Abstract
Objective
Despite recent advances in our molecular understanding of Spitz-type tumors, the clinical behavior of these lesions remains unclear. We thus set out to define the clinical outcome of classic Spitz nevi, atypical Spitz tumors (ASTs), and spitzoid melanomas.
Design
From 1987 through 2002, data on all lesions containing the term “Spitz” or “Spitz” [AND] “melanoma” were retrieved from the pathology database at Massachusetts General Hospital, and the cases were followed up for their outcome.
Setting
The study was performed at a university-affiliated tertiary health care center in Boston, Massachusetts.
Patients
A total of 157 patients with Spitz-type melanocytic lesions and follow-up information were identified.
Main Outcome Measures
Sentinel lymph node biopsy results, metastases, or fatality were assessed.
Results
There were 68 classic Spitz nevi, 76 ASTs, 10 spitzoid melanomas, and 3 melanomas that arose in Spitz nevi. Spitz nevi were diagnosed at a younger age than ASTs (mean age, 26.4 years vs 33.7 years) (P=.01), though both occurred earlier than melanomas (mean age, 50.4 years, P<.001). Sentinel lymph node biopsy findings were positive in 1 of 6 and 4 of 8 patients with ASTs and spitzoid melanomas, respectively. After a median follow-up of 9.1 years, only 1 patient with an AST, who had a separate intermediate-thickness melanoma, developed distant metastasis. There were 6 documented invasive melanomas among 144 patients with classic Spitz nevi or ASTs (observed/expected ratio, 8.03) (P=.01).
Conclusions
Atypical Spitz tumors are associated with minimal lethal potential, an increased melanoma risk, and a moderate risk of metastasis to regional nodes. It makes clinical sense to minimize aggressive treatment but to offer careful surveillance for rare relapses and subsequent melanomas.
Spitz (spindle and/or epithelioid cell) tumors/nevi were first described in 1948 by Sophie Spitz as “juvenile melanomas.”1,2 The incidence of Spitz tumor is estimated to range from 1.4 to 1.66 per 100 000, affecting all age groups but uncommon beyond the 4th and 5th decades of life.3,4 Clinically, these lesions appear as well-circumscribed pink, tan, or brown papules or nodules with an average diameter of 8 mm that may involve any part of the body. Even early on, the clinical outcome of Spitz tumors has been of tremendous interest because a reasonable subset of these lesions appear to metastasize and yet not prove fatal—hence, the earlier term, benign juvenile melanomas.1,2
There are several elaborate criteria for the histopathologic diagnosis of Spitz tumors. Discriminating them from the benign or malignant variants, especially in their atypical forms, has been a great challenge in dermatopathology and has resulted in many controversies in the diagnosis and management of these not-so-rare pigmented lesions.4–8 Spatz et al9 proposed a grading system for risk stratification of Spitz tumors and categorized them into low, intermediate, or high risk based on a combination of clinical and pathologic parameters. Another similar and pragmatic approach classified Spitz tumors into 3 categories: Spitz tumor without atypicality (classic Spitz nevus [CSN]), atypical Spitz tumor (AST), and spitzoid malignant melanoma (SMM).10 However, in 1 blinded study, a panel of 10 pathologists reviewed 30 Spitz-type tumors (including Spitz tumors without atypicality, ie, CSN, ASTs, and spitzoid melanomas) and failed to reach consensus on objective morphologic criteria.7
In the face of histopathologic controversies, it is also possible to refocus analysis on clinical outcome rather than histologic discrimination. Clinical, histopathologic, and outcome studies of ASTs have suffered from short follow-up periods and/or small case series size. In aggregate, several studies have used sentinel lymph node biopsy (SLNB) to demonstrate nodal involvement in about 40% of cases.11–21 In 1 recent series of 67 patients with AST, 27 of 57 patients who underwent SLNB had detectable microscopic disease in the sentinel node (47%)12; however, all 27 patients with a positive SLNB finding were alive and disease free at median follow-up of 43.8 months. Taken together, these studies point to an emerging divide between the intense histologic and molecular analysis applied to these lesions and the prognostic relevance of Spitz-type tumors to patients. We thus set out to evaluate the clinical outcomes of patients with Spitz-type tumors over the course of 30 years in a large, retrospective, single-institution case series.
