Abstract
Aim
The aim of this study was to evaluate the efficacy of isoproterenol and prednisolone in the treatment of subcutaneous lipomas.
Methods
The first experiment evaluated in vitro lipolysis induced by isoproterenol 10−6 M alone and across a range of prednisolone concentrations to determine the optimal dose to maximize lipolysis. The second experiment evaluated lipolysis in a lipoma and subcutaneous fat by in vivo microdialysis in five subjects to isoproterenol 10−6 M with and without prednisolone 10−6 M. These five subjects and five additional subjects had a lipoma treated five times a week for 4 weeks in a 4-cm grid with 0.2 ml injections of 10−6 M isoproterenol and 10−6 M prednisolone. Lipoma size was followed monthly for 1 year or until surgical removal.
Results
Prednisolone increased in vitro lipolysis to isoproterenol and 10−6 M was the optimal concentration of both drugs. Lipomas responded with less lipolysis to isoproterenol than subcutaneous fat during microdialysis, and prednisolone treatment increased lipolysis in both lipomas and subcutaneous fat. Injection treatment of the lipomas decreased their volume 50%. All but one lipoma grew after treatment. Eight of the 10 subjects elected for surgical treatment, and the histology of the lipomas was normal fat tissue.
Conclusions
Prednisolone and isoproterenol in combination increased lipolysis, and injections of the combination into lipomas decreased their volume 50% over 4 weeks. Eight of the 10 subjects elected for surgical removal.
Keywords: adipose tissue, cellular pharmacology, clinical trial, drug mechanism, fat, obesity therapy
Introduction
Lipomas are benign subcutaneous tumours that grow at slightly faster rates than the surrounding fat tissue. Maximal rates of lipolysis and lipogenesis in lipomas seem to be the same as in normal fat tissue [1]. The ability to regulate glycolysis is impaired in lipomas and this is manifested by a lack of feedback inhibition on phosphofructokinase by citrate. This, in contrast to normal fat tissue, allows glycolysis to operate maximally in lipomas under all conditions creating an excess of glyceride–glycerol. This lack of feedback inhibition has been postulated to be secondary to a defect in the regulatory site of phosphofructokinase that does not affect the catalytic site [1].
The traditional treatment of lipomas has been surgical excision, but this therapy is often limited by a poor aesthetic outcome because of deformities and scarring. Attempts have been made to treat lipomas with injections of phosphatidylcholine combined with deoxycholate, but this therapy gives an average size reduction of <50% and is associated with inflammation and pain that lasts for 3 days after the injection [2].
Isoproterenol is a non-selective β-adrenergic agonist that is approved for subcutaneous injection [3]. β-adrenergic stimulation of subcutaneous adipose tissue by the injection of isoproterenol causes local fat reduction without systemic toxicity, presumably by stimulating lipolysis locally [4]. β-adrenergic stimulation of lipolysis, however, downregulates the β-2 receptor causing tachyphylaxis through phosphorylation of the specific β-2 receptor agonist-dependent β-adrenergic receptor kinase and sequestration of the receptors away from the cell surface [5,6]. This effect has been measured in humans using microdialysis. The glycerol release in response to β-2 adrenergic stimulation decreases after 30 min of stimulation, and this effect is important physiologically as well as pharmacologically, because glycerol release is also reduced by 41% in response to 60 min of exercise at 50% of the maximal aerobic power [7–9].
Corticosteroids have been shown to increase the appearance of β-2 receptors on murine fat cells and increase the efficiency of coupling between the occupied receptors and other components of the adenylate cyclase system at low concentrations (2.5 nM–10 µM) [10]. This prevention of β-2 adrenergic receptor downregulation by low doses of corticosteroids has been confirmed in human subcutaneous fat cells [11,12]. Studies with human lymphocytes confirmed that corticosteroids block downregulation of the β-2 adrenergic receptor [13]. Although there is reason to believe that corticosteroid injections might increase lipolysis by preventing downregulation to endogenous β-adrenergic stimulation, the results of steroid injections to lipomas have not been reported and the efficacy is not known.
As lipolysis in lipomas is reported to be normal, it is possible that the association of isoproterenol with prednisolone (a corticosteroid that is also approved for subcutaneous injection) to prevent downregulation of the β-2 adrenergic receptor will decrease the size of lipomas more effectively than isoproterenol alone and without the pain associated with phosphatidylcholine and deoxycholate. The aim of this study was to test the efficacy of isoproterenol combined with prednisolone for the treatment of lipoma.
Materials and Methods
Study 1—In Vitro Studies to Determine Dose
To determine the dose of prednisolone required to prevent β adrenergic receptor downregulation with 1 µM isoproterenol [3], we measured lipolysis in fat cells after a 24-h incubation with either 1 µM isoproterenol alone or 1 µM isoproterenol with prednisolone concentrations of 10−2, 10−3, 10−4, 10−5, 10−6, 10−7, 10−8 and 10−9 M. The dose of prednisolone chosen for the microdialysis experiments was one log order above the lowest dose (to account for possible dilution in the tissue) of prednisolone that maximally prevented downregulation of the β-adrenergic receptor.
