Abstract
Malignant peritoneal mesothelioma (MPM) is a rare and aggressive neoplasm that is largely resistant to traditional anti-cancer therapies. For years it has been considered a terminal condition and once diagnosed, patients generally survived less than a year despite aggressive treatment. Although rare, the worldwide incidence of MPM continues to rise, in part due to its association with asbestos exposure. Patients usually present with non-specific symptoms of abdominal distension and pain making the diagnosis challenging. In recent years, aggressive cytoreductive surgery with the administration of hyperthermic intraperitoneal chemotherapy (HIPEC) has improved survival in patients with MPM treated at multiple centers worldwide. This review article briefly highlights the presentation, diagnosis, and natural history of MPM. We then explore the available treatment options with primary focus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy.
Introduction
Malignant mesothelioma is a rare, aggressive malignancy that arises from serosal surfaces such as the pleura, peritoneum, pericardium, and tunica vaginalis. It is found more commonly in males and classically effects middle aged to elderly patients with a mean age of 58 years[1]. The link between malignant mesothelioma and exposure to asbestos was first recognized in 1960[2]. Malignant mesothelioma is a rapidly fatal disease and if left untreated, survival rarely exceeds a year[3]. Although first described in 1908[4], advances in traditional oncologic therapies such as palliative surgery, systemic chemotherapy, and whole body radiation have produced little, if any, survival benefit[5-8].
Approximately 10-20% of malignant mesotheliomas arise from the peritoneum. Patients with malignant peritoneal mesothelioma (MPM) generally present with vague abdominal symptoms and most ultimately die from a bowel obstruction or cachexia. Over the past fifteen years, significant improvements in overall survival and disease-free survival have consistently been reported by treating MPM patients with aggressive cytoreductive surgery (CS) combined with perioperative hyperthermic intraperitoneal chemotherapy (HIPEC). Median overall survival (OS) between 36 and 92 months, with 5-year survival rates as high as 59%, have been published[9]. In addition, long-term survivors without evidence of recurrence have been reported suggesting a potential cure in a small subset of patients. However, most of our knowledge regarding the management of MPM with CS plus HIPEC comes from single institutional phase I/II studies[10-18], primarily because the rarity of the disease makes large multi-institutional randomized trials nearly impossible. Beyond the single institutional experiences, only one meta-analysis[9] and one multi-institutional registry analysis[19] are available to analyze the efficacy of this procedure. However, by comparing results from historic controls and traditional therapies, it is becoming increasingly evident that CS plus HIPEC is the best treatment option for long-term survival in patients with MPM.
Epidemiology
The overall incidence of malignant mesothelioma is significantly higher in men than in females, most likely related to occupational exposure to asbestos. It generally occurs in middle aged and elderly patients with a mean age of 58 years[1]. Age-adjusted rates of mesothelioma in the 1990’s in the United States were approximately 20 per million for males and 4 per million for females[20]. In contrast, the incidence of mesothelioma in Great Britain is 70.9 per million in men which is one of the highest rates in the world[21]. The peritoneum is the second most common site of mesothelioma comprising of 10-20% of all malignant cases. Approximately 250-400 cases of MPM are diagnosed in the United States each year[20,22].
Multiple studies have confirmed the association between asbestos exposure (specifically amphibole fibers) and malignant mesothelioma[3,22-25]. Temporal trends of asbestos use and rates of malignant mesothelioma suggest a latency period of 20 to 40 years. As such, asbestos exposure peaked in the early 1970’s in the United States and age-adjusted rates of malignant mesothelioma appear to have peaked in the mid 1990’s[20]; however, the worldwide incidence of malignant mesothelioma continues to rise and is not expected to peak for another 15 to 20 years[26]. Peak incidences of mesothelioma in several European countries have been estimated: 2010 in Norway[27]; between 2011 and 2015 in Great Britain[21]; between 2012 and 2024 in Italy[28]; and 2030 in France[29]. The association between asbestos exposure and MPM is much weaker compared to pleural mesothelioma[22,24] especially in females. Only between 29-58% of males with MPM report known asbestos exposure compared to 2-23% in females[25,30]. In addition, lung fiber analysis suggests that 75% of MPM in men were asbestos-related, where as only 33% of MPM in women were asbestos-related[31]. Exposure to erionite, thorotrast, simian virus 40 have also been implemented in malignant mesothelioma, however their exact roles remain unclear[23,32]. In some rural areas of Turkey, malignant mesothelioma is endemic due to environmental exposure from asbestos-contaminated soil[33]. Some genetic studies of Turkish families living in these remote villages in Turkey suggest that some individuals may also possess a genetic predisposition to developing mesothelioma[34].
Clinical Presentation and Natural History
Classically, patients who are diagnosed with MPM present with ascites, abdominal pain, abdominal distension, fatigue and/or an abdominal mass. Some patients present with a new abdominal wall hernia relating to the intra-abdominal ascites and pressure. Lymph node and distant metastases are rare but patients often present with advanced disease resulting in bowel obstruction and widespread peritoneal dissemination[35]. In addition, a subset of patients with MPM present primarily with medical symptoms including fevers, diarrhea, weight loss, abdominal pain, and fatigue[36]. Thrombocytosis and hypercoagulability are also commonly associated with MPM. Prior to modern treatment options, median survival for patients with MPM was between 6 and 8.2 months[37-38]. The most common causes of death from MPM are bowel obstruction, cachexia, and pulmonary thromboembolism[36,39].
