Abstract
Objectives
Cell migration is an important step in pulpal wound healing. Although components in the resin-based dental materials are known to have adverse effects on pulp wound healing including proliferation and mineralization, their effects on cell migration have been scarcely examined. Here, we investigated effects of 2-Hydroxyethyl methacrylate (HEMA) on migration of dental pulp stem cells (DPSC) in vitro.
Methods
Cell viability was assessed using MTT assay, and cell migration was evaluated using wound scratch assay and transwell migration assay at non-cytotoxic doses. Western blotting was used to examine pathways associated with migration such as focal adhesion kinase (FAK), mitogen-activated protein kinase (MAPK), and glycogen synthase kinase 3 (GSK3).
Results
There were no drastic changes in the cell viability below 3mM HEMA. When DPSC were treated with HEMA at 0.5, 1.0, and 2.5mM, cell migration was diminished. HEMA-treated DPSC exhibited the loss of phosphorylated focal adhesion kinase (FAK) in a dose-dependent manner. The HEMA-mediated inhibition of cell migration was associated with phosphorylation of p38 but not GSK3, ERK or JNK pathways. When we inhibited the p38 signaling pathway using a p38 inhibitor, migration of DPSC was suppressed.
Conclusion
HEMA inhibits migration of dental pulp cells in vitro, suggesting that poor pulpal wound healing under resin-based dental materials may be due, in part, to inhibition of cell migration by HEMA.
Keywords: HEMA, dental pulp stem cells, migration, pulpal wound healing, p38
INTRODUCTION
2-Hydroxyethyl methacrylate (HEMA) is an important component in dental composite resins and dentin adhesive materials used in dental restorations. HEMA has both hydrophilic and hydrophobic functional groups which, in combination, make it an excellent material to form an interface that allows for mediating the bonding between hydrophilic collagenous dentin and hydrophobic resin materials (1). As such, it plays an important role in increasing bonding strength of dental composite restoration materials.
Although the use of HEMA in dental materials is biomechanically advantageous due to its ability to increase the bonding strength, the biocompatibility of HEMA to pulp tissues is a concerning issue. Evidence shows that pulp tissues have a poor repairing capacity in the presence of dentin adhesives in vivo (2,3,4) which suggests that direct contact of the dentin adhesives and their components to pulp tissues leads to adverse effects on pulpal wound healing. Because HEMA accounts for 30–50% of dentin adhesives, cytotoxicity of HEMA has been well documented in vitro (5,6). HEMA has been shown to induce apoptosis and cause DNA fragmentation in both human peripheral blood mononuclear cells and mouse macrophages in a dose dependent manner (7). Furthermore, HEMA has been shown to induce an intense pulpal inflammatory response (8), and chronic exposure to the monomer is known to suppress immunological functions in vivo (9). Recently, HEMA has been shown to inhibit odontogenic differentiation of stem cells derived from deciduous teeth (10).
Pulpal wound healing is a complex process that is orchestrated by discrete but overlapping multiple steps of migration, proliferation, and mineralization of cells in dental pulp (11). Although migration is one of the required steps during the pulp wound healing process before the dental pulp mesenchymal cells become repopulated and mineralized in the wounded area, roles of dental materials, particularly HEMA, on migration of dental pulp cells are poorly characterized. The aim of this study is to examine the effects of HEMA on the migration of dental pulp stem cells (DPSC).
MATERIALS AND METHODS
Reagents and antibodies
p-FAK(Y397) (#44-624G) was purchased from Invitrogen (Carlsbad, CA). FAK (#558),GSK3α/β (#7291), pGSK3α/β(Y297/Y216) (#81496), GAPDH (#25778) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). p-p38(T180/Y182) (#4511), p38 (#9212), p-Erk1/2 (T202/Y204) (#4370), Erk1/2 (#9102), p-JNK(T183/Y185) (#4668), and JNK (#9528) were purchased from Cell Signaling Technology, Inc. (Danvers, MA). 2-Hydroxyethyl methacrylate (HEMA; CAS: 868-77-9) was purchased from Sigma-Aldrich (St. Louis, MO). PD169316 (4-(4-Fluorophenyl)-2-(4-nitrophenyl)-5-(4-pyridyl)-1H-imidazole), p38-Inhibitor, was purchased from CalBioChem/EMD Biosciences (San Diego, CA).