Differentiated human adipocytes adherent to the bottom of a 96-well plate were washed, treated with 150 µL of media containing prednisolone or a media control and incubated at 37 ° C, 5% CO2 for 5 h. After incubation, a 100-µL aliquot was removed and combined with 100 ml glycerol reagent to create a colorimetric assay dependent on the amount of glycerol released during incubation with the test compounds. Samples were then read in a spectrophotometer plate reader at 540 nm and the results were plotted based on the standard curve. Eight replicates were conducted for each condition. Data were analysed by t-tests and expressed as the mean ± standard error of the mean (s.e.m.).
Study 2—Clinical Study: Microdialysis
We conducted a clinical study (ClinicalTrials.gov ID # NCT 00624416) with microdialysis in 10 individuals to test (i) whether prednisolone treatment (48 and 24 h prior to microdialysis) would potentiate lipolysis and (ii) whether repeated infusions of isoproterenol into subcutaneous adipose tissue and subcutaneous lipomas would result in reduced lipolysis.
Ten individuals between the ages of 18 and 60 years with a body mass index between 20 and 40 kg/m2 were recruited for this study. Eligible subjects had at least one lipoma 2.54 cm or greater in diameter, were weight stable (±5 kg) and had a stable exercise routine or were sedentary (<60 min of planned exercise per week) for 3 months prior to entry into the study. Subjects with a current or previous history of type 1 diabetes, cardiovascular disease, elevated blood pressure (>140/90), kidney disease, liver disease, untreated thyroid disease, alcohol or substance abuse, smokers and pregnant women were excluded. Subjects taking a β-adrenergic stimulator, a β-adrenergic blocker or glucocorticoids were excluded.
Screening included a medical history and physical examination and measures of height, weight, blood pressure, pulse, temperature and heart health (electrocardiogram). A 24-h urine collection was performed to assess free urinary cortisol and blood was collected for a chemistry panel (glucose, creatinine, potassium, uric acid, albumin, calcium, magnesium, creatine phosphokinase, alanine leucine transaminase, alkaline phosphatase, iron, cholesterol, triglycerides, low-density lipoprotein cholesterol and high-density lipoprotein cholesterol) and a complete blood count (haemoglobin, haematocrit, mean cell volume, platelet count, white blood cell count, granulocyte number, neutrophil number, basophil number and eosinophil number). Five subjects passing screening underwent microdialysis of the lipoma and subcutaneous fat tissue in the same region. Five additional subjects, who qualified for the study but had a lipoma in an area not suitable for microdialysis, participated only in the treatment part of the study.
Microdialysis
Two microdialysis studies were conducted approximately 1 week apart. Microdialysis study 1 was the baseline condition. Microdialysis study 2 had pretreatment with 0.2 to 0.4 cc prednisolone 10−6 M (0.07–0.14 mg) injected into the lipoma 48 and 24 h prior.
During each study, two microdialysis catheters (CMA 60, CMA, Solna, Sweden) with a 30-mm-long membrane were introduced into subcutaneous tissue under local anaesthesia. One probe was inserted into a subcutaneous lipoma and one probe was inserted into normal subcutaneous adipose tissue adjacent to the lipoma. The two probes were at least 5 cm apart. The probes were connected to a microdialysis pump (CMA 107 Microdialysis Pump, CMA, Solna, Sweden) and continuously perfused with sterile Ringer solution (139 mmol/l sodium, 2.7 mmol/l potassium, 0.9 mmol/l calcium, 140.5 mmol/l chloride, 2.4 mmol/l bicarbonate, 5.6 mmol/l glucose; Baxter Pharmaceuticals, Deerfield, IL, USA) supplemented with ethanol (1.7 g/l) to estimate changes in adipose tissue blood flow (ATBF), as previously described [14]. ATBF was assessed by the ethanol outflow-to-inflow ratio (expressed in %): [ethanol concentration in dialysate]/[ethanol concentration in perfusate] × 100.
Initially, the probe and tubing were flushed for 10 min at 10µl/min and the dialysate collected was discarded. The microdialysis system was then calibrated [14,15]. To begin, the flow rate was set to 2.0µl/min for 30 min. This dialysate was also discarded. Next, the flow rate was reduced to 0.5µl/min for 30 min and a single dialysate fraction was collected. The flow rate was then increased to 2.0µl/min and two 15-min dialysate fractions were collected. These samples were used to determine baseline in vivo lipolysis. After this baseline period was completed, the perfusion flow rate remained 2.0 µl/min for the duration of the experimental period.
To assess the effect of a β-adrenergic agonist on lipolysis and local blood flow, 1 µmol/l isoproterenol hydrochloride (Isuprel, Hospira, Lake Forest, IL, USA) in Ringer solution was perfused for 45 min. The microdialysis system was then washed with Ringers solution for 60 min before a second 45 min perfusion with isoproterenol. Dialysate fractions were collected every 15 min.
The microvials were stored in specialized microvial racks at −80 ° C until analysed. Dialysate concentrations of glycerol were measured in duplicate by an automated spectrophotometric kinetic enzymatic analyser (CMA 600, CMA, Solna, Sweden) and ethanol by colorimetric assay as previously described [15,16].