Diagnosis and Pathology
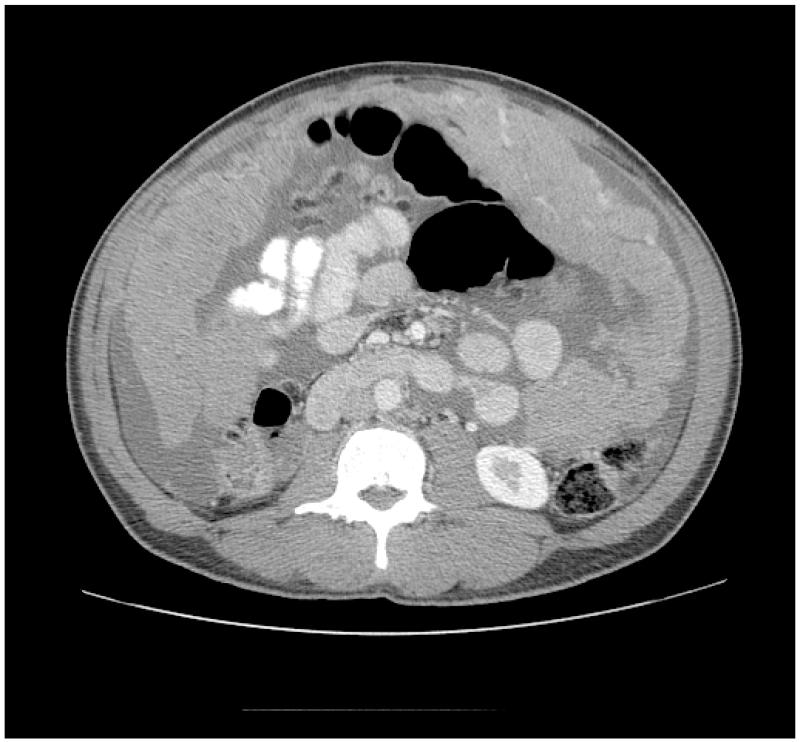
Computed tomography (CT) with intravenous, oral and rectal contrast is the imaging modality of choice and often reveals serous ascites, peritoneal and/or omental thickening/nodularity and an abdominal mass(es), although the full extent of disease is difficult to appreciate because of its diffuse nature (see figure 1). Magnetic resonance imaging and positron emission tomography provide little additional information and their roles in evaluating MPM remains unclear. In addition to imaging, tumor markers are helpful in some patients. CA 125 and CA 15.3 tumor markers have been shown to have a sensitivity of 53% and 49%, respectively, in patients with MPM[40] and when elevated can be used as a marker for response to treatment and disease progression/recurrence. New tumor markers are being evaluated and appear to be more specific for mesothelioma, including; mesothelin[41], soluble mesothelin related proteins (SMRP)[42] and osteopontin[43].
Figure 1.
CT image of malignant peritoneal mesothelioma showing significant omental thickening and ascites.
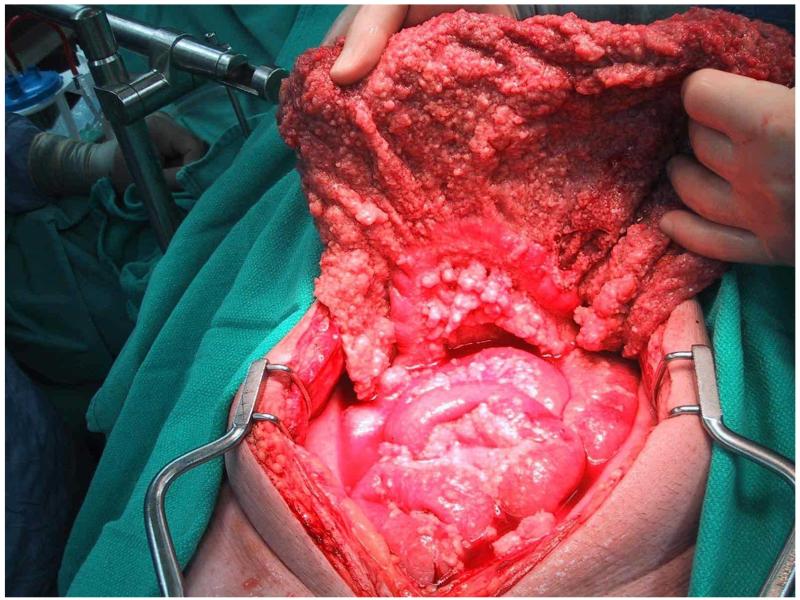
The pathologic diagnosis of MPM can be challenging and is sometimes made by peritoneal fluid cytology or radiographic guided biopsy but definitive diagnosis most often requires laparoscopy or laparotomy. Macroscopically, MPM is characterized by thousands of white nodules on the peritoneal surface (see figure 2). When performing diagnostic procedures, biopsies and laparoscopic ports should be placed through the linea alba because of case reports of tumor implantation within needle tracts and abdominal incisions[44].
Figure 2.
Malignant peritoneal mesothelioma characterized by small nodules covering peritoneal surfaces most often resulting in diffuse intra-abdominal dissemination, omental thickening and serous ascites.
The cytologic and histologic features of MPM have been described in recent articles[45-46]. The tumor arises from mesothelial cells lining the peritoneal cavity. Three broad subtypes of MPM have been described: epithelioid, mixed/biphasic, and sarcomatoid. Epithelioid MPM is by far the most commonly found subtype, diagnosed in approximately 75-92% of cases; while mixed/biphasic comprises 8-22% [47-48]. Sarcomatoid MPM is very rare. This distinction is important because biphasic and sarcomatoid MPM are extremely resistant to treatment and are associated with a poor prognosis[10,14-16]. In addition, nuclear/nucleolar size, chromatin pattern, mitotic index, atypical mitosis, pattern of necrosis, depth of bowel invasion, and lymph node involvement are histomorphologic parameters with prognostic value[48]. There are many immunohistochemical markers that aid pathologists in distinguishing MPM from nongynecologic adenocarcinomas and papillary serous carcinoma. The most useful positive immunohistochemical markers are calretinin, WT-1, and D2-40, while common negative markers include CEA, MOC-31, BG8, B72.3, and Ber-EP4[46].
Traditional Treatment Strategies
Historically, palliative surgery and systemic chemotherapy have yielded disappointing results when treating MPM patients with median survival ranging from 6 to 15 months[1,8,37-39,49]. Response rates for single-agent chemotherapy are only 10-15% while combination chemotherapy regimens claim response rates up to 25%[50]. MPM is also largely resistant to whole abdominal radiation with similar survival rates[51]. In addition, intraperitoneal chemotherapy without surgical debulking has not significantly influenced the natural history of MPM yielding median survival between 5 and 9 months[52-53]. In a more recent series, Manzini, et al.[36] analyzed the natural history of MPM in 81 patients. Thirty patients (37%) were treated with surgery but only seven (9%) were treated with concomitant HIPEC. Over half (45 patients) were treated with systemic chemotherapy and 21 patients (26%) were treated with surgery and systemic chemotherapy. The median survival in this group of patients was only 13 months.