METHODS
IDENTIFICATION OF ELIGIBLE CASES
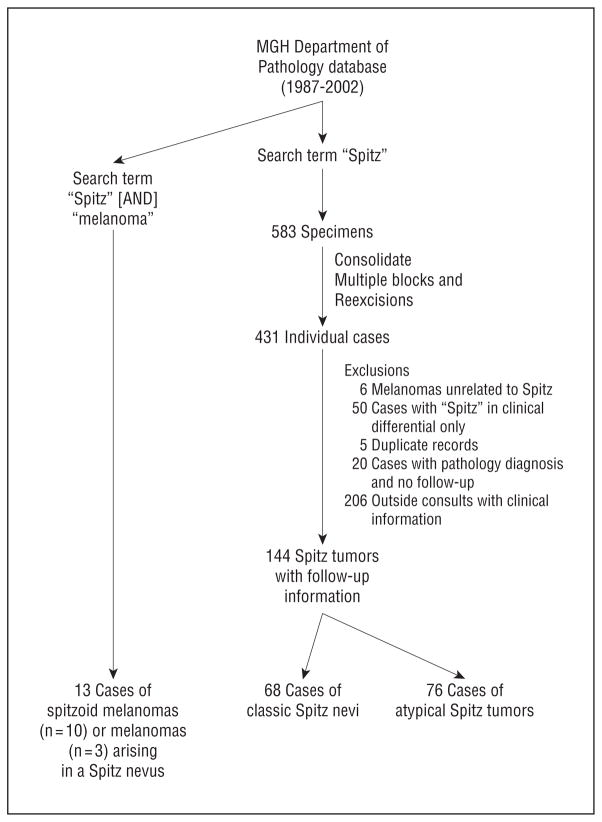
After obtaining institutional review board approval at Massachusetts General Hospital (MGH) (legacy 2008P000860), we applied the search terms “Spitz” or “Spitz” [AND] “melanoma” to the database of the Department of Pathology at MGH, and limited the search period to 1987 through 2002 (Figure 1) so as to allow at least a 6-year follow-up window. Although retrospective in nature, the index cases were entered into our series through the pathology database and were forward tracked through medical records to examine outcome. Collectively, 583 pathology reports created during this time interval included the word “Spitz” either in the clinical diagnosis field, final pathology diagnosis, or the pathology notes and/or comments. Each final pathology report was then evaluated by a single pathologist (A.S.). After examination of each report, duplicate records and re-excision specimens were eliminated, and 431 lesions from distinct individuals were identified.
Figure 1.
Identification of 157 Spitz-type tumors. MGH indicates Massachusetts General Hospital, Boston.
Of these 431 individuals, 144 patients had records in the Partners Longitudinal Medical Record (LMR) system, which includes laboratory values and medical records from 8 Harvard-affiliated hospitals, and thus demographic characteristics, birth date, and date of diagnosis could be confirmed on these cases. The excluded cases were 50 cases with the word “Spitz” in their clinical differential diagnosis rather than in the pathology report, 5 duplicate records, 6 melanomas unrelated to Spitz, 20 cases with the pathology diagnosis only and no clinical follow-up, and 206 pathology consult slides only, with no follow-up or treatment information at MGH (Figure 1). Using the search terms of “Spitz” [AND] “melanoma”, we also identified 13 cases of SMM or malignant melanomas arising in association with spitzoid lesions. All other details regarding SLNB procedures and second melanomas were confirmed with a pathology report.
For comparison, the ages at diagnosis for 2345 melanomas evaluated during the study period (1987 through 2002) were also retrieved through the MGH cancer registry.
