Abstract
Bisphenol A (BPA) is a high volume production chemical used in polycarbonate plastics, epoxy resins, thermal paper receipts, and other household products. The neural effects of early life BPA exposure, particularly to low doses administered orally, remain unclear. Thus, to better characterize the dose range over which BPA alters sex specific neuroanatomy, we examined the impact of perinatal BPA exposure on two sexually dimorphic regions in the anterior hypothalamus, the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the anterioventral periventricular (AVPV) nucleus. Both are sexually differentiated by estradiol and play a role in sex specific reproductive physiology and behavior. Long Evans rats were prenatally exposed to 10, 100, 1000, 10,000 mg/kg bw/day BPA through daily, noninvasive oral administration of dosed-cookies to the dams. Offspring were reared to adulthood. Their brains were collected and immunolabeled for tyrosine hydroxylase (TH) in the AVPV and calbindin (CALB) in the SDN-POA. We observed decreased TH-ir cell numbers in the female AVPV across all exposure groups, an effect indicative of masculinization. In males, AVPV TH-ir cell numbers were significantly reduced in only the BPA 10 and BPA 10,000 groups. SDN-POA endpoints were unaltered in females but in males SDN-POA volume was significantly lower in all BPA exposure groups. CALB-ir was significantly lower in all but the BPA 1000 group. These effects are consistent with demasculinization. Collectively these data demonstrate that early life oral exposure to BPA at levels well below the current No Observed Adverse Effect Level (NOAEL) of 50 mg/kg/day can alter sex specific hypothalamic morphology in the rat.
Keywords: Endocrine disruption, Estrogen, Hypothalamus, Sexually dimorphic, Dopamine
1. Introduction
Numerous attempts have been made to characterize the impact of early life bisphenol A (BPA) exposure on sexually dimorphic brain development in rodents because of growing concern that similar effects may occur in humans (He et al., 2012; Palanza et al., 2008; Richter et al., 2007; Wolstenholme et al., 2011). Two hypothalamic regions which have garnered considerable attention, because of their well characterized estrogen-dependent structural and functional sex differences (Simerly, 2002), are the sexually dimorphic nucleus of the preoptic area (SDN-POA) and the anteroventral periventricular (AVPV) nucleus. Males and females are born with the same number of neurons in both regions, but estradiol-mediated selective cell death mediated by ERα (Patchev et al., 2004) during neonatal life rapidly induces morphological sex differences (Wright et al., 2010). Remarkably, estradiol has opposite effects on cell survival in each region such that the SDN-POA is larger in males, and the AVPV is larger in females. The female AVPV also contains more dopaminergic neurons than males (Davis et al., 1996b; Simerly, 2002; Simerly et al., 1985a). Previous studies examining BPA-related impacts on SDN-POA and AVPV volume and composition have yielded discordant results (He et al., 2012; Kwon et al., 2000; Nagao et al., 1999; Patisaul et al., 2006, 2007; Rubin et al., 2006). Inconsistencies in the data likely result, at least in part, from experimental design differences including exposure duration, dose, route of BPA administration, and critical differences in neural structure between rats and mice (Bonthuis et al., 2010). To improve data continuity, study design-related guidelines for BPA research have recently been issued including statistical control for litter effects, examination over a wide dose range, use of oral administration, employment of concurrent positive controls, and performing all evaluations blinded to the exposure groups (Goodman et al., 2006; Hengstler et al., 2011; Hunt et al., 2009; Richter et al., 2007). Here we evaluated the impact of perinatal BPA exposure on SDN-POA and AVPV structure in rats of both sexes using these design guidelines to enhance the currently available data and provide results across a wider dose range than has been done previously.
In mammals, including humans, there are numerous structural and functional sex differences throughout the brain, particularly within the hypothalamus and surrounding structures (Bonthuis et al., 2010; De Vries, 2004; Simerly, 2002), which underpin physiological and behavioral sexual dimorphisms. The SDN-POA and AVPV are ideal regions to examine because the estrogendependent mechanisms by which they sexually differentiate, both structurally and functionally, are well understood and can be predictably manipulated by exogenous hormone administration (Davis et al., 1996a; Gilmore et al., 2012; Gorski et al., 1978, 1980; Sickel and McCarthy, 2000; Simerly, 1989; Simerly et al., 1985b; Yang et al., 2004). Thus, they are well defined targets for endocrine disrupting chemicals (EDCs) such as BPA. The SDN-POA is physically larger in males while the AVPV is larger in females; morphometric differences resulting from the presence or absence of perinatal estrogens (Bleier et al., 1982; Bloch and Gorski, 1988; Murakami and Arai, 1989). The specific cellular mechanisms by which estradiol can be pro-apoptotic in the AVPV but antiapoptotic in the SDN-POA remain to be fully characterized, but likely involve region specific proinflammatory cytokine and caspase signaling pathways (Wright et al., 2010).
The number of preoptic dopaminergic neurons, identified by the presence of tyrosine hydroxylase (TH; the rate-limiting enzyme for dopamine biosynthesis), is also sexually dimorphic in rats (Patisaul et al., 2006; Simerly et al., 1985b). In females, the region comprising the AVPV and the periventricular region just caudal to it contains approximately three times as many TH immunoreactive cells as the comparable male region (Simerly, 1989). Perinatal exposure to estradiol or an aromatizable androgen such as testosterone propionate results in masculinization of this region in females, and reduced numbers of immunoreactive TH (TH-ir) cells (Simerly, 1989; Simerly et al., 1985b). In contrast, the rat SDN-POA is 2–4 times larger in males than females (Gorski et al., 1978, 1980); a trait that develops in the first two weeks of life and results from a higher apoptotic rate in females (Davis et al., 1996a; Yang et al., 2004). In males, neonatal castration lowers circulating testosterone, from which neural estrogen is derived, and SDN-POA volume is consequently reduced (Davis et al., 1996a; Gorski et al., 1978, 1980; Sickel and McCarthy, 2000). Similarly, perinatal exposure to estradiol masculinizes the female SDN-POA resulting in increased volume (Dohler et al., 1982, 1984; Gorski et al., 1978). Calbindin-D28 (CALB), a calcium-binding protein and potential neuroprotectant, is a reliable marker to define the borders of the SDN-POA (Patisaul et al., 2007; Sickel and McCarthy, 2000). Thus, CALB immunolabeling (CALB-ir) was used here to identify both the borders of the SDN-POA and cell density within it.
These physical sex differences are reflective of functional ones. Both the SDN and AVPV are thought to play a role in the display of male sex behaviors (Rhees et al., 1999; Roselli et al., 2004), and lesion studies have demonstrated the critical importance of the AVPV in generating the preovulatory gonadatropin surge in females (Gerall et al., 1980; Wiegand and Terasawa, 1982). Although still highly controversial, in humans and sheep, a smaller SDN volume has been linked to the defeminization of sexual behavior and mate choice in males (Roselli et al., 2004). In rodents, early life BPA exposure has been shown to impact both male reproductive behavior (Jones et al., 2010) and female fertility (Cabaton et al., 2011); effects consistent with AVPV and SDN perturbation.