Recent randomized trials using modern combination chemotherapy regimens to treat pleural mesothelioma[49,54] have sparked renewed interest in the systemic treatment of MPM and several phase I/II studies have shown modest clinical improvements. Jänne, et al.[55] recently reported outcomes on 73 patients with MPM from multiple institutions in the United States who were treated with pemetrexed with or without cisplatin. Disease control (complete response, partial response or stable disease) was achieved in 71% of patients and median survival was 13.1 months in patients who received the combined regimen. These results are comparable to a multi-institutional European study[56] that analyzed 109 patients with MPM who received pemetrexed with or without cisplatin or carboplatin. The disease control rate was 68% with one-year survival of 47%. While median OS for patients receiving combined therapy had not been reached, median survival for patients receiving pemetrexed alone was 10.3 months. As part of another phase II trial[57], pemetrexed plus gemcitabine yielded a 50% disease control rate with a time to disease progression of 10.4 months, a median OS of 26.8 months and a two-year survival of 50% in twenty patients with MPM not amendable to curative surgery.
Cytoreductive Surgery with Intraperitoneal Hyperthermic Chemotherapy
Over the past twenty years, the locoregional therapeutic approach of aggressive surgical cytoreduction with the perioperative administration of HIPEC has gained increasing attention in treating pseudomyxoma peritonei and peritoneal carcinomatosis from a variety of malignancies including ovarian, appendiceal, colorectal, and gastric cancers. Because MPM is also confined to the peritoneal cavity, this treatment modality is being utilized with curative intent in patients diagnosed with MPM. The rationale of CS plus HIPEC stems from considering the peritoneum as a unique organ system that can be treated by local resection. Aggressive cytoreduction involves complete lysis of adhesions, omentectomy, peritonectomies, and various organ resections when required to eliminate as much macroscopic disease as possible. Microscopic residual disease is then treated with intraperitoneal heated chemotherapy at doses much higher than could be given systemically[58-59]. It is generally accepted that intraperitoneal chemotherapy only penetrates a few millimeters into tissue which underscores the importance of aggressive surgical cytoreduction of all macroscopic disease when safely possible. Hyperthermia has direct cytotoxic effects and increases the depth of chemotherapy penetration[60-62]. A variety of administration technique of HIPEC exist but at our institution it involves placing two inflow and two outflow cannulas into the peritoneal cavity before temporarily closing the midline laparotomy skin incision. Using a perfusion pump the chemotherapeutic agent is pumped in and out of the abdominal cavity maintaining a constant temperature (40° Celsius) and flow rate (1 liter/minute) while gentle external massage is used to facilitate chemoperfusate flow throughout the peritoneal cavity. After 60 to 120 minutes, the abdomen is drained, the midline incision reopened, the cannulas removed, and the abdomen closed in the usual fashion. Regimens involving early postoperative intraperitoneal chemotherapy (EPIC) require cannulas, ports, or drains to be left within the abdominal cavity.
Wake Forest University Medical Center is one of the largest peritoneal malignancy programs in the United States and we recently published our results treating MPM with CS and HIPEC[10]. Over a fifteen year period, thirty-eight patients with MPM were treated with CS and HIPEC. Our initial intraoperative chemoperfusions utilized mitomycin C, however for the past seven years, we have used cisplatin. The mean age was 55 years and the male to female ratio was 2:1. Twenty-two patients (65%) were considered to be disease free after undergoing either R0/1 or R2a cytoreduction (residual tumor ≤ 5mm). Median OS was 40.8 months and 3- and 5-year survival of 56% and 17%, respectively. Although not statistically significant, we found a trend towards better OS in patients treated with cisplatin (40.8 months vs 10.8 months). In fact, there was a significant difference in 3-year survival between patients perfused with cisplatin compared to mitomycin (80% vs 42%). This is the first study to suggest that the chemoperfusion regimen of HIPEC may make a difference in clinical outcomes. The longest surviving patient in our series is alive without evidence of disease over 14 years after his procedure, suggesting that cure may be possible in a subset of cases.
Other recently published single institutional series have reported similar results treating MPM with CS and HIPEC (see table 1). Brigand, et al.[11] from France, reported results treating fifteen MPM patients with CS and HIPEC. Cytoreduction of all macroscopic disease (CC-0) or residual nodules less than 2.5mm (CC-1) was achieved in 73% of patients. Patients were perfused with cisplatin and mitomycin C. Mild perioperative complications occurred in six patients (40%) but no patient experienced a complication requiring reintervention and there were no postoperative deaths. Median OS was 36 months with 1-, 3-, and 5-year survival rates of 69%, 43%, and 29% respectively. Patients who received a CC-0 or CC-1 resection had a median OS of 37.8 months and a 5-year survival of 43.8%, compared to patients who underwent a CC-2 CC-3 resection (residual nodules greater than 2.5mm) whose median OS was 6.5 months and 5-year survival was 0%.
Table 1.
Survival results from selected series using cytoreductive surgery and hyperthermic intraperitoneal chemotherapy to treat malignant peritoneal mesothelioma.
| Study | n= | Median OS (months) |
Survival | |
|---|---|---|---|---|
| 3-year | 5-year | |||
| Blackham 2010[10] | 38 | 41 | 56% | 17% |
| Brigand 2006[11] | 15 | 36 | 43% | 29% |
| Baratti 2010[12] | 83 | 44 | * | 50% |
| Feldman 2003[13] | 49 | 92 | 59% | 59% |
| Yan 2006[14] | 100 | 52 | 55% | 46% |
| Kluger 2010[15] | 47 | 55 | 62% | 49% |
| Tudor 2010[16] | 20 | 30 | 46% | * |
| Yan 2009[19] | 401 | 53 | 60% | 47% |
not reported
A recent report from the National Cancer Institute in Milan, Italy[12] analyzed results treating 83 MPM patients with CS and HIPEC. Patients were perfused with either cisplatin + mitomycin C or cisplatin + doxorubicin. Grade III-V complications occurred in 27.7% of patients and operative mortality was 2.4%. Median OS was 44 months with 5- and 10-year survival rates of 49.5% and 45.5% respectively. This same group from Italy studied outcomes of thirty-eight patients who developed progressive disease following CS and HIPEC[63]. Median time to progression was 9 months and median survival from progression was 8 months. In thirty-one patients (81.6%) only peritoneal progression was found with small bowel and its mesentery involved in all cases except four. Disease progression was noted in 21% of anatomical regions where CC-0 cytoreduction were performed, while it was seen in 78% and 71% of regions where CC-1 and CC-2/3 resections were achieved. They noted a trend toward better outcomes in patients treated with repeat CS with or without HIPEC compared to systemic chemotherapy.