Cell cultures
Primary DPSC were kindly provided by Dr. Songtao Shi (Herman Ostrow School of Dentistry, USC). Cells were cultured in α-MEM (Invitrogen) supplemented with 10%FBS (Invitrogen) and 5μg/mL Gentamycin Sulfate (Gemini Bio-Products, West Sacramento, CA).
MTT assay
Actively proliferating DPSC cells were plated at 4×103 cells per well in 96-well plates. One day after plating, cells were treated with different amounts of HEMA. After two days, the cell viability was measured using MTT Cell Proliferation Assay Kit (ATCC, Manassas, VA) according to manufacturer’s protocol. Briefly, MTT reagents were added and incubated for 4 hours. After confirmation of purple precipitates under the microscope, detergents were added and cells were further incubated in the dark overnight. The colorimetric analysis was performed using the microplate reader at 570nm. For each HEMA concentration, we performed the assay in triplicate.
Scratch Assay
DPSC were grown to 90% confluency in 100mm culture dishes. Cells were then serum starved in 0.1%FBS (Invitrogen) for 24 hours. Medium was changed with the indicated HEMA amounts, and a scratch was made with a 200μL pipet tip. The initial scratched areas were uniform across the different samples and permanently marked; the marked areas were photographed at 0, 6, and 15 hours after the scratch. The photographs were taken using an Olympus DP72 microscope. The migration of the cells was determined by measuring the distance between the wounded edges. Three different areas were measured for each time point.
Boyden Chamber Assay
DPSC (2×104cells per well) were plated on a porous membrane (pore size: 8μm; Corning, Cat. #3422) with or without HEMA (0, 0.5, 1.0, 2.5mM) for 48 hours. Cells were fixed with 10% formaldehyde and stained with 2.5%Crystal violet overnight. Cells on the top of the transwell were removed, and the cells that migrated through the transwell membrane were photographed. Cells were counted and the cell numbers were normalized to the control. This assay was performed in triplicate. The same procedures were followed when checking migration patterns of DPSC in the presence of a p38-inhibitor at the indicated amounts.
Western blot
Cells were lysed using lysis buffer (1%Triton X-100, 20mM Tris-HCl (pH7.5), 150mM NaCl, 1mM EDTA, 1mM EGTA, 2.5mM sodium pyrophosphate, 1mM β-glycerolphosphate, 1mM sodium orthovandate and PMSF). Lysates (40–50μg) from cells were fractionated by 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis and transferred onto Immobilon protein membrane (Millipore, Billerica, MA). Immobilized membrane was incubated with primary antibodies and probed with the respective secondary antibodies. The membrane was exposed to the HyGLO Chemiluminescent HRP antibody detection reagent (Denville Scientific, South Plainfield, NJ) and scanned using ChemiDoc System (Bio-Rad, Hurcules, CA, USA).
Statistical analysis
The results are expressed as means ± standard deviation. For the comparison, the outcome measurements were compared to the control group using the Student t-test. p-values that are less than 0.05 are considered significant.
