Abstract
Determining how neurons integrate different streams of information is critical to understanding circuit computational functions. In this issue of Neuron, Harnett et al. (2013) show that voltage-gated K+ channels control multiple layers of dendritic integration in layer 5 pyramidal neurons.
A basic but enduring problem facing neuroscientists is to understand the computations performed by the brain at the cellular level. How do neurons integrate tens of thousands of synaptic inputs, which are widely dispersed across varied and complex dendritic architectures to produce meaningful output? The spatial dispersion of inputs, together with fundamental physical properties of dendrites that act to severely filter synaptic conductances, means that synaptic inputs do not simply sum linearly. Rather, a given synapse’s location and relative timing greatly impacts its ability to influence the neuron’s action potential (AP) output.
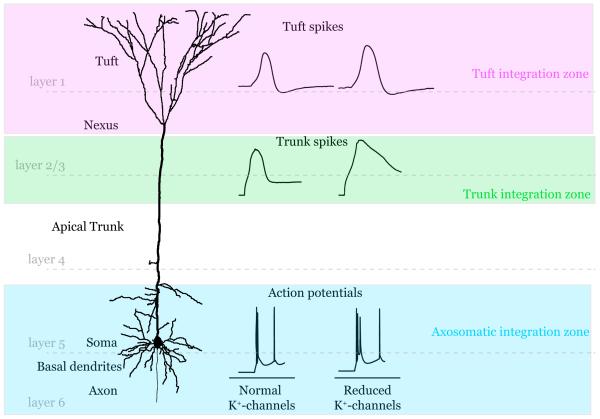
This problem acutely affects cortical layer 5 pyramidal neurons (L5), which have dendrites spanning all six layers of cortex (Figure 1). These cells are the major source of cortical output and so are decisive integrators in the cortical column. Previous reports have shown that active dendritic conductances can be recruited to produce regenerative events (spikes) to boost the propagation of synaptic signals to the axosomatic area where classical action potentials are initiated (Figure 1) (Larkum et al., 2009; Larkum et al., 1999; Schiller et al., 2000; Williams and Stuart, 2002). Dendritic spikes carried by voltage-gated Na+ and Ca2+ currents, along with regenerative N-methyl-D-aspartic acid (NMDA) receptor currents, have led to a multi-layered compartmental model for dendritic integration (Figure 1). Such a model is intriguing given the general cortical design where feedforward sensory information is delivered to middle layers (layer 4) while top-down feedback internal representations of context, feature, attention etc. arrive at layer 1 (Gilbert and Sigman, 2007). How then do these different streams of information interact? The different compartments of integration must somehow convene to provide contextualized output. Larkum et al., 2009 addressed this issue, showing that while individual branches of dendrites in the apical dendritic tuft produce NMDA receptor-mediated spikes in isolation, when multiple branches are activated together they can elicit a Ca2+ spike in the dendritic trunk which can then propagate to the axosomatic initiation zone to affect AP output (Figure 1).
Figure 1.
Schematic diagram of the integration zones in a reconstructed layer 5 pyramidal neuron. Representative traces from signals in each zone are shown before and after K+-channel blockade.
In this this issue of Neuron, Harnett et al. have extended these findings, using a remarkable array of challenging electrophysiological and imaging techniques to describe a multi-layer integration scheme where regenerative signals are compartmentalized by voltage-gated K+ channels (Harnett et al., 2013). Blocking these channels decreased the threshold for initiating spikes in multiple compartments to enhance their coupling. Moreover, they show that these principles apply in vivo during a sensory-motor object localization task.
In the first set of experiments, recording at the soma and the base of the apical dendritic tuft (termed the nexus, Figure 1), they confirmed previous findings by injecting supra-threshold current into the nexus which resulted in large amplitude spikes initiated in the distal dendritic trunk which then forward propagated to the axosomatic integration zone to set off a classical action potential (Larkum and Zhu, 2002; Williams and Stuart, 2002). As previously proposed, this suggests that, in addition to the axosomatic integration zone, the distal apical trunk nonlinearly integrates synaptic signals from the tuft (Larkum et al., 2009; Williams and Stuart, 2002).