Lipoma Treatment
Following the microdialysis study, 10 subjects entered into a 4-week lipoma treatment with isoproterenol 10−6 M and prednisolone 10−6 M. Subjects had one lipoma injected with 0.2 cc of isoproterenol–prednisolone solution (0.04 mg isoproterenol and 0.07 mg prednisolone) in one or more sites in the lipoma depending on its size, 5 days a week for 4 weeks. Prior studies documented that diffusion of 0.2 cc of a β-adrenergic stimulator in fat tissue is approximately spherical in a 2-cm radius from the needle tip based on measurement of the size of the red circle on the skin after injection [4]. Thus, injections were spaced in a 4-cm2 grid to cover the entirety of the lipoma. Although most lipomas required only one injection, a lipoma with a large volume could require multiple injections (two injections if >5 cm in diameter and three injections if >7.5 cm in diameter, but most lipomas are 2.5–5 cm in diameter). The isoproterenol–prednisolone solution was injected daily 5 days per week at the Pennington Center and the lipoma was inspected. These injections were scheduled for week days, but up to two missed visits were made up on the weekend.
On a weekly basis, blood pressure, pulse rate, questioning about possible adverse events and assessment of feelings about the lipoma and injection treatments using a Visual Analogue Scale (VAS) were done. The size of the lipoma was measured at the start of the treatment and at the weekly clinic visits to document any changes in the dimensions when vital signs were measured. On the final clinic treatment visit at week 4, the 24-h urine for free cortisol collected at baseline was repeated. If the lipoma remained at the end of the treatment period and the subject desired surgical treatment, the lipoma was examined histologically after surgical excision. The lipomas were removed by Dr. Thomas Guillot, a board certified plastic surgeon. If the subject elected did not have the lipoma surgically excised, the subject was asked to return to the clinic monthly for up to a year to monitor the lipoma size. During these visits, the subject was asked to rate his or her feelings towards the lipoma and the treatment injections using a VAS. Many subjects with one lipoma had one or more additional lipomas in other locations.
As the therapeutic dose of isoproterenol is 10 mg, the dose used in this experiment was a very small fraction of the dose that would be expected to give systemic changes in pulse or blood pressure. The dose of prednisolone was much below 5 mg/day. The body puts out the equivalent of 5 mg of prednisolone per day and the body reduces its output to compensate for any dose below 5 mg. Thus, the prednisolone should not give any systemic effects, but only act locally where injected.
Statistics
This was a pilot study and no power analysis was performed. The interstitial glycerol concentration in dialysate collected from subcutaneous adipose tissue and subcutaneous lipomas by microdialysis during 45 min infusion of isoproterenol (1 µM) with or without injection of prednisolone 24 h prior were compared by unpaired t-test. The volume of the lipoma at the end of the 4-week treatment period was compared by Student’s t-test. The lipolytic response to isoproterenol in subcutaneous versus lipoma fat was compared by unpaired t-test. Lipolysis with isoproterenol was compared to isoproterenol with prednisolone in lipomas and subcutaneous fat by paired t-test. Serum cortisol and 24 h urine-free cortisol were compared to baseline by t-test to detect any safety concerns from prednisolone injections.
Results
Study 1—In Vitro Studies to Determine Dose
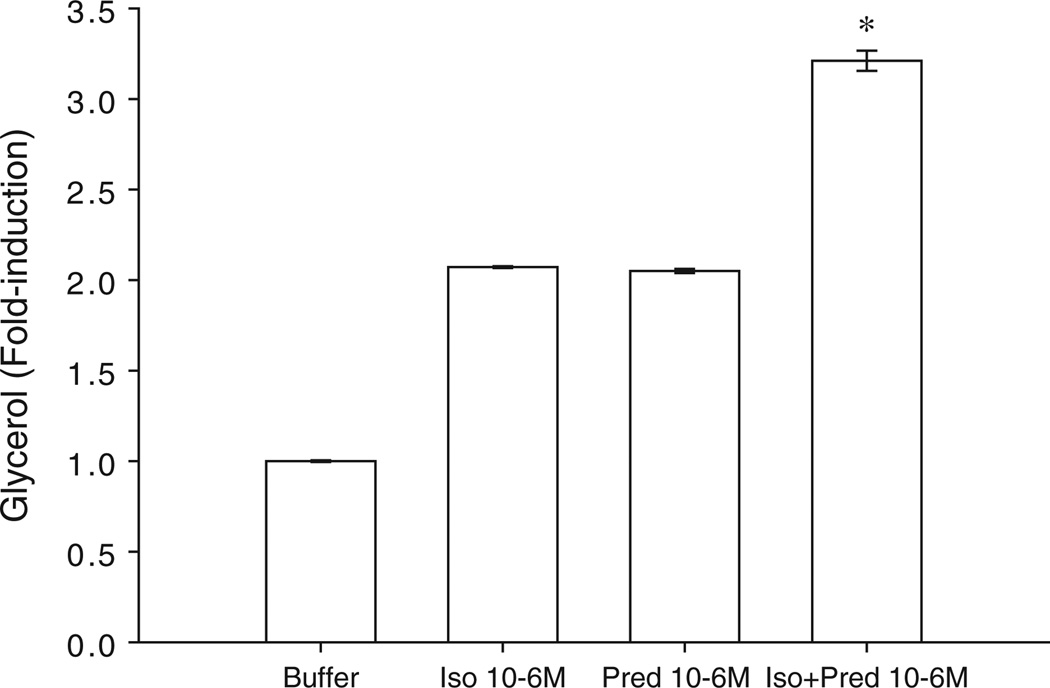
Pretreatment of cultured adipocytes with prednisolone 10−6 M produced a twofold increase in lipolysis that was equivalent to pretreatment with 10−6 M isoproterenol (figure 1). Isoproterenol in combination with prednisolone, however, increased lipolysis more than threefold above control and more than onefold above pretreatment with either isoproterenol alone or prednisolone alone. These concentrations were carried forward to the clinical studies of in vivo lipolysis by microdialysis and lipoma therapy.
