Abstract
Neuroinflammation contributes to the pathogenesis of early brain injury (EBI) after subarachnoid hemorrhage (SAH). Cytotoxic events following SAH, such as extracellular accumulation of adenosine triphosphate (ATP), may activate the P2X purinoceptor 7 (P2X7R)/cryopyrin inflammasome axis, thus inducing the proinflammatory cytokines IL-1β/IL-18 secretion. We therefore hypothesized that inhibition of P2X7R/cryopyrin inflammasome axis would ameliorate neuroinflammation after SAH. In the present study, SAH was induced by the endovascular perforation in rats. Small interfering RNAs (siRNAs) of P2X7R or cryopyrin were administered intracerebroventricularly 24 hours before SAH. Brilliant Blue G (BBG), a non-competitive antagonist of P2X7R, was administered intraperitoneally 30 minutes following SAH. Post-assessments including SAH severity score, neurobehavioral test, brain water content, Western blot and immunofluorescence, were performed. Administration of P2X7R and cryopyrin siRNA as well as pharmacologic blockade of P2X7R by BBG ameliorated neurological deficits and brain edema at 24 hours following SAH. Inhibition of P2X7R/cryopyrin inflammasome axis suppressed caspase-1 activation, which subsequently decreased maturation of IL-1β/IL-18. To investigate the link between P2X7R and cryopyrin inflammasome in vivo, Benzoylbenzoyl-ATP (BzATP), a P2X7R agonist, was given to lipopolysaccharide (LPS) primed naive rats with scramble or cryopyrin siRNAs. In LPS-primed naïve rats, BzATP induced caspase-1 activation and mature IL-1β release was neutralized by cryopyrin siRNA. Thus, the P2X7R/cryopyrin inflammasome axis may contribute to neuroinflammation via activation of caspase-1 and thereafter mature IL-1β/IL-18 production following SAH. Therapeutic interventions targeting P2X7R/cryopyrin pathway may be a novel approach to ameliorate EBI following SAH.
Keywords: subarachnoid hemorrhage, early brain injury, cryopyrin, P2X purinoceptor 7, inflammation, edema
Introduction
Subarachnoid hemorrhage (SAH) is associated with a mortality rate of 32% in the United States and 8%∼20% of SAH survivors will depend on continuous ambulatory care (Connolly et al., 2012; Nieuwkamp et al., 2009). Recent authoritative opinions suggest that early brain injury (EBI) represents a key mechanism of SAH pathophysiology (Macdonald et al., 2007; Sehba et al., 2012). Growing evidence pointed that inflammation contributed to injury progression after aneurysm rupture (Murakami et al., 2011). Therefore, a novel, safe, and effective anti-inflammatory drug might be a promising strategy to improve the outcome of SAH patients.
The nucleotide-binding domain, leucine-rich repeat containing (NLR) recently received attention, because of its role in innate immune regulation and genetic linkage to human inherited autoinflammatory syndromes (Jha and Ting, 2009). Among >20 NLR members, the most widely studied is the cryopyrin (also called NLRP3 or NALP3), which is activated by endogenous stimuli, including uric acid crystals, adenosine triphosphate (ATP), asbestos and silica (Franchi et al., 2012). Upon activation, cryopyrin recruits apoptosis-associated speck-like protein containing a CAED (caspase recruitment domain) (ASC) and pro-caspase-1 to form an inflammasome as a platform to activate pro-caspase-1 (Lamkanfi and Dixit, 2011). Activated caspase-1, in turn, cleaves cytosolic pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into their mature forms. An uncontrolled secretion of mature IL-1β/IL-18 could lead to neuronal damage (Felderhoff-Mueser et al., 2005). IL-1β is well accepted as a pivotal cytokine in SAH-induced neuroinflammation (Greenhalgh et al., 2012). Consistently, we suggested that caspase-1 inhibition may constitute a new strategy to attenuate EBI via preventing the activation of pro-IL-1β (Sozen et al., 2009). We observed that caspase-1 inhibition prevented neurogenic pulmonary edema after SAH in mice (Suzuki et al., 2009). Thus, cryopyrin may mediate an inflammatory response via the caspase-1/IL-1β pathway after SAH.
Recently, the transmembrane P2X purinoceptor 7 (P2X7R) has been shown to play a crucial role in the posttranslational processing of pro-IL-1β/IL-18 via activating the cryopyrin inflammasome (Beynon et al., 2012; Surprenant and North, 2009). P2X7R and cryopyrin inhibition limited infarction size and prevented heart failure in mural acute myocardial infarction (Mezzaroma et al., 2011). Rapid expanding literature suggested that P2X7R antagonists have potential to treat diverse central nervous system (CNS) diseases, including traumatic brain injury, ischemia and seizure (Arbeloa et al., 2012; Kim and Kang, 2011; Kimbler et al., 2012). However, the specific role of P2X7R in SAH has not yet been established.
In this study, we hypothesized that activation of the P2X7R/cryopyrin inflammasome axis is vital for SAH-induced EBI. We first investigated the time course of brain expression of cryopyrin, mature IL-1β and P2X7R after SAH. Next, we used gene silencing approach to test the role of P2X7R and cryopyrin in a rat SAH model. To test the P2X7R/cryopyrin inflammasome axis in vivo, we further examined whether cryopyrin siRNA reversed the P2X7R agonist-induced inflammatory response in lipopolysaccharide (LPS) primed rats. Finally, of particular translational significance, we observed the therapeutic effect of the pharmacological P2X7R antagonist, brilliant blue G (BBG), in SAH model.
Material and methods
Adult male Sprague-Dawley rats (SD 280–320 g) were housed in a room with a 12/12 hours light/dark cycle and with constant temperature and humidity control. Experimental procedures and animal care were approved by the Institutional Animal Care and Use Committee (IACUC) at Loma Linda University.
Reagents
P2X7R antagonist, BBG and agonist, (Benzoylbenzoyl-ATP) BzATP; LPS; as well as Cryopyrin and P2X7R siRNA were purchased from SIGMA-Aldrich (St Louis, MO). Scramble siRNA was purchased from Dharmacon/Thermo Fisher Scientific (Lafayette, CO).
SAH rat model
SAH was induced using the endovascular perforation model (Duris et al., 2011; Shiba et al., 2012). Briefly, rats were anesthetized with 3% isoflurane in 70/30% medical air/oxygen. The animals were transorally intubated and a small rodent respirator was used to maintain an adequate respiration (Harvard Apparatus, Holliston, MA). The external carotid artery (ECA) was ligated, cut, and shaped into a 3mm stump. A sharpened 4-0 monofilament nylon suture was gently inserted into the internal carotid artery (ICA) from the ECA stump until feeling resistance, and then advanced 5mm to perforate the bifurcation of the anterior and middle cerebral artery. Immediately after puncture, the suture was withdrawn, and the ICA was opened for reperfusion to produce SAH. Sham animals were subjected to the same procedure with the exception that the suture was withdrawn once resistance was felt, without vessel perforation. The incision was then closed, and rats were housed individually following recovery from anesthesia.
