its concurrent validity as a measure of adjustment before onset.
Advances in neuroimaging and genetics offer new variables and/or outcomes for analysis. It may be, for example, that our poor-deteriorating group shows genetic associations similar to the “deficit syndrome” group identified by Fanous et al. (2008) or the “cognitive deficit” subtype identified by Holliday et al. (2009). This group may also be relatively enriched in structural chromosomal abnormalities that have been associated with early intellectual impairment in patients with the diagnosis of schizophrenia (St. Clair, 2009; Walsh et al., 2008). Alternatively, removal of the extreme classes in these or similar data may enhance the biological homogeneity of the remaining majority of schizophrenia cases thereby highlighting associations that are masked in more heterogeneous samples.
In summary, our findings add weight to the notion that information about premorbid social and academic adjustment can be a useful discriminator among groups of people with schizophrenia, and support the use of latent class and similar minimally-supervised modeling methods to discern such subgroups without precise a priori hypotheses. In further work we plan to refine these subgroups and examine their relationship to genetics and imaging variables.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health.
References
- Addington J, van Mastrigt S, Addington D. Patterns of premorbid functioning in first-episode psychosis: initial presentation. Schizophrenia Research. 2003;62:23–30. doi: 10.1016/s0920-9964(02)00408-5. [DOI] [PubMed] [Google Scholar]
- Allen DN, Frantom LV, Strauss GP, van Kammen DP. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophrenia Research. 2005;75:389–397. doi: 10.1016/j.schres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Amminger GP, Pape S, Rock D, Roberts SA, Ott SL, Squires-Wheeler E, et al. Relationship Between Childhood Behavioral Disturbance and Later Schizophrenia in the New York High-Risk Project. American Journal of Psychiatry. 1999;156(4):525–530. doi: 10.1176/ajp.156.4.525. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Revised 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Asparouhov T, Muthén BO. Wald test of mean equality for potential latent class predictors in mixture modeling. Unpublished technical paper. 2007 http://www.statmodel.com.
- Bailer J, Brauer W, Rey E-R. Premorbid adjustment as predictor of outcome in schizophrenia: results of a prospective study. Acta Psychiatrica Scaninavica. 1996;93:368–377. doi: 10.1111/j.1600-0447.1996.tb10662.x. [DOI] [PubMed] [Google Scholar]
- Basso MR, Nasrallah HA, Olson SC, Bornstein RA. Cognitive deficits distinguish patients with adolescent- and adult-onset schizophrenia. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1997;10(2) [PubMed] [Google Scholar]
- Bechard-Evans L, Iyer S, Lepage M, Joober R, Malla A. Investigating cognitive deficits and symptomatology across pre-morbid adjustment patterns in first-episode psychosis. Psychological Medicine. 2010;40:479–459. doi: 10.1017/S0033291709991097. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Textbook of Psychiatry. New York City: The Macmillan Company; 1924. [Google Scholar]
- Brill N, Reichenberg A, Weiser M, Rabinowitz J. Validity of the Premorbid Adjustment Scale. Schizophrenia Bulletin. 2008;34(5):981–983. doi: 10.1093/schbul/sbm128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M, Caspi A, Moffitt TE, Harrington H, Taylor A, Murray RM, et al. Evidence for Early-Childhood, Pan-Developmental Impairment Specific to Schizophreniform Disorder. Arhcives of General Psychiatry. 2002;59(5):449–456. doi: 10.1001/archpsyc.59.5.449. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T. Childhood Cognitive Functioning in Schizophrenia Patients and Their Unaffected Siblings: A Prospective Cohort Study. Schizophrenia Bulletin. 2000;26(2):379–393. doi: 10.1093/oxfordjournals.schbul.a033460. [DOI] [PubMed] [Google Scholar]
- Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of Premorbid Adjustment in Chronic Schizophrenia. Schizophrenia Bulletin. 1982;8(3):470–484. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- Clark SL, Muthén B. Relating Latent Class Analysis Results to Variables not Included in the Analysis. Submitted for publication. 2009 http://www.statmodel.com.