For the present study, Long Evans pups were perinatally exposed to a wide range of BPA doses (10, 100, 1000, 10,000 µg/kg/ day) through daily, non-invasive oral administration of dosedcookies to dams. This dose range spanned the current no observable adverse effect level (NOAEL) of 50 mg/kg bw/day and the current reference dose (tolerable daily intake) of 50 µg/kg bw/ day (Chapin et al., 2008; Geens et al., 2012; NTP, 1982). Despite well recognized metabolic differences between rats and humans, the lowest dose used here likely produces dam serum levels at the top end of, or just above, the human-relevant range (Doerge et al., 2011), although the boundaries of this range remain a point of contention (FAO/WHO, 2011; Hengstler et al., 2011; Sathyanarayana et al., 2011; Taylor et al., 2010; Teeguarden et al., 2011; Twaddle et al., 2010). Exposure to the pups (through gestation and lactation) is likely lower. Prior estimates (reviewed in (Chapin et al., 2008; FAO/WHO, 2011) suggest that gestational exposure is 1.6–18.5 times lower than that of the dam, and lactational transfer is even lower. Daily intake of BPA by humans is not well characterized but estimated to be approximately 0.4–1.4 µg/kg/ day (Chapin et al., 2008; FAO/WHO, 2011).
2. Methods
2.1. Animal care and exposure
Animal care and exposure of pregnant Long Evans (LEs) dams was conducted at the University of Rochester. All experimental procedures were performed in accordance with Society for Neuroscience guidelines and University of Rochester animal care and utilization committees (UCAR).
Sixty-four timed-pregnant LE female rats (Charles River, Raleigh, NC) were orally exposed to one of four BPA dose levels, BPA 10 (n = 12), BPA 100 (n = 12), BPA 1000 (n = 10), and BPA 10,000 (n = 11) µg/kg bw/day, corn-oil vehicle (n = 11), or 17β-estradiol (n = 2). This small number of estradiol-exposed dams was included to verify the sensitivity of LE rats to estrogenic compounds using this specific exposure paradigm. Prior studies have clearly established that exposure to ≥2 µg/kg bw/day 17β -estradiol during early development is sufficient to masculinize the size and CALB-ir content of the female rat SDN-POA (Dohler et al., 1984; Gilmore et al., 2012; Gorski et al., 1978) as well asTH-ircell number in the female AVPV (Patisaul et al., 2006; Simerly, 1989; Simerly et al., 1985a).
Dams arrived on gestational day (GD) 4 and were housed under a 12-h light cycle at 74 °F and 30–70% humidity in thoroughly washed polysulfone cages on woodchip bedding, fed Purina 5001 rodent chow (Purina Lab Diet, Richmond, IN), and provided with filtered tap water in glass water bottles ad libitum. BPA doses and the corn-oil vehicle were delivered daily to pregnant dams via a quartered, Nilla® Wafer cookie from GD 12 to postnatal day (PND) 10 using procedures similar to those described previously (Patisaul et al., 2013). Thus, developing rat pups were exposed in utero and during lactation for a total exposure period of 21 days.
Corn-oil vehicle or corn-oil/BPA dose (~0.2 cm3 adjusted for bw) was applied daily to quartered, standard-sized (roughly 1″ to 1–1/4″ in circumference prior to quartering) Nilla® Wafers using a fresh, sterile 1cc syringe for each dose. The corn-oil/corn-oil BPA solution was readily absorbed by the wafer ensuring that the animal received the entire dose. Each animal had a separate, labeled weigh-boat in which the dosed cookie was transferred. The cookies were placed in the cage, away from the nesting location of the female by lifting the wire rack at a small angle (enough to accommodate the cookie) and dropped onto the bedding. Each dam was observed daily during this exposure regimen to ensure complete wafer consumption. The average time for dams to fully consume the wafer was approximately three minutes. All pups were weaned on PND 21 and randomly assigned to one of four experimental groups. Four males and four females per litter were used to generate the data.
2.2. Tissue collection and preparation
Animals were sacrificed between PNDs 65–68. Animals were deeply anesthetized with sodium pentobarbital and transcardially perfused with 0.9% NaCl followed by 400 ml 4% paraformaldehyde in 0.01 M sodium phosphate buffer (pH 7.4). Females were sacrificed in estrous (verified by vaginal cytology (Becker et al., 2005)) and weight was recorded for all animals at the time of sacrifice. Brains were removed and postfixed in 30% sucrose/4% paraformaldehyde for 3–4 h, then cryoprotected in 30% sucrose/ PBS solution for 24–72 h (Hoffman and Le, 2004). Brains were rapidly frozen on dry ice, shipped to NCSU for processing and stored at −80 °C. Each brain was coronally sectioned at 50 µm using a freezing slide microtome, divided into four series of alternating sections comprising the SDN-POA and AVPV and stored free-floating in a cryoprotectant antifreeze solution (30% sucrose, 30% ethylene glycol, 10% polyvinylpyrrolidone, 5% glycerol in 0.1 M sodium phosphate buffer) at −20 °C until staining (Hoffman and Le, 2004).
2.3. Immunohistochemistry
SDN-POA sections were immunolabeled for CALB, and sections comprising the AVPV and the periventricular region just caudal to it were immunolabeled for TH as detailed in our prior publications (Patisaul et al., 2006, 2007). Selected sections were 100 µm apart in the SDN-POA, and 200 µm in the AVPV region. Briefly, all sections were thoroughly washed at 4 °C in 0.02 M potassium phosphate buffered saline (KPBS), and endogenous peroxidase activity was quenched with a 15min wash in 0.5% H2O2 in DK-LKPBS (2% normal donkey serum and 0.3% Triton X in 0.2 M KPBS). The sections were then washed and incubated in DK-LKPBS overnight at 4 °C. SDN-POA sections were incubated in a primary antibody solution directed against CALB (Mouse Monoclonal Anti-Calbindin-D-28K (Cat # C9848, Sigma, St. Louis, MO), 1:100,000 in DK-LKPBS; Sigma, St. Louis, MO), and AVPV sections were incubated in a primary antibody directed against TH (Mouse Monoclonal Anti-Tyrosine Hydroxylase (Cat # 22941, Immunostar, Hudson, WI), 1:80,000 in DK-LKPBS) for 72 h at 4 °C. All sections were then washed and placed in a biotinylated donkey anti-mouse immunoglobulin G (IgG) secondary antibody (1:200, Jackson ImmunoResearch Laboratories, West Grove, PA) for 90 min at room temperature. The signal was amplified using an avidin-biotin complex kit (Vector Labs, Burlingame, CA) and developed using DAB chromagen (Vector Labs, Burlingame, CA). After a final set of washes, the sections were serially mounted onto Fisherbrand Superfrost Plus slides (Fisher, Pittsburgh, PA) and allowed to dry overnight. The sections were then dehydrated via washes of increasing ethanol stringency and cleared in xylene (Fisher, Pittsburgh, PA) for two hours. Slides were coverslipped with DPX mountant (Electron Microscopy Services, Hatfield, PA).