Experimental Design
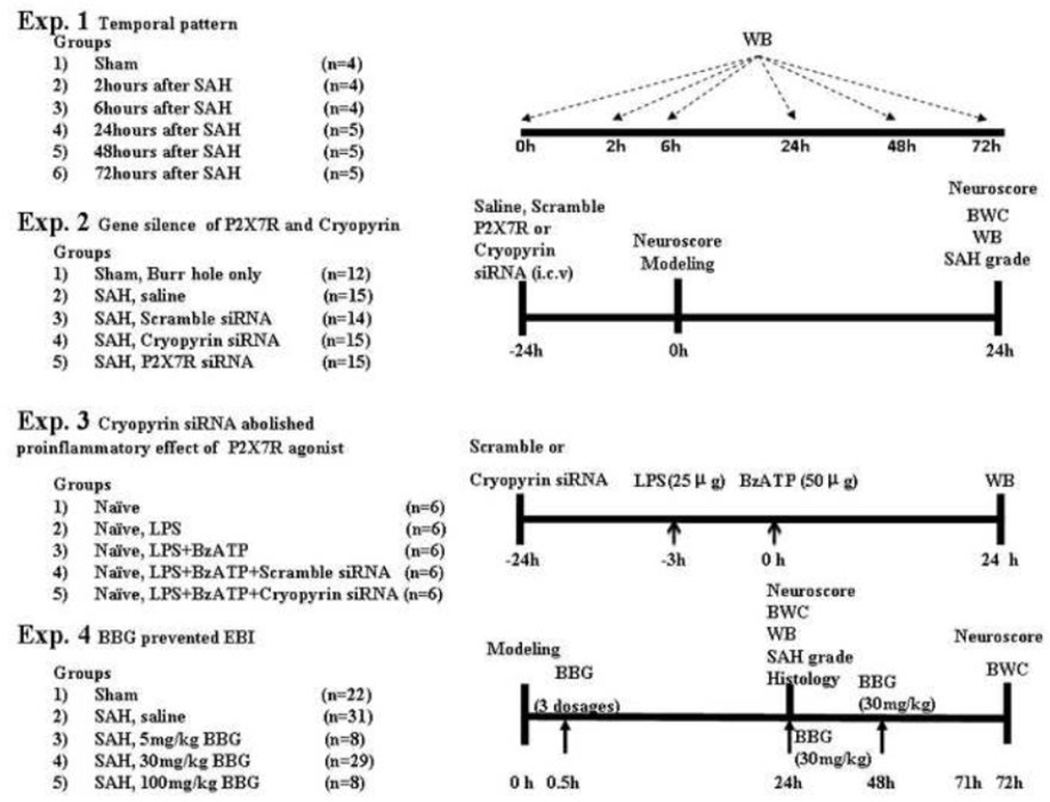
Four separate experiments were conducted (Fig. 1, Experiments 1 to 4).
FIGURE 1.
Experimental design and animal group classification. SAH = subarachnoid hemorrhage; WB = Western blotting; BWC = brain water content; LPS = Lipopolysaccharide; P2X7R= P2X purinoceptor 7; BzATP= Benzoylbenzoyl ATP; BBG= Brilliant blue G.
Experiments 1,2,4 were conducted using the endovascular perforation SAH model, and experiment 3 was conducted using the LPS primed naïve rats.
Experiment 1: Twenty-seven rats were divided into 6 groups (sham, and SAH after 2, 6, 24, 48, and 72 hours). The temporal expression of cryopyrin, P2X7R and mature IL-1β were detected by Western blot.
Experiment 2: Seventy-one rats were randomly divided into 5 groups: sham, vehicle (SAH+saline, intracerebroventricular injection), scramble small interfering RNA (siRNA) (500pmol/2µL), cryopyrin siRNA (500pmol/2µL) and P2X7R siRNA (500pmol/2µL). The effect of cryopyrin siRNA and P2X7R siRNA were evaluated using SAH grading, neurological score, brain water content and Western blot (24 hours).
Experiment 3: Thirty rats were randomly divided into 5 groups: naïve, LPS, LPS+BzATP, LPS+BzATP+Scramble siRNA, and LPS+BzATP+Cryopyrin siRNA. Naïve rats received an intracerebroventricular priming dose of LPS (25µg/5µL per rat) as previous described (Guerra et al., 2011). 3 hours later, BzATP, a P2X7R agonist, (50µg/5µL per rat) was administrated intracerebroventricularly (Chu et al., 2012). Cryopyrin siRNA or scramble siRNA (500pmol/2µL) was injected 24 hours prior to BzATP injection.
Experiment 4: Ninety-eight rats were randomly divided into 5 groups: sham, vehicle (SAH+saline, intraperitoneally injection), Brilliant Blue G (BBG) (5mg/kg), BBG (30mg/kg) and BBG (100mg/kg). For 72 hours study, BBG (30mg/kg) was administered daily by intraperitoneally injection. Postassessment included SAH grading, neurological score, brain water content, Western blot and immunofluorescence (24 hours and 72 hours).
Intracerebroventricular infusion
Rats were placed in a stereotaxic apparatus under 2% isoflurane anesthesia during the whole procedure. The needle of a 10µL Hamilton syringe (Microliter #701; Hamilton Company, Reno, NV) was inserted into the left lateral ventricle through a burr hole on the skull, using the following coordinates relative to bregma: 1.5mm posterior; 1.0mm lateral; 3.2mm below the horizontal plane of bregma (He et al., 2012; Suzuki et al., 2010). To improve the gene silence efficiency, two different sequences targeting cryopyrin siRNA were pooled: cryopyrin (a) sense, 5’-GAUCCUAUUUGAAGAGUGU-3’; antisense, 5’-ACACUCUUCAAAUAGGAUC-3’; (b)sense, 5’-GAUCAACCUCUCUACCAGA-3’; antisense, 5’-UCUGGUAGAGAGGUUGAUC-3’. In addition, two different sequences targeting P2X7R siRNA were pooled (a) sense,5’-CAGUGAAUGAGUACUACUA-3’; antisense, 5’-UAGUAGUACUCAUUCACUG-3’ (b) sense,5’-CUCUUGAGGAGCGCCGAAA-3’; antisense, 5’-UUUCGGCGCUCCUCAAGAG-3’. The nonsilencing RNA (Dharmacon/Thermo Fisher Scientific, Lafayette, CO) was used as the control siRNA. 500pmol SiRNA in 2µL sterile saline was injected intracerebroventricularly by a pump at a rate of 0.5µL/min at 24 hours before SAH induction. The needle was kept in place 15 minutes after finishing of the infusion. In the sham group, a hole was drilled on the skull at the same position, but needle insertion was not performed. Finally, the burr hole was plugged with a bone wax and the incision was closed with sutures.
In experiment 3, LPS (25µg/rat, E.coli B5) and BzATP (50µg/rat) was administrated by intracerebroventricular infusion into left lateral ventricle.