Figure 1.
Effect of prednisolone pretreatment on isoproterenol-mediated lipolysis in vitro in cultured human adipocytes (n = 8).
Study 2—Clinical Study: Microdialysis
Lipoma Versus Normal Fat Tissue
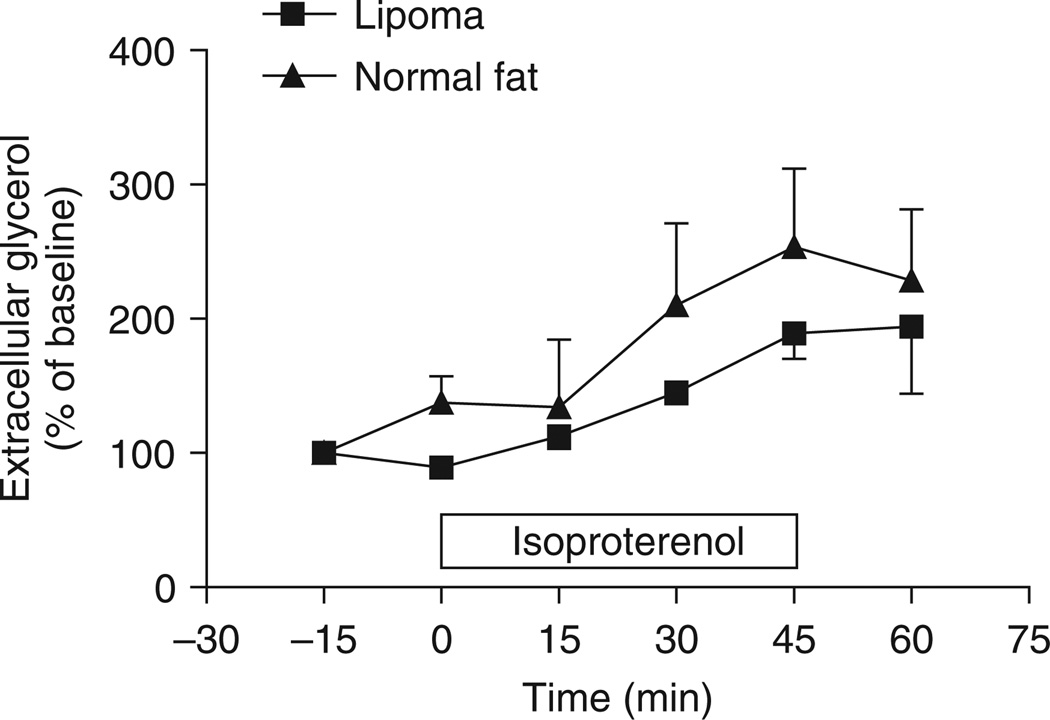
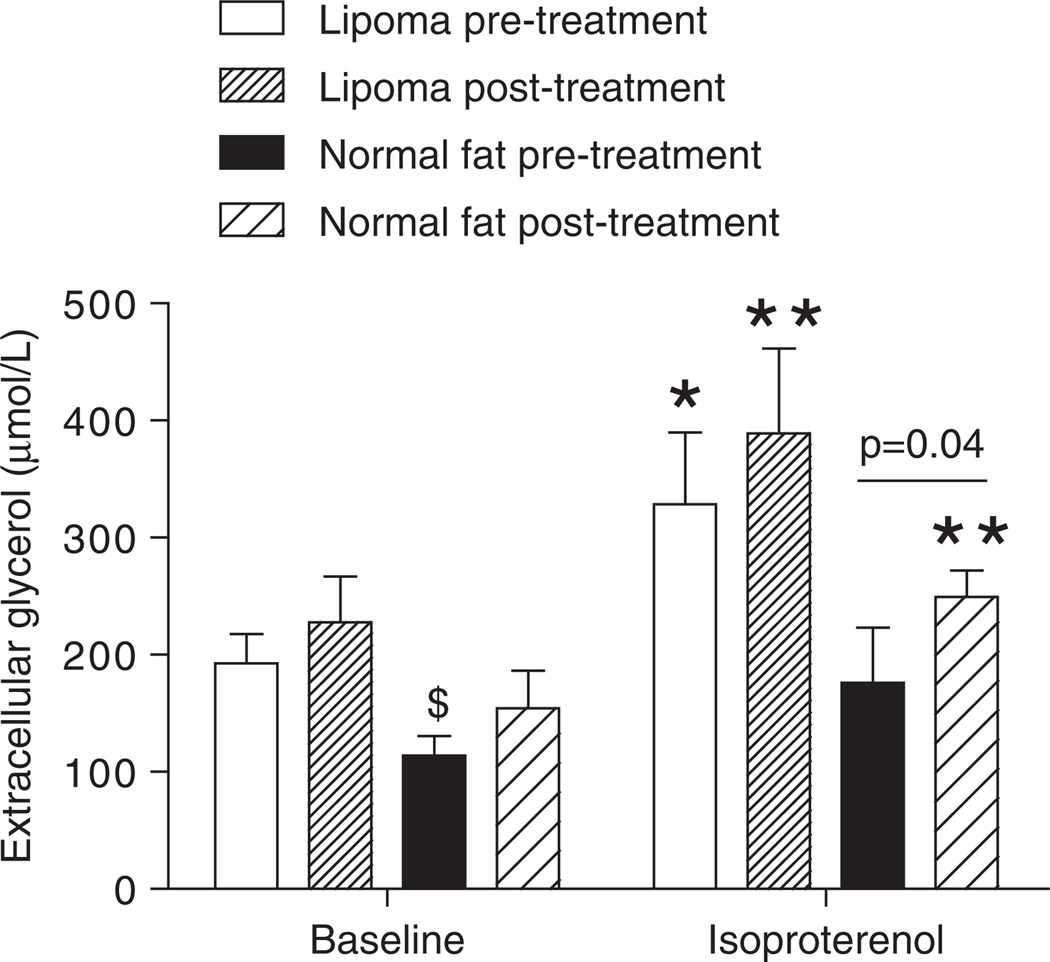
In vivo, the lipoma was partly resistant to the lipolytic effect of isoproterenol compared to normal abdominal subcutaneous fat (figure 2). The area under the curve tended to be reduced in lipoma versus normal fat (lipoma: 153.2 ± 32.1 vs. normal fat: 300.1 ± 62.4 arbitrary units, p = 0.07). Interestingly, the extracellular glycerol concentration measured in the lipoma was 1.7-fold higher at baseline and 1.9-fold higher during the isoproterenol infusion than in the normal abdominal subcutaneous fat (p = 0.03) (figure 3). This difference in extracellular glycerol concentration occurred in the absence of major changes in ATBF at baseline (lipoma: 50.5 ± 3.6 vs. normal fat: 52.1 ± 9.5%).
Figure 2.
Time-course of isoproterenol-mediated lipolysis in vivo in human subcutaneous adipose tissue assessed by microdialysis. The −15 and 0 time points represent the baseline glycerol measurement. Isoproterenol 1 µmol/l was infused for 45 min as represented by the white box on the x-axis. The data are expressed in percentage of baseline extracellular glycerol concentrations.
Figure 3.
Effect of prednisolone pretreatment on isoproterenol-mediated lipolysis in vivo in human subcutaneous adipose tissue and lipoma. *p < 0.05, **p < 0.01 baseline versus isoproterenol; $p < 0.05 lipoma versus normal fat. Each bar represents the average extracellular glycerol concentration measured over 30 min of baseline and 45 min of isoproterenol infusion.
Effect of Prednisolone Treatment
Isoproterenol increased lipolysis both in the lipoma and in the normal fat before and after pretreatment with prednisolone (figure 3). ATBF did not change significantly in response to isoproterenol infusion and prednisolone treatment both in the lipoma and normal fat (data not shown). Similar to what was observed in vitro, pretreatment with prednisolone tended to increase baseline lipolysis in both tissues and potentiated isoproterenol-mediated lipolysis in the normal fat (249.1 ± 21.1 vs. 176.3 ± 39.6 µmol/l, p = 0.04). This effect did not reach significance in the lipoma (328.7 ± 61.3 vs. 389.1 ± 72.5 µmol/L, not significant). Interestingly, prednisolone pretreatment did not prevent isoproterenol-mediated desensitization of β-adrenergic receptors during two consecutive 45-min infusions of isoproterenol performed at a 1-h interval (data not shown).