2.4. Quantification of CALB-ir and SDN-POA volume
Quantification of SDN-POA CALB-ir cells and determination of SDN-POA nuclear volume, was accomplished via unbiased stereology (Glaser and Glaser, 2000; Schmitz and Hof, 2005) as we have done previously (Patisaul et al., 2007). All analyses were done using Stereologer™ (Stereology Resource Center, Inc., MD) on a Leica DM2500P scope (Leica Microsystems, Wetzlar, Germany). The borders of the SDN-POA were clearly defined by CALB immunolabeling and confirmed using the Adult Rat Stereotaxic Atlas (Paxinos and Watson, 2007). For each section, SDN-POA borders were traced at low magnification (5×) and then analyzed at high magnification (63×). Volume was calculated using Calviari’s Principle. CALB-ir cells were counted using the optical fractionator. The complete nucleus was contained within 2–3 sections per animal. The final post-processing tissue thickness of the sections was measured to be approximately 22.7 µm, therefore the frame height was set below that threshold at 20 µm with a guard height of 1 µm. To quantify the dense population of cells, the frame area was set at 15 µm2 (3.873 µm × 3.873 µm), and framing spacing was 50 µm. The volume of the CALB-ir subregion of the SDN was quantified with a region point counting area per point of 1000 µm2. The mean coefficient of error for CALB-ir cells counted with the optical fractionator was 0.14 and for CALB-SDN volume was 0.09. Images were captured using a Qimaging Retiga 2000R 12-bit color camera (QImaging, Surry, British Columbia, Canada) mounted on a Leica DM5000B scope (Leica Microsystems, Wetzlar, Germany) using MCID Core Image software program (InterFocus Imaging Ltd., Cambridge, England).
2.5. Quantification of tyrosine hydroxylase (TH) immunoreactivity in the AVPV
Quantification of TH-ir was done with the optical fractionator as described above for CALB-ir cells in the SDN-POA. TH-ir cells were contained within 3–4 sections per animal. The final postprocessing tissue thickness of the sections was measured to be approximately 22.7 µm, therefore the frame height was set at 20µm with a guard height of 1 µm. The frame area was 2500 µm2 (50 µm × 50 µm), and framing spacing was 50 µm. The region point counting area per point was set at 1000 µm2. The mean coefficient of error for TH-ir nuclei counted with the optical fractionator was 0.09. Images were captured using a Qimaging Retiga 2000R 12-bit color camera (QImaging, Surry, British Columbia, Canada) mounted on a Leica DM5000B scope (Leica Microsystems, Wetzlar, Germany) using MCID Core Image software program (InterFocus Imaging Ltd., Cambridge, England).
2.6. Statistical analysis
Data analysis was performed using published guidelines established for assessing low-dose endocrine disruptor data (Haseman et al., 2001). Control and 17β-estradiol exposed females were compared by a Student’s t-test (pooled variance) for each measure to confirm the sensitivity of the animal model to the masculinizing influence of estradiol. For each endpoint, control and BPA exposed animals of both sexes were compared by two-way analysis of variance (ANOVA) with exposure group and sex as factors, and followed up with a one-way ANOVA within sex. Significant effects were followed up by protected Fisher’s least significant difference (LSD) post hoc analysis. Two sample t-tests (separate variance) were performed within each exposure group to address if sex difference was preserved between male and female groups. All analyses were completed using SYSTAT software (SYSTAT, Systat Software Inc., Richmond, CA) and in all cases effects were considered significant at p ≤ 0.05.