Other cancer centers have combined HIPEC with early postoperative intraperitoneal chemotherapy (EPIC). In their most recent publication on MPM, Feldman, et al.[13] at the National Cancer Institute in the United States, describe combining CS plus cisplatin-based HIPEC and a single intraperitoneal administration of 5-fluorouracil and paclitaxel between postoperative days 7 and 10. Their mean operative time was 6.5 hours and an RD0 or RD1 cytoreduction (less than 100 residual lesions all less than 5mm) was achieved in 47% of patients. Post-operative complications occurred in 25% of patients with two patients requiring reoperation for fascial dehiscence or gastric perforation. Median PFS was 17 months and median OS was 92 months with 5-year estimated survival of 59%.
The experience treating 100 patients with MPM at the Washington Cancer Institute by Sugarbaker and colleagues was recently reported[14]. While their HIPEC protocol has evolved over time, their current protocol involves intraperitoneal cisplatin and doxorubicin at the time of cytoreduction followed by EPIC using paclitaxel on post-operative days 1 through 5. Sixty-nine patients underwent adequate cytoreduction. Mean duration of surgery was 7.9 hours and mean hospital stay was 22 days. Grade III and IV morbidity rates were 24% and 11% respectively. Post-operative mortality was 5%. Median OS was 52 months with 1-, 3-, 5-, 7-year survival rates of 78%, 55%, 46% and 39%, respectively. In a report analyzing only patients treated with their current regimen[64], sixty-two patients had a median OS of 79 months with 3- and 5- year survival rates of 58% and 50% respectively.
Kluger, et al.[15] from Columbia University in New York recently proposed a two-stage procedure to reduce the morbidity and mortality of CS plus HIPEC. They reported results from forty-seven patients with MPM who received partial cytoreduction followed by eight weekly intraperitoneal courses of alternating cisplatin ± gemcitabine and doxorubicin, followed by four weeks of intraperitoneal gamma interferon. Patients were then treated with a second laparotomy and complete cytoreduction if necessary, followed by HIPEC using cisplatin and mitomycin C. Eight patients did not undergo the second procedure and four additional patients did not undergo intraoperative HIPEC. The mean length of stay for both operations was 16 days. In total, perioperative complications occurred in 16 patients (34%) while major complications occurred in 24% of patients. One patient died following the first operative (2% overall mortality). Median OS was 54.9 months and 3- and 5- year survival rates were 62% and 49% respectively. Seventeen patients were also treated with whole abdominal radiation, however due to treatment-related complications, radiation therapy was discontinued.
In 2008, a multi-institutional data registry on MPM was established and outcome data was recently published including 401 patients treated at eight institutions worldwide[19]. The mean age was 50 years and 56% were men. Median follow-up was 33 months. One hundred eighty-seven patients (46%) underwent complete cytoreduction. As expected a variety of different HIPEC regimens were used at each institution. Thirty-three patients (8%) did not receive intraoperative HIPEC. The mean operation duration was 8 hours. The mean hospital length of stay was 22 days. One hundred eighty-eight patients (46%) suffered a perioperative complication; including 127 patients (31%) who had grade III/IV complications. Post-operative mortality was 2%. Overall median survival was 53 months with 3- and 5-year survival rates of 60% and 47% respectively.
Prognostic Factors
While the available studies include relatively small numbers of patients and the treatment regimens differ at most institutions, several clinical, operative, and histopathologic factors have consistently shown prognostic value. Completeness of cytoreduction has been associated with improved survival in all major studies[10-17,63]. This underscores the importance of patient selection pre-operatively, as well as surgical technique and clinical experience. Most centers have identified histologic subtype as a significant predicator of survival. Specifically, biphasic and sarcomatoid MPM are very resistant to treatment and patient prognosis is extremely poor[10,14-16,64]. Mitotic count ≥ 5 per high power field[12,17] and nuclear grade[17] have also been associated with survival. Nuclear size was recognized as the dominant histopathologic prognostic factor by Yan et al.[64]. The 3-year survival rate with nuclear size <20μm was 100% while 3-year survival in patients with nuclear size > 40μm was 0%. Finally, although uncommon, lymph node metastasis correlates with a poor prognosis and some authors have recommended lymph node sampling at the time of cytoreduction[12,14].
From the multi-institutional MPM data registry,[19] variables associated with improved survival were identified on univariate analysis and included: age ≥ 50, female gender, epithelial subtype, absence of lymph node metastasis, absence of extra-abdominal metastasis, CC-0 or CC-1 cytoreduction, peritoneal cancer index of ≥ 20, use of HIPEC, transfusion of ≤ 5 units, and absence of cardiac complication. Only epithelial subtype, absence of lymph node metastasis, completeness of cytoreduction and use of HIPEC were independently associated with improved outcomes in multivariate analysis. Interestingly, the HIPEC delivery technique (open vs closed), the chemoperfusion regimen and the use of EPIC were not associated with significant differences in survival.
Using the data from this multi-institutional registry, a TMN staging system has recently been proposed for MPM[65]. In this proposed system (see Table 2), the T factor is based on the peritoneal cancer index (PCI) which assesses tumor size (0 to 3) in 13 intraabdominal anatomical regions yielding a score between 0 and 39. Applying this staging system to the data registry showed five-year survival rates for Stage I, II, and III of 87%, 53%, and 29% respectively. While this proposed staging system requires further validation, it provides clinicians and patients with valuable prognostic information that otherwise would not exist.
Table 2.