Study 2—Clinical Study: Lipoma Treatment
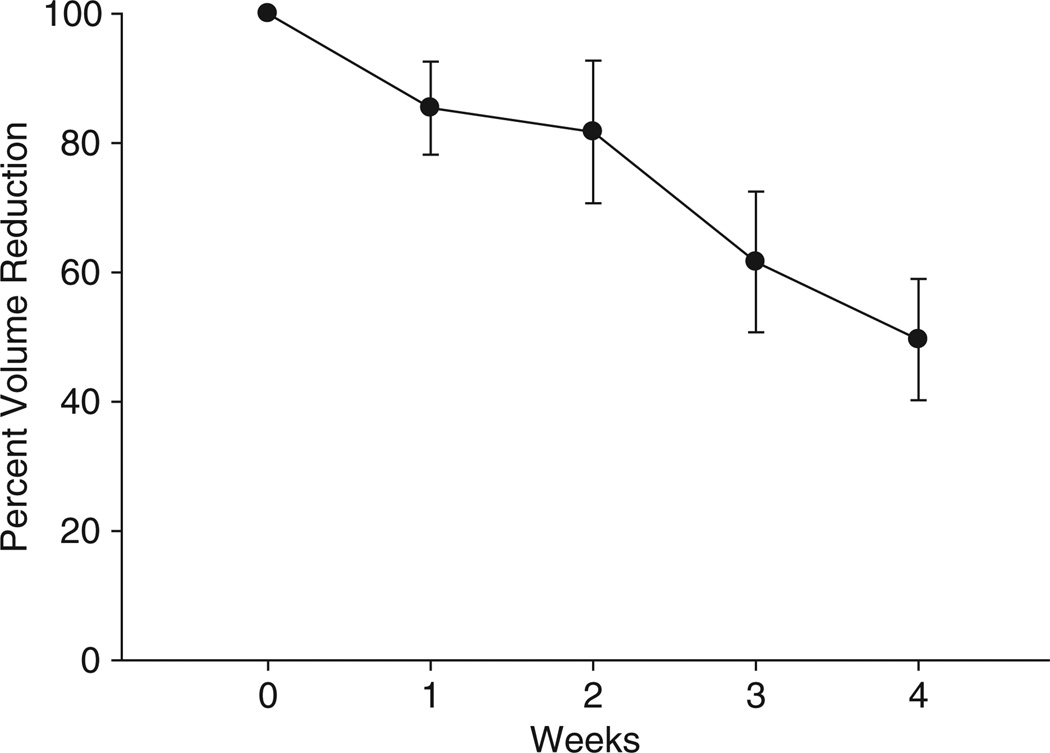
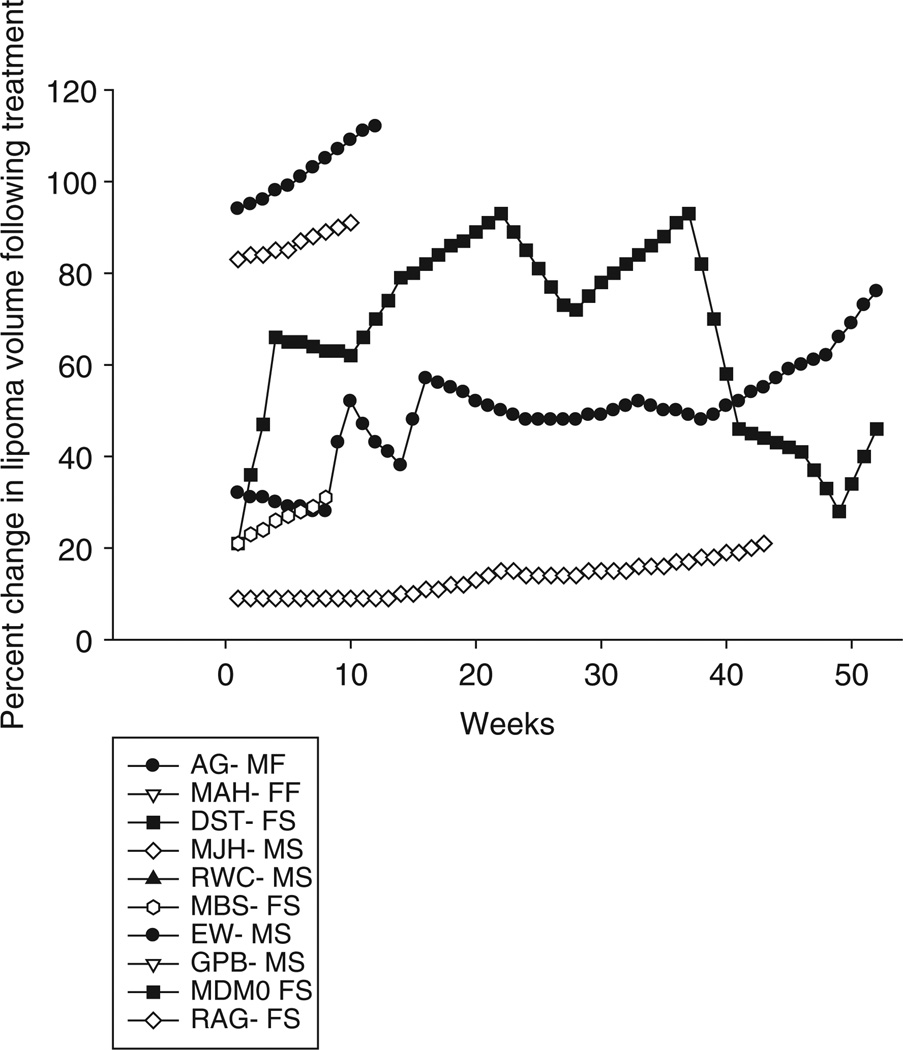
Four weeks of treatment with prednisolone 10−6 M and isoproterenol 10−6 M injected 5 days a week in a 4-cm grid gave a 50% reduction in volume of the lipomas (p < 0.002), but there was substantial variability with one lipoma only losing 10% of its volume from baseline and another losing as much as 90% of its volume (figure 4). At the completion of the treatment, we measured lipoma volume weekly for 1 year (figure 5). All lipomas, with the exception of one, increased in volume after the 4 weeks of treatment. The single case that did not have an increase in lipoma volume showed variability which may have been because of a small lipoma making accurate measurement difficult. All except two subjects elected to have the lipoma removed. The histology showed normal appearing lipoma tissue. The urinary free cortisol values did not change, there was no change in vital signs and there were no adverse events reported. Despite a 50% reduction in lipoma size, the subjects elected for surgical removal in all but two cases.