3. Results
3.1. Confirmation of strain sensitivity
Both regions were completely masculinized by 17β-estradiol, observations which confirm the sensitivity of the LE rat to oral estrogen during this critical window of development and demonstrate that it is an appropriately sensitive animal model for examining BPA effects. Estradiol exposed females (n = 2) had significantly fewer AVPV TH-ir neurons then unexposed controls (t(9.000) = 3.820, p ≤ 0.004). Similarly, SDN-POA volume (t (7.000) = 7.763, p≤ 0.001) and CALB-ir cell number (t(7.000) = 7.763, p ≤ 0.02) was significantly increased in estradiol exposed females.
3.2. Impact of BPA on SDN-POA volume and CALB-ir
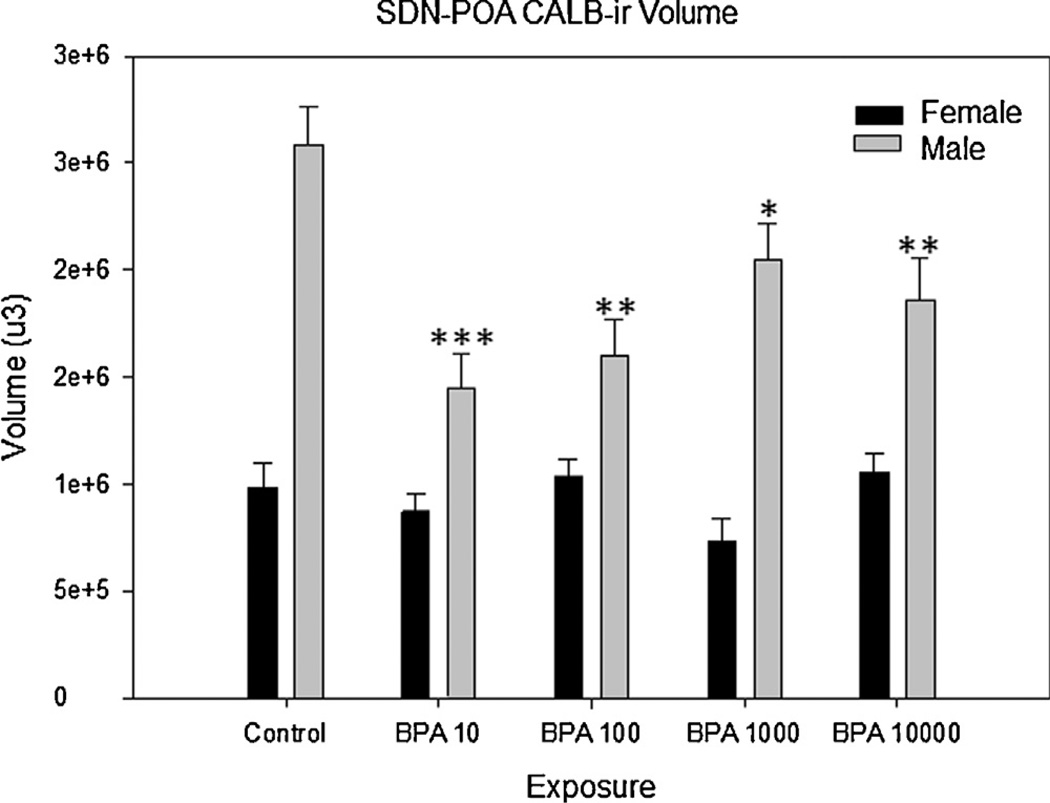
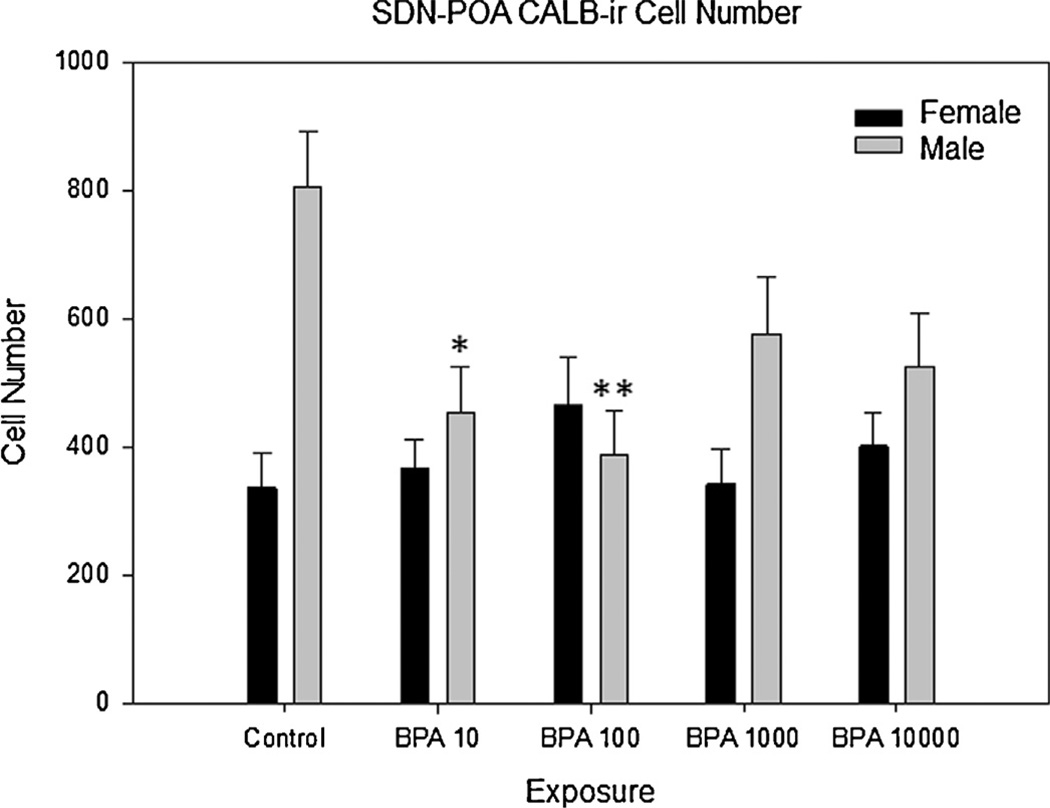
As anticipated (Davis et al., 1996a; Dohler et al., 1984; Gorski et al., 1978; Patisaul et al., 2007; Sickel and McCarthy, 2000), control males had both a significantly larger SND-POA (t(9.310) = 6.251, p≤ 0.001) and a greater number of CALB-ir cells within it (t(9.812) = 4.201, p ≤ 0.002) compared to control females. Two-way ANOVA revealed an interaction between exposure group and sex for both SDN volume (F(4,84) = 5.719, p ≤ 0.001) and CALB-ir cell numbers (F(4,81) = 3.890, p ≤ 0.006). Significant effects of exposure were only present in males. There was a main effect of BPA exposure on male SDN-POA volume (F(4,38) = 6.557, p ≤ 0.001; Fig. 1), and post hoc analysis revealed that SDN-POA volume was significantly smaller in all BPA exposed groups compared to unexposed controls (Fig. 3). Volumetric effects were greatest in the BPA 10 µg/kg/day (p ≤ 0.001) and BPA 100 µg/kg/day (p ≤ 0.001) groups, with mean volume decreased 45% and 39%, respectively. There was also a main effect of exposure on the number of CALB-ir cells in the male SDN-POA (F(4,40) = 3.9445, p ≤ 0.009) with numbers significantly decreased in all BPA exposed groups compared to the unexposed controls (p ≤ 0.003 for all) except the BPA 1000 group (p = 0.07; Fig. 2). Two sample t-tests within each group revealed that the sex difference in SDN-POA volume was preserved in all exposure groups (BPA 10: t(13.577) = 3.174, p ≤ 0.007; BPA 100: t(17.424) = 3.631, p ≤ 0.002; BPA 1000: t(10.013) = 7.415, p≤ 0.001; BPA 10,000: t(8.399) = 4.098, p ≤ 0.003). The sex difference in CALB-ir cell density, however, was only preserved in the BPA 1000 exposure group (t(11.552) = 2.376, p ≤ 0.036), and lost in the other groups.
Fig 1.
Bisphenol-A exposure on SDN-POA CALB-ir volume in adult male and female rats. There was a main effect of BPA exposure on the volume of the SDN-POA observed between control male and all male exposure groups (p ≤ 0.001). No significant change was observed in female exposure groups. All exposure groups are in µg/kg/day. S.E. is indicated by error bars. Control M, F (n = 8, 7); BPA 10 M, F (n = 10, 13); BPA 100 M, F (n = 9, 13); BPA 1000 M, F (n = 9, 7); BPA 10,000 M, F (n = 7, 11); statistically significant change within exposure groups as compared to control animals within the same sex are as indicated: *p < 0.01–0.05, **p < 0.001– 0.01, ***p < 0.001.
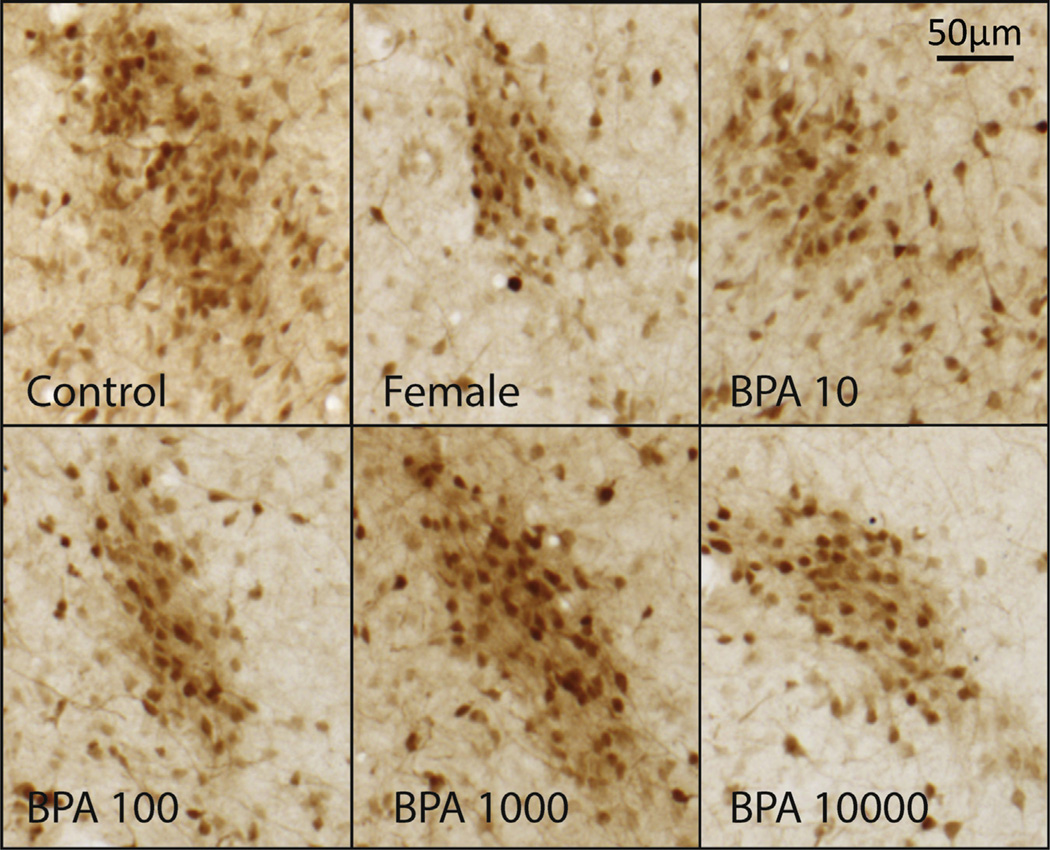
Fig 3.
