Abstract
Cell-based regenerative therapies, based on in vitro propagation of stem cells, offer tremendous hope to many individuals suffering from degenerative diseases that were previously deemed untreatable. Due to the self-renewal capacity, multilineage potential, and immunosuppressive property, mesenchymal stem cells (MSCs) are considered as an attractive source of stem cells for regenerative therapies. However, poor growth kinetics, early senescence, and genetic instability during in vitro expansion and poor engraftment after transplantation are considered to be among the major disadvantages of MSC-based regenerative therapies. A number of complex inter- and intracellular interactive signaling systems control growth, multiplication, and differentiation of MSCs in their niche. Common laboratory conditions for stem cell culture involve ambient O2 concentration (20%) in contrast to their niche where they usually reside in 2–9% O2. Notably, O2 plays an important role in maintaining stem cell fate in terms of proliferation and differentiation, by regulating hypoxia-inducible factor-1 (HIF-1) mediated expression of different genes. This paper aims to describe and compare the role of normoxia (20% O2) and hypoxia (2–9% O2) on the biology of MSCs. Finally it is concluded that a hypoxic environment can greatly improve growth kinetics, genetic stability, and expression of chemokine receptors during in vitro expansion and eventually can increase efficiency of MSC-based regenerative therapies.
1. Introduction
The promising role of stem cell therapy is becoming more conceivable in addressing the unmet needs of treating degenerative diseases through conventional medicine. Diseases such as diabetes, myocardial infarction, spinal cord injury, stroke, and Parkinson's and Alzheimer's diseases have become more prevalent with increasing life expectancy. It has been estimated that in the United States alone, approximately 128 million individuals would benefit from regenerative stem cell therapy during their lifetime [1].
Self-renewal and multipotency are the key hallmarks of stem cells, permitting them to act as the fundamental units maintaining growth, homeostasis and repair of many tissues. These two key features establish stem cells as the most promising tool for regenerative medicine [2, 3]. Among the different types of stem cells, mesenchymal stem cells (MSCs) or multipotent mesenchymal stromal cells [4] are considered as a potential tool to treat degenerative diseases. This is due to their multipotent differentiative capacity [5–7] with trophic activity [8, 9], potent immunosuppressive effects [10–12], and ability to induce vascularisation [13]. Moreover, MSCs can be efficiently isolated from tissues such as bone marrow, adipose tissue, umbilical cord, and dental pulp [14–17]. These properties have fascinated and encouraged researchers to push the frontiers of regenerative medicine, utilizing MSCs to treat a large variety of pathologies, including traumatic lesions, stroke, autoimmune diseases, musculoskeletal and cardiac disorders [18–21].
Despite the various sources, concentration of MSCs within tissues is very low [22, 23], and it is not possible to isolate 50–200 million MSCs (typically used in clinical trials) from a donor for each therapy [24–29]. Thus, in vitro expansion of MSCs has become an inevitable option [23]. In several clinical trials, MSCs expanded in vitro are being transplanted to find out their efficacy in treating degenerative diseases, reducing acute rejection of transplanted organs, and in preventing and treating graft-versus-host disease [25, 29–32]. Sometimes the expanded cells are induced to differentiate into a particular cell type and then the predifferentiated cells are transplanted for the regeneration of particular tissues or organs [33]. After transplantation, tissue-specific migration and engraftment ensure the success of cell-based regenerative therapy.
From isolation to engraftment, the MSCs usually pass through two different environmental conditions. One is the in vitro culture condition (from isolation to transplantation) and the other is the in vivo or physiological condition (before isolation and after transplantation) (Figure 1). At present, most of the expansion procedures of MSCs are performed under ambient O2 concentration, where cells are exposed to 20% O2, which is approximately 4–10 times more than the concentration of O2 in their natural niches [35, 36]. The higher O2 concentration might cause environmental stress to the in vitro cultured MSCs. Moreover, in recent years, several studies have presented clear evidence regarding the negative influence of ambient O2 concentration on MSCs, including early senescence, longer population doubling time, DNA damage [37, 38], and poor engraftment following transplantation [33, 39]. All these have shown the influential effect of O2 concentration on MSCs biology and raised serious concern over its therapeutic efficiency and biosafety.
Figure 1.

Steps involved in MSCs-based therapy.
Numerous in vitro studies have been conducted in the last two decades to analyze the complex processes involved in stem cell maintenance. However, the role of physiologically normoxic (hypoxic) conditions (usually 2–9% O2 concentration) on stem cell biology received very little attention [40]. Thus, this paper discusses the differences between in vitro MSC culture in ambient and hypoxic conditions. Finally this paper also highlights how MSCs cultured in vitro in hypoxic conditions can offer a solution for MSCs-based therapy.
2. Stem Cell Niche
In both in vitro and in vivo conditions, the fate and function of stem cells depend upon their intrinsic genetic program and the local microenvironment, often referred to as the “stem cell niche” [41]. The stem cell niche concept was proposed by Schofield in 1978 [42], and several researchers have tried to elucidate the confusion and controversy over it [43–45]. “Stem cell niche” can be defined as the anatomical compartment composed of cellular and acellular components that orchestrate both systemic and local signals to control the rate of stem cell proliferation, to determine the fate of stem cell daughters, and to protect stem cells from exhaustion or death [46–48]. The cellular and acellular components of the stem cell niche can be divided into four main groups of key factors, namely, the regulatory molecules (O2, nutrients, and cytokines), other cells (3D context, cell-cell contacts, autocrine, and paracrine signals), extracellular matrix (immobilized and released factors, structure, topology, and stiffness), and physical factors (flow shear, compression, stretch, and electrical signals) [41].
2.1. The Hypoxic Embryonic Stem Cell Niche
In mammals, from fertilization to parturition, cells within the embryo face continuously change in O2 concentration [49]. During the time of blastocyst implantation, O2 levels within the lumen of the uterus remain as low as 1-2% [50]. In human tissues, O2 has a diffusion distance of approximately 150 μm [51, 52], which regulates the O2 supply during development and implantation of the blastocyst [53]. However, after development of the circulatory system until 8–10 weeks of gestation, the placental O2 levels remain lower (approximately 2-3%) than those in the surrounding endometrium and reach physiological O2 concentration at the 12-13th week of gestation [54, 55]. Therefore, embryos go through hypoxic O2 concentrations while passing through different developmental periods. Among all the embryonic stages, blastocyst which resides in a hypoxic environment has been recognized as the main source of pluripotent embryonic stem cells (ESCs).