Proposed staging system for malignant peritoneal mesothelioma.
| Tumor (T) | |
| T1 | PCI 1-10 |
| T2 | PCI 11-20 |
| T3 | PCI 21-30 |
| T4 | PCI 31-39 |
|
| |
| Lymph Nodes (N) | |
| N0 | No lymph node metastases |
| N1 | Positive lymph node metastases |
|
| |
| Distant Metastasis (M) |
|
| No extra-abdominal metastatic disease | |
| M0 | |
| M1 | Extra-abdominal metastatic disease |
| Stage |
Tumor (T) |
Lymph Nodes (N) |
Distant Metastasis (M) |
Estimated
5-year Survival |
|---|---|---|---|---|
| I | T1 | N0 | M0 | 87% |
| II | T2-3 | N0 | M0 | 53% |
| III | T4 | N0/1 | M0/1 | 29% |
| T1-4 | N1 | M0/1 | ||
| T1-4 | N0/1 | M1 |
PCI—peritoneal cancer index
Challenges and Future Directions
The improved outcomes from CS plus HIPEC in treating MPM are accompanied with significant challenges. One of the greatest challenges in treating MPM is selecting which patients are able to undergo optimal treatment prior to laparotomy. Unlike other forms of peritoneal carcinomatosis, MPM often does not spare the small bowel making resectability difficult to assess prior to surgery. As described above, patients who cannot receive adequate cytoreduction because of disease location or extent have poorer outcomes. For this reason, Yan et al.[66] analyzed preoperative CT scans in patients who underwent adequate cytoreduction compared to suboptimal cytoreduction. They found significantly larger tumors in the epigastric region and proximal small bowel in patients who underwent suboptimal surgical cytoreduction. Based on these results, they developed a prediction model where patients who have tumor > 5 cm in the epigastric region and have nodular thickening of the small bowel with mesentery involvement have a 100% probability of a suboptimal resection, whereas patients who lack these two CT scan findings have a 94% probability of adequate cytoreduction.
Another significant challenge in treating MPM patients is that adequate cytoreduction is technically demanding and results in significant morbidity for patients. An extensive learning curve exists in performing CS plus HIPEC and experience over time has been associated with improved survival rates[67]. In fact, a recent study[68] suggests that as many as 130 procedures may be required before peak performance (graded by percentage of complete cytoreduction) is reached.
Morbidity from CS plus HIPEC has been reported to be as high as 46%[19]. Despite the high morbidity, quality of life studies suggest that most patients are able to attain acceptable quality of life with return of functional status and reduced pain between 3 and 6 months after surgery[69-70]. At experienced centers, improved patient selection and refined surgical technique will continue to improve clinical outcomes and decrease the morbidity and mortality of this procedure. As more medical oncologist and surgeons become increasingly aware and knowledgeable about CS and HIPEC and its potential benefits in treating MPM patients, more patients will be diagnosed earlier and referred to peritoneal surface malignancy programs. As a result, more surgical oncologists will become proficient in this procedure and it will become more widely available.
Lastly, due to the rarity of MPM, there is an absence of “level 1 evidence” comparing efficacy and safety of CS plus HIPEC compared to traditional modalities. Selection bias is an obvious consequence of the available phase I/II single institutional studies. In addition, each institution utilizes a different perioperative HIPEC regimen, making direct comparisons between studies difficult. Clearly, randomized controlled trials are needed; however, they will be difficult, if not impossible to obtain. Years will be required at multiple centers to recruit enough patients as evident by the largest available study[19] on MPM in which twenty years of experience at eight international institutions were required to accumulate 400 patients. Additionally, based on the current evidence it would be difficult, if not unethical, to randomize patients between a potentially curative procedure and a palliative traditional therapy. In the meantime, quality multi-institutional prospective studies are vital to establish consistent prognostic criteria to improve patient selection and identify the most efficacious HIPEC regimen. Multi-modality studies are also needed to establish the role of systemic chemotherapy in addition to CS plus HIPEC in patients with MPM.
Summary
A recent consensus statement regarding MPM was published based on questionnaire responses from health care providers who attended the 5th International Workshop on Peritoneal Surface Malignancies[71]. In summary, it recommends that patients with MPM should be referred to a peritoneal malignancy program to be evaluated for CS plus HIPEC. Patients should undergo pre-operative chest and abdominal CT scans to assess the extent of disease and determine resectability. As histology is a strong prognostic factor, the diagnosis should be confirmed by highly qualified centers with adequate experience with the disease. Tissue biopsy should be obtained via laparoscopy and if disease is found incidentally during another operation, cytoreduction should not be performed so as to preserve the integrity of the peritoneal barrier until definitive cytoreduction with HIPEC can be performed. Patient age, histologic subtype, mitotic count, nuclear grade or size, deep tissue invasion, previous debulking, performance status, and completeness of resection are the most significant prognostic factors that influence clinical outcomes in patients with MPM treated with CS and HIPEC. Based on current evidence, systemic chemotherapy with cisplatin plus pemetrexed or gemcitabine may be considered as adjuvant therapy. A subset of inoperable candidates may theoretically benefit from systemic chemotherapy and afterwards undergo a second surgical evaluation. While no standard chemoperfusion regimen is recommended, most experts agree that a combined regimen including cisplatin is preferred. While several centers use EPIC and/or whole abdominal radiation, the contribution of such therapies in addition to HIPEC is unknown. Follow-up visits should be performed every 3-4 months during the first two years and every 6 months thereafter and should include an abdominal CT scan and CA125 level for those patients with elevated levels pre-operatively.
Once considered a uniformly fatal condition, MPM is now a treatable malignancy by utilizing aggressive CS and HIPEC. While this aggressive therapy can be associated with significant morbidity, clinical outcomes have consistently revealed improved survival rates. Increased awareness and acceptance is needed among oncology care providers and prospective multi-institutional studies are required to better define patient selection criteria, prognostic factors, the most efficacious HIPEC regimen, and the role of neoadjuvant/adjuvant systemic chemotherapy. In the meantime, based on the available evidence, CS plus HIPEC should be considered standard of care for patient diagnosed with MPM.