Neurological score
Neurobehavioral deficits were blindly evaluated with the modified Garcia test and with the beam balance test at 24 hours and 72 hours after SAH (Mu et al., 2013). For the modified Garcia test, the evaluation consisted of six tests that can be scored either from 0–3 or from 1–3. These six tests include: spontaneous activity; symmetry in the movement of all four limbs; forepaw outstretching; climbing; body proprioception; and response to whisker stimulation. The total score ranged from 3 to 18. For beam balance, the rats walked on a narrow wooden beam for 1 minute. The score ranged from 0 to 4 according walking distance. The mean score of three consecutive trials was calculated. Higher scores indicate greater function.
SAH severity score
SAH severity score was blindly assessed as previously described (Sugawara et al., 2008). The basal cistern was divided into six segments and each segment was allotted a score from 0 to 3 according to the amount of blood clot in the segment. The animals received a total score ranging from 0 to 18. Only animals experiencing severity score >8 were included in this work.
Brain Water Content Measurement
Brain edema was evaluated by the wet weight / dry weight method (Guo et al., 2012). The rats were decapitated at 24 hours or at 72 hours after SAH. Brains were immediately divided into the left and right cerebral hemispheres, cerebellum and brain-stem, and first weighed (wet weight). Brain specimens were dried at 100°C for 72 hours in an oven and second weighed (dry weight). The following formula was used to calculate the percentage of water content: ([wet weight - dry weight]/wet weight)×100% (He et al., 2012).
Western Blot
Western blot analysis was performed as previously described (Manaenko et al., 2013). Briefly, the left hemispheres (perforation side) were homogenized, and aliquots of each fraction were used to measure the protein concentration of each sample using a detergent compatible assay (Bio-Rad, Hercules, CA). Equal amounts of protein samples (50µg) were subjected to a SDS-PAGE gel, electrophoresed, and transferred to a nitrocellulose membrane. Membranes were then blocked with a blocking buffer for 1 hour, followed by incubation overnight at 4°C with the primary antibodies: rabbit polyclonal anti-interleukin (IL)-1β antibody (1:1000), rabbit polyclonal anti-caspase-1 (1:200) (Millipore, Temecula, CA), mouse monoclonal anti-myeloperoxidase (MPO, 1:800), rabbit polyclonal anti-Cryopyrin (1:1000), rabbit polyclonal anti-P2X7R (1:1000), rabbit polyclonal anti-IL-18 (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA). Immunoblots were processed with appropriate secondary antibodies (1:2000, Santa Cruz Biotechnology, Santa Cruz, CA) for 1 hour at room temperature, and bands were detected with a chemiluminescence reagent kit (ECL Plus; Amersham Bioscience, Arlington Heights, IL). For loading control, β-Actin (1:4000, Santa Cruz Biotechnology, Santa Cruz, CA) was blotted on the same membrane. Blot bands were quantified by densitometry with Image J software.
Immunofluorescence
24 hours after SAH, rats were perfused with 300ml of ice-cold phosphate buffer solution under deep anesthesia, followed by infusion of 200 ml. The brains were harvested and immersed in 10% formalin at 4°C for 24 hours, then in 30% sucrose until saturation. The brains were cut into 10µm thick coronal sections in cryostat (CM3050S; Leica Microsystems, Bannockburn, IL) (Simard et al., 2012). The sections were incubated overnight at 4°C with the rabbit anti-MPO (1:300; Dako Cytomation Inc., Carpinteria, CA) followed by incubation with appropriate FITC-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA). The sections were visualized with a fluorescence microscope (Olympus BX51).
Statistics
Statistical analysis was performed using GraphPad Prism 5 and SPSS 16.0 software. Data were expressed as mean ± standard error of the mean. Statistical differences between two groups were analyzed using chi-square tests. Multiple comparisons (except neurological score) were statistically analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. For neurological score, data were expressed as median ± 25th-75th percentiles and were test by Kruskal-Wallis one-way ANOVA on rank, followed by the Steel-Dwass multiple comparisons tests. Statistically significance was defined as p<0.05.
Results
Mortality and SAH Severity Scores
The whole mortality of SAH in the present study was 17.72%, 28 of 158 rats. The mortality was not significantly different among the groups in experiment 2 and 4, respectively (data not shown). In addition, there was no significant difference in average SAH grading score among the groups in each experiment (data not shown). No mortality was recorded in the sham-operated rats.
Temporal patterns of cryopyrin, P2X7R and mature IL-1β were detected in left hemisphere following SAH
Western blot was performed to determine the protein expression of cryopyrin, mature IL-1β and P2X7R at 2, 6, 24, 48 and 72 hours in left hemisphere (perforation side) after SAH. Result showed that cryopyrin level increased as early as 2 hours after SAH, and reached peak around 24 hours in which cryopyrin level was 2.6 times more than sham group ( p <0.05). Following the peak, cryopyrin level declined, returning close to its baseline level by 72 hours (Fig. 2A, B). Additionally, the expression of mature IL-1β was significantly increased in left hemisphere 24 hours post-SAH compared to sham animals (p<0.01). Following this peak, the level of mature IL-1β remained higher than the sham animals at 48 hours and at 72 hours post SAH (Fig. 2A, C). However, compared to the sham group, P2X7R level did not increase during 72 hours following SAH (Fig. 2A, D).
FIGURE 2.
Expression profile of P2X purinoceptor 7 (P2X7R)/cryopyrin inflammasome components after subarachnoid hemorrhage (SAH). Western blot assay (A) for the expression profiles of cryopyrin, mature IL-1β and P2X7R in the left hemisphere (perforation side) within 72 hours after SAH. Quantification of cryopyrin (B), mature IL-1β (C) and P2X7R (D) expression is shown, respectively. n = 4 rats per group and per time point. Error bars represent mean ± standard error of the mean. *p<0.05 vs. Sham.