Recently, a new type of pluripotent stem cell has been generated by reprogramming human adult somatic cells. Pluripotency of this cell type is comparable to human ESCs and commonly referred as “induced pluripotent stem cells” (iPSCs) [56]. Hypoxic culture environments have shown to enhance the generation of these iPSCs too [57, 58].
2.2. The Hypoxic Environment of the Mesenchymal Stem Cell Niche
Like ESCs, MSCs also reside in low O2 concentrations. In mammals including humans, MSCs are located in perivascular niches close to the vascular structure in almost all tissues [17, 59, 60]. Despite residing near the blood vessels, in different tissues where they are found, the O2 concentrations are low [61, 62]. In adult human tissues, O2 concentration varies widely (Table 1) depending on the vascularisation and the type of microenvironment within the respective organ, and they are considerably lower than the inhaled ambient O2 concentration (21%). The partial pressure or O2 concentration of inspired air gradually decreases after it enters the lungs and then in the blood flowing from the alveolar capillaries that carry O2, towards the organs and tissues for their oxygenation. By the time O2 reaches the organs and tissues, O2 concentration drops to 2%–9%, with a mean of 3% [40, 63].
Table 1.
Oxygen concentration in different organs and tissues.
| Name of the tissue or organ | Oxygen concentration | References |
|---|---|---|
| Lung parenchyma | 4% to 14% | [64, 65] |
| Circulation | 4% to 14% | [63, 66] |
| Liver | 4% to 14% | [64, 67] |
| Kidneys | 4% to 14% | [64, 68] |
| Heart | 4% to 14% | [69, 70] |
| Brain | 0.5% to 8% | [71–73] |
| Eye (retina, corpus vitreous) | 1% to 5% | [74, 75] |
| Bone marrow | 1% to 6% | [35, 36, 76] |
| Adipose tissue | 2% to 8 % | [62] |
As the concentrations of O2 in blastocysts and the MSCs niches are very low [73, 76, 77], this could be an important clue for maintaining the self-renewal property and plasticity of MSCs.
3. Comparison between Culture in Hypoxic and Ambient Environments
Since 1963, when the isolation and self-renewing properties of mouse bone marrow cells were first reported [78, 79], until now most of the research efforts have been focused on the identification of molecular markers [4, 80, 81]. This has allowed the isolation of different types of tissue-specific stem or progenitor cells [82–85] and has also assisted to define the differentiation of stem or progenitor cells into a particular cell type [86, 87]. Moreover, the development of specific methods for functional stem cell isolation and identification is highly important, in order to study the molecular mechanisms behind the multipotentiality and self-renewable capacity of stem cells and also for the establishment of stem cell-based regenerative therapeutics. This trend has overshadowed the importance of O2 concentration, a key environmental factor that might play a vital role on stem cell fate and function [40]. Unfortunately till now in most laboratories, stem cells are typically cultured under the ambient O2 concentration without paying attention to the metabolic milieu of the niche in which they grow or normally reside [88]. However, in recent years, scientists have started to manipulate the O2 concentration in cell cultures by maintaining a niche-like hypoxic environment. Though the effect of hypoxic culture conditions on the proliferation and differentiation potential of MSCs has been reviewed by few researchers [77, 89], the effect of hypoxia on the genetic stability, early senescence, and site-specific migration of MSCs has not been reviewed in depth. Thus, on the basis of recent research outcomes, the effect of different O2 concentration on MSCs biology is further discussed.
3.1. Proliferation of MSCs
Capability for self-renewal is a key feature of stem cells. An increased proliferation rate is necessary for more efficient use of stem cells in regenerative therapies. Fehrer et al. (2007) demonstrated that bone marrow-derived MSCs (BM-MSCs) cultured in 3% O2 concentration showed significantly increased in vitro proliferative lifespan, with approximately 10 additional population doublings (PDs) (28.5 ± 3.8 PD in 20% O2 and 37.5 ± 3.4 PD in 3% O2) before reaching senescence compared to cells cultured in the ambient O2 environment [38]. In addition, early passaged MSCs cultured in hypoxic conditions also exhibit increased proliferative lifespan along with significant difference in population doubling [37]. Furthermore, it is possible to harvest more than 1 × 109 MSCs from the first five passages cultured in 3% O2, whereas in ambient condition only 2 × 107 cells can be obtained [37]. Higher in vitro expansion rate in hypoxic conditions has also been reported by several other researchers [90–93]. Such in vitro culture environment also allows to maintain a higher proportion of rapidly self-renewing MSCs for a longer period of time [94]. However, proliferation of MSCs was reduced significantly in 1% or less O2 concentration [95].
3.2. Plasticity of MSCs
Besides higher growth kinetics, maintaining plasticity is also an important factor for prospective use of MSCs in regenerative medicine. Trilineage (osteogenic, chondrogenic, and adipogenic) mesenchymal differentiation is a unique biological property of MSCs [4]. Several researchers reported the effect of different culture O2 concentrations on the trilineage differentiation of MSCs. In an elegantly designed experiment, Raheja et al. (2010) seeded and induced MSCs for differentiation under an atmosphere of 5% carbon dioxide (CO2) along with 1 of 4 O2 concentrations (1%, 2%, 5%, and 21%). According to their results, MSCs differentiated into osteoblast most rapidly in 21% O2, and O2 below 5% showed reduced differentiation potential. However, no statistically significant difference in osteogenic marker was reported when O2 was between 5% and 21% [96]. In addition, Basciano et al. (2011) have reported improved osteoblastic and adipogenic differentiation potential of early passaged (P2) MSCs in 5% O2 concentration [90]. Several other recent reports support that the multilineage differentiation potential of MSCs can be maintained under hypoxic (1–5% O2 concentration) environment [91, 92, 95, 97]. Increased adipogenic and osteogenic differentiation potentials of adipose tissue-derived MSCs precultured in hypoxic environment have also been reported [98]. In contrast, few researchers showed reduction in the differentiation potential of MSCs when maintained and induced for differentiation in 1% O2 concentration [99, 100].
3.3. Genetic Stability of MSCs
Genetic instability of MSCs is another major problem that is directly related to the biosafety of stem cell therapy. For instance, aneuploidy, DNA breakdown, and telomere shortening can be observed in cultured MSCs [37, 101, 102]. However, Tarte et al. (2010) reported that aneuploidy in cultured MSCs is donor dependent rather than its dependence on the culture environment [102]. In contrast, Estrada et al. (2012) have shown a negative effect of ambient O2 concentration on cultured MSCs responsible in bringing about DNA damage and aneuploidy. However, this effect was minimized by expanding MSCs in a physiological O2 concentration [37]. There is scientific evidence that aneuploidy is a major cause of tumorigenesis [103, 104] which raised concerns regarding the biosafety of MSCs cultured in ambient O2 condition.