SDN-POA CALB-ir cells in exposed males compared to control males and females. Representative images (10×) depicting the density and distribution of CALB-ir within the SDN-POA of vehicle treated males (control) and females (females) and males exposed to BPA (10, 100, 1000, and 10,000 µg/kg bw/day).
Fig 2.
Bisphenol-A exposure on SDN-POA CALB-ir cell number in adult male and female rats. There was a significant overall effect of BPA exposure (p ≤ 0.009) in male exposure groups BPA 10 and BPA 100 as compared to control males. All exposure groups are in µg/kg/day. S.E. is indicated by error bars. Control M, F (n = 7, 9); BPA 10 M, F(n = 11,13); BPA 100 M, F (n = 12, 5); BPA 1000 M, F(n = 7, 9); BPA 10,000 M, F (n = 8, 10); statistically significant change within exposure groups as compared to control animals within the same sex are as indicated: *p < 0.01–0.05, **p < 0.001–0.01, ***p < 0.001, †p = 0.072.
3.3. Impact of BPA on AVPV TH-ir
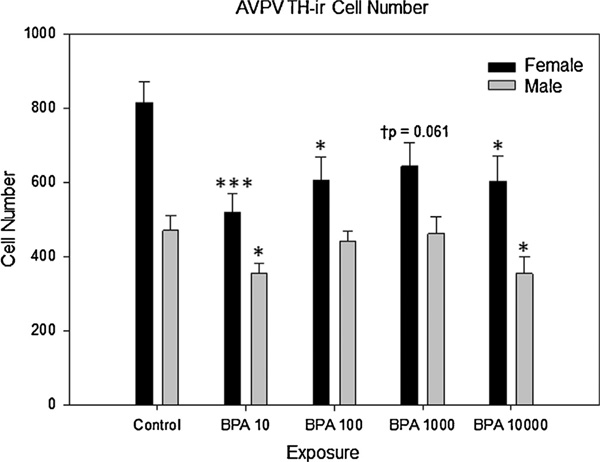
As expected (Davis et al., 1996b; Patisaul et al., 2006; Simerly et al., 1985a), AVPV TH-ir was sexually dimorphic in the unexposed controls (t(15.201) = 4.807, p ≤ 0.001), with females having nearly twice as many TH-ir neurons than males (Fig. 4). Two-way ANOVA revealed a main effect of sex (F(4,92) = 4.694, p ≤ 0.002) and a main effect of exposure (F(1,92) = 42.214, p ≤ 0.001) but no significant interaction between sex and exposure. Because TH-ir is sexually dimorphic, subsequent analyses were performed within sex. One way ANOVA within sex revealed a main effect of BPA exposure on AVPV TH-ir cell numbers in females (F(4,52) = 3.142, p ≤ 0.02) and males (F(4,39) = 4.153, p ≤ 0.007) (Fig. 5). TH-ir cell numbers were significantly lower in all BPA exposed females (p ≤ 0.05 for all) with the exception of the BPA 1000 group (†p ≤ 0.06). In males, TH-ir cell numbers were only significantly impacted in the BPA 10 (p≤0.03) and BPA 10,000 (p ≤ 0.013) exposure groups, with fewer TH-ir cells compared to controls. Two sample t-tests within each exposure group revealed that the sex difference in TH-ir cell density was preserved in all exposure groups (BPA 10: t(19.743) 2.779, p≤0.01; BPA 100: t(16.069) = 2.423,p ≤ 0.03; BPA 1000: t(16.270) = 2.670,p ≤ 0.02; BPA 10,000: t(18) = 2.943, p ≤ 0.01).
Fig 4.
Early developmental bisphenol-A exposure on AVPV-TH-ir cell number in adult male and female rats. There was a main effect of BPA exposure on AVPV-TH-ir cell number observed between control female and all female exposure groups (p ≤ 0.022), with the exception of BPA 1000 (†p = 0.061). In male exposure groups, BPA exposure significantly effected (p ≤ 0.007) AVPV-TH-ir cell number in BPA 10 and BPA 10,000 groups as compared to control males. All exposure groups are in µg/ kg/day. S.E. is indicated by error bars. Control M,F(n = 9,10); BPA 10M,F(n = 9,13); BPA 100 M, F (n = 10, 12); BPA 1000 M, F (n = 8, 11); BPA 10,000 M, F (n = 8, 11); statistically significant change within exposure groups as compared to control animals within the same sex are as indicated: *p < 0.01–0.05, ***p < 0.001.
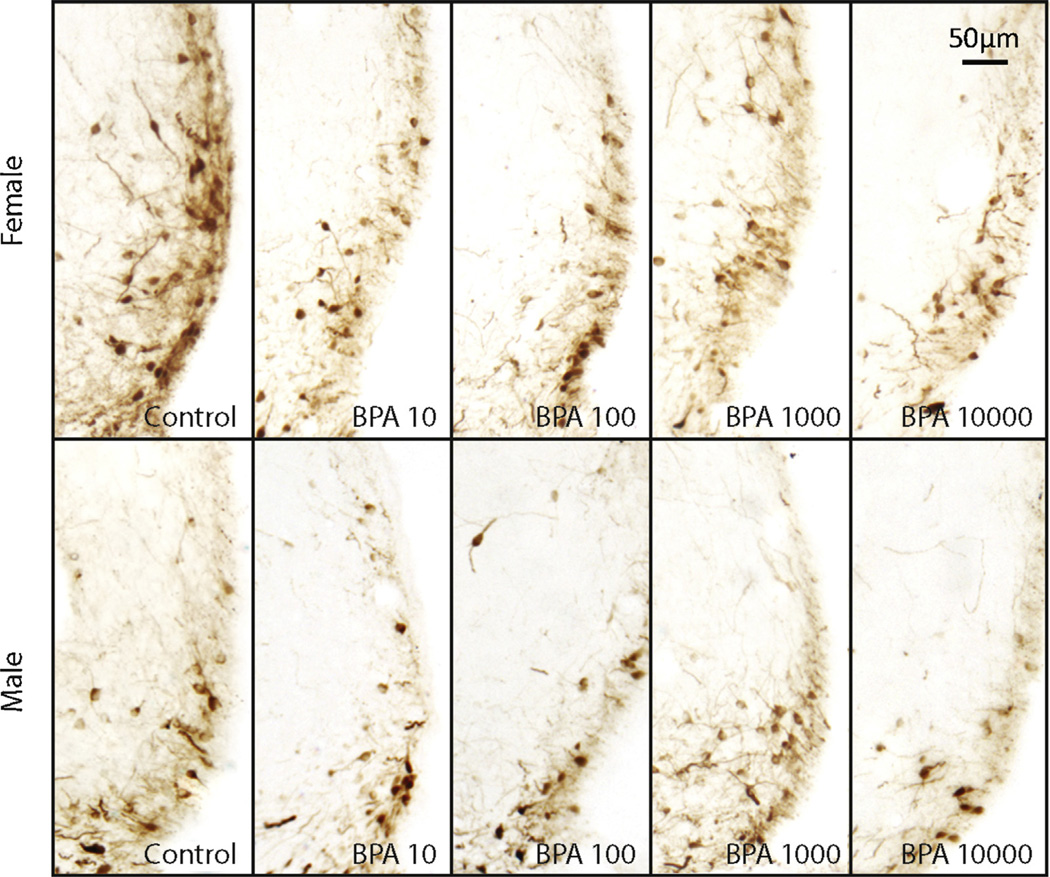
Fig 5.