RESULTS
HEMA inhibits migration of DPSC
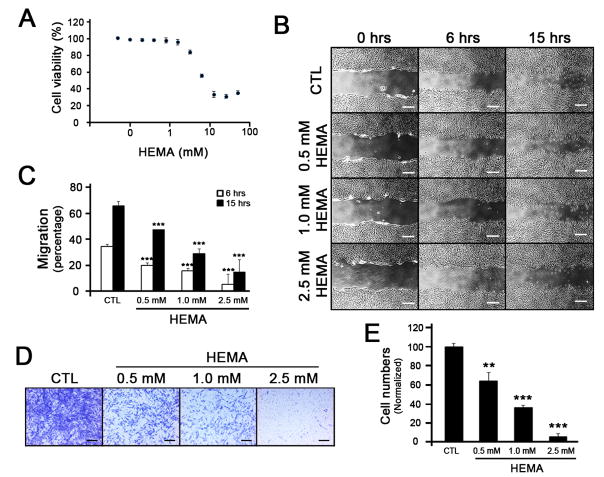
To examine effects of HEMA on migration of DPSC, we first determined viability of DPSC in response to HEMA treatment. After normalization to the control, we found that the viability of DPSC decreased dramatically after about 3mM HEMA (Fig. 1A). Using non-cytotoxic HEMA concentrations (below 3mM), migratory patterns of DPSC were observed. During the course of 6 and 15 hours after scratches were made, inhibition of cell migration was evident in a dose-dependent manner (Fig. 1B and 1C). To further confirm anti-migratory effects of HEMA, we utilized the transwell migration assay that is more stringent and devoid of proliferation effects. When treated with HEMA, the migration of DPSC decreased markedly (Fig. 1D). Quantification of the migrated cells revealed that the migration was inhibited in a dose-dependent manner (Fig. 1E), indicating that HEMA inhibits migration of DPSC at non-cytotoxic doses.
Figure 1. Effects of HEMA on migration of DPSC using wound scratch assay.
(A) DPSC were treated with different doses of HEMA for 2 days, and MTT assay was performed to examine cell viability. Each dose was triplicated and the error bars indicate standard deviation. (B) Cells grown up to 90% confluency were serum-starved for 24 hours, and scratches were made with a 200 μL pipet tip. The medium containing different doses of HEMA was changed, and photographs were taken at the indicated hours. The white bar represents 4 μm. (C) The migrated distance was measured and presented as bar graphs. Three different fields were taken and the standard deviations were obtained. *** p < 0.001. (D) DPSC were plated on the top of the transwell membrane with different amounts of HEMA. Cells were allowed to migrate through the transwell membrane for 2 days. Cells were stained with Crystal Violet, and the cells on the top of the transwell membrane were removed. The migrated cells on the bottom of the transwell membrane were photographed using a microscope. The black bar represents 2 μm. (E) The numbers of migrated cells were counted and plotted as a bar graph. ** p < 0.01, *** p < 0.001.
Suppression of FAK and p38 phosphorylation is associated with HEMA-induced inhibition of cell migration
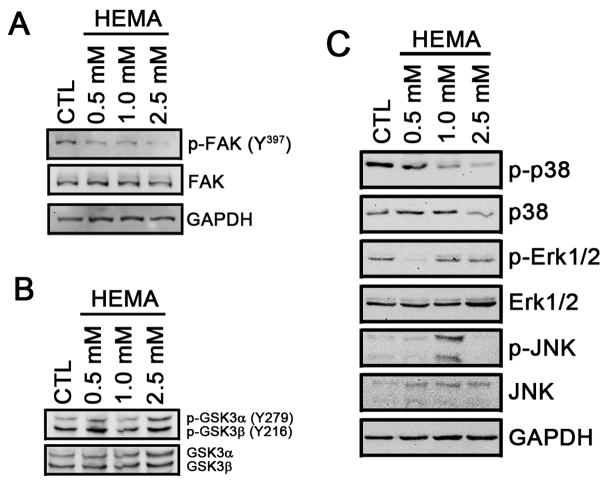
Activation of FAK by autophosphorylation at Tyr397 is an important initial signaling pathway in promoting cell migration. Therefore, we examined the phosphorylation status of FAK in response to HEMA treatment. In untreated DPSC, FAK is highly phosphorylated at Tyr397 (Fig. 2A) which was consistent with active migration (Fig. 1). When cells were treated with HEMA, the phosphorylation of FAK was suppressed (Fig. 2A). HEMA had no effects on phosphorylation of GSK3α and GSK3β (Fig. 2B), suggesting anti-migratory effects of HEMA is GSK3-independent. When the phosphorylation status of proteins involved in the MAP kinase pathways were examined, there was a dose-dependent reduction of phosphorylated p38 in response to HEMA treatment while Erk1/2 and JNK showed no correlation (Fig. 2C). These data suggest that HEMA-induced inhibition of cell migration is associated with FAK and p38 pathways.