3.4. Engraftment of MSCs
Engraftment is an important part of MSC therapy. Modest engraftment capacity following transplantation of MSCs cultured in ambient condition has been reported in some clinical trial reports [33, 39]. Unpretentious therapeutic outcomes of clinical trials by using MSCs have also been reported in several review articles and meta-analysis [105–107]. Moreover, failure of in vivo engraftment of bone marrow (BM)-MSCs into nonhematopoietic tissue has been reported previously [108–110]. Various strategies can be employed to overcome this problem. For instance, in a recent publication, Jin et al. (2011) reported that the 1st passage of mouse BM-MSCs had shown better engraftment and differentiation potential to cardiomyocytes in vivo, compared to the 5th passage mouse BM-MSCs [111]. In addition, murine MSCs preconditioned in hypoxic environment showed enhanced skeletal muscle regeneration at day 7 and improved blood flow and vascular formation compared to MSCs maintained in normoxic condition [112]. Furthermore, expression of chemokine receptors CXCR4, CXCR7, and CX3CR1 was upregulated when MSCs were exposed to hypoxia or a reagent that mimics the response to hypoxia [94, 113–115]. These chemokine receptors play an important role in damaged-tissue-specific trafficking and homing of MSCs [113, 115–118].
4. Biochemical and Molecular Changes due to Hypoxia
O2 concentration in the stem cell niche (usually 2–9% O2) is considered a driver of cell function [40]. Hypoxia plays a vital role in maintaining homeostasis within the body from the very beginning of embryonic development. It helps facilitate proper embryonic development, maintain stem cell pluripotency, induce differentiation, and regulate the signalling of multiple cascades, including angiogenesis [119]. In hypoxic conditions, usually these functions are regulated by several transcription factors such as hypoxia-inducible factors (HIFs), prolyl-hydroxylases (PHDs), factor-inhibiting HIF-1 (FIH-1), activator protein 1 (AP-1), nuclear factor (NF)-κB, p53, and c-Myc [120]. Although interaction among all of the transcription factors is required for cellular response, HIFs (especially HIF-1) are the key regulators of cellular response to hypoxia [121].
4.1. Regulation of Transcription by HIF-1 during Direct Sensing of Changes in Oxygen
The HIF-1β subunit of a heterodimeric transcription factor HIF-1 (HIF-1α and HIF-1β) [122, 123] is nonresponsive to oxygen, whereas HIF-1α is an oxygen labile protein. Therefore, under ambient condition the HIF-1α subunit is usually synthesized and degraded rapidly, whereas under hypoxic conditions, its breakdown is delayed [122, 124]. Degradation of HIF-1α under ambient culture condition (Figure 2) is regulated by HIF-1 prolyl-hydroxylases (HPHs) [125]. HIF-1 prolyl-hydroxylases (HPHs) in the presence of O2, iron, and α-ketoglutarate hydroxylate the proline residues 402 and 564 of the oxygen-dependent degradation domain (ODD) of HIF1α [126, 127], which in turn induce a conformational change of HIFα, thus allowing Von Hippel-Lindau protein (VHL) to bind with it [62]. Consequently, VHL binds to a complex that serves as E3 ubiquitin ligase (E3UL) and ubiquitinylate HIF-1α for degradation in proteasome [63, 128, 129].
Figure 2.

Regulation of transcription by HIF-1 during ambient and hypoxic condition. HIF: hypoxia-inducible factor; HPH: HIF-1 prolyl-hydroxylases; VHL: Von Hippel-Lindau; E3UL: E3 ubiquitin ligase; HRE; hypoxia-response element; GLUT; glucose transporter: LDH: lactate dehydrogenase; PDK, pyruvate dehydrogenase kinase (see text for details).
In contrast, under hypoxic conditions, the prolyl-hydroxylation process is suppressed due to lack of O2 that allows HIF-1α accumulation and nuclear translocation to occur [124]. After nuclear translocation, it binds with HIF-1β to form the heterodimer. Then the HIF-1 heterodimer binds to a hypoxia-response element (HRE) in the target genes, associated with coactivators such as CBP/p300, and regulates the transcription (Figure 2) of as many as 70 genes involved in metabolism, angiogenesis, invasion/metastasis, and cell fate [130].
4.2. Reduction of Reactive Oxygen Species by Suppressing Mitochondrial Respiration during Hypoxia
Relatively recent discoveries also support the role of HIF-1α on metabolic regulation by suppressing mitochondrial respiration. In hypoxic conditions, stabilized HIF-1α translocates into the nucleus and binds to HIF-1β to form the heterodimer, which in turn binds to the target gene-specific HREs to transcriptionally activate genes that code for glucose transporters (GLUT), glycolytic enzymes, and lactate dehydrogenase-A (LDH-A) to facilitate anaerobic respiration [130, 131]. Besides suppression of mitochondrial respiration, HIF-1α promotes the expression of pyruvate dehydrogenase kinase (PDK) that prevents the conversion of pyruvate into acetyl CoA [131] inhibiting the enzymatic activity of pyruvate dehydrogenase (PDH) (Figure 3). This results in the reduction of mitochondrial O2 consumption, and as a consequence, the production of reactive oxygen species (ROS) is lowered [132, 133]. In addition, HIF-1α in a hypoxic condition causes the production of cytochrome c that also ensures optimum ATP production and cell integrity, by minimizing ROS [134].
Figure 3.

Suppression of mitochondrial respiration by HIF-1α in hypoxic environment. HIF: hypoxia-inducible factor; HRE: hypoxia-response element: GLUT: glucose transporter; LDH: Lactate dehydrogenase; PDH: pyruvate dehydrogenase; PDK: pyruvate dehydrogenase kinase; TCA: tricarboxylic acid; ETC: electron transport chain; ROS: reactive oxygen species (see text for details).
4.3. Induction of Notch Target Genes by Hypoxia
The Notch signaling pathway is an important pathway that regulates the stem cells fate [135]. Crosstalk between hypoxia and activated Notch signaling (Figure 4) has been reported by several researchers [34, 136]. In hypoxic conditions, HIF-1α can regulate cell fate by activation of Notch down-stream genes (e.g., Hes and Hey) necessary to maintain proliferation of stem cells. During this crosstalk, in response to ligand presentation from neighboring cells, Notch receptors undergo proteolytic activation that is mediated by two proteases (tumour necrosis factor and γ-secretase). Due to the proteolytic activity, Notch intracellular domain (NIC) is released and translocated into the nucleus. There, NIC binds to HIF-1α to build heterodimer which binds to recombination-signal binding protein-Jk (RBP-Jk), CBP/p300 proteins, and RBP-Jk response element (RRE) in the Notch target genes to activate them (e.g., Hes and Hey genes) [34, 136].