AVPVTH-ir cells in males (top panels) and females (bottom panels). Representative images (10×) depicting the density and distribution ofTH-ir neurons in the vehicle controls and BPA exposed (10, 100, 1000, and 10,000 µg/kg bw/day) animals of both sexes.
4. Discussion
Perinatal BPA exposure via oral exposure to the dam altered the structure and composition of the rat SDN-POA and AVPV within a dose range encompassing the current reference dose of 50 µg/ kg bw/day. Effects were region and sex specific with evidence of demasculinization in the male SDN-POA, and evidence of defeminization in the female AVPV. All effects were significant at the lowest dose employed (10 µg/kg bw/day). Despite well recognized metabolic differences between rats and humans, this dose likely produces serum levels at the top end of the humansrelevant range (Chapin et al., 2008; Doerge et al., 2011), although the bounds of this range remain unresolved (FAO/WHO, 2011; Hengstler et al., 2011; Sathyanarayana et al., 2011; Taylor et al., 2010; Teeguarden et al., 2011; Twaddle et al., 2010). Importantly, the effects occurred at exposure levels below those needed to increase uterine weight, suggesting that the brain may be a more sensitive endpoint when considering the potential of BPA, and other endocrine disruptors, of interfering with the organizational role of estrogen on hormone sensitive structures. Why the brain is more responsive than the uterus is unclear but differences in the milieu of co-factors and co-repressors needed for estrogen dependent transcription, activation of non-classical signaling pathways, or induction of epigenetic changes which enhance estrogen receptor activity are plausible (McCarthy et al., 2009; Yeo et al., 2013). Collectively, these data suggest that steroid hormone sensitive brain regions may be vulnerable to endocrine disruption by BPA resulting in altered sex specific morphology.
SDN-POA effects were only observed in males, which is consistent with a prior report from our research group (Patisaul et al., 2007). An important difference, however, is the direction of the effect. In the prior study, male Sprague Dawley (SD) rats were subcutaneously injected with 250 µg BPA every 12 h over the first two days of life, which is approximately equivalent to 42 mg/kg bw per day, and 4-fold higher than the highest dose used in the present study. At this higher dose, SDN-POA volume was unchanged but the number of CALB-ir cells was significantly increased. Other research groups have also observed that SDN-POA volume is unaltered by high dose developmental BPA exposure. For example, neonatal injections of 300,000 µg/kg bw/day (Nagao et al., 1999), or perinatal oral exposure to 200,000–400,000 µg/kg bw/day failed to alter male SDN-POA volume in SD rats (Kwon et al., 2000; Takagi et al., 2004). Here, however, we found that low dose perinatal BPA exposure, reduced, rather than increased, CALB-ir neuron numbers, and male SDN-POA volume was also diminished. These effects were most pronounced at the lowest doses used (10 and 100 µg/kg/day bw orally).
One possible explanation for these dose-dependent differences on SDN-POA morphometrics across studies is that the direction of the effect reverses across the dose curve; with demasculinization occurring at low doses and resistance to change or subtle hypermasculinization occurring at higher doses. Collectively, our data suggest that the inflection point is around 100 µg/kg bw/day. The mechanism by which this dose-specific response reverses direction remains unclear but likely involves a dose-dependent interaction with estrogen signaling. In the female SDN-POA, the masculinizing effect of estradiol can be induced by selective agonism of ERa but not ERb, demonstrating the importance of ERa for enhancing volume (Patchev et al., 2004). BPA may inhibit estrogen signaling at low doses, resulting in increased apoptosis, but augment estrogen signaling at high doses, thereby enhancing cell preservation in this region.
The dose-dependent volumetric change hypothesis posed above is supported by all available data published to date on the rat SDN-POA, with the exception of a recent study, which reported evidence for hypermasculinization at 2.5 µg/kg/day bw; a dose lower than any used in the present study (He et al., 2012). Key design elements in that study may account for this discrepant finding, one of which is the route of oral dosing employed. For their study, He and colleagues (2012) used orogastric gavage (to the dams during gestation and then directly to the pups until weaning) which is a popular oral exposure route because it ensures precise dosing, but can be stressful to the animals (Balcombe et al., 2004). Significant differences in pup weight and survival between litters born to vehicle gavaged and naïve (ungavaged) controls were observed (Ferguson et al., 2011), suggesting that gavage-related stress might have confounded toxicant-related effects on hormone sensitive brain endpoints, including the SDN-POA. Subsequent work will be needed to more clearly establish how early life stress, such as the stress associated with gavage or other forms of handling, interact with exposures to endocrine disruptors and other toxicants to alter brain morphology and sex specific organization.
For the present study, BPA was administered to the dams using a food treat, which eliminates the potential confound of dosing stress but relies on lactational transfer for the pups to be effectively dosed after birth. In humans, BPA has been shown accumulate in fetal liver tissue (Nahar et al., 2012), serum, and amniotic fluid (Engel et al., 2006; Ikezuki et al., 2002) demonstrating the capacity for gestational exposure. Of note, fetal BPA concentrations were shown to have a greater variance in retention time, greater mean retention time, and a longer terminal half-life than that of dams (e.g., half-life from 6 to 48 h collection was approximately 37.2 times greater in fetuses than in dam blood) (Chapin et al., 2008; FAO/WHO, 2011). In rats, however, lactational transfer of BPA appears to be less efficient, with pup serum concentrations 300 times lower than the exposed dams (Chapin et al., 2008; Doerge et al., 2010). It is therefore possible that, in the present study, BPA exposure occurred primarily during gestation. If so, then these data would suggest that the observed decreases in male SDN-POA volume and CALB content result primarily from exposure prior to birth. In female rats, postnatal exposure to diethylstilbestrol (DES) is more effective at masculinizing the SDN-POA than prenatal exposure (Tarttelin and Gorski, 1988). Subsequent work established that the hormone-sensitive period for SDN-POA sexual differentiation begins on GD 18 (Rhees et al., 1990a) and ends abruptly on PND 5 (Rhees et al., 1990b), with males being more sensitive to hormone manipulation than females. In the present study, lower BPA exposure during postnatal life due to poor lactational transfer could account for why no statistically significant effects of BPA exposure were observed in the female SDN-POA. Moreover, in the study reporting increased male SDN-POA volume at 2.5 µg/kg bw/day (He et al., 2012), animals were exposed during gestation but then also gavaged directly up to the point of sacrifice at weaning. This post-natal exposure may be why low dose BPA enhanced SDN-POA volume in their exposure paradigm but not ours.
In contrast to the SDN-POA, effects in the AVPV were observed in both sexes. In the male AVPV, hypermasculinization of TH-ir numbers occurred only in the lowest and highest BPA exposure groups (10 µg/kg/day and 10,000 µg/kg/day) suggesting a non-monotonic dose response. In females, evidence of masculinization was observed across all BPA exposed groups, but did not achieve statistical significance in the BPA 1000 group (†p ≤ 0.06). These results are in accord with a prior study, using CD1 mice exposed via mini-pumps to 25 or 250 ng/kg bw/BPA from GD 16 to PND 16, which also found masculinization of the female AVPV (Rubin et al., 2006). Similar to what was observed in the SDN-POA, at higher doses, the sex specific outcome appears to reverse. We have previously shown that s.c. injection of 250 µg BPA every 12 h over the first two days of life, has no effect on total TH-ir numbers in females, but significantly increases TH-ir levels in males (Patisaul et al., 2006). This effect is consistent with the hypothesis that BPA blocks estrogen action at low doses but augments it at higher doses. Interactions with ERβ may also play a role. Neonatal exposure to an ERβ selective agonist masculinizes the female AVPV (Bodo et al., 2006; Patchev et al., 2004), and ERβKO males have been shown to possess an abnormally high number of TH-ir neurons in the AVPV (Bodo et al., 2006). At birth, ERβ expression is higher in the male AVPV, but this sex difference equalizes by PND 2 (Cao and Patisaul, 2011). Exposure to 50 µg/kg bw or 50mg/kg bw/BPA by s.c. injection from birth to PND 2 significantly eliminates AVPV ERβ expression levels in both sexes by PND 4 suggesting that altered ER expression could underlie the morphometric changes reported here.