Gene Silencing of cryopyrin or P2X7R decreased their protein expression respectively and cleaved caspase-1 and subsequent production of mature IL-1β/ IL-18 upon SAH
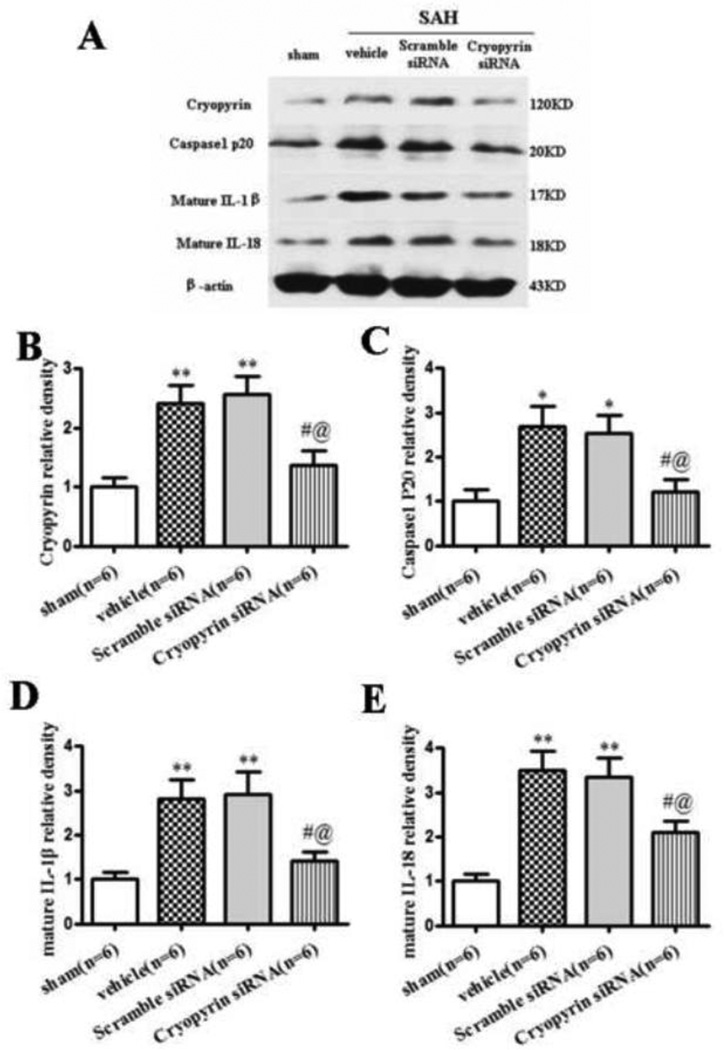
Upregulation of cryopyrin was observed at 24 hours following SAH in the vehicle group and scramble siRNA group, when compared to sham (p<0.01). Silencing efficacy by Western blot showed 43.4% and 46.5% decrease of cryopyrin level in the cryopyrin siRNA group compared with vehicle and scramble siRNA group (p<0.05, Fig. 3A, B). The protein expression of cleaved caspase-1 (p<0.05) and mature IL-1β/IL-18 (p<0.01) were significantly increased in the vehicle and the scramble siRNA group compared to sham animals. Cryopyrin siRNA treatment significantly abolished caspase-1 activation and subsequent mature IL-1β/IL-18 secretion at 24 hours after SAH (p<0.05) (Fig. 3A, C–E). There was no difference between animals that received scramble siRNA and the vehicle group.
FIGURE 3.
Cryopyrin siRNA mixture inhibited inflammation at 24 hours after SAH. Representative (A) Western blot and therapeutic effects of cryopyrin siRNA pre-SAH injection on cryopyrin (B), cleaved caspase-1(P20) (C), mature IL-1β (D) and mature IL-18 (E) levels in left hemisphere at 24 hours following SAH. Error bars represent mean ± standard error of the mean. *p<0.05 vs. Sham; **p<0.01 vs. Sham; #p<0.05 vs. Vehicle; @p<0.05 vs. Scramble siRNA.
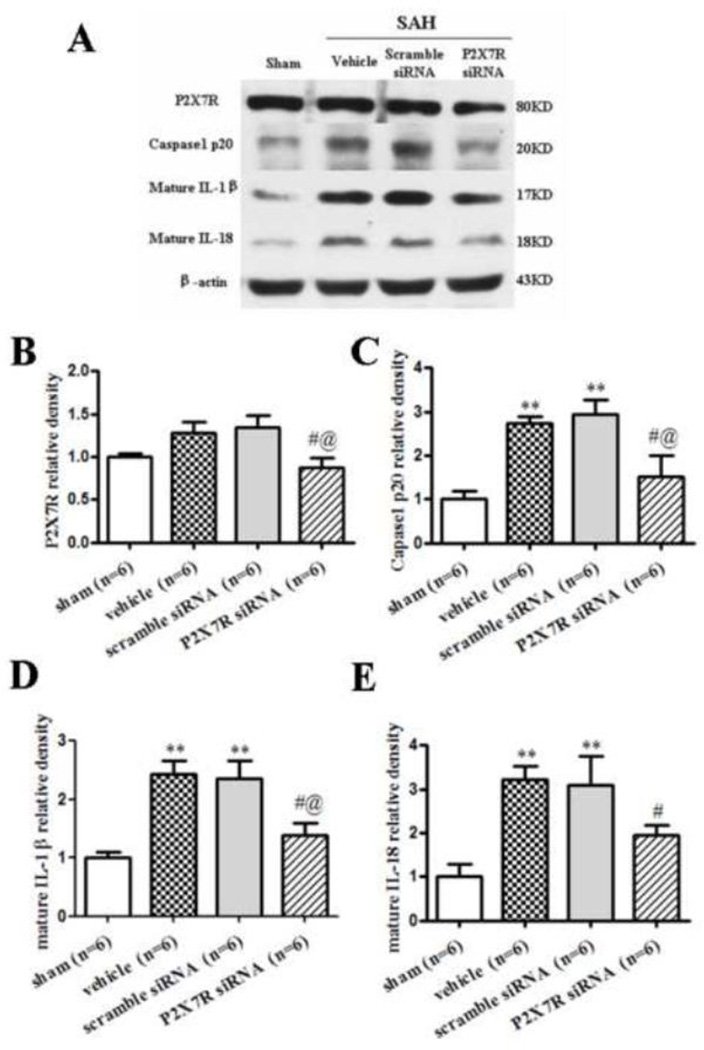
Western blot results revealed that P2X7R expression did not increase at 24 hours after SAH, whereas 32.0% and 34.9% decrease in P2X7R siRNA group compared with vehicle and scramble siRNA group (p<0.05, Fig. 4A, B). P2X7R siRNA significantly reduced cleaved caspase-1 and mature IL-1β/IL-18 (p<0.05, Fig. 4A, C–E).
FIGURE 4.
P2X7R siRNA mixture inhibited inflammation at 24 hours after SAH. Representative (A) Western blot and therapeutic effects of P2X7R siRNA pre-SAH injection on P2X7R (B), cleaved caspase-1(P20) (C), mature IL-1β (D) and mature IL-18 (E) levels in left hemisphere 24 hours following SAH. n = 6 rats per group. Error bars represent mean ± standard error of the mean. *p<0.05 vs. Sham; **p<0.01 vs. Sham; #p<0.05 vs. Vehicle; @p<0.05 vs. Scramble siRNA.
Gene Silencing of cryopyrin or P2X7R improved neurobehavioral functions and reduced brain edema
Neurobehavioral functions (Fig. 5A) and brain water content (Fig. 5B) were evaluated at 24 hours following SAH. No neurological impairment was evident at 24 hours after siRNA injection (before SAH induction). The results revealed that vehicle and scramble siRNA treated animals developed sensorimotor deficits compared to sham animals at 24 hours after SAH (p<0.01). Cryopyrin siRNA and P2X7R siRNA administration ameliorated sensorimotor deficits (p<0.05 vs. vehicle and scramble siRNA).
FIGURE 5.
Effects of P2X7R/cryopyrin knockdown on neurological functions and brain water content at 24 hours after SAH. Cryopyrin siRNA and P2X7R siRNA improved neurological functions (A), reduced brain edema (B) at 24 hours following SAH. Box=25th-75th interquarile percentiles, horizontal line=median, vertical line=range (A) or Error bars represent mean ± standard error of the mean (B). *p<0.05 vs. Sham; **p<0.01 vs. Sham; #p<0.05 vs. Vehicle; @p<0.05 vs. Scramble siRNA.