References
- 1.Sridhar K, Doria R, Raub W, Thurer R, Saldana M. New strategies are needed in diffuse malignant mesothelioma. Cancer. 1992;70:2969–2979. doi: 10.1002/1097-0142(19921215)70:12<2969::aid-cncr2820701239>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Wagner J, Sleggs C, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in south east England: clinicopathological experience of 272 cases. Thorax. 1997;52:507–512. doi: 10.1136/thx.52.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller J, Wynn H. A malignant tumor arising from the endothelium of the peritoneum and producing a mucoid ascitic fluid. J Pathol Bacteriol. 1908;12:267. [Google Scholar]
- 5.Chailleux E, Dabouis G, Pioche D, de Lajartre M, de Lajartre A, Rembeaus A, Germaud P. Prognostic factors in diffuse malignant pleural mesothelioma. A study of 167 patients. Chest. 1988;93:159–162. doi: 10.1378/chest.93.1.159. [DOI] [PubMed] [Google Scholar]
- 6.Pass H, Kranda K, Temeck B, Feuerstein I, Steinberg S. Surgically debulked malignant pleural mesothelioma: results and prognostic factors. Ann Surg Oncol. 1997;4:215–222. doi: 10.1007/BF02306613. [DOI] [PubMed] [Google Scholar]
- 7.Janne P. Chemotherapy for malignant pleural mesothelioma. Clin Lung Cancer. 2003;5:98–106. doi: 10.3816/CLC.2003.n.023. [DOI] [PubMed] [Google Scholar]
- 8.Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women’s Hospital experience over two decades, 1965-1985. J Clin Oncol. 1988;6:147–153. doi: 10.1200/JCO.1988.6.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Yan T, Welch L, Black D, Sugarbaker P. A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann of Oncol. 2007;18:827–834. doi: 10.1093/annonc/mdl428. [DOI] [PubMed] [Google Scholar]
- 10.Blackham AU, Shen P, Stewart JH, Russel GB, Levine EA. Cytoreductive surgery with intraperitoneal chemotherapy for malignant peritoneal mesothelioma: mitomycin vs. cisplatin. Ann Surg Oncol. 2010;17:2720–2727. doi: 10.1245/s10434-010-1080-6. [DOI] [PubMed] [Google Scholar]
- 11.Brigand C, Monneuse O, Mohamed F, Sayag-Beaujard A, Isaac S, Gilly F, Glehen O. Peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: Results of a prospective study. Ann Surg Oncol. 2006;13:405–412. doi: 10.1245/ASO.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 12.Baratti D, Kusamura S, Cabras AD, Laterza B, Balestra MR, Deraco M. Lymph Node Metastases in Diffuse Malignant Peritoneal Mesothelioma. Ann Surg Oncol. 2010;17:45–53. doi: 10.1245/s10434-009-0756-2. [DOI] [PubMed] [Google Scholar]
- 13.Feldman A, Libutti S, Pingpank J, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560–4567. doi: 10.1200/JCO.2003.04.150. [DOI] [PubMed] [Google Scholar]
- 14.Yan TD, Yoo D, Sugarbaker PH. Significance of lymph node metastasis in patients with diffuse malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:948–953. doi: 10.1016/j.ejso.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Kluger MD, Taub RN, Hesdorffer M, Jin Z, Chabot JA. Two-stage operative cytoreduction and intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma: Operative morbidity and mortality in phase I and II trials. Eur J Surg Oncol. 2010;36:997–1003. doi: 10.1016/j.ejso.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Tudor EC, Chua TC, Liauw W, Morris DL. Risk Factors and Clinicopathological Study of Prognostic Factors in the Peritoneal Mesothelioma. Am Surg. 2010;76:400–405. [PubMed] [Google Scholar]
- 17.Nonaka D, Kusamura S, Baratti D, et al. Diffuse malignant mesothelioma of the peritoneum: a clinicopathological study of 35 patients treated with locoregionally at a single institution. Cancer. 2005;104:2181–2188. doi: 10.1002/cncr.21239. [DOI] [PubMed] [Google Scholar]
- 18.Elias D, Bedard V, Bouzid T, Duvillard P, Kohneh-Sharhi N, Raynard B, Goere D. Malignant peritoneal mesothelioma: Treatment with maximal cytoreductive surgery plus intraperitoneal chemotherapy. Gastroenterol Clin Biol. 2007;31:784–788. doi: 10.1016/s0399-8320(07)73964-7. [DOI] [PubMed] [Google Scholar]
- 19.Yan TD, Deraco M, Baratti D, Kusamura S, Elias D, Glehen O, Filly FN, Levine EA, Shen P, Mohamed F, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: Multi-institutional experience. J Clin Oncol. 2009;27:6237–6242. doi: 10.1200/JCO.2009.23.9640. [DOI] [PubMed] [Google Scholar]
- 20.Price B, Ware A. Mesothelioma Trends in the United States: An update based on Surveillance, Epidemiology and End Results Program data for 1973 through 2003. Am J Epidemiol. 2004;159:107–112. doi: 10.1093/aje/kwh025. [DOI] [PubMed] [Google Scholar]
- 21.McElvenny DM, Darnton AJ, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond) 2005;55:79–87. doi: 10.1093/occmed/kqi034. [DOI] [PubMed] [Google Scholar]
- 22.Moolgavkar S, Meza R, Turim J. Pleural and peritoneal mesothelioma in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control. 2009;20:935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 23.Boffetta P. Epidemiology of peritoneal mesothelioma: a review. Ann Onc. 2007;18:985–990. doi: 10.1093/annonc/mdl345. [DOI] [PubMed] [Google Scholar]
- 24.Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: An update based on SEER data for 1973 through 2005. Crit Rev Toxicol. 2009;39:576–588. doi: 10.1080/10408440903044928. [DOI] [PubMed] [Google Scholar]
- 25.Spirtas R, Heineman E, Bernstein L, Beebe G, Keehn R, Stark A, Harlow B, Benichou J. Malignant mesothelioma: Attributable risk of asbestos exposure. Occup Environ Med. 1994;51:804–811. doi: 10.1136/oem.51.12.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson B, Lake R. Advances in malignant mesothelioma. NEJM. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 27.Ulvestad B, Kjaerheim K, Møller B, Andersen A. Incidence trends of mesothelioma in Norway, 1965-1999. Int J Cancer. 2003;107:94–98. doi: 10.1002/ijc.11357. [DOI] [PubMed] [Google Scholar]