Prior studies exploring the impact of early life BPA exposure on brain development and gene expression have produced inconsistent and conflicting data (Palanza et al., 2008; Richter et al., 2007; Wolstenholme et al., 2011) which has confounded risk assessment. Although a number of study design elements, including differences in exposure duration, dose, route of BPA administration, and critical species differences in neural structure between rats and mice (Bonthuis et al., 2010), likely account for the discordance in the literature, concerns about the sensitivity of some rat strains to gonadal steroid-derived effects have also been raised (Long et al., 2000; Richter et al., 2007; Steinmetz et al., 1998). Thus, we exposed a very small group of females (n = 2) to 17β-estradiol to expressly confirm the estrogen sensitivity of the LE rat strain used for this study. As expected based on numerous prior studies using LE rats (Cao et al., 2012; Fader et al., 1998; Ford et al., 2004; Laws et al., 2000), all endpoints were fully masculinized in the estradiolexposed females, including SDN-POA volume (Dohler et al., 1984; Gilmore et al., 2012; Gorski et al., 1978; Patchev et al., 2004) and decreased TH-ir cell number in the AVPV (Patisaul et al., 2006; Simerly, 1989). Assessment of strain sensitivity by including a concurrent positive control for the predicted effect is critical when attempting to evaluate the impact of BPA, especially when no significant effects are observed.
The potential for human-relevant exposure to result in adverse health outcomes remains a subject of active debate (Beronius et al., 2010; Hengstler et al., 2011; Vandenberg et al., 2009). It is important to emphasize that species differences make organizational neuroendocrine effects in animals difficult to apply to human risk assessment. In humans, androgen rather than estrogens is thought to be most important for masculinizing the brain during development (Grumbach, 2002; Wallen, 2005). This difference makes it challenging to infer how endocrine disrupting compounds, like BPA, might impact the sexual differentiation of the human hypothalamus or other brain regions. Our data reveal that the rat anterior hypothalamus is sensitive to endocrine disruption by BPA at oral doses below the current reference dose, suggesting that neural effects in humans are plausible. Animals were exposed perinatally, a critical period that is entirely prenatal in humans. Prenatal exposure to BPA has been demonstrated in humans (Braun et al., 2011,2009; Calafat et al., 2008) and associated with elevated anxiety and hyperactivity in young girls. Collectively, these epidemiological data support the possibility that developmental BPA exposure has adverse, sex specific, neural effects in humans.
Acknowledgments
This work was supported by NIEHS 1RC2 ES018736. The authors would like to acknowledge Jinyan Cao, Meghan Radford, Emily Sluzas, and Sandra Losa-Ward for their invaluable contributions to tissue processing, as well as their editorial comments. We would also like to thank the animal care staff at the University of Rochester.
Footnotes
Conflict of interest
None.
References
- Balcombe JP, Barnard ND, Sandusky C. Laboratory routines cause animal stress. Contemp Top Lab Anim Sci Am Assoc Lab Anim Sci. 2004;43:42–51. [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Beronius A, Ruden C, Hakansson H, Hanberg A. Risk to all or none? A comparative analysis of controversies in the health risk assessment of bisphenol. A Reprod Toxicol. 2010;29:132–146. doi: 10.1016/j.reprotox.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Bleier R, Byne W, Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982;212:118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- Bloch GJ, Gorski RA. Estrogen/progesterone treatment in adulthood affects the size of several components of the medial preoptic area in the male rat. J Comp Neurol. 1988;275:613–622. doi: 10.1002/cne.902750409. [DOI] [PubMed] [Google Scholar]
- Bodo C, Kudwa AE, Rissman EF. Both estrogen receptor-alpha and -beta are required for sexual differentiation of the anteroventral periventricular area in mice. Endocrinology. 2006;147:415–420. doi: 10.1210/en.2005-0834. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF. Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol. 2010;31:341–358. doi: 10.1016/j.yfrne.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, et al. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119:131–137. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, et al. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect. 2009;117:1945–1952. doi: 10.1289/ehp.0900979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Mickens JA, McCaffrey KA, Leyrer SM, Patisaul HB. Neonatal bisphenol A exposure alters sexually dimorphic gene expression in the postnatal rat hypothalamus. Neurotoxicology. 2012;33:23–36. doi: 10.1016/j.neuro.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Patisaul HB. Sexually dimorphic expression of hypothalamic estrogen receptors alpha and beta and Kiss1 in neonatal male and female rats. J Comp Neurol. 2011;519:2954–2977. doi: 10.1002/cne.22648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, Gray LE, Jr, Hayward SW, Lees PS, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A Birth defects research part B. Develop Reprod Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996a;734:10–18. [PubMed] [Google Scholar]
- Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996b;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol Appl Pharmacol. 2011;255:261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Vanlandingham M, Twaddle NC, Delclos KB. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol Lett. 2010;199:372–376. doi: 10.1016/j.toxlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci Lett. 1982;33:295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- Dohler KD, Coquelin A, Davis F, Hines M, Shryne JE, Gorski RA. Pre- and postnatal influence of testosterone propionate and diethylstilbestrol on differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res. 1984;302:291–295. doi: 10.1016/0006-8993(84)90242-7. [DOI] [PubMed] [Google Scholar]
- Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod Toxicol. 2006;21:110–112. doi: 10.1016/j.reprotox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Fader AJ, Hendricson AW, Dohanich GP. Estrogen improves performance of reinforced T-maze alternation and prevents the amnestic effects of scopolamine administered systemically or intrahippocampally. Neurobiol Learn Mem. 1998;69:225–240. doi: 10.1006/nlme.1998.3820. [DOI] [PubMed] [Google Scholar]
- FAO/WHO. Toxicological and health aspects of bisphenol A: report of joint FAO/WHO expert meeting and report of stakeholder meeting on bisphenol A. World Health Organization. 2011 [Google Scholar]
- Ferguson SA, Law CD, Jr, Abshire JS. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol Sci Off J Soc Toxicol. 2011;124:149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- Ford MM, Eldridge JC, Samson HH. Determination of an estradiol dose-response relationship in the modulation of ethanol intake. Alcohol Clin Exp Res. 2004;28:20–28. doi: 10.1097/01.ALC.0000108647.62718.5A. [DOI] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Gerall AA, Dunlap JL, Sonntag WE. Reproduction in aging normal and neonatally androgenized female rats. J Comp Physiol Psychol. 1980;94:556–563. doi: 10.1037/h0077690. [DOI] [PubMed] [Google Scholar]
- Gilmore RF, Varnum MM, Forger NG. Effects of blocking developmental cell death on sexually dimorphic calbindin cell groups in the preoptic area and bed nucleus of the stria terminalis. Biol Sex Differ. 2012;3:5. doi: 10.1186/2042-6410-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser JR, Glaser EM. Stereology, morphometry, and mapping: the whole is greater than the sum of its parts. J Chem Neuroanat. 2000;20:115–126. doi: 10.1016/s0891-0618(00)00073-9. [DOI] [PubMed] [Google Scholar]