At 24 hours post-SAH, brain edema in the left hemisphere was significantly increased in the vehicle and the scramble siRNA group compared to the sham group (vehicle, 79.64 ± 0.10 vs. sham, 79.15 ± 0.06, p<0.05; scramble siRNA, 79.61 ± 0.07 vs. sham, 79.15 ± 0.06, p<0.05). Cryopyrin siRNA and P2X7R siRNA treatment significantly decreased brain edema in left hemisphere compared to vehicle group and the scramble siRNA group (cryopyrin siRNA, 79.30 ± 0.11 vs vehicle, 79.64 ± 0.10, p<0.05; cryopyrin siRNA, 79.30 ± 0.11 vs. scramble siRNA, 79.61 ± 0.07, p<0.05; and P2X7R siRNA, 79.35 ± 0.07 vs. vehicle, 79.64 ± 0.10, p<0.05; P2X7R siRNA, 79.35 ± 0.07 vs. scramble siRNA, 79.61 ± 0.07, p<0.05). In the right hemisphere, brain edema was significantly increased in the vehicle and scramble siRNA group compared to the sham group (vehicle, 79.45 ± 0.08 vs. sham, 79.09 ± 0.07, p<0.05; scramble siRNA, 79.45 ± 0.04 vs. sham, 79.09 ± 0.07, p<0.05). Cryopyrin siRNA significantly decreased brain edema in right hemisphere compared to vehicle group and scramble siRNA group (cryopyrin siRNA, 79.21 ± 0.04 vs. vehicle, 79.45 ± 0.08, p<0.05; cryopyrin siRNA, 79.21 ± 0.04 vs. scramble siRNA, 79.45 ± 0.04, p<0.05). Otherwise, P2X7R siRNA treatment showed a trend towards reducing edema in the right hemisphere; however, without statistical significance.
Cryopyrin siRNA reduced the level of cleaved caspase-1 and mature IL-1β induced by BzATP in LPS primed rats
LPS robustly induced cryopyrin and pro-forms of the cytokines production (Shenoy et al., 2012). We observed LPS alone priming significantly increased cryopyrin level in naïve rats (p<0.05, Fig. 6A, B), but failed to induce production of cleaved caspase-1 and mature IL-1β (Fig. 6A, C, D). BzATP, a P2X7R agonist, enabled the increase of cleaved caspase-1 level as well as the level of mature IL-1β upon LPS priming (p<0.01). Cryoprin siRNA prevented the proinflammatory effect of BzATP (p<0.05, Fig. 6B–D). Scramble siRNA had no preventive effect on BzATP-induced activation of caspase-1 and IL-1β.
FIGURE 6.
Cryopyrin siRNA reduced inflammation induced by Benzoylbenzoyl-ATP (BzATP) in lipopolysaccharide (LPS) primed rats. Representative Western blot (A) and cryopyrin siRNA abolished augment of cleaved caspase-1(P20) and mature IL-1β induced by BzATP in LPS primed model. LPS alone significantly increased cryopyrin level, while failed to induce production of cleaved caspase-1 and mature IL-1β. BzATP enable a large increase in expression of cleaved caspase-1 and mature IL-1β upon LPS primed and cryoprin siRNA abolished the proinflammatory response of BzATP (Fig 6 B–D). n = 6 rats per group. Error bars represent mean ± standard error of the mean. *p<0.05 vs. naive; **p<0.01 vs. naive; &p<0.05 vs. LPS; @p<0.05 vs. LPS+BzATP; #p<0.05 vs. LPS+BzATP+Scramble siRNA.
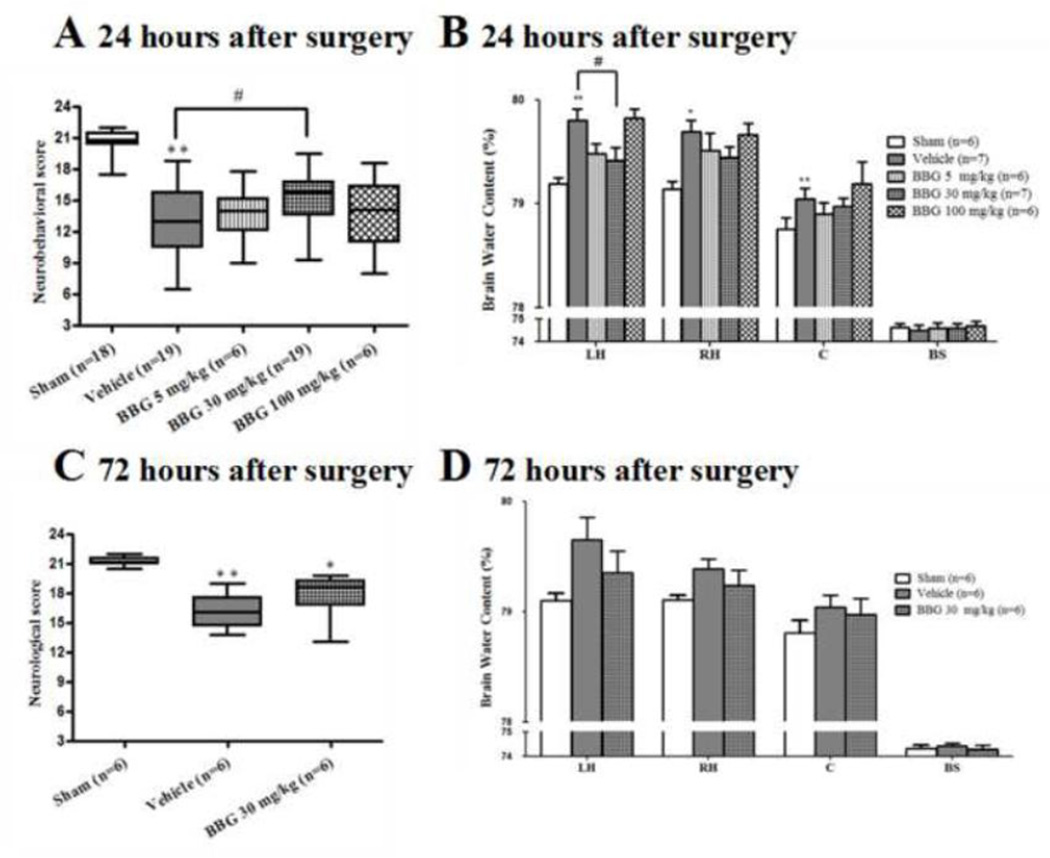
BBG improved neurobehavioral functions and attenuated brain edema after SAH
Post-SAH administration of 30mg/kg BBG significantly improved neurobehavioral deficits at 24 hours after SAH (p<0.05 vs. vehicle); a lower (5mg/kg) or higher (100mg/kg) dose of BBG did not significantly improve neurobehavioral outcome (Fig. 7A). Progressively up to 72 hours post SAH, we observed BBG (30mg/kg) treatment improved neurobehavioral impair without significant difference compare to vehicle group (Fig. 7C).
FIGURE 7.