Figure 4.
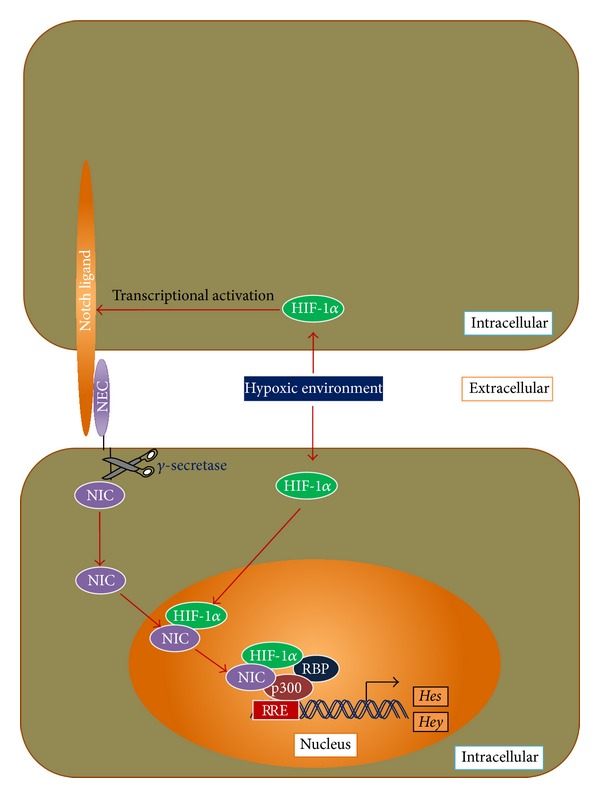
Crosstalk between hypoxia and notch signaling, and regulation of stem cell proliferative gene expression. HIF: hypoxia-inducible factor; NEC: notch extracellular domain; NIC: notch intracellular domain; RBP: recombination-signal binding protein. (Modified from Gustafsson et al., (2005) [34] and Sainson and Harris (2006) [136]; see text for details).
4.4. Upregulation of Chemokine Receptors by Hypoxia
The success of cell-based therapies highly depends upon the engraftment of the transplanted cells. The engraftment of the transplanted cells to the target organ is mediated through interaction between chemotactic factors (released by the organ) and their receptors on the surface of the transplanted cells. Though there are controversies over the expression of chemokine receptors and their migration towards target organs [137], in recent years, several articles have also reported that interaction between chemokines (SDF-1, fractalkine), and their receptors (e.g., CXCR4, CXCR7, and CX3CR1) play a vital role in chemotaxis, viability, and homing of MSCs both in vitro and in vivo [113, 138]. Moreover, expression of chemokine receptors on MSCs increases in the presence of HIF-1α [113]. The above information indicates that HIF1-α obtained stability in hypoxic condition prior to it being translocated into the nucleus, where it binds to HIF-1β to form the heterodimer. After that, the heterodimer binds to the gene-specific HRE associated with coactivators such as CBP/p300 [130] and upregulates the expression of chemokine receptors CXCR4, CXCR7, and CX3CR1. These chemokine receptors then respond to chemokines (e.g., SDF-1, fractalkine) secreted from diseased tissues or organs that finally facilitate the chemotaxis of the transplanted MSCs to the target site (Figure 5).
Figure 5.

Upregulation of the expression of chemokine receptors by HIF-1α in hypoxic environment to facilitate target organ-specific chemotaxis. HIF: hypoxia-inducible factor; HRE: hypoxia-response element (see text for details).
5. Hypoxic Culture Conditions as a Solution for MSC-Based Regenerative Therapy
The above discussions supported the positive role of hypoxic culture environments for MSCs and provided answers to solve problems related to cell-based therapies. In a hypoxic environment, HIF-1α prevents the TCA cycle and results in lower ROS (Figure 3). Lower ROS generation resulted in slowing the rate of telomere shortening [139, 140], and as a consequence replicative senescence might be delayed. Moreover, a hypoxic environment upregulates the expression of Notch target genes (e.g., Hes and Hey genes), responsible for cell proliferation (Figure 4). Therefore, the higher proliferation rate along with more population doubling in hypoxic conditions [37, 38, 92] may be due to the lowered ROS generation and overexpression of Notch target genes (e.g., Hes and Hey).
Maintaining genetic stability is another challenge during in vitro expansion of MSCs. Increased rates of aneuploidy, double-stranded DNA breakdown, and faster telomere shortening have been reported for MSCs cultured in ambient condition [37]. Gordon et al. (2012) reviewed the causes and consequences behind aneuploidy. They have defined defective spindle assembly checkpoint, centrosome amplification, and merotelic attachments as major causes behind aneuploidy [141]. Moreover, Wang et al. (2012) have described ROS as the causative factor of defective spindle assembly checkpoint, centrosome amplification and merotelic attachments [142]. ROS also acts in acceleration of telomere shortening and DNA breakdown [143, 144]. In addition, correlation between telomere shortening and aneuploidy in embryonic and hepatocellular carcinoma cells has been reported in recently published articles [145, 146]. The above discussion supports that higher ROS production due to the increased mitochondrial respiration during expansion of MSCs in ambient O2 concentration (Figure 3) might be the cause behind genetic instability in them. However, during hypoxia, cells go through anaerobic respiration, and as a result lower the ROS concentration within the cells (Figure 3). This might help in reducing the DNA damage, telomere shortening, and aneuploidy which in return may increase the biosafety of stem cell-based therapy.
Hypoxic culture conditions may also provide a solution for more efficient engraftment. Recently, it has been reported that early passaged mouse BM-MSCs showed better engraftment than late passaged mouse BM-MSCs in in vivo model [111]. Moreover, hypoxic preconditioned murine MSCs also showed enhanced skeletal muscle regeneration and improved blood flow and vascular formation compared to MSCs maintained in normoxic condition [112]. Furthermore, hypoxic conditions cause MSCs to grow faster [37] while maintaining a higher proportion of rapidly self-renewing cells [94]. In addition to that, a hypoxic environment increases the expression of chemokine receptors CXCR4, CXCR7, and CX3CR1 [113, 114], and they may facilitate tissue-specific trafficking of MSCs (Figure 5). From the above information, it can be anticipated that adequate numbers of MSCs with a higher fraction of rapidly self-renewing cells and highly expressed chemokine receptors on their surface can be obtained from the early passages of hypoxic cultures, and that MSCs might increase the efficiency of damaged-tissue-specific migration and engraftment following transplantation. Therefore, culturing MSCs in hypoxic conditions can also be considered as a solution for tissue-specific engraftment.