- Goodman JE, McConnell EE, Sipes IG, Witorsch RJ, Slayton TM, Yu CJ, et al. An updated weight of the evidence evaluation of reproductive and developmental effects of low doses of bisphenol A. Critic Rev Toxicol. 2006;36:387–457. doi: 10.1080/10408440600758317. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;143:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Harlan RE, Jacobson CD, Shryne JE, Southam AM. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980;193:529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- Haseman JK, Bailer AJ, Kodell RL, Morris R, Portier K. Statistical issues in the analysis of low-dose endocrine disruptor data. Toxicol Sci. 2001;61:201–210. doi: 10.1093/toxsci/61.2.201. [DOI] [PubMed] [Google Scholar]
- He Z, Paule MG, Ferguson SA. Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol Teratol. 2012;34:331–337. doi: 10.1016/j.ntt.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Hengstler JG, Foth H, Gebel T, Kramer PJ, Lilienblum W, Schweinfurth H, et al. Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A. Critic Rev Toxicol. 2011;41:263–291. doi: 10.3109/10408444.2011.558487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman GE, Le WW. Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides. 2004;25:425–431. doi: 10.1016/j.peptides.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod. 2009;81:807–813. doi: 10.1095/biolreprod.109.077008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Human Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Jones BA, Shimell JJ, Watson NV. Pre- c results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Horm Behav. 2010 doi: 10.1016/j.yhbeh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Kwon S, Stedman DB, Elswick BA, Cattley RC, Welsch F. Pubertal development and reproductive functions of Crl:CD BR Sprague-Dawley rats exposed to bisphenol A during prenatal and postnatal development. Toxicol Sci Off J Soc Toxicol. 2000;55:399–406. doi: 10.1093/toxsci/55.2.399. [DOI] [PubMed] [Google Scholar]
- Laws SC, Carey SA, Ferrell JM, Bodman GJ, Cooper RL. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Long X, Steinmetz R, Ben-Jonathan N, Caperell-Grant A, Young PC, Nephew KP, et al. Strain differences in vaginal responses to the xenoestrogen bisphenol A. Environ Health Perspect. 2000;108:243–247. doi: 10.1289/ehp.00108243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Wright CL, Schwarz JM. New tricksby anold dogma: mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav. 2009;55:655–665. doi: 10.1016/j.yhbeh.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Arai Y. Neuronal death in the developing sexually dimorphic periventricular nucleus of the preoptic area in the female rat: effect of neonatal androgen treatment. Neurosci Lett. 1989;102:185–190. doi: 10.1016/0304-3940(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Nagao T, Saito Y, Usumi K, Kuwagata M, Imai K. Reproductive function in rats exposed neonatally to bisphenol A and estradiol benzoate. Reprod Toxicol. 1999;13:303–311. doi: 10.1016/s0890-6238(99)00017-9. [DOI] [PubMed] [Google Scholar]
- Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol. 2012;27(2):116–123. doi: 10.1002/jbt.21459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTP. Carcinogenesis Bioassay of Bisphenol A (CAS No. 80-05-7) in F344 Rats and B6C3F1 Mice (Feed Study) Natl Toxicol Program Tech Rep Ser. 1982;215:1–116. [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environ Res. 2008;108:150–157. doi: 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Patchev AV, Gotz F, Rohde W. Differential role of estrogen receptor isoforms in sexspecific brain organization. FASEB J. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–118. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster((R)) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27:124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. London: Academic Press; 2007. [Google Scholar]
- Rhees RW, Al-Saleh HN, Kinghorn EW, Fleming DE, Lephart ED. Relationship between sexual behavior and sexually dimorphic structures in the anterior hypothalamus in control and prenatally stressed male rats. Brain Res Bull. 1999;50:193–199. doi: 10.1016/s0361-9230(99)00191-4. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990a;21:781–786. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res. 1990b;52:17–23. doi: 10.1016/0165-3806(90)90217-m. [DOI] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Larkin K, Resko JA, Stellflug JN, Stormshak F. The volume of a sexually dimorphic nucleus in the ovine medial preoptic area/anterior hypothalamus varies with sexual partner preference. Endocrinology. 2004;145:478–483. doi: 10.1210/en.2003-1098. [DOI] [PubMed] [Google Scholar]
- Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of altered brain sexual differentiation in mice exposed perinatally to low, environmentally relevant levels of bisphenol A. Endocrinology. 2006;147:3681–3691. doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana S, Braun JM, Yolton K, Liddy S, Lanphear BP. Case report: high prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ health Perspect. 2011;119:1170–1175. doi: 10.1289/ehp.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Sickel MJ, McCarthy MM. Calbindin-D28K immunoreactivity is a marker for a subdivision of the sexually dimorphic nucleus of the preoptic area of the rat: developmental profile and gonadal steroid modulation. J Neuroendocrinol. 2000;12:397–402. doi: 10.1046/j.1365-2826.2000.00474.x. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Ann Rev Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Gorski RA. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985a;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985b;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Steinmetz R, Mitchner NA, Grant A, Allen DL, Bigsby RM, Ben-Jonathan N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology. 1998;139:2741–2747. doi: 10.1210/endo.139.6.6027. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shibutani M, Masutomi N, Uneyama C, Takahashi N, Mitsumori K, et al. Lack of maternal dietary exposure effects of bisphenol A and nonylphenol during the critical period for brain sexual differentiation on the reproductive/endocrine systems in later life. Arch Toxicol. 2004;78:97–105. doi: 10.1007/s00204-003-0517-0. [DOI] [PubMed] [Google Scholar]
- Tarttelin MF, Gorski RA. Postnatal influence of diethylstilbestrol on the differentiation of the sexually dimorphic nucleus in the rat is as effective as perinatal treatment. Brain Res. 1988;456:271–274. doi: 10.1016/0006-8993(88)90227-2. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, et al. Similarity of bisphenol A pharmacokinetics in Rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect. 2010;119(4):422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, et al. Twenty-four hour human urine and serum profiles of bisphenol a during high-dietary exposure. Toxicol Sci. 2011;123:48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- Twaddle NC, Churchwell MI, Vanlandingham M, Doerge DR. Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:3011–3020. doi: 10.1002/rcm.4733. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm Behav. 2011;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CL, Schwarz JS, Dean SL, McCarthy MM. Cellular mechanisms of estradiolmediated sexual differentiation of the brain. Trends Endocrinol Metab. 2010;21:553–561. doi: 10.1016/j.tem.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SL, Chen YY, Hsieh YL, Jin SH, Hsu HK, Hsu C. Perinatal androgenization prevents age-related neuron loss in the sexually dimorphic nucleus of the preoptic area in female rats. Develop Neurosci. 2004;26:54–60. doi: 10.1159/000080712. [DOI] [PubMed] [Google Scholar]
- Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, et al. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci U S A. 2013;110(11):4315–4320. doi: 10.1073/pnas.1300959110. [DOI] [PMC free article] [PubMed] [Google Scholar]