DIAGNOSTIC CRITERIA
Inclusive criteria for histopathological diagnosis of Spitz tumors and their discrimination from atypical and malignant forms have been previously published.10 Briefly, tumor characteristics are categorized into 3 groups: (1) organizational criteria, including diameter, depth, ulceration, circumscription, pagetoid spread, confluence, cellular density, zonation, maturation, asymmetry, and Kamino bodies; (2) proliferation criteria, including mitotic rate, deep mitoses, and proliferation index (in some cases); and (3) cytologic criteria, including quality of cytoplasm, nucleus to cytoplasm ratio, chromatin pattern, nuclear membranes, and nucleoli.
However, our study period precedes publication of these criteria, and the dermatopathologists in the present study used some of the above features combined with their overall impression to discriminate benign from atypical from malignant spitzoid melanocytic tumors. After each final pathology report was reviewed, cases without features of atypia in the final pathology diagnosis—regardless of the involvement of the epidermis, dermis, or both—were classified as CSN, and cases for which any form of atypia was mentioned in the final diagnosis were included in the AST category. Cases with an unequivocal final diagnosis of invasive SMM or other type of invasive melanoma arising in association with Spitz-type tumors that included synoptic reports and evaluations of melanoma prognostic factors were included in SMM category.
CALCULATING THE OBSERVED/EXPECTED RATIO
The number of melanomas observed among our 144 cases during their follow-up period was compared with the number of expected cutaneous melanomas calculated using the National Cancer Institute’s Surveillance, Epidemiology and End Result (SEER) data (www.seer.cancer.gov). SeerStat, version 6.5.1 statistical software was used to calculate age-adjusted incidence rates of malignant melanoma separately for white men and white women by year from 1973 through 2006; rates for the 2007–2010 period were extrapolated using a regression model. For each individual in our data set, we calculated the cumulative sum of the yearly incidence rate beginning with the individual’s birth year and up through the year of last known follow-up or year of melanoma diagnosis, if affected. We then took the sum of these values across all individuals as the expected number of melanomas in this cohort. A 95% confidence interval (CI) was calculated using a bootstrap approach.
RESULTS
Of the 144 confirmed cases of Spitz tumors, there were 57 and 87 cases diagnosed in men and women, respectively. The mean age of diagnosis was 30.2 years (age range, 3–77 years), and the mean follow-up period for our cohort was 109 months (9.1 years; range, 1–206 months); 5 patients had a full skin evaluation and surgical treatment at MGH but decided to follow up with their local dermatologists (follow up, ≤1 month). From the pathologic diagnoses, 68 were CSN (47.2%), and 76 were ASTs (52.8%). There were also 13 cases of Spitz-associated melanomas (10 SMMs and 3 melanomas arising in Spitz nevi).
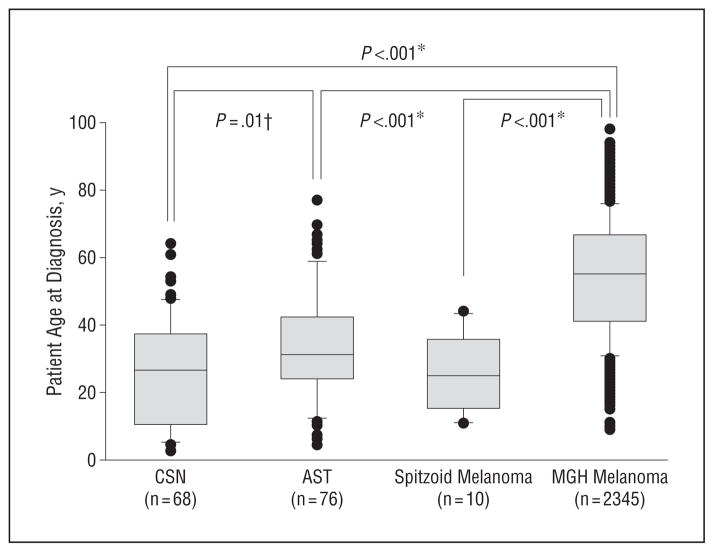
We first examined differences between the demographic characteristics and clinical features of Spitz tumors with and without atypia. As shown in Figure 2, CSN occurred significantly earlier in life than their atypical counterparts (mean age, 26.4 years vs 33.7 years) (P=.01), although 12 of 76 ASTs were diagnosed in patients younger than 18 years (15.8%). The mean patient age at diagnosis for 2345 melanomas seen at the MGH during the study period was 50.4 years. This was significantly older than age at diagnosis for CSN, ASTs, or SMMs (P<.001 for all).