Figure 2. HEMA-induced inhibition of cell migration is mediated through suppression of FAK and p38 phosphorylation.
DPSC were treated with different doses of HEMA, and western blotting was performed to examine phosphorylation of FAK (A), GSK (B) or MAPK (C). GAPDH was used as a control.
Inhibition of p38 MAPK suppresses migration of DPSC
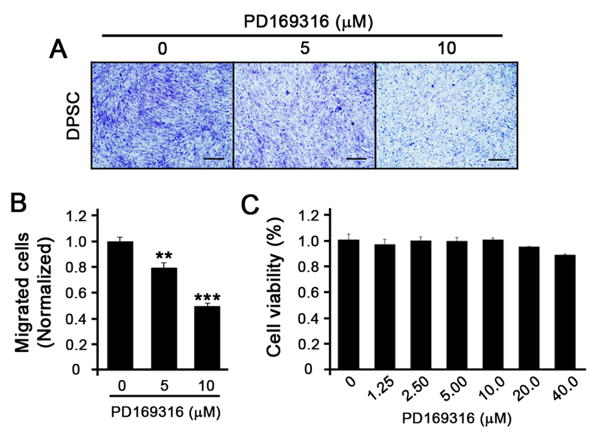
To determine the role of p38 in the migration of DPSC, we treated DPSC with PD169316, a known p38-specific inhibitor, and performed the transwell migration assay. PD169316-treated DPSC showed dose-dependent decrease in migration (Fig. 3A and 3B). To rule out the cytotoxic effects of the doses that we used to perform the migration assay, we examined the cell viability with different doses of PD169316 and found that, at 5 and 10μM, no changes in cell viability were observed (Fig. 3C). These data suggest that anti-migratory effect of HEMA on DPSC may be mediated, in part, through the p38 signaling pathway.
Figure 3. Inhibition of p38 suppresses the migration of DPSC.
(A) DPSC were plated on the top of the transwell membrane with different amounts of p38 inhibitor, PD169316. Cells were allowed to migrate through the transwell membrane for 2 days. Cells were stained with Crystal Violet, and the cells on the top of the transwell membranes were removed. The migrated cells on the bottom of the transwell membrane were photographed using a microscope. The black bar represents 2 μm. (B) The numbers of migrated cells were counted and plotted as a bar graph. ** p < 0.01, *** p < 0.001. (C) DPSC were treated with different doses of PD169316 for 2 days, and MTT assay was performed to examine cell viability. Each group of cells was performed in triplicate and the error bars indicate standard deviation.
DISCUSSION
Although the cytotoxic effects of HEMA on dental pulp cells have been well documented, its effect on cell migration has not been examined. Here, we provide evidence that HEMA exerts anti-migratory effects on dental pulp cells, and that such effects are not the result of cellular cytotoxicity. We also showed that its anti-migratory effects are associated with inhibition of FAK and p38 MAPK phosphorylation.
One of the main goals of pulp therapy is to regenerate hard tissue barriers on iatrogenic or pathologic pulp exposures to protect pulp tissues from further exogenous insults. The utilization of dentin adhesives was initially considered to be a viable treatment option for pulp exposure provided that bacterial contamination is well-isolated (12,13,14). In contrast, more recent studies suggest that the utilization of dentin adhesives for direct pulp capping causes adversary effects to pulp healing in vivo (2,3,4). Indeed, several in vivo studies found that no dentinal bridges were found under the dentin adhesives even in the absence of bacterial contamination (15,16,17,18), indicating that dentin adhesives are generally not well-tolerated for direct pulp capping procedures.