6. Conclusion
MSCs have tremendous potential in regenerative medicine. However, poor growth kinetics, genetic instability, and poor engraftment after transplantation are seen as drawbacks in their translation from bench side to bed side. The above information suggests hypoxic culture conditions (2–5% O2 concentration) as a promising solution to overcome these problems. Tissue development and regeneration process solely depend upon the sequential steps of stem cell renewal, specialization, and assembly that are coordinated by the cascades of environmental factors in its niche, rather than with one single dominating factor. Thus, success in cell-based regenerative therapies requires a holistic view of stem cell regulation. Besides maintaining MSCs in physiological oxygen condition, there is a need to develop new techniques to analyze in vivo conditions of the stem cell niche, so that the appropriate in vitro modelling can yield novel information for niche-directed cell-based therapies.
Conflict of Interests
No competing financial interests exist.
Acknowledgment
The work is part of a project supported by the University of Malaya, High Impact Research-Ministry of Higher Education, Malaysia (UM.C/HIR/MOHE/DENT/01).
References
- 1.Harris DT. Non-haematological uses of cord blood stem cells. British Journal of Haematology. 2009;147(2):177–184. doi: 10.1111/j.1365-2141.2009.07767.x. [DOI] [PubMed] [Google Scholar]
- 2.Rehman J. Empowering self-renewal and differentiation: the role of mitochondria in stem cells. Journal of Molecular Medicine. 2010;88(10):981–986. doi: 10.1007/s00109-010-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wagers AJ. The stem cell niche in regenerative medicine. Cell Stem Cell. 2012;10(4):362–369. doi: 10.1016/j.stem.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Govindasamy V, Ronald VS, Abdullah AN, et al. Differentiation of dental pulp stem cells into islet-like aggregates. Journal of Dental Research. 2011;90(5):646–652. doi: 10.1177/0022034510396879. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. Journal of Immunology. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 7.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. Journal of Cellular Biochemistry. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 9.Zhang M, Mal N, Kiedrowski M, et al. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. The FASEB Journal. 2007;21(12):3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4):1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 11.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunology and Cell Biology. 2006;84(5):413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 12.Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110(10):3499–3506. doi: 10.1182/blood-2007-02-069716. [DOI] [PubMed] [Google Scholar]
- 13.Martens TP, See F, Schuster MD, et al. Mesenchymal lineage precursor cells induce vascular network formation in ischemic myocardium. Nature Clinical Practice Cardiovascular Medicine. 2006;3(1):S18–S22. doi: 10.1038/ncpcardio0404. [DOI] [PubMed] [Google Scholar]
- 14.Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. British Journal of Haematology. 2000;109(1):235–242. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- 15.Govindasamy V, Abdullah AN, Ronald VS, et al. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. Journal of Endodontics. 2010;36(9):1504–1515. doi: 10.1016/j.joen.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Lund P, Pilgaard L, Duroux M, Fink T, Zachar V. Effect of growth media and serum replacements on the proliferation and differentiation of adipose-derived stem cells. Cytotherapy. 2009;11(2):189–197. doi: 10.1080/14653240902736266. [DOI] [PubMed] [Google Scholar]
- 17.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. Journal of Bone and Mineral Research. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 18.Kosztowski T, Zaidi HA, Quiñones-Hinojosa A. Applications of neural and mesenchymal stem cells in the treatment of gliomas. Expert Review of Anticancer Therapy. 2009;9(5):597–612. doi: 10.1586/era.09.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nature Medicine. 2006;12(4):459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 20.Richardson SM, Hoyland JA, Mobasheri R, Csaki C, Shakibaei M, Mobasheri A. Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. Journal of Cellular Physiology. 2010;222(1):23–32. doi: 10.1002/jcp.21915. [DOI] [PubMed] [Google Scholar]
- 21.Siegel G, Schäfer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(9):S45–S49. doi: 10.1097/TP.0b013e3181a285b0. [DOI] [PubMed] [Google Scholar]
- 22.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6(1):7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Faustini M, Bucco M, Chlapanidas T, et al. Nonexpanded mesenchymal stem cells for regenerative medicine: yield in stromal vascular fraction from adipose tissues. Tissue Engineering C. 2010;16(6):1515–1521. doi: 10.1089/ten.TEC.2010.0214. [DOI] [PubMed] [Google Scholar]
- 25.Connick P, Kolappan M, Patani R, et al. The mesenchymal stem cells in multiple sclerosis (MSCIMS) trial protocol and baseline cohort characteristics: an open-label pre-test: post-test study with blinded outcome assessments. Trials. 2011;12, article 62 doi: 10.1186/1745-6215-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trachtenberg B, Velazquez DL, Williams AR, et al. Rationale and design of the transendocardial injection of autologous human cells (bone marrow or mesenchymal) in chronic ischemic left ventricular dysfunction and heart failure secondary to myocardial infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. American Heart Journal. 2011;161(3):487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 27.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. Journal of the American Medical Association. 2012;308(22):2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirana S, Stratmann B, Prante C, et al. Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. International Journal of Clinical Practice. 2012;66(4):384–393. doi: 10.1111/j.1742-1241.2011.02886.x. [DOI] [PubMed] [Google Scholar]
- 29.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. Journal of the American Medical Association. 2012;307(11):1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 30.Tewarie RSN, Hurtado A, Bartels RH, Grotenhuis A, Oudega M. Stem cell-based therapies for spinal cord injury. Journal of Spinal Cord Medicine. 2009;32(2):105–114. doi: 10.1080/10790268.2009.11760761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ringdén O, Uzunel M, Rasmusson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81(10):1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 32.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. The Lancet. 2008;371(9624):1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 33.Mohamadnejad M, Pournasr B, Bagheri M, et al. Transplantation of allogeneic bone marrow mesenchymal stromal cell-derived hepatocyte-like cells in homozygous familial hypercholesterolemia. Cytotherapy. 2010;12(4):566–568. doi: 10.3109/14653240903511143. [DOI] [PubMed] [Google Scholar]