Figure 2.
Box and whisker plot showing age distribution of cases (dark circles) at diagnosis of classic Spitz nevi (CSN), atypical Spitz tumors (ASTs), spitzoid melanomas, and all melanomas diagnosed at Massachusetts General Hospital, Boston (MGH), from 1987 through 2002. The gray boxes represent all patients within the 25th to 75th percentiles; error bars, the 10th and 90th percentiles; and horizontal lines, median age at diagnosis.
*Differences in the median ages at onset among spitzoid melanomas, ASTs, and all MGH melanomas were compared using the Mann-Whitney test.
†The distribution of CSN and ASTs passed normality, and the means were compared using the t test.
More than half the lesions occurred on the extremities (81/144, 56.3%) (Table 1), 52 of 144 being located on the lower extremities (36.1%). There were no apparent site differences between CSN and ASTs (P=.22). Although women were slightly overrepresented among ASTs compared with CSN (65.8% vs 54.4%), this was not statistically significant (P=.22).
Table 1.
Characteristics of Classic and Atypical Spitz Tumor Casesa
| Characteristic | Classic Spitz | Atypical Spitz | P Value | |
|---|---|---|---|---|
| All cases (n = 144) | 68 (47.2) | 76 (52.3) | NA | |
| Age, mean, y | 26.5 | 33.7 | .01 (t test) | |
| Sex | ||||
| Female | 37 (54.4) | 50 (65.8) |
|
P = .22 (χ2 test) |
| Male | 31 (45.6) | 26 (34.2) | ||
| Site | ||||
| Head and/or neck | 13 (19.1) | 14 (18.4) |
|
P = .23 (χ2 test) |
| Back | 12 (17.6) | 12 (15.8) | ||
| Chest | 2 (2.9) | 0 | ||
| Upper extremities | 12 (17.6) | 17 (22.4) | ||
| Abdomen | 3 (4.4) | 1 (1.3) | ||
| Buttock and/or hip | 5 (7.4) | 1 (1.3) | ||
| Lower extremities | 21 (30.8) | 31 (40.8) | ||
Abbreviation: NA, not applicable.
Unless otherwise noted, data are reported as number (percentage) of tumors.
Sentinel lymph node biopsies were performed on 6 of 76 patients with ASTs and on none with CSN (Table 2). Only 1 person had microscopic nodal involvement, and this patient was diagnosed as having an AST on his left knee at age 22 years. After the sentinel lymphadenectomy, he underwent a completion lymph node dissection, and no additional metastatic nodes were identified. Subsequently, he received high-dose interferon and remained disease free at last follow-up, 8.8 years after AST diagnosis. None of the patients who underwent SLNB for their ASTs had a prior melanoma.
Table 2.