- Corcoran C, Davidson L, Sills-Shahar R, Nickou C, Malaspina D, Miller T, et al. A qualitative research study of the evolution of symptoms in individuals identified as prodromal to psychosis. Psychiatric Quarterly. 2003;74(4):313–332. doi: 10.1023/a:1026083309607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeQuardo JR, Tandon R, Goldman R, Meador-Woodruff JH, McGrath-Giroux M, Brunberg JA, et al. Ventricular Enlargement, Neuropsychological Status, and Premorbid Function in Schizophrenia. Biological Psychiatry. 1994;35:517–524. doi: 10.1016/0006-3223(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Dickinson D, Goldberg TE, Gold JM, Elvevåg B, Weinberger DR. Cognitive Factor Structure and Invariance in People With Schizophrenia, Their Unaffected Siblings, and Controls. Schizophrenia Bulletin. in press doi: 10.1093/schbul/sbq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D, Ramsey ME, Gold JM. Overlooking the Obvious: A Meta-analytic Comparison of Digit Symbol Coding Tasks and Other Cognitive Measures in Schizophrenia. Archives of General Psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanous AH, Neale MC, Webb BT, Straub RE, O'Neill FA, Walsh D, et al. Novel Linkage to Chromosome 20p Using Latent Classes of Psychotic Illness in 270 Irish High-Density Families. Biological Psychiatry. 2008;64(2):121–127. doi: 10.1016/j.biopsych.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Farmer AE, McGuffin P, Spitznagel EL. Heterogeneity in Schizophrenia: A Cluster-Analytic Approach. Psychiatry Research. 1983;8:1–12. doi: 10.1016/0165-1781(83)90132-4. [DOI] [PubMed] [Google Scholar]
- Fleischhaker C, Schulz E, Tepper K, Martin M, Hennighausen K, Remschmidt H. Long-Term Course of Adolescent Schizophrenia. Schizophrenia Bulletin. 2005;31(3):769–780. doi: 10.1093/schbul/sbi014. [DOI] [PubMed] [Google Scholar]
- Garmezy N. Process and reactive schizophrenia: Some conceptions and issues. Schizophrenia Bulletin. 1970;2:30–74. [Google Scholar]
- Gleeson JF, Rawlings D, Jackson HJ, McGorry PD. Agreeableness and Neuroticism as Predictors of Relapse After First-Episode Psychosis: A Prospective Follow-Up Study. Journal of Nervous and Mental Disease. 2005;193(3):160–169. doi: 10.1097/01.nmd.0000154841.99550.d3. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Weinberger DR. A case against subtyping in schizophrenia. Schizophrenia Research. 1995;17(2):147–152. doi: 10.1016/0920-9964(95)00060-y. [DOI] [PubMed] [Google Scholar]
- Haas GL, Sweeney JA. Premorbid and Onset Features of First-Episode Schizophrenia. Schizophrenia Bulletin. 1992;18(3):373–386. doi: 10.1093/schbul/18.3.373. [DOI] [PubMed] [Google Scholar]
- Haim R, Rabinowitz J, Bromet E. The Relationship of Premorbid Functioning to Illness Course in Schizophrenia and Psychotic Mood Disorders During Two Years Following First Hospitalization. Journal of Nervous and Mental Disease. 2006;194(10):791–795. doi: 10.1097/01.nmd.0000240158.39929.e3. [DOI] [PubMed] [Google Scholar]
- Holliday EG, McLean DE, Nyholt DR, Mowry BJ. Susceptibility Locus on Chromosome 1q23–25 for a Schizophrenia Subtype Resembling Deficit Schizophrenia Identified by Latent Class Analysis. Archives of General Psychiatry. 2009;66(10):1058–1067. doi: 10.1001/archgenpsychiatry.2009.136. [DOI] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: implications for genetic research. Molecular Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Jung T, Wickrama KAS. An introduction to latent class growth analysis and growth mixture modeling. Social and Personality Psychology Compass. 2007;1:1–9. [Google Scholar]
- Kay SR, Opler LA, Fizbein A. Positive and Negative Syndrome Scale. North Tonawanda, New York: Multi-Health System Inc; 1986. [Google Scholar]
- Krauss H, Marwinski K, Held T, Rietschel M, Freyberger HJ. Reliability and validity of the premorbid adjustment scale (PAS) in a German sample of schizophrenic and schizoaffective patients. European Archives of Psychiatry and Clinical Neuroscience. 1998;248:277–281. doi: 10.1007/s004060050050. [DOI] [PubMed] [Google Scholar]
- Levitt JJ, O'Donnell BF, McCarley RW, Nestor PG, Shenton ME. Correlations of Premorbid Adjustment in Schizophrenia With Auditory Event-Related Potential and Neuropsychological Abnormalities. American Journal of Psychiatry. 1996;153(10):1347–1349. doi: 10.1176/ajp.153.10.1347. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J-P, Grochowski S, Hyman RB. Five factor model of schizophrenia: Replication across samples. Schizophrenia Research. 1995;14(3):229–234. doi: 10.1016/0920-9964(94)00041-6. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Marenco S, Weinberger DR. The neurodevelopmental hypothesis of schizophrenia: Following a trail of evidence from cradle to grave. Development and Psychopathology. 2000;12:501–527. doi: 10.1017/s0954579400003138. [DOI] [PubMed] [Google Scholar]
- Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in First-Episode Schizophrenia: A Meta-Analytic Review. Neurospychology. 2009;23(3):315–336. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- Muthén B, Muthén LK. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcoholism: Clinical and Experimental Research. 2000;24:882–891. [PubMed] [Google Scholar]
- Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, et al. A Prospective Study of Childhood Neurocognitive Functioning in Schizophrenic Patients and Their Siblings. American Journal of Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- Quitkin F, Rifkin A, Klein DF. Neurologic Soft Signs in Schizophrenia and Character Disorders. Archives of General Psychiatry. 1976;33(7):845–853. doi: 10.1001/archpsyc.1976.01770070075008. [DOI] [PubMed] [Google Scholar]
- Rabinowitz J, Smedt GD, Harvey PD, Davidson M. Relationship Between Premorbid Functioning and Symptom Severity as Assessed at First Episode of Psychosis. American Journal of Psychiatry. 2002;159:2021–2026. doi: 10.1176/appi.ajp.159.12.2021. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RSE, Murray RM, et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 2010;167(2):160–169. doi: 10.1176/appi.ajp.2009.09040574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rund BR, Melle I, Friis S, Larsen TK, Midboe LJ, Opjordsmoen S, et al. Neurocognitive Dysfunction in First-Episode Psychosis: Correlates With Symptoms, Premorbid Adjustment, and Duration of Untreated Psychosis. American Journal of Psychiatry. 2004;161:466–472. doi: 10.1176/appi.ajp.161.3.466. [DOI] [PubMed] [Google Scholar]
- Schmael C, Georgi A, Krumm B, Buerger C, Deschner M, Moethen MM, et al. Premorbid adjustment in schizophrenia -- An important aspect of phenotype definition. Schizophrenia Research. 2007;92:50–62. doi: 10.1016/j.schres.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Schwartz G. Estimating the dimension of a model. The Annals of Statistics. 1978;6:461–464. [Google Scholar]
- Sham PC, Castle DJ, Wessely S, Farmer AE, Murray RM. Further exploration of a latent class typology of schizophrenia. Schizophrenia Research. 1996;20:105–115. doi: 10.1016/0920-9964(95)00091-7. [DOI] [PubMed] [Google Scholar]
- Silverstein ML, Mavrolefteros G, Close D. Premorbid Adjustment and Neuropsychological Performance in Schizophrenia. Schizophrenia Bulletin. 2002;28(1):157–165. doi: 10.1093/oxfordjournals.schbul.a006918. [DOI] [PubMed] [Google Scholar]
- St. Clair D. Copy Number Variation in Schizophrenia. Schizophrenia Bulletin. 2009;35(1):9–12. doi: 10.1093/schbul/sbn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens JH, Richard P, McHugh PR. Suicide in patients hospitalized for schizophrenia: 1913–1940. Journal of Nervous and Mental Disease. 1999;187:10–14. doi: 10.1097/00005053-199901000-00003. [DOI] [PubMed] [Google Scholar]
- Ucok A, Polat A, Cakir S, Genc A. One year outcome in first episode schizophrenia. Predictors of relapse. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:37–43. doi: 10.1007/s00406-005-0598-2. [DOI] [PubMed] [Google Scholar]
- Ucok A, Polat A, Genc A, Cakir S, Turan N. Duration of untreated psychosis may predict acute treatment response in first-episode schizophrenia. Journal of Psychiatric Research. 2004;38:163–168. doi: 10.1016/s0022-3956(03)00104-3. [DOI] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, et al. Rare Structural Variants Disrupt Multiple Genes in Neurodevelopmental Pathways in Schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang CP, Brown CH, Bandeen-Roche K. Residual diagnostics for growth mixture models: Examining the impact of preventive intervention on multiple trajectories of aggressive behavior. Journal of the American Statistical Association. 2005;100(3):1054–1076. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale -- Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- Weickert TW, Goldberg TE, Gold JM, Bigelow LB, Egan MF, Weinberger DR. Cognitive Impairments in Patients With Schizophrenia Displaying Preserved and Compromised Intellect. Archives of General Psychiatry. 2000;57:907–913. doi: 10.1001/archpsyc.57.9.907. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Levitt P. Neurodevelopmental origins of schizophrenia. In: Weinberger DR, Harrison P, editors. Schizophrenia. 3rd ed. Oxford: Wiley-Blackwell; 2011. [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in Schizophrenia: A Meta-Analytic Review. American Journal of Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]