Formation of hard tissue barriers, or reparative dentin, is mediated by the pulp wound healing process. This is a complex process comprised of multiple discrete but overlapping steps of migration, proliferation, and mineralization of pulp cells (11). Therefore, ordered orchestration of these steps yields reparative dentin formation and successful outcome of pulp therapy. Our data suggests that HEMA inhibited the migration of cells (Fig. 1). Cell migration is an important step in pulpal wound healing and is indispensable particularly when dental pulp mesenchymal cells are expected to migrate into the wounded areas before they repopulate and differentiate to form reparative dentin. Clinical studies have demonstrated that exposed pulp areas capped with dentin adhesives containing HEMA lacked reactionary dentin deposition without the presence of odontoblast-like cells or newly-formed odontoblast layers, suggesting that pulp cells were not only failed to undergo mineralization but also inhibited to migrate to the exposed areas in vivo (16). This finding, along with our study, suggests that HEMA may be associated with inhibition of cell migration in areas of exposed pulp.
Our data showed that HEMA-mediated inhibition of cell migration occurred at sub-cytotoxic doses (Fig. 1). The primary cellular responses to HEMA cytotoxicity in vitro are suggested to be apoptosis as demonstrated in multiple cell types including peripheral blood mononuclear cells, gingival fibroblasts, gingival epithelial cells and pulp cells (7,19,20). However, there is a lack of direct evidence that apoptotic responses actually occur in vivo (e.g., TUNEL positive cells). This implies that dentin adhesives may have sub-apoptotic effects in vivo and that inhibition of cell migration may play a more important role in poor pulpal wound healing in response to dentin adhesives in vivo.
Previous reports examining migration of pulp cells showed that cell migration is mediated through different signaling pathways (21,22,23). In particular, recent studies suggest that GSK3 is essential for cell polarization and migration (24). However, we did not find any link between GSK3 and HEMA-mediated migration inhibition (Fig. 2B). Rather, HEMA inhibited phosphorylation of FAK (Fig. 2A). FAK is known to play a crucial role in cell motility (25). FAK-deficient mice exhibited a general defect of mesoderm development and reduced cell motility of embryonic cells in vitro (26). Conversely, ‘SuperFAK,’ a constitutively active form of FAK, has been shown to increase the migration of cells (27), indicating that the loss of phosphorylated FAK in HEMA-treated cells is an important signal that leads to the inhibition of cell migration.
Previous studies showed that MAP kinases including p38, Erk and JNK are known to play important roles in cell migration (21, 23,28), and our results suggest that p38 MAP kinase is involved in HEMA-mediated inhibition of DPSC migration (Fig. 3). Conflicting results have been shown in other studies where HEMA treated cells exhibited increased phosphorylation of MAP kinases including p38 (29). Increased phosphorylation of Erk1/2 was also noted when cells were treated with HEMA (30). The differences in MAPK phosphorylation may be attributed to treatment durations (hours vs. days), cell culture conditions (DMEM vs. α-MEM) and cell types (glandular cells or fibroblasts vs. dental pulp stem cells). On the other hand, it has been demonstrated that increased phosphorylation of p38 is directly associated with increased FAK phosphorylation and cell migration (31). Indeed, inhibition of the p38 signaling pathway has been shown to suppress enhanced migration of pulp cells by hepatocyte-growth factor (23). It would be worthwhile to examine the phosphorylation status of MAPKs in pulp cells in vivo.
In this study, we demonstrated that HEMA inhibits the migration of DPSC at non-cytotoxic doses and such inhibition was associated with the FAK and p38 signaling pathways. Therefore, mitigating these signaling pathways may provide therapeutic options to improve pulp regeneration in the presence of the resin-based dental materials containing HEMA.
Acknowledgments
This study was supported in part by the grants (K08DE17121, K03DE30304 to R.H.K.) from NIDCR/NIH and the Faculty Research Grant (to R.H.K.) from the UCLA Council on Research of the Academic Senate.