Patients With Spitz-Type Tumors and Sentinel Lymph Node Biopsies
| Sex/Age, y | Follow-up, mo | Location | Pathology Finding | SLNB | Additional Treatments | Statusa |
|---|---|---|---|---|---|---|
| F/40.0 | 117 | Abdomen | Combined lentiginous junctional dysplastic/desmoplastic Spitz nevus with severe atypia | Neg | None | Alive, NED |
| F/25.5 | 1 | L arm | Severely atypical spindle cell and epithelioid cell nevus (Spitz) | Neg | None | Alive, NED |
| M/15.7 | 2 | Mid back | Severely atypical compound Spitz nevus | Neg | None | Alive, NED |
| M/18.9 | 57 | R cheek | Atypical compound Spitz tumor | Neg | None | Alive, NED |
| F/67.2 | 106 | R upper thigh | Desmoplastic dermal Spitz nevus with atypia | Neg | None | Alive, NED |
| M/22.4 | 105 | L knee | Severely atypical Spitz tumor | Pos (1/3 L groin) | CLND (0/17 L groin) + IFN | Alive, NED |
Abbreviations: CLND, completion lymph node dissection; IFN, interferon; L, left; Mel, melanoma; NED, no evidence of disease; Neg, negative; Pos, positive; R, right; SLNB, sentinel lymph node biopsy finding.
No patient had developed melanoma by last follow-up.
For evaluating the outcomes of CSN and ASTs, we had a median follow-up period of 109 months among our cohort of 144 patients. During this interval, there was only a single case of distant metastases, in a 36-year-old man who had a moderately atypical compound Spitz nevus on the right ear and a concomitant intermediate-thickness, superficial spreading melanoma (SSM) on the scalp. Both the primary SSM and the metastases consisted of similar epithelioid cells. Although the metastases most likely developed from the bona fide melanoma, it is difficult to completely exclude the rare possibility that the disseminated disease had emerged from the patient’s Spitz tumor rather than from the melanoma. The collective experience, however, suggests that Spitz-type tumors, regardless of their atypical features, are not associated with an aggressive outcome.
As detailed in Table 3, there were 7 invasive melanomas and 1 in situ melanoma at an unrelated site among 7 patients with Spitz-type tumors. These preceded the melanoma in 3 of the cases, and 5 of the 7 Spitz-type tumors had atypical features. Given the relatively large cohort size (n = 144), we were able to calculate the expected number of primary invasive melanomas over the entire monitored period (ie, age at last follow-up) using SEER-derived incidence data. Since we could not fully confirm 1 of the melanomas by pathology report, and since the SEER database does not fully capture in situ melanomas, we used the 6 invasive melanomas among our cohort for the analysis. Matching for sex, date of birth and age at last follow-up, we obtained an observed/ expected ratio of 8.03 (95% CI, 2.61–14.9) (P=.01). This suggests that the presence of either a Spitz nevus or an AST may be a risk factor for melanoma.
Table 3.
Patients With Spitz-Type Tumors and Non-Spitz–Type Melanomas
| Sex/Age at First Dx, y | First Dx | Location of First Dx | Age at Second Dx, y | Second Dx | Location of Second Dx | Age at Last f/u, y | Status at Last f/u |
|---|---|---|---|---|---|---|---|
| F/35.0 | Acral lentiginous MIS | L thumb | 35.6 | Compound epithelioid nevus (Spitz) with atypia | Back | 48.6 | Alive, NED |
| M/36.0 | SSM (1.2 mm, level 4, nonulcerated) | R posterior scalp | 36.0 | Compound nevus (combined blue and Spitz types) with focal moderate cytologic atypia and dermal sclerosis | R ear | 38.8 | Stage IV disease |
| F/17.8 | SSM (1.14 mm, level 3, nonulcerated) | R thigh | 26.6 | Atypical sclerosing epithelioid cell proliferation most consistent with epithelioid cell nevus (Spitz) | R knee | 36.0 | Alive, NED |
| M/57.5 | SSM (0.24 mm, level 2) | L lower earlobe | 58.4 | Combined dermal and compound spindle cell and epithelioid cell nevus (Spitz nevus) with severe atypia | R back | 69.1 | Alive, NED |
| Melanoma (pathology report unavailable) | L forearm | 67.5 | NA | NA | NA | NA | |
| F/24.5 | Compound spindle cell and epithelioid cell nevus (Spitz) | Mid back | 30.6 | SSM (0.52 mm, level 2, nonulcerated) | R lower eyelid | 35.4 | Alive, NED |
| M/62.1 | Combined dermal nevus and dermal Spitz nevus with moderate atypia | R arm | 69.7 | SSM (0.32 mm, level 3) | L back | 70.8 | Alive, NED |
| F/18.7 | Junctional spindle cell and/or epithelioid cell nevus (Spitz) | R ankle | 29.7 | SSM (0.85 mm, level 3, nonulcerated) | L upper arm | 29.8 | Alive, NED |
Abbreviations: Dx, diagnosis; f/u, follow up; L, left; MIS, melanoma in situ; NED, no evidence of disease; NA, not applicable; R, right; SSM, superficial spreading melanoma.