- 34.Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires Notch signaling to maintain the undifferentiated cell state. Developmental Cell. 2005;9(5):617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Antoniou ES, Sund S, Homsi EN, Challenger LF, Rameshwar P. A theoretical simulation of hematopoietic stem cells during oxygen fluctuations: prediction of bone marrow responses during hemorrhagic shock. Shock. 2004;22(5):415–422. doi: 10.1097/01.shk.0000142185.88094.88. [DOI] [PubMed] [Google Scholar]
- 36.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO2 distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophysical Journal. 2001;81(2):685–696. doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Estrada JC, Albo C, Benguría A, et al. Culture of human mesenchymal stem cells at low oxygen tension improves growth and genetic stability by activating glycolysis. Cell Death and Differentiation. 2012;19(5):743–755. doi: 10.1038/cdd.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehrer C, Brunauer R, Laschober G, et al. Reduced oxygen tension attenuates differentiation capacity of human mesenchymal stem cells and prolongs their lifespan. Aging Cell. 2007;6(6):745–757. doi: 10.1111/j.1474-9726.2007.00336.x. [DOI] [PubMed] [Google Scholar]
- 39.Schächinger V, Erbs S, Elsässer A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. New England Journal of Medicine. 2006;355(12):1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 40.Simon MC, Keith B. The role of oxygen availability in embryonic development and stem cell function. Nature Reviews Molecular Cell Biology. 2008;9(4):285–296. doi: 10.1038/nrm2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vunjak-Novakovic G, Scadden DT. Biomimetic platforms for human stem cell research. Cell Stem Cell. 2011;8(3):252–261. doi: 10.1016/j.stem.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. A hypothesis. Blood Cells. 1978;4(1-2):7–25. [PubMed] [Google Scholar]
- 43.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–2299. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 44.Quiñones-Hinojosa A, Sanai N, Soriano-Navarro M, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. The Journal of Comparative Neurology. 2005;494(3):415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- 45.Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94(2):251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 46.Buravkova L, Andreeva E, Rylova J, Grigoriev A. Anoxia. Rijeka, Croatia: InTech; 2011. Resistance of multipotent mesenchymal atromal cells to anoxia in vitro; pp. 61–80. [Google Scholar]
- 47.Jones DL, Wagers AJ. No place like home: anatomy and function of the stem cell niche. Nature Reviews Molecular Cell Biology. 2008;9(1):11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 48.Yin T, Li L. The stem cell niches in bone. Journal of Clinical Investigation. 2006;116(5):1195–1201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fischer B, Bavister BD. Oxygen tension in the oviduct and uterus of rhesus monkeys, hamsters and rabbits. Journal of Reproduction and Fertility. 1993;99(2):673–679. doi: 10.1530/jrf.0.0990673. [DOI] [PubMed] [Google Scholar]
- 50.Yedwab GA, Paz G, Homonnal TZ, David M, Kraicer P. The temperature, pH, and partial pressure of oxygen in the cervix and uterus of women and uterus of rats during the cycle. Fertility and Sterility. 1976;27(3):304–309. doi: 10.1016/s0015-0282(16)41722-x. [DOI] [PubMed] [Google Scholar]
- 51.Folkman J, Hahnfeldt P, Hlatky L. Cancer: looking outside the genome. Nature Reviews Molecular Cell Biology. 2000;1(1):76–79. doi: 10.1038/35036100. [DOI] [PubMed] [Google Scholar]
- 52.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nature Reviews Cancer. 2004;4(11):891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 53.Gassmann M, Fandrey J, Bichet S, et al. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(7):2867–2872. doi: 10.1073/pnas.93.7.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jauniaux E, Watson AL, Hempstock J, Bao Y-P, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. American Journal of Pathology. 2000;157(6):2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstetrics and Gynecology. 1992;80(2):283–285. [PubMed] [Google Scholar]
- 56.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S. Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell. 2009;5(3):237–241. doi: 10.1016/j.stem.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nature Methods. 2011;8(5):424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Zannettino ACW, Paton S, Arthur A, et al. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. Journal of Cellular Physiology. 2008;214(2):413–421. doi: 10.1002/jcp.21210. [DOI] [PubMed] [Google Scholar]
- 61.Snow JW, Abraham N, Ma MC, Abbey NW, Herndier B, Goldsmith MA. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99(1):p. 394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 62.Pasarica M, Sereda OR, Redman LM, et al. Reduced adipose tissue oxygenation in human obesity evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58(3):718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nature Reviews Neuroscience. 2004;5(6):437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- 64.Ivanovic Z. Hypoxia or in situ normoxia: the stem cell paradigm. Journal of Cellular Physiology. 2009;219(2):271–275. doi: 10.1002/jcp.21690. [DOI] [PubMed] [Google Scholar]
- 65.Wild JM, Fichele S, Woodhouse N, Paley MNJ, Kasuboski L, Van Beek EJR. 3D volume-localized pO2 measurement in the human lung with 3He MRI. Magnetic Resonance in Medicine. 2005;53(5):1055–1064. doi: 10.1002/mrm.20423. [DOI] [PubMed] [Google Scholar]
- 66.McKinley BA, Butler BD. Comparison of skeletal muscle PO2, PCO2, and pH with gastric tonometric PCO2 and pH in hemorrhagic shock. Critical Care Medicine. 1999;27(9):1869–1877. doi: 10.1097/00003246-199909000-00027. [DOI] [PubMed] [Google Scholar]
- 67.Jungermann K, Kietzmann T. Role of oxygen in the zonation of carbohydrate metabolism and gene expression in liver. Kidney International. 1997;51(2):402–412. doi: 10.1038/ki.1997.53. [DOI] [PubMed] [Google Scholar]
- 68.Welch WJ, Baumgärtl H, Lübbers D, Wilcox CS. Nephron pO2 and renal oxygen usage in the hypertensive rat kidney. Kidney International. 2001;59(1):230–237. doi: 10.1046/j.1523-1755.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- 69.Mik EG, Van Leeuwen TG, Raat NJ, Ince C. Quantitative determination of localized tissue oxygen concentration in vivo by two-photon excitation phosphorescence lifetime measurements. Journal of Applied Physiology. 2004;97(5):1962–1969. doi: 10.1152/japplphysiol.01399.2003. [DOI] [PubMed] [Google Scholar]
- 70.Roy S, Khanna S, Wallace WA, et al. Characterization of perceived hyperoxia in isolated primary cardiac fibroblasts and in the reoxygenated heart. Journal of Biological Chemistry. 2003;278(47):47129–47135. doi: 10.1074/jbc.M308703200. [DOI] [PubMed] [Google Scholar]
- 71.Erecińska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respiration Physiology. 2001;128(3):263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 72.Hemphill JC, III, Smith WS, Sonne DC, Morabito D, Manley GT. Relationship between brain tissue oxygen tension and CT perfusion: feasibility and initial results. American Journal of Neuroradiology. 2005;26(5):1095–1100. [PMC free article] [PubMed] [Google Scholar]