Footnotes
The authors deny any conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swift EJ, Jr, Perdigão J, Heymann HO. Bonding to enamel and dentin: a brief history and state of the art, 1995. Quintessence Int. 1995 Feb;26(2):95–110. [PubMed] [Google Scholar]
- 2.Hebling J, Giro EM, Costa CA. Biocompatibility of an adhesive system applied to exposed human dental pulp. J Endod. 1999 Oct;25(10):676–82. doi: 10.1016/s0099-2399(99)80354-9. [DOI] [PubMed] [Google Scholar]
- 3.Kiba H, Hayakawa T, Nakanuma K, Yamazaki M, Yamamoto H. Pulpal reactions to two experimental bonding systems for pulp capping procedures. J Oral Sci. 2000;42:69–74. doi: 10.2334/josnusd.42.69. [DOI] [PubMed] [Google Scholar]
- 4.Trope M, McDougal R, Levin L, May KN, Jr, Swift EJ., Jr Capping the inflamed pulp under different clinical conditions. J Esthet Restor Dent. 2002;14:349–357. doi: 10.1111/j.1708-8240.2002.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 5.Hanks CT, Strawn SE, Wataha JC, Craig RG. Cytotoxic effects of resin components on cultured mammalian fibroblasts. J Dent Res. 1991;70(11):1450–5. doi: 10.1177/00220345910700111201. [DOI] [PubMed] [Google Scholar]
- 6.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–80. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 7.Paranjpe A, Bordador LC, Wang MY, Hume WR, Jewett A. Resin monomer 2-hydroxyethyl methacrylate (HEMA) is a potent inducer of apoptotic cell death in human and mouse cells. J Dent Res. 2005;84:172–7. doi: 10.1177/154405910508400212. [DOI] [PubMed] [Google Scholar]
- 8.Costa CAS, Teixeira HM, Nascimento ABL, Hebling J. Biocompatibility of an adhesive system and 2-hydroxyethylmethacrylate. ASDC J Dent Child. 1999;66(5):337–42. [PubMed] [Google Scholar]
- 9.Andersson J, Dahlgren U. Effects on mouse immunity of long-term exposure in vivo to minute amounts of HEMA. Eur J Oral Sci. 2011;119:109–114. doi: 10.1111/j.1600-0722.2011.00818.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Effects of HEMA and TEDGMA on the in vitro odontogenic differentiation potential of human pulp stem/progenitor cells derived from deciduous teeth. Dent Mater. 2011;27:608–17. doi: 10.1016/j.dental.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Schröder U. Effects of calcium hydroxide-containing pulp-capping agents on pulp cell migration, proliferation, and differentiation. J Dent Res. 1985 Apr;64(Spec No):541–8. doi: 10.1177/002203458506400407. [DOI] [PubMed] [Google Scholar]
- 12.Cox CF, Suzuki S, Suzuki SH. Biocompatibility of dental adhesives. J Calif Dent Assoc. 1995;23:35–41. [PubMed] [Google Scholar]
- 13.Cox CF, Hafez AA, Akimoto N, Otsuki M, Suzuki S, Tarim B. Biocompatibility of primer, adhesive and resin composite systems on non-exposed and exposed pulps of non-human primate teeth. Am J Dent. 1998 Jan;11(Spec No):S55–63. [PubMed] [Google Scholar]
- 14.Akimoto N, Momoi Y, Kohno A, Suzuki S, Otsuki M, Suzuki S, Cox CF. Biocompatibility of Clearfil Liner Bond 2 and Clearfil AP-X system on nonexposed and exposed primate teeth. Quintessence Int. 1998;29:177–188. [PubMed] [Google Scholar]
- 15.Pereira JC, Segala AD, Costa CA. Human pulpal response to direct pulp capping with an adhesive system. Am J Dent. 2000 Jun;13(3):139–47. [PubMed] [Google Scholar]
- 16.de Souza Costa CA, Lopes do Nascimento AB, Teixeira HM, Fontana UF. Response of human pulps capped with a self-etching adhesive system. Dent Mater. 2001 May;17(3):230–40. doi: 10.1016/s0109-5641(00)00076-2. [DOI] [PubMed] [Google Scholar]