Effects of Brilliant blue G (BBG) on neurological functions and brain water content at 24 hours and 72 hours after SAH. BBG significantly improved neurological score (A) and reduced brain edema (B) 24 hours following SAH, but there was no significance difference at 72 hours (C and D). Box=25th–75th interquarile percentiles, horizontal line= median, vertical line=range (A, C). Error bars represent mean ± standard error of the mean (B, D). *p<0.05 vs. Sham; **p<0.01 vs. Sham; #p<0.05 vs. Vehicle.
At 24 hours post SAH, brain edema significantly increased by 0.61% in the left hemisphere (p<0.01), 0.56% in the right hemisphere (p<0.05), and 0.29% (p<0.01) in the cerebellum compared to sham animals, while no change was found in the brain stem (Fig. 7B). The brain edema in the left hemisphere significantly improved with the medium dosage of BBG (30 mg/kg) compared to vehicle administration (30 mg/kg, 79.42 ± 0.12 vs. vehicle, 79.80 ± 0.11, p<0.05; Fig. 7B). There were no significant differences between BBG (5 mg/kg) and vehicle group in brain water contents of each area (Fig. 7B). To our surprise, brain edema was similar between BBG (100mg/kg) and vehicle group. There was no significant difference in brain edema between all groups at 72 hours after SAH (Fig. 7D). Because the BBG (30mg/kg) group showed significant improvements in neurological score and brain edema compared to the vehicle group, the medium dosage was used for further analysis.
BBG decreased cleaved caspase-1 and the subsequent production of mature IL-1βIL-18 following SAH
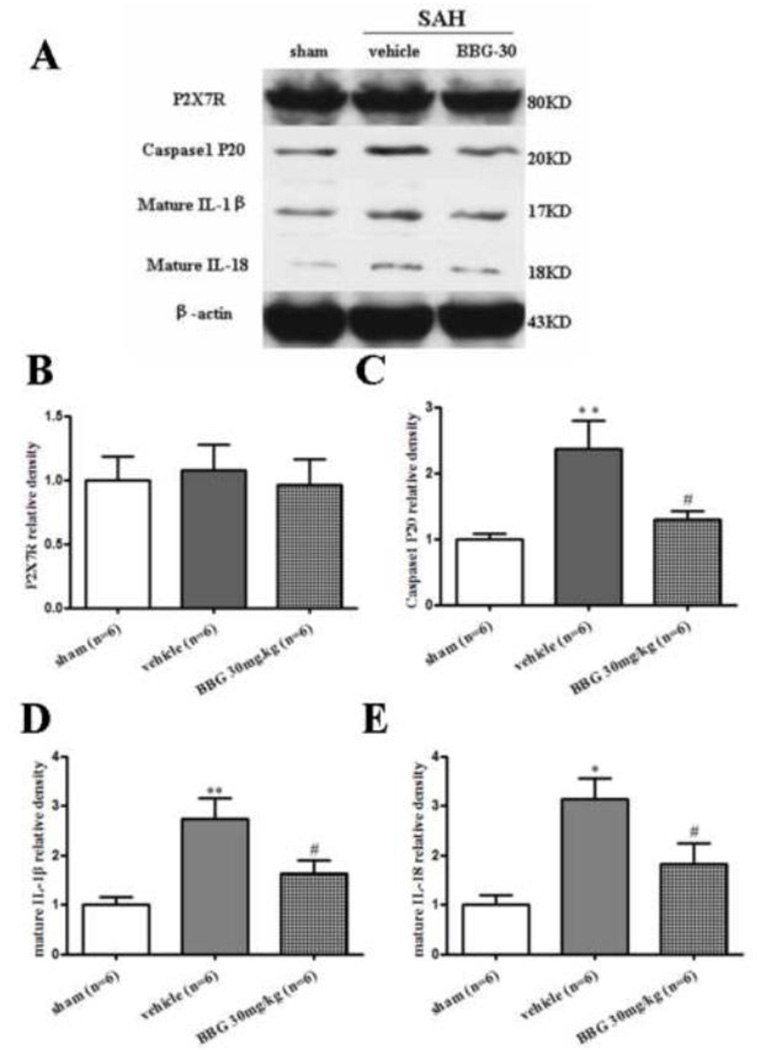
The protein expression of cleaved caspase-1 and mature IL-1β/IL-18 were significantly increased at 24 hours after SAH (p<0.05 vs. sham), while administration of BBG (30mg/kg) reduced the expression of cleaved caspase-1 and mature IL-1β/IL-18 at 24 hours post-SAH (p<0.05 vs. vehicle group, Fig. 8A, C–E). The P2X7R levels in the vehicle and BBG groups were not significantly changed compared with the sham group at 24 hours following SAH (Fig. 8A, B).
FIGURE 8.
BBG inhibited inflammation at 24 hours after SAH. Representative (A) Western blot and therapeutic effects of BBG post-SAH injection on P2X7R (B), cleaved caspase-1(P20) (C), mature IL-1β (D) and mature IL-18 (E) levels in left hemisphere 24 hours following SAH. n = 6 rats per group. Error bars represent mean ± standard error of the mean. *p<0.05 vs. Sham; **p<0.01 vs. Sham; #p<0.05 vs. Vehicle.
BBG suppressed neutrophil infiltration in the cortex at 24 hours after SAH
To further confirm the anti-inflammation role of BBG, we evaluated the neutrophil infiltration by Western blot and staining of MPO. Western blot revealed that MPO levels were significantly increased at 24 hours following SAH and BBG significantly reduced MPO intensity compared to vehicle group (p<0.05, Fig. 9A, B). Similarly, MPO staining showed less neutrophils in the cortical region after BBG treatment at 24 hours. Sham animals displayed no neutrophils in the brain (Fig. 9C).
FIGURE 9.
BBG significantly suppressed neutrophil infiltration at 24 hours after SAH. Representative Western blots (A) and effects of BBG on myeloperoxidase (MPO) level (B) in left hemisphere at 24 hours after SAH. n = 6 rats per group. Representative photograph of immunofluorescence staining (C) for MPO showing that the MPO-positive cells were increased in vehicle group and decreased in BBG treatment (30 mg/kg) 24 hours after SAH. Scale bars: 50µm. Error bars represent mean ± standard error of the mean. *p<0.05 vs. Sham; #p<0.05 vs. Vehicle.
Discussion
Our present study showed the impact of P2X7R/cryopyrin inflammasome axis in the pathogenesis of EBI in a rat SAH model. We made the following main observations in this study: I. Cryopyrin expression significantly increased 24 hours after SAH, which is the priming step for the formation of the cryopyrin inflammasome. II. Silencing of either cryopyrin or P2X7R via siRNA administration attenuated neurobehavioral deficit and brain edema, possibly by inhibiting caspase-1-induced maturation of IL-1β/IL-18 after SAH. III. Cryopyrin siRNA abolished the pro-inflammatory effect of BzATP, a P2X7R agonist, in LPS primed rats, indicating P2X7R is associated with activation of cryopyrin inflammasome in vivo. IV. In the rodent SAH model, BBG significantly improved neurological function and decreased brain edema via inhibiting caspase-1/IL-1β, IL-18 pathway. These data suggested the P2X7R/cryopyrin inflammasome axis plays a novel role in the pathogenesis of SAH.