- 73.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. Journal of cellular physiology. 2009;220(3):562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 74.Buerk DG, Shonat RD, Riva CE, Cranstoun SD. O2 gradients and countercurrent exchange in the cat vitreous humor near retinal arterioles and venules. Microvascular Research. 1993;45(2):134–148. doi: 10.1006/mvre.1993.1013. [DOI] [PubMed] [Google Scholar]
- 75.Yu D-Y, Cringle SJ. Retinal degeneration and local oxygen metabolism. Experimental Eye Research. 2005;80(6):745–751. doi: 10.1016/j.exer.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Eliasson P, Jönsson J-I. The hematopoietic stem cell niche: low in oxygen but a nice place to be. Journal of Cellular Physiology. 2010;222(1):17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- 77.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7(2):150–161. doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 78.Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197(4866):452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- 79.Siminovitch L, McCulloch EA, Till JE. The distribution of colony-forming cells among spleen colonies. Journal of Cellular and Comparative Physiology. 2005;62(3):327–336. doi: 10.1002/jcp.1030620313. [DOI] [PubMed] [Google Scholar]
- 80.Honczarenko M, Le Y, Swierkowski M, Ghiran I, Glodek AM, Silberstein LE. Human bone marrow stromal cells express a distinct set of biologically functional chemokine receptors. Stem Cells. 2006;24(4):1030–1041. doi: 10.1634/stemcells.2005-0319. [DOI] [PubMed] [Google Scholar]
- 81.Kim EJ, Kim N, Cho SG. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Experimental & Molecular Medicine. 2013;45(1):p. e2. doi: 10.1038/emm.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barker N, Van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 83.Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435(7044):948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- 84.Yan X, Owens DM. The skin: a home to multiple classes of epithelial progenitor cells. Stem Cell Reviews. 2008;4(2):113–118. doi: 10.1007/s12015-008-9022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Buhrin H-J, Battula VL, Treml S, Schewe B, Kanz L, Vogel W. Novel markers for the prospective isolation of human MSC. Annals of the New York Academy of Sciences. 2007;1106:262–271. doi: 10.1196/annals.1392.000. [DOI] [PubMed] [Google Scholar]
- 86.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology. 2009;71:241–260. doi: 10.1146/annurev.physiol.010908.163145. [DOI] [PubMed] [Google Scholar]
- 87.Zheng H, Ying H, Wiedemeyer R, et al. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 2010;17(5):497–509. doi: 10.1016/j.ccr.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441(7097):1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 89.Hung S-C. Effects of hypoxic culture on bone marrow mesenchymal stem cells: from bench to bedside. Formosan Journal of Surgery. 2013;46(2):35–38. [Google Scholar]
- 90.Basciano L, Nemos C, Foliguet B, et al. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biology. 2011;12, article 12 doi: 10.1186/1471-2121-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grayson WL, Zhao F, Bunnell B, Ma T. Hypoxia enhances proliferation and tissue formation of human mesenchymal stem cells. Biochemical and Biophysical Research Communications. 2007;358(3):948–953. doi: 10.1016/j.bbrc.2007.05.054. [DOI] [PubMed] [Google Scholar]
- 92.Nekanti U, Dastidar S, Venugopal P, Totey S, Ta M. Increased proliferation and analysis of differential gene expression in human Wharton’s jelly-derived mesenchymal stromal cells under hypoxia. International Journal of Biological Sciences. 2010;6(5):499–512. doi: 10.7150/ijbs.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weijers EM, Van Den Broek LJ, Waaijman T, Van Hinsbergh VWM, Gibbs S, Koolwijk P. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Engineering A. 2011;17(21-22):2675–2685. doi: 10.1089/ten.tea.2010.0661. [DOI] [PubMed] [Google Scholar]
- 94.Saller MM, Prall WC, Docheva D, et al. Increased stemness and migration of human mesenchymal stem cells in hypoxia is associated with altered integrin expression. Biochemical and Biophysical Research Communications. 2012;423(2):379–385. doi: 10.1016/j.bbrc.2012.05.134. [DOI] [PubMed] [Google Scholar]
- 95.Holzwarth C, Vaegler M, Gieseke F, et al. Low physiologic oxygen tensions reduce proliferation and differentiation of human multipotent mesenchymal stromal cells. BMC Cell Biology. 2010;11, article 11 doi: 10.1186/1471-2121-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Raheja LF, Genetos DC, Yellowley CE. The effect of oxygen tension on the long-term osteogenic differentiation and MMP/TIMP expression of human mesenchymal stem cells. Cells Tissues Organs. 2010;191(3):175–184. doi: 10.1159/000235679. [DOI] [PubMed] [Google Scholar]
- 97.López Y, Seshareddy K, Trevino E, Cox J, Weiss ML. Evaluating the impact of oxygen concentration and plating density on human wharton’s jelly-derived mesenchymal stromal cells. Open Tissue Engineering and Regenerative Medicine Journal. 2011;4(1):82–94. [Google Scholar]
- 98.Valorani MG, Montelatici E, Germani A, et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Proliferation. 2012;45(3):225–238. doi: 10.1111/j.1365-2184.2012.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hung S-P, Ho JH, Shih Y-RV, Lo T, Lee OK. Hypoxia promotes proliferation and osteogenic differentiation potentials of human mesenchymal stem cells. Journal of Orthopaedic Research. 2012;30(2):260–266. doi: 10.1002/jor.21517. [DOI] [PubMed] [Google Scholar]
- 100.Yang D-C, Yang M-H, Tsai C-C, Huang T-F, Chen Y-H, Hung S-C. Hypoxia inhibits osteogenesis in human mesenchymal stem cells through direct regulation of RUNX2 by TWIST. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0023965.e23965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bochkov NP, Vinogradova MS, Volkov IK, Voronina ES, Kuleshov NP. Statistical analysis of clone formation in cultures of human stem cells. Bulletin of Experimental Biology and Medicine. 2011;151(4):498–501. doi: 10.1007/s10517-011-1366-0. [DOI] [PubMed] [Google Scholar]
- 102.Tarte K, Gaillard J, Lataillade J-J, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood. 2010;115(8):1549–1553. doi: 10.1182/blood-2009-05-219907. [DOI] [PubMed] [Google Scholar]