Next, with electrodes placed at the nexus and tuft, simulated subthreshold synaptic input into the tuft was dramatically attenuated by the time it arrived at the nexus due to dendritic filtering. And unlike the trunk spikes, tuft spikes did not propagate well. When current was injected close to the nexus, tuft spikes were able to then detonate dendritic trunk spikes. However in more distal tuft regions, the tuft spike only decrementally spread to the nexus, failing to induce trunk spikes. The local tuft spikes were prevented by tetrodotoxin, suggesting that they were initiated by voltage-gated Na+ channels. Harnett et al. provided support for this finding with glutamate uncaging/Ca2+ imaging experiments showing that activation of multiple dendritic spines resulted in large amplitude Ca2+ influx into the stimulated branches. These NMDA receptor-dependent signals too, however, failed to actively propagate to the trunk.
Therefore the tuft can be considered yet another integration zone, capable of amplifying local excitatory input through regenerative spiking. However, these spikes cannot overcome electrical compartmentalization to propagate to the dendritic trunk and axosomatic integrative zones. How then do distal tuft inputs influence neuronal output? Recently, the same group obtained in vivo two-photon imaging results showing that large, synchronous, tuft-wide, Ca2+ transients are induced during sensory-motor behaviour in mice (Xu et al., 2012). These could be induced experimentally by pairing trunk spikes with tuft depolarization, leading to increased frequency and duration of dendritic trunk Ca2+ spikes, which influenced AP output. Guided by previous findings in hippocampal CA1 pyramidal neuron dendrites showing that dendritic signalling is controlled by voltage-gated K+ channels (Cai et al., 2004; Hoffman et al., 1997; Losonczy et al., 2008) the authors reasoned that these may also compartmentalize signals between L5 integration zones.
In outside-out patches from the trunk and tuft, the authors mapped the expression pattern and measured the properties of both transient (rapidly inactivating) and sustained (slowly/non-inactivating) voltage-gated K+ channels. The data revealed a similar distribution pattern for both currents throughout the apical dendritic trunk and tuft. They then investigated the pharmacological profile of the currents, finding two drugs (quinidine and barium), which, at the concentrations used, appeared to selectively reduce both types of K+ currents. These K+-channel blockers were then used to determine in which ways K+-channels affected excitability for each compartment.
With recording electrodes in the soma and nexus, K+-channel blockers boosted trunk spikes initiated with nexus current injection, which induced repetitive AP firing. Blockers did not, however, affect AP firing induced by somatic current injection, demonstrating specific K+-channel control spiking in the dendritic trunk. This finding was supported by an additional set of experiments where subthreshold current injections into the soma, to simulate barrages of synaptic input, were paired with simulated synaptic input to the trunk. The enhanced trunk electrogenesis upon K+-channel block was found to increase AP output.
Recording simultaneously in the trunk and the tuft, K+-channel block decreased the threshold current required for trunk spike initiation and enhanced their propagation into the tuft, allowing full invasion of tuft branches. Signals traveling from the tuft to the trunk were also enhanced, with blockers again reducing the threshold current required to induce tuft spikes, which were increased in both amplitude and duration. Simulated subthreshold synaptic input delivered simultaneously into the tuft and trunk generated plateau potentials in the tuft, which then spread to the trunk. This same group had recently shown that such signals are induced during whisking behaviour during an object localization task in mouse L5 neurons (Xu et al., 2012). Here, while performing the same task in the presence of K+-channel blockers, the authors found increased occurrence, amplitude and duration of tuft Ca2+ signals evoked by whisker-object contact.
K+-channels therefore contribute to the electrical compartmentalization of both the dendritic trunk and tuft. Because K+-channels inactivate with depolarization, the authors suggested that activation of multiple compartments might lead to their interaction. They tested this in triple whole-cell recordings at the soma, trunk and tuft. While the rate of axonal firing induced with somatic current injection was mostly unaffected by subthreshold trunk or tuft excitatory input, pairing tuft and trunk inputs generated large plateau potentials which altered the pattern of neuronal output, inducing high frequency burst firing.
In summary, the paper by Harnett et al. presents a convincing case for voltage-gated K+ channel regulation the interaction between dendritic integration compartments in cortical pyramidal neurons. These findings provide a mechanism for nonlinear dendritic integration of incoming sensory information with intrinsic feedback information streams in an individual neuron, demonstrating the importance of active dendritic properties in shaping cortical output. Tuft inputs can produce regenerative signals but these do not actively forward propagate, limiting their ability to influence on trunk spike initiation and thus axonal output. K+-channel inactivation during multi-compartment excitation can allow for such forward propagation. While Harnett et al.’s in vivo results introduce some object localization data, it will be interesting to see if and how these mechanisms are engaged with different behaviours. Such active dendritic integration schemes may play a general role in integrating sensory information with top-down influences encoding attention, expectation, perception and action command in other cortical areas (Gilbert and Sigman, 2007).