Figure 4.
Effect of 4 weeks of prednisolone 10−6 M and isoproterenol 10−6 M injections on lipoma volume. The average volume reduction in the 10 lipomas was approximately 50% (p < 0.002) with a range of 10–90%.
Figure 5.
Changes in lipoma volume following a 4-week treatment with prednisolone and isoproterenol. All lipomas, with the exception of one, increased in volume, and all subjects except two elected to have the lipoma surgically excised prior to the end of the 1-year follow-up. Subject initials followed by gender—M, male; F, female and subject outcome; S, surgery; F, finished follow-up.
Discussion
In this study, we determined the effectiveness of a combined β-2 adrenergic agonist and corticosteroid injection for the treatment of lipomas. The in vitro studies confirmed that pretreatment of adipocytes with prednisolone increases the response to isoproterenol, presumably through modulations at a postreceptor level. This finding is consistent with earlier studies reporting a lipolytic effect of cortisol in human adipose tissue [17,18]. The in vitro studies provided important information regarding the doses of combination isoproterenol and prednisolone injections to use both in the in vivo studies with microdialysis and in lipoma treatment. The potentiating effect of prednisolone on isoproterenol-mediated lipolysis was also observed in vivo. Interestingly, extracellular glycerol concentrations reflecting lipolysis were about twofold higher at baseline and during isoproterenol infusion in lipoma compared to normal subcutaneous abdominal adipose tissue. However, the lipolytic responsiveness of the lipoma tended to be lower compared to normal fat. The mechanisms behind this relative resistance to lipolytic stimulation should be further explored.
During the treatment phase, there were no adverse events reported, urinary free cortisol did not change and there were no changes in vital signs. The 4-week treatment with the isoproterenol and prednisolone injection (5 days/week) decreased the volume of the lipomas by approximately 50%. We did observe substantial variation between subjects in the reduction of the lipoma. In one case, the lipoma volume decreased by approximately 90%, whereas in another case the reduction was approximately 10%. Despite the substantial reduction in lipoma volume and excellent safety, 80% of the participants elected to have surgical removal of their residual lipomas. Those that delayed or did not have surgery had regrowth of their lipoma with one exception that may have been because of difficulties in measuring the lipoma. Thus, subjects with lipomas are interested in disappearance rather than just a substantial reduction in size. Although this treatment may be appropriate to shrink the size of a very large lipoma to make it more surgically amenable, this injection method is unlikely to replace surgery, if its efficacy cannot be increased. It is of note that the two subjects who elected not to have surgery had lipomas measuring <3 cm in all directions (2.2 × 1.7 cm and 2 × 1 cm) on an extremity that responded to treatment with a 70–80% reduction in volume, whereas all other subjects had lipomas that exceeded 3 cm in at least one measurement direction. Thus, if there is a place for this treatment, it appears from this data to be small lipomas located on an extremity that respond with a >70%reduction in volume.
Whether a longer treatment regimen or repeated shorter bouts of treatment would result in greater efficacy will need to await further trials. The daily nature of the injections is clearly a drawback to the treatment used in this study. Future attempts using a longer acting β-adrenergic stimulator and steroid might make this treatment much more attractive for patients. Especially, if a longer acting preparation was more efficacious and allowed for progressive lipoma volume reduction with repeated treatments at much longer intervals. Thus, although this medical treatment of lipoma was safe, the acceptance by the patients was not sufficiently great for them not to desire surgery. Thus, further research is needed to arrive at a more effective injection therapy with similar safety to the formulation tested in this study if a medical treatment for lipoma is to compete with surgical therapy which is now the treatment gold standard.
Acknowledgements
The authors would like to thank Aubrey Windham, Jennifer Rood, Crystal Traylor and nurses of the Pennington Biomedical Research Center inpatient and outpatient units. The authors would also like to thank Lawrence E. Klein, MD who performed the dermatopathology evaluation of the lipomas that were surgically removed in this study.
This study was supported by a grant from Lithera and primary cultured human adipose stem cells used in this work were obtained from the Molecular Mechanisms Core Facility directed by Dr Jeff Gimble and partially supported by a Clinical Nutrition Research Unit Center Grant # 1P30 DK072476 entitled ‘Nutritional Programming: Environmental and Molecular Interactions’ sponsored by National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of Interest
L. M. R. and F. G. helped in the design, conduction/data collection, analysis and writing of the manuscript. C. M. helped in the design, conduction/data collection and analysis of the manuscript. J. D. helped in the design of the study. Y. Y. helped in the analysis and writing of the manuscript. T. G. helped in the conduction/collection of the data.