In addition, there were 13 Spitz-related melanomas: 10 of these were classified as spitzoid melanomas, while 3 were more common types of melanoma (2 SSMs and 1 nodular melanoma [NM]) that appeared to be contiguous to a Spitz nevus (Table 4). The mean age at diagnosis for this group of Spitz-related melanomas was 28.0 years. The 10 spitzoid melanomas were also diagnosed significantly earlier than the MGH melanomas (Figure 2) (P<.001 for patient age 35 years vs 55 years). In total, 8 of 13 patients in this group underwent SLNB sampling: 4 results were positive, and 1 patient with an NM that was contiguous to a Spitz nevus subsequently developed a regional axillary metastasis 35 months after the initial diagnosis. Another 29-year-old man developed a second primary SSM after an initial spitzoid melanoma. All 13 patients were alive without evidence of disease at last follow-up (mean follow-up, 110 months; range, 20–218 months).
Table 4.
Spitzoid and Spitz-Related Melanomas
| Sex/Age, y | Follow-up, mo | Location | Pathologic Finding | SLNB | Additional Treatment | Status at Last Follow-up |
|---|---|---|---|---|---|---|
| M/11 | 115 | R shoulder | Spitzoid melanoma (3.0 mm, level 4) | Yes (1/4-0/1 R jugular, 1/3 R axillary) | CLND (0/5) + IFN | Alive, NED |
| F/15 | 62 | L ankle | Spitzoid melanoma (1.58 mm, level 4) | Yes (0/1) | None | Alive, NED |
| F/16 | 26 | L neck | Spitzoid melanoma (5.2 mm, level 4) | Yes (0/1) | None | Alive, NED |
| M/19 | 139 | R ear | Spitzoid melanoma (1.7 mm, level 4) | Yes (0/2) | None | Alive, NED |
| M/21 | 20 | R leg | Spitzoid melanoma (1.0 mm, level 3) | No | None | Alive, 2nd primary SSM (0.24mm, level 2) on back |
| F/29 | 218 | R calf | Spitzoid melanoma (1.8 mm, level 4) | No | None | Alive, NED |
| M/31 | 141 | R ankle | Spitzoid melanoma (2.08 mm, level 4) | Yes (0/2) | None | Alive, NED |
| M/35 | 36 | L shoulder | Minimal deviation melanoma of the Spitz type | No | None | Alive, NED |
| M/40 | 186 | L forearm | Spitzoid melanoma (2.3 mm, level 4) | Yes (2/4: 2/2 L epitrochlear node; 0/2 L axillary) | CLND (epitrochlear, no nodes) + IFN | Alive, NED |
| F/44 | 91 | L foot | Spitzoid melanoma (1.0 mm, level 3) | Yes (2/2 L groin) | CLND (0/17) + IFN | Alive, NED |
| M/17 | 113 | L thigh | SSM (0.28 mm, level 2) contiguous to a Spitz nevus | No | None | Alive, NED |
| F/27 | 148 | L scapula | NM (0.9 mm, level 3) contiguous to a Spitz nevus | No | L axilla relapse at 35 mo Treated with IFN+ Melacine a | Alive, NED |
| F/52 | 147 | R foot | SSM (0.8 mm, level 4) contiguous to a Spitz nevus | Yes (1/1 R groin) | None | Alive, NED |
Abbreviations: CLND, completion lymph node dissection; f/u, follow-up; IFN, interferon; L, left; Mel, melanoma; NED, no evidence of disease; R, right; SLNB, sentinel lymph node biopsy finding.