- 103.Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Current Opinion in Cell Biology. 2006;18(6):658–667. doi: 10.1016/j.ceb.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Ozery-Flato M, Linhart C, Trakhtenbrot L, Izraeli S, Shamir R. Large-scale analysis of chromosomal aberrations in cancer karyotypes reveals two distinct paths to aneuploidy. Genome Biology. 2011;12(6, article R61) doi: 10.1186/gb-2011-12-6-r61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121(2):325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 106.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of Internal Medicine. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 107.Lipinski MJ, Biondi-Zoccai GGL, Abbate A, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. Journal of the American College of Cardiology. 2007;50(18):1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 108.Vassilopoulos G, Wang P-R, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422(6934):901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 109.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 110.Wang X, Willenbring H, Akkari Y, et al. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422(6934):897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 111.Jin J, Zhao Y, Tan X, Guo C, Yang Z, Miao D. An improved transplantation strategy for mouse mesenchymal stem cells in an acute myocardial infarction model. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0021005.e21005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leroux L, Descamps B, Tojais NF, et al. Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a wnt4-dependent pathway. Molecular Therapy. 2010;18(8):1545–1552. doi: 10.1038/mt.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu H, Liu S, Li Y, et al. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PloS ONE. 2012;7(4) doi: 10.1371/journal.pone.0034608.e34608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hung S-C, Pochampally RR, Hsu S-C, et al. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS ONE. 2007;2(5, article e416) doi: 10.1371/journal.pone.0000416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsai C-C, Yew T-L, Yang D-C, Huang W-H, Hung S-C. Benefits of hypoxic culture on bone marrow multipotent stromal cells. American Journal of Blood Research. 2012;2(3):p. 148. [PMC free article] [PubMed] [Google Scholar]
- 116.Wynn RF, Hart CA, Corradi-Perini C, et al. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104(9):2643–2645. doi: 10.1182/blood-2004-02-0526. [DOI] [PubMed] [Google Scholar]
- 117.Sordi V, Malosio ML, Marchesi F, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood. 2005;106(2):419–427. doi: 10.1182/blood-2004-09-3507. [DOI] [PubMed] [Google Scholar]
- 118.Song C-H, Honmou O, Furuoka H, Horiuchi M. Identification of chemoattractive factors involved in the migration of bone marrow-derived mesenchymal stem cells to brain lesions caused by prions. Journal of Virology. 2011;85(21):11069–11078. doi: 10.1128/JVI.05318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin Q, Kim Y, Alarcon RM, Yun Z. Oxygen and cell fate decisions. Gene Regulation and Systems Biology. 2008;2008(2):43–51. doi: 10.4137/grsb.s434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kenneth NS, Rocha S. Regulation of gene expression by hypoxia. Biochemical Journal. 2008;414(1):19–29. doi: 10.1042/BJ20081055. [DOI] [PubMed] [Google Scholar]
- 121.Stamati K, Mudera V, Cheema U. Evolution of oxygen utilization in multicellular organisms and implications for cell signalling in tissue engineering. Journal of Tissue Engineering. 2011;2(1) doi: 10.1177/2041731411432365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fong G-H. Mechanisms of adaptive angiogenesis to tissue hypoxia. Angiogenesis. 2008;11(2):121–140. doi: 10.1007/s10456-008-9107-3. [DOI] [PubMed] [Google Scholar]
- 123.Wang GL, Jiang B-H, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weidemann A, Johnson RS. Biology of HIF-1α . Cell Death and Differentiation. 2008;15(4):621–627. doi: 10.1038/cdd.2008.12. [DOI] [PubMed] [Google Scholar]
- 125.Jaakkola P, Mole DR, Tian Y-M, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 126.Hon W-C, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF-1α by pVHL. Nature. 2002;417(6892):975–978. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 127.Metzen E, Berchner-Pfannschmidt U, Stengel P, et al. Intracellular localisation of human HIF-1α hydroxylases: implications for oxygen sensing. Journal of Cell Science. 2003;116(7):1319–1326. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- 128.Ivan M, Kondo K, Yang H, et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 129.Maxwell PH, Wlesener MS, Chang G-W, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 130.Brahimi-Horn MC, Pouysségur J. Oxygen, a source of life and stress. FEBS Letters. 2007;581(19):3582–3591. doi: 10.1016/j.febslet.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 131.Lavrentieva A, Majore I, Kasper C, Hass R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Communication and Signaling. 2010;8:p. 18. doi: 10.1186/1478-811X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim J-W, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3(3):177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 133.Semenza GL. Regulation of oxygen homeostasis by hypoxia-Inducible factor 1. Physiology. 2009;24(2):97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 134.Arciuch VGA, Elguero ME, Poderoso JJ, Carreras MC. Mitochondrial regulation of cell cycle and proliferation. Antioxidants and Redox Signaling. 2012;16(10):1150–1180. doi: 10.1089/ars.2011.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schweisguth F. Regulation of notch signaling activity. Current Biology. 2004;14(3):R129–R138. [PubMed] [Google Scholar]
- 136.Sainson RC, Harris AL. Hypoxia-regulated differentiation: let’s step it up a Notch. Trends in Molecular Medicine. 2006;12(4):141–143. doi: 10.1016/j.molmed.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 137.Ponte AL, Marais E, Gallay N, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 138.Shi M, Li J, Liao L, et al. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92(7):897–904. doi: 10.3324/haematol.10669. [DOI] [PubMed] [Google Scholar]
- 139.Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279(5349):349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 140.Richter T, Zglinicki TV. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. Experimental Gerontology. 2007;42(11):1039–1042. doi: 10.1016/j.exger.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 141.Gordon DJ, Resio B, Pellman D. Causes and consequences of aneuploidy in cancer. Nature Reviews Genetics. 2012;13(3):189–203. doi: 10.1038/nrg3123. [DOI] [PubMed] [Google Scholar]
- 142.Wang CY, Liu LN, Zhao ZB. The role of ROS toxicity in spontaneous aneuploidy in cultured cells. Tissue and Cell. 2012;45(1):47–53. doi: 10.1016/j.tice.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 143.Barzilai A, Yamamoto K-I. DNA damage responses to oxidative stress. DNA Repair. 2004;3(8-9):1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 144.Guachalla LM, Rudolph KL. ROS induced DNA damage and checkpoint responses: influences on aging? Cell Cycle. 2010;9(20):4058–4060. doi: 10.4161/cc.9.20.13577. [DOI] [PubMed] [Google Scholar]
- 145.Treff NR, Su J, Taylor D, Scott RT. Telomere dna deficiency is associated with development of human embryonic aneuploidy. PLoS Genetics. 2011;7(6) doi: 10.1371/journal.pgen.1002161.e1002161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Plentz RR, Schlegelberger B, Flemming P, et al. Telomere shortening correlates with increasing aneuploidy of chromosome 8 in human hepatocellular carcinoma. Hepatology. 2005;42(3):522–526. doi: 10.1002/hep.20847. [DOI] [PubMed] [Google Scholar]