J. D. has financial relationship and stock ownership involvement with the research. He is the CEO and the founder of the study sponsor, Lithera. F. G. has board membership and consulting from Lithera.
References
- 1.Atkinson JN, Galton DJ, Gilbert C. Regulatory defect of glycolysis in human lipoma. Br Med J. 1974;1:101–102. doi: 10.1136/bmj.1.5898.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechara FG, Sand M, Sand D, et al. Lipolysis of lipomas in patients with familial multiple lipomatosis: an ultrasonography-controlled trial. J Cutan Med Surg. 2006;10:155–159. doi: 10.2310/7750.2006.00040. [DOI] [PubMed] [Google Scholar]
- 3.Moro C, Crampes F, Sengenes C, et al. Atrial natriuretic peptide contributes to physiological control of lipid mobilization in humans. FASEB J. 2004;18:908–910. doi: 10.1096/fj.03-1086fje. [DOI] [PubMed] [Google Scholar]
- 4.Greenway FL, Bray GA, Heber D. Topical fat reduction. Obes Res. 1995;3(Suppl. 4):561S–568S. doi: 10.1002/j.1550-8528.1995.tb00228.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson M. The beta-adrenoceptor. Am J Respir Crit Care Med. 1998;158:S146–S153. doi: 10.1164/ajrccm.158.supplement_2.13tac110. [DOI] [PubMed] [Google Scholar]
- 6.Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990;265:3202–3211. [PubMed] [Google Scholar]
- 7.Arner P, Kriegholm E, Engfeldt P. In situ studies of catecholamine-induced lipolysis in human adipose tissue using microdialysis. J Pharmacol Exp Ther. 1990;254:284–288. [PubMed] [Google Scholar]
- 8.Stallknecht B, Bulow J, Frandsen E, Galbo H. Desensitization of human adipose tissue to adrenaline stimulation studied by microdialysis. J Physiol. 1997;500(Pt 1):271–282. doi: 10.1113/jphysiol.1997.sp022017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marion-Latard F, De Glisezinski I, Crampes F, et al. A single bout of exercise induces beta-adrenergic desensitization in human adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2001;280:R166–R173. doi: 10.1152/ajpregu.2001.280.1.R166. [DOI] [PubMed] [Google Scholar]
- 10.Lai E, Rosen OM, Rubin CS. Dexamethasone regulates the beta-adrenergic receptor subtype expressed by 3T3 L1 preadipocytes and adipocytes. J Biol Chem. 1982;257:6691–6696. [PubMed] [Google Scholar]
- 11.Lacasa D, Agli B, Giudicelli Y. Permissive action of glucocorticoids on catecholamine-induced lipolysis: direct “in vitro” effects on the fat cell beta-adrenoreceptor-coupled-adenylate cyclase system. Biochem Biophys Res Commun. 1988;153:489–497. doi: 10.1016/s0006-291x(88)81121-5. [DOI] [PubMed] [Google Scholar]
- 12.Reynisdottir S, Wahrenberg H, Bylin G, Arner P. Effect of glucocorticosteroid treatment on beta-adrenoceptor subtype function in adipocytes from patients with asthma. Clin Sci (Lond) 1993;85:237–244. doi: 10.1042/cs0850237. [DOI] [PubMed] [Google Scholar]
- 13.Brodde OE, Brinkmann M, Schemuth R, O’Hara N, Daul A. Terbutaline-induced desensitization of human lymphocyte beta 2-adrenoceptors. Accelerated restoration of beta-adrenoceptor responsiveness by prednisone and ketotifen. J Clin Invest. 1985;76:1096–1101. doi: 10.1172/JCI112063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellander G, Linde B, Bolinder J. Evaluation of the microdialysis ethanol technique for monitoring of subcutaneous adipose tissue blood flow in humans. Int J Obes Relat Metab Disord. 1996;20:220–226. [PubMed] [Google Scholar]
- 15.Barbe P, Millet L, Galitzky J, Lafontan M, Berlan M. In situ assessment of the role of the beta 1-, beta 2- and beta 3-adrenoceptors in the control of lipolysis and nutritive blood flow in human subcutaneous adipose tissue. Br J Pharmacol. 1996;117:907–913. doi: 10.1111/j.1476-5381.1996.tb15279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millet L, Barbe P, Lafontan M, Berlan M, Galitzky J. Catecholamine effects on lipolysis and blood flow in human abdominal and femoral adipose tissue. J Appl Physiol. 1998;85:181–188. doi: 10.1152/jappl.1998.85.1.181. [DOI] [PubMed] [Google Scholar]
- 17.Ottosson M, Lonnroth P, Bjorntorp P, Eden S. Effects of cortisol and growth hormone on lipolysis in human adipose tissue. J Clin Endocrinol Metab. 2000;85:799–803. doi: 10.1210/jcem.85.2.6358. [DOI] [PubMed] [Google Scholar]
- 18.Divertie GD, Jensen MD, Miles JM. Stimulation of lipolysis in humans by physiological hypercortisolemia. Diabetes. 1991;40:1228–1232. doi: 10.2337/diab.40.10.1228. [DOI] [PubMed] [Google Scholar]