- 28.Marinaccio A, Montanaro F, Mastrantonio M, Uccelli R, Altavista P, Nesti M, Costantini AS, Gorini G. Predictions of mortality from pleural mesothelioma in Italy: A model based on asbestos consumption figures supports results from age-period-cohort models. Int J Cancer. 2005;115:142–147. doi: 10.1002/ijc.20820. [DOI] [PubMed] [Google Scholar]
- 29.Banaei A, Auvert B, Goldberg M, Gueguen A, Luce D, Goldberg S. Future trends in mortality of French men from mesothelioma. Occup Environ Med. 2000;57:488–494. doi: 10.1136/oem.57.7.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemminki K, Li X. Time trends and occupational risk factors for peritoneal mesothelioma in Sweden. J Occup Environ Med. 2003;45:451–455. doi: 10.1097/01.jom.0000052960.59271.d4. [DOI] [PubMed] [Google Scholar]
- 31.Roggli V, Sharma A, Butnor K, Sporn T, Vollmer R. Malignant mesothelioma and occupational exposure to asbestos: A clinicopathological correlation of 1445 cases. Ultrastruct Pathol. 2002;26:55–65. doi: 10.1080/01913120252959227. [DOI] [PubMed] [Google Scholar]
- 32.Strickler H, Goedert J, Devesa S, Lahey J, Fraumeni J, Rosenberg P. Trends in U.S. pleural mesothelioma incidence rates following simian virus 40 contamination of early poliovirus vaccines. J Natl Cancer Inst. 2003;95:38–45. doi: 10.1093/jnci/95.1.38. [DOI] [PubMed] [Google Scholar]
- 33.Metintas S, Metintas M, Ucgun I, Oner U. Malignant Mesothelioma due to environmental exposure to asbestos: Follow-up Turkish cohort living in a rural area. Chest. 2002;122:55–65. doi: 10.1378/chest.122.6.2224. [DOI] [PubMed] [Google Scholar]
- 34.Roushdy-Hammady I, Siegel J, Emri S, Testa JR, Carbone M. Genetic-susceptibility factor and malignant mesothelioma in the Cappadocian region of Turkey. Lancet. 2001;357:444–445. doi: 10.1016/S0140-6736(00)04013-7. [DOI] [PubMed] [Google Scholar]
- 35.Ribak J, Lilus R, Suzuki Y, Penner L, Selikoff I. Malignant mesothelioma in a cohort of asbestos insulation workers: Clinical presentation, diagnosis, and cause of death. Br J Ind Med. 1998;45:182–187. doi: 10.1136/oem.45.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzini V, Recchia L, Cafferata M, Porta C, Siena S, Giannetta L, Morellis F, Oniga F, Bearz A, Torri V, Cinquini M. Malignant peritoneal mesothelioma: A multicenter study on 81 cases. Ann Oncol. 2010;21:348–353. doi: 10.1093/annonc/mdp307. [DOI] [PubMed] [Google Scholar]
- 37.Piccigallo E, Jeffers L, Reddy R, Caldironi M, Parenti A, Schiff E. Malignant peritoneal mesothelioma. A clinical and laparoscopic study of ten cases. Dig Dis Sci. 1988;5:633–639. doi: 10.1007/BF01798369. [DOI] [PubMed] [Google Scholar]
- 38.van Gelder T, Hoogsteden H, Versnel M, de Beer P, Vandenbroucke J, Planteydt H. Malignant peritoneal mesothelioma: a series of 19 cases. Digestion. 1989;43:222–227. doi: 10.1159/000199880. [DOI] [PubMed] [Google Scholar]
- 39.Antman KH, Pomfret EA, Aisner J, MacIntyre J, Osteen RT, Greenberger JS. Peritoneal mesothelioma: natural history and response to chemotherapy. J Clin Oncol. 1983;1:386–391. doi: 10.1200/JCO.1983.1.6.386. [DOI] [PubMed] [Google Scholar]
- 40.Baratti D, Kusamura S, Martinetti A, Seregni E, Oliva D, Laterza B, Deraco M. Circulating CA125 in patients with peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol. 2007;14:500–508. doi: 10.1245/s10434-006-9192-8. [DOI] [PubMed] [Google Scholar]
- 41.Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J, Alexander R, Willingham M, Pastan I, Onda M. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res. 2006;12:447–453. doi: 10.1158/1078-0432.CCR-05-1477. [DOI] [PubMed] [Google Scholar]
- 42.Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N, Winzell P, Hellstrom KE, Hellstrom I. Soluble mesothelin-related protein--a blood test for mesothelioma. Lung Cancer. 2005;49:S109–111. doi: 10.1016/j.lungcan.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Pass HI, Lott D, Lonardo F, Harbut M, Liu Z, Tang N, Carbone M, Webb C, Wali A. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 44.Muensterer OJ, Averbach AM, Jacquet P, Otero SE, Sugarbaker PH. Malignant peritoneal mesothelioma. Case-report demonstrating pitfalls of diagnostic laparoscopy. Int Surg. 1997;82:240–243. [PubMed] [Google Scholar]
- 45.Patel N, Taylor C, Levine E, Trupiano J, Geisinger K. Cytomorphologic features of primary peritoneal mesonthelioma in effusion, washing, and fine-needle aspiration biopsy specimens: Examination of 49 cases at one institution, including post-intraperitoneal hyperthermic chemotherapy findings. Am J Clin Pathol. 2007;128:414–422. doi: 10.1309/DV1JYBL8LLYYT4J5. [DOI] [PubMed] [Google Scholar]
- 46.Husain AN, Colby TV, Ordóñez NG, Krausz T, Borczuk A, Cagle PT, Chirieac LR, Churg A, Galateau-Salle F, Gibbs AR, Gown AM, Hammar SP, Litzky LA, Roggli VL, Travis WD, Wick MR. Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009 Aug;133:1317–1331. doi: 10.5858/133.8.1317. [DOI] [PubMed] [Google Scholar]
- 47.Kannerstein M, Churg J. Peritoneal mesothelioma. Hum Pathol. 1977;8:83–94. doi: 10.1016/s0046-8177(77)80067-1. [DOI] [PubMed] [Google Scholar]
- 48.Cerruto D, Brun E, Chang D, Sugarbaker P. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med. 2006;130:1654–1661. doi: 10.5858/2006-130-1654-PSOHPI. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Carbonero R, Paz-Ares L. Systemic chemotherapy in the management of malignant peritoneal mesothelioma. Eur J Surg Oncol. 2006;32:676–681. doi: 10.1016/j.ejso.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 50.Hassan R, Alexander R, Antman K, Boffetta P, Churg A, Coit D, Hausner P, Kennedy R, Kindler H, Metintas M, Mutti L, Onda M, Pass H, Premkumar A, Roggli V, Sterman D, Sugarbaker P, Taub R, Verschraegen C. Current treatment options and biology of peritoneal mesothelioma: meeting summary of the first NIH peritoneal mesothelioma conference. Ann Oncol. 2006;17:1615–1619. doi: 10.1093/annonc/mdl060. [DOI] [PubMed] [Google Scholar]