The inflammasome is a large multimolecular complex that controls maturation and release of the pro-inflammatory cytokines (Kayagaki et al., 2011). Among all inflammasomes, the cryopyrin inflammasome is the best characterized. Upon activation, cryopyrin inflammasomes assemble, comprising the NLR protein cryopyrin, the adapter ASC and pro-caspase-1. The complex results in the autoactivation of pro-caspase-1, which in turn activates pro-IL-1β/IL-18 (Lamkanfi and Dixit, 2011). Aberrant cryopyrin inflammasome activation leads to an uncontrolled production of mature IL-1β/IL-18, which plays a pivotal role in a number of human diseases (Strowig et al., 2012; Wen et al., 2012). In the CNS, cryopyrin inflammasome is essential for the modulation of proinflammatory cytokines (Halle et al., 2008; Inoue et al., 2012).
The activation of the cryopyrin inflammasome requires two distinct signals: the first signal (priming) leads to synthesis of pro-IL-1β/IL-18 and cryopyrin itself, and the second signal (activation) is required to assemble the cryopyrin inflammasome (Supplementary Fig. 1) (Bauernfeind et al., 2009). The first signal is usually provided by Toll-like receptor 4/nuclear factor-kappa B (TLR4/NF-κB) (Bauernfeind et al., 2009), which has been found up-regulated following SAH (Wang et al., 2011). In the present study, cryopyrin level was found to increase at 24 hours after SAH, suggesting that cryopyrin is involved in EBI. The peak at 24 hours after SAH induction indicated a relatively wide therapeutic time window. Besides, LPS can activate the TLR4/NF-κB signaling pathway. We observed that LPS alone was able to increase the level of cryopyrin in the brain, but failed to increase the level of cleaved capase-1 and mature IL-1β, which is in agreement with a previous report (Mezzaroma et al., 2011). Thus, the first signal (cryopyrin priming) is necessary, but not sufficient and a second stimulus is required for cryopyrin inflammasome activation (Qiao et al., 2012).
Generally, three distinct mechanisms are in second signal for cryopyrin inflammasome activation: K+ efflux, mitochondria reactive oxygen species and lysosomal destabilization (Bird, 2012). More recently, increased intracellular Ca2+ was found to activate cryopyrin inflammasome (Lee et al., 2012). K+ efflux and Ca2+ influx can be triggered by activation of P2X7R (Surprenant and North, 2009). P2X7R is a unique member of the P2 family of nucleotide receptors for it pro-inflammatory effects, in that they trigger the release of interleukins. It is reported that P2X7R is a key player in IL-1β maturation and exteriorization (Ferrari et al., 2006). Caspase-1 activation is necessary for P2X7R-dependent release of mature IL-1β. Additionally, P2X7R is ATP-gated ion channel. Extracellular ATP, a damage associated molecular pattern, is normally kept within cells unless there is a setting of acute cellular injury (Wang et al., 2004). ATP can also be released from platelets and erythrocytes that infiltrate the lesioned tissue (Beigi et al., 1999; Edwards et al., 2001). A previous study reported that extracellular ATP can activate the cryopyrin inflammasome via P2X7R, leading to the activation of caspase-1 and release of mature IL-1β (Schroder and Tschopp, 2010). P2X7R-activated cryopyrin inflammasome formation promoted the expression of mature IL-1β. LPS-primed P2X7R−/− macrophages restored to secrete mature IL-1β, when pretreated with the inflammasome activator (Solle et al., 2001). Taken together, it is quite logical to consider that P2X7R is an upstream signaling for cryopyrin inflammasome activation after SAH. To investigate P2X7R/cryopyrin inflammasome axis in vivo, we assessed engagement of P2X7R resulted in cryopyrin inflammasome activation in LPS primed naïve rats. BzATP, a P2X7R agonist, stimulated activation of caspase-1 and secretion of mature IL-1β. Pretreatment of cryopyrin siRNA abrogated proinflammatory effect of BzATP, which indicates a link between P2X7R and cryopyrin inflammasome activation in parenchymal area.
At the onset of SAH, ATP is released into the extracellular space from damaged neurons and nonneuronal cells. Elevated extracellular ATP stimulates K+ efflux via P2X7R, subsequently triggering assembly of cryopyrin inflammasome. Upon cryopyrin inflammasome activation, procaspase-1 is converted to cleaved caspase-1 that then cleaves pro-IL-1β/IL-181 to the mature and secretion form, eventually mediating inflammation. In present study, our result revealed that P2X7R and cryopyrin knockdown by their siRNA ameliorated SAH-induced cerebral edema formation and effectively improved neurological impairment by reducing cleaved caspase-1 and mature IL-1β/IL-18.
Of particular translational significance, we furthermore observed protective effect of BBG in SAH rat model. BBG is a noncompetitive and selective antagonist of P2X7R, which is at least 1000-fold more potent at P2X7R than other P2X receptors in rats (Jiang et al., 2000). Previously, BBG has been described in cerebral ischemia, traumatic brain injury and spinal cord injury with therapeutic effect (Arbeloa et al., 2012; Kimbler et al., 2012; Peng et al., 2009). The low toxicity and high selectivity make BBG an ideal candidate for blocking the potential adverse effect of P2X7R activation after SAH. BBG administration had no significant effect on neurological outcome and brain edema in sham-operated mice (Kimbler et al., 2012). Our experiment aimed to extend previous reports showing BBG inhibited the inflammatory response via P2X7R/cryopyrin axis after SAH. We found BBG improved neurological recovery and attenuated brain edema, which directly inhibited activation of caspase-1 and subsequent cytokines. In addition, BBG decreased the number of infiltrating neutrophils, suggesting protection of BBG was likely involved in suppression of inflammatory response after SAH. Therefore, we suggest that BBG might be an excellent candidate for in advanced preclinical stroke research. No difference in brain edema between BBG (100 mg/kg) and vehicle group is unexpected, as BBG is believed as non-toxic drug. It could be explained 100mg/kg is approximately 10 times than no toxicity dosage (up to 12 mg/kg per day) in healthy animals (Peng et al., 2009). We also cannot exclude the possibility of some undiscovered side effect of BBG.
Several potential weaknesses of the present study deserve mention. First, although it is known that extracellular ATP engages P2X7R to trigger K+ efflux, leading assemble of cryopyrin inflammasome, we did not show K+ efflux. Second, potential weakness of this model is that the ATP overflow after SAH has not been measured, although extracellular ATP is believed to increase as result of leakage from damaged or dying cells after SAH. Third, the cellular sources of P2X7R or cryopyrin expression, and IL-1β and IL-18 production were not examined in the present study. Fourth, we also did not explore the physiology role of P2X7R and cryopyrin in normal rats. Finally, a caveat of the present study is that P2X7R triggering inflammation may be involved in distinct pathways, such as Ca2+, mitogen-activated protein kinase and phospholipase D.