Manufactured by Corixa Corporation, Seattle, Washington.
COMMENT
The long-term clinical outcome of patients with Spitz tumors has been somewhat controversial given the propensity of Spitz tumors for regional metastasis. In this study of 144 patients with Spitz-type tumors (68 CSN and 76 ASTs) and 13 Spitz-related melanomas, we did not detect any deaths after a mean follow-up period of 9.1 years except for 1 patient who had a concurrent, intermediate-thickness SSM. Thus, in our series, Spitz-type tumors were associated with a highly favorable prognosis. Compared with other analyses,11–16 the present study is larger and has longer follow-up and consonant findings. Although fatalities have been reported for patients with Spitz-type tumors,7 mortal outcome appears uncommon and unpredictable in light of present clinical, pathologic, and molecular information.
A somewhat intriguing result is the increased risk of melanoma among patients with either CSN or AST. We detected an 8.0-fold excess risk for melanoma when individuals were matched to the SEER registry for date of birth, age and sex. Unfortunately, the small number of cases precludes us from differentially estimating CSN and AST risks with any great precision. In our analysis, we calculated the expected number of melanomas by the age at last follow-up. Thus, we assessed for an association between Spitz tumors and melanoma without regard to order of occurrence (ie, Spitz then melanoma vs melanoma then Spitz). Nevertheless, this association raises the clinical hypothesis that Spitz tumors may share similar risk factors with melanoma and that the early presence of Spitz tumors may be a possible risk marker for subsequent melanomas. However, unlike common nevi, Spitz-type tumors do not occur in large numbers on most patients; therefore, CSN and ASTs are less likely to exhibit the quantitative risk gradient by nevus count reported for common and atypical moles.22 One confounding factor in the analysis is the ongoing surveillance experienced by many in the cohort. Therefore, part of the apparent risk that we found may reflect increased surveillance and sampling. Although this finding requires replication, it does support the notion that Spitz-type tumors are demonstrable markers of risk for cutaneous melanoma.
There are several dimensions to the relationship between Spitz tumors and melanoma. First, the cells within the proliferation may adopt atypical spindle and epithelioid morphologic characteristics, giving rise to the more canonical group of spitzoid melanomas, which can morphologically overlap with ASTs. Second, SLNBs have been used in the recent past under the assumption that this staging procedure would distinguish ASTs from true melanomas. In our series, 5 of 14 patients who underwent SLNB had microscopic disease (36%) (1 of 6 ASTs, 3 of 7 spitzoid melanomas, and 1 of 1 melanoma arising in a Spitz nevus), which is in line with the 40% estimate from the literature.11–16 None of these patients developed widespread metastases in our interval of observation. Unlike microscopic nodal disease in the more common forms of melanoma, which reproducibly predicts higher mortality, the utility of SLNB with Spitz tumors is unclear because the predictive value of finding nodal disease is uncertain.
Another less commonly recognized event is the coincidence of SSMs and NMs and Spitz tumors. Magro et al23 recently described a distinct superficial atypical variant of Spitz tumors that can evolve into SSMs. Based on the histomorphologic criteria, including dermal mitoses in 7 of 19 patients, they included 19 cases in this provisional category. In 3 patients, SLNBs were performed, and the results were negative. The patients were all alive and disease free during a 2-year clinical follow-up period. Whether to place this newly suggested category in a borderline group, such as a subtype of AST, or frank melanoma requires larger studies and extended follow-up to verify their actual natural history and behavior.
Although there may be some bias