The widespread applicability of a commonly organized, cell-based integration design is exciting but more work remains in describing the basic principles involved. The precise nature and timing of the various input streams and their subcellular localization are yet to be resolved. The extreme electrical compartmentalization in the tuft suggests that presynaptic inputs must temporally and spatially coordinated to initiate spikes. Are the related inputs required to initiate spikes clustered early in development or by experience to bind behaviourally relevant information onto dendritic branches (Makino and Malinow, 2011)? The nature of the tuft spikes is still in question given differences in between the present study (mixed Na+ and NMDA receptor-dependent and previous work (mediated predominately by NMDA receptors) (Larkum et al., 2009) and the role of synaptic inhibition still needs to be incorporated into the compartmentalized integration framework.
The next step in characterizing the K+-channel contribution to dendritic integration will be to uncover the molecular identity of channels involved. The kinetics, pharmacology and expression level of K+-channels clearly differed between the soma and apical dendrite/dendritic tuft recordings, likely indicating a different complement of pore-forming and/or auxiliary subunits. However, while the density of both the transient and sustained components appeared relatively constant throughout the apical trunk and tufts, a more thorough investigation into the location-dependent properties of activation and inactivation seem warranted given the important role of their inactivation proposed for the coupling of tuft inputs and integration zones. This data could reveal subtle compartmental or distance-dependent differences in auxiliary subunit composition as found for CA1 dendrites (Sun et al., 2011). After identifying the primary and auxiliary subunits, their genetic knockdown may help to define their role in behaviourally relevant dendritic integration.
An important K+ channel feature is their high degree of modulation (Shah et al., 2010). Expression levels and location, along with their voltage-dependence and timing can be rapidly modified in dendrites in response to activity and neuromodulation through posttranslational modifications (Hoffman and Johnston, 1999). This active modulation of K+-channel function could dynamically regulate compartmentalization and thus the integration of information pathways.
Finally, combining the techniques used by Harnett et al. with mouse models of CNS disorders, it is possible to examine the disease implications of aberrant dendritic excitability and synaptic integration. Investigations into the molecular mechanisms behind CNS disorders have uncovered synaptic dysfunction in diverse diseases such as autism, schizophrenia, depression and Alzheimer’s disease. However dendritic integration of synaptic signals, linking synaptic molecular pathways and higher-ordered circuit functions are also likely effected, either by propagating synaptic errors to integration and cortical circuit and network abnormalities or through direct disease mechanisms acting on voltage-or ligand gated channel proteins and their regulation, providing potential treatment options.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Harnett MT, Xu NL, Magee JC, Williams SR. Potassium channels control the interaction between active dendritic integration compartments in layer 5 cortical pyramidal neurons. Neuron. 2013 doi: 10.1016/j.neuron.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Johnston D. Neuromodulation of dendritic action potentials. J Neurophysiol. 1999;81:408–411. doi: 10.1152/jn.1999.81.1.408. [DOI] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Nevian T, Sandler M, Polsky A, Schiller J. Synaptic integration in tuft dendrites of layer 5 pyramidal neurons: a new unifying principle. Science. 2009;325:756–760. doi: 10.1126/science.1171958. [DOI] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:6991–7005. doi: 10.1523/JNEUROSCI.22-16-06991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature. 1999;398:338–341. doi: 10.1038/18686. [DOI] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. Compartmentalized versus global synaptic plasticity on dendrites controlled by experience. Neuron. 2011;72:1001–1011. doi: 10.1016/j.neuron.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller J, Major G, Koester HJ, Schiller Y. NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature. 2000;404:285–289. doi: 10.1038/35005094. [DOI] [PubMed] [Google Scholar]
- Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends Neurosci. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Maffie JK, Lin L, Petralia RS, Rudy B, Hoffman DA. DPP6 Establishes the A-Type K(+) Current Gradient Critical for the Regulation of Dendritic Excitability in CA1 Hippocampal Neurons. Neuron. 2011;71:1102–1115. doi: 10.1016/j.neuron.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science. 2002;295:1907–1910. doi: 10.1126/science.1067903. [DOI] [PubMed] [Google Scholar]
- Xu NL, Harnett MT, Williams SR, Huber D, O’Connor DH, Svoboda K, Magee JC. Nonlinear dendritic integration of sensory and motor input during an active sensing task. Nature. 2012;492:247–251. doi: 10.1038/nature11601. [DOI] [PubMed] [Google Scholar]