In conclusion, we postulated that the P2X7R/cryopyrin inflammasome axis may be one such target that could be effective for modulating the inflammatory response after SAH. Hence, blockade of P2X7R/cryopyrin inflammasome axis can exert potentially anti-inflammatory effects in CNS, such as BBG.
Supplementary Material
Hightlights.
The expression of cryopyrin significantly increased at 24 hours after SAH.
Cryopyrin or P2X7R siRNA inhibited caspase-1/IL-1β, IL-18 pathway after SAH.
P2X7R is associated with activation of cryopyrin inflammasome in brain.
BBG improved neurological deficit via inhibiting caspase-1/IL-1β, IL-18 pathway.
P2X7R/cryopyrin axis plays a novel role in the pathogenesis of SAH.
Acknowledgement
This study was supported by NIH NS053407 to JH Zhang and by Natural Science Foundation of China (No.81171096); Natural Science Foundation of Zhejiang province, China (No.Z2090200) to JM Zhang.
Abbreviations
- SAH
subarachnoid hemorrhage
- EBI
early brain injury
- NLR
nucleotide-binding domain, leucine-rich repeat containing
- ATP
adenosine triphosphate
- ASC
apoptosis-associated speck-like protein containing a CAED
- IL-1β
interleukin-1β
- CNS
central nervous system
- P2X7R
P2X purinoceptor 7
- siRNA
small interfering RNA
- LPS
lipopolysaccharide
- BBG
brilliant blue G
- SD
Sprague-Dawley
- IACUC
Institutional Animal Care and Use Committee
- BzATP
Benzoylbenzoyl-ATP
- ANOVA
analysis of variance
- MPO
myeloperoxidase
- TLR4
Toll-like receptor 4
- NF-κB
nuclear factor-kappa B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None.
Reference
- Arbeloa J, et al. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigi R, et al. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- Beynon V, et al. Activated human CD4+CD45RO+ memory T-cells indirectly inhibit NLRP3 inflammasome activation through downregulation of P2X7R signalling. PLoS One. 2012;7:e39576. doi: 10.1371/journal.pone.0039576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L. Innate immunity: Linking mitochondria and microbes to inflammasomes. Nat Rev Immunol. 2012;12:229. doi: 10.1038/nri3195. [DOI] [PubMed] [Google Scholar]
- Chu K, et al. Inhibition of P2X7 receptor ameliorates transient global cerebral ischemia/reperfusion injury via modulating inflammatory responses in the rat hippocampus. J Neuroinflammation. 2012;9:69. doi: 10.1186/1742-2094-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly ES, Jr, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- Duris K, et al. Sampling of CSF via the Cisterna Magna and Blood Collection via the Heart Affects Brain Water Content in a Rat SAH Model. Transl Stroke Res. 2011;2:232–237. doi: 10.1007/s12975-010-0063-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J, et al. Chemiluminescence detection of ATP release from red blood cells upon passage through microbore tubing. Analyst. 2001;126:1257–1260. doi: 10.1039/b100519g. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, et al. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28:487–493. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Ferrari D, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- Franchi L, et al. Sensing and reacting to microbes through the inflammasomes. Nat Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhalgh AD, et al. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech. 2012;5:823–833. doi: 10.1242/dmm.008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra MC, et al. Lipopolysaccharide modulates astrocytic S100B secretion: a study in cerebrospinal fluid and astrocyte cultures from rats. J Neuroinflammation. 2011;8:128. doi: 10.1186/1742-2094-8-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, et al. Inhibition of carbonic anhydrase reduces brain injury after intracerebral hemorrhage. Transl Stroke Res. 2012;3:130–137. doi: 10.1007/s12975-011-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle A, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, et al. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012;43:484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, et al. NLRP3 inflammasome induces chemotactic immune cell migration to the CNS in experimental autoimmune encephalomyelitis. Proc Natl Acad Sci U S A. 2012;109:10480–10485. doi: 10.1073/pnas.1201836109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183:7623–7629. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, et al. Brilliant blue G selectively blocks ATP-gated rat P2X(7) receptors. Mol Pharmacol. 2000;58:82–88. [PubMed] [Google Scholar]
- Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- Kim JE, Kang TC. The P2X7 receptor-pannexin-1 complex decreases muscarinic acetylcholine receptor-mediated seizure susceptibility in mice. J Clin Invest. 2011;121:2037–2047. doi: 10.1172/JCI44818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbler DE, et al. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187:597–602. doi: 10.4049/jimmunol.1100229. [DOI] [PubMed] [Google Scholar]
- Lee GS, et al. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123–127. doi: 10.1038/nature11588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, et al. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- Manaenko A, et al. PAR-1 antagonist SCH79797 ameliorates apoptosis following surgical brain injury through inhibition of ASK1-JNK in rats. Neurobiol Dis. 2013;50:13–20. doi: 10.1016/j.nbd.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzaroma E, et al. The inflammasome promotes adverse cardiac remodeling following acute myocardial infarction in the mouse. Proc Natl Acad Sci U S A. 2011;108:19725–19730. doi: 10.1073/pnas.1108586108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu J, et al. Delayed hyperbaric oxygen therapy induces cell proliferation through stabilization of cAMP responsive element binding protein in the rat model of MCAo-induced ischemic brain injury. Neurobiol Dis. 2013;51:133–143. doi: 10.1016/j.nbd.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Murakami K, et al. Subarachnoid Hemorrhage Induces Gliosis and Increased Expression of the Pro-inflammatory Cytokine High Mobility Group Box 1 Protein. Transl Stroke Res. 2011;2:72–79. doi: 10.1007/s12975-010-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkamp DJ, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8:635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- Peng W, et al. Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury. Proc Natl Acad Sci U S A. 2009;106:12489–12493. doi: 10.1073/pnas.0902531106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, et al. TLR-induced NF-kappaB activation regulates NLRP3 expression in murine macrophages. FEBS Lett. 2012;586:1022–1026. doi: 10.1016/j.febslet.2012.02.045. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Sehba FA, et al. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy AR, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336:481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- Shiba M, et al. Imatinib mesylate prevents cerebral vasospasm after subarachnoid hemorrhage via inhibiting tenascin-C expression in rats. Neurobiol Dis. 2012;46:172–179. doi: 10.1016/j.nbd.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Simard JM, et al. Heparin Reduces Neuroinflammation and Transsynaptic Neuronal Apoptosis in a Model of Subarachnoid Hemorrhage. Transl Stroke Res. 2012;3:155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solle M, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- Sozen T, et al. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strowig T, et al. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- Sugawara T, et al. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008;167:327–334. doi: 10.1016/j.jneumeth.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333–359. doi: 10.1146/annurev.physiol.70.113006.100630. [DOI] [PubMed] [Google Scholar]
- Suzuki H, et al. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, et al. Caspase-1 inhibitor prevents neurogenic pulmonary edema after subarachnoid hemorrhage in mice. Stroke. 2009;40:3872–3875. doi: 10.1161/STROKEAHA.109.566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. P2X7 receptor inhibition improves recovery after spinal cord injury. Nat Med. 2004;10:821–827. doi: 10.1038/nm1082. [DOI] [PubMed] [Google Scholar]
- Wang Z, et al. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H, et al. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.