- 51.Antman KH, Blum RH, Greenberger JS, Flowerdew G, Skarin AT, Canellos GP. Multimodality therapy for malignant mesothelioma based on a study of natural history. Am J Med. 1980;68:356–362. doi: 10.1016/0002-9343(80)90103-5. [DOI] [PubMed] [Google Scholar]
- 52.Langer CJ, Rosenblum N, Hogan M, Nash S, Bagchi P, LaCreta FP, Catalano R, Comis RL, O’Dwyer PJ. Intraperitoneal cisplatin and etoposide in peritoneal mesothelioma: Favorable outcome with a multimodality approach. Cancer Chemother Pharmacol. 1993;32:204–208. doi: 10.1007/BF00685836. [DOI] [PubMed] [Google Scholar]
- 53.Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol. 1992;118:547–550. doi: 10.1007/BF01225271. [DOI] [PubMed] [Google Scholar]
- 54.Fennell DA, Gaudino G, O’Byrne KJ, Mutti L, van Meerbeeck J. Advances in the systemic therapy of malignant pleural mesothelioma. Nat Clin Pract Oncol. 2008;5:136–147. doi: 10.1038/ncponc1039. [DOI] [PubMed] [Google Scholar]
- 55.Jänne P, Wozniak A, Belani C, Keohan M, Ross H, Polikoff J, Mintzer D, Taylor L, Ashland J, Ye Z, Monberg M, Obasaju C. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: Outcomes of an expanded access program. Clin Lung Cancer. 2005;7:40–46. doi: 10.3816/CLC.2005.n.020. [DOI] [PubMed] [Google Scholar]
- 56.Carteni G, Manegold C, Garcia G, Siena S, Zielinski C, Amadori D, Liu Y, Blatter J, Visseren-Grul C, Stahel R. Malignant peritoneal mesothelioma—Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009;64:211–218. doi: 10.1016/j.lungcan.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 57.Simon G, Verschraegen C, Janne P, Langer C, Dowlati A, Gadgeel S, Kelly K, Kalemkerian G, Traynor A, Peng G, Gill J, Obasaju, Kindler H. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: Final report of a phase II trial. J Clin Oncol. 2008;26:3567–3572. doi: 10.1200/JCO.2007.15.2868. [DOI] [PubMed] [Google Scholar]
- 58.Katz MH, Barone RM. The rationale of perioperative intraperitoneal chemotherapy in the treatment of peritoneal surface malignancies. Surg Oncol Clin N Am. 2003;12:673–688. doi: 10.1016/s1055-3207(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 59.Yan TD, Coa CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol. 2010;15:109–116. doi: 10.4251/wjgo.v2.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sticca RP, Dach BW. Rationale for hyperthermia with intraoperative intraperitoneal chemotherapy agents. Surg Oncol Clin N Am. 2003;12:689–701. doi: 10.1016/s1055-3207(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 61.van de Vaart PJ, van der Vange N, Zoetmulder FA, van Goethem AR, van Tellingen O, ten Bokkel Huinink WW, Beijnen JH, Bartelink H, Begg AC. Intraperitoneal cisplatin with regional hyperthermia in advanced ovarian cancer: Pharmacokinetics and cisplatin-DNA adduct formation in patients and ovarian cancer cell lines. Eur J Cancer. 1998;34:148–154. doi: 10.1016/s0959-8049(97)00370-5. [DOI] [PubMed] [Google Scholar]
- 62.Los G, Smals OA, van Vugt MJ, van der Vlist M, den Engelse L, McVie JG, Pinedo HM. A rationale for carboplatin treatment and abdominal hyperthermia in cancers restricted to the peritoneal cavity. Cancer Res. 1992;52:1252–1258. [PubMed] [Google Scholar]
- 63.Baratti D, Kusamura S, Cabras AD, Dilco P, Laterza B, Deraco M. Diffuse malignant peritoneal mesothelioma: Failure analysis following cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) Ann Surg Oncol. 2009;16:463–472. doi: 10.1245/s10434-008-0219-1. [DOI] [PubMed] [Google Scholar]
- 64.Yan T, Brun E, Cerruto C, Haveric N, Chang D, Sugarbaker P. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol. 2007;14:41–49. doi: 10.1245/s10434-006-9169-7. [DOI] [PubMed] [Google Scholar]
- 65.Yan TD, Deraco M, Elias D, Glehen O, Levine EA, Moran BJ, Morris DL, Chua TC, Piso P, Sugarbaker PH, Peritoneal Surface Oncology Group A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer. 2010 doi: 10.1002/cncr.25640. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 66.Yan TD, Haveric N, Carmignani CP, Chang D, Sugarbaker PH. Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer. 2005;103:839–849. doi: 10.1002/cncr.20836. [DOI] [PubMed] [Google Scholar]
- 67.Levine E, Stewart J, Russell G, Geisinger K, Loggie B, Shen P. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: Experience with 501 patients. J Am Coll Surg. 2007;204:943–953. doi: 10.1016/j.jamcollsurg.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 68.Smeenk RM, Verwaal VJ, Zoetmulder FA. Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg. 2007;94:1408–1414. doi: 10.1002/bjs.5863. [DOI] [PubMed] [Google Scholar]
- 69.Schmidt U, Dahlke MH, Klempnauer J, Schlitt HJ, Piso P. Perioperative morbidity and quality of life in long-term survivors following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2005;31:53–58. doi: 10.1016/j.ejso.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 70.McQuellon RP, Danhauer SC, Russell GB, Shen P, Fenstermaker J, Stewart JH, Levine EA. Monitoring health outcomes following cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol. 2007;14:1105–1113. doi: 10.1245/s10434-006-9304-5. [DOI] [PubMed] [Google Scholar]
- 71.Deraco M, Bartlett D, Kusamura S, Baratti D. Consensus statement on peritoneal mesothelioma. J Surg Oncol. 2008;98:268–272. doi: 10.1002/jso.21055. [DOI] [PubMed] [Google Scholar]