- 17.Koliniotou-Koumpia E, Tziafas D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J Dent. 2005 Sep;33(8):639–47. doi: 10.1016/j.jdent.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Silva GA, Lanza LD, Lopes-Júnior N, Moreira A, Alves JB. Direct pulp capping with a dentin bonding system in human teeth: a clinical and histological evaluation. Oper Dent. 2006 May-Jun;31(3):297–307. doi: 10.2341/05-65. [DOI] [PubMed] [Google Scholar]
- 19.Chang HH, Guo MK, Kasten FH, Chang MC, Huang GF, Wang YL, Wang RS, Jeng JH. Stimulation of glutathione depletion, ROS production and cell cycle arrest of dental pulp cells and gingival epithelial cells by HEMA. Biomaterials. 2005 Mar;26(7):745–53. doi: 10.1016/j.biomaterials.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Reichl FX, Esters M, Simon S, Seiss M, Kehe K, Kleinsasser N, Folwaczny M, Glas J, Hickel R. Cell death effects of resin-based dental material compounds and mercurials in human gingival fibroblasts. Arch Toxicol. 2006 Jun;80(6):370–7. doi: 10.1007/s00204-005-0044-2. [DOI] [PubMed] [Google Scholar]
- 21.Kajiya M, Shiba H, Komatsuzawa H, Ouhara K, Fujita T, Takeda K, Uchida Y, Mizuno N, Kawaguchi H, Kurihara H. The antimicrobial peptide LL37 induces the migration of human pulp cells: a possible adjunct for regenerative endodontics. J Endod. 2010;36(6):1009–13. doi: 10.1016/j.joen.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 22.Man J, Shelton RM, Cooper PR, Scheven BA. Low-intensity Low-frequency Ultrasound Promotes Proliferation and Differentiation of Odontoblast-like Cells. J Endod. 2012;38(5):608–13. doi: 10.1016/j.joen.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Su Y, Xie W, Wang C, Peng L, Zhou X, Ye L. JNK/P38 Mitogen-activated Protein Kinase Used for Hepatocyte Growth Factor-induced Proliferation, Differentiation, and Migration in Human Dental Papilla Cells. J Endod. 2012;38(9):1207–13. doi: 10.1016/j.joen.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Sun T, Rodriguez M, Kim L. Glycogen synthase kinase 3 in the world of cell migration. Dev Growth Differ. 2009 Dec;51(9):735–42. doi: 10.1111/j.1440-169X.2009.01141.x. [DOI] [PubMed] [Google Scholar]
- 25.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005 Jan;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 26.Ili D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995 Oct 12;377(6549):539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 27.Gabarra-Niecko V, Keely PJ, Schaller MD. Characterization of an activated mutant of focal adhesion kinase: ‘SuperFAK’. Biochem J. 2002 Aug 1;365(Pt 3):591–603. doi: 10.1042/BJ20020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang C, Jacobson K, Schaller MD. MAP kinases and cell migration. J Cell Sci. 2004 Sep 15;117(Pt 20):4619–28. doi: 10.1242/jcs.01481. [DOI] [PubMed] [Google Scholar]
- 29.Samuelsen JT, Dahl JE, Karlsson S, Morisbak E, Becher R. Apoptosis induced by the monomers HEMA and TEGDMA involves formation of ROS and differential activation of the MAP-kinases p38, JNK and ERK. Dent Mater. 2007 Jan;23(1):34–9. doi: 10.1016/j.dental.2005.11.037. Epub 2006 Jan 23. [DOI] [PubMed] [Google Scholar]
- 30.Spagnuolo G, D’Antò V, Valletta R, Strisciuglio C, Schmalz G, Schweikl H, Rengo S. Effect of 2-hydroxyethyl methacrylate on human pulp cell survival pathways ERK and AKT. J Endod. 2008 Jun;34(6):684–8. doi: 10.1016/j.joen.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 31.Lee J, Jung ID, Chang WK, Park CG, Cho DY, Shin EY, Seo DW, Kim YK, Lee HW, Han JW, Lee HY. p85 beta-PIX is required for cell motility through phosphorylations of focal adhesion kinase and p38 MAP kinase. Exp Cell Res. 2005 Jul 15;307(2):315–28. doi: 10.1016/j.yexcr.2005.03.028. [DOI] [PubMed] [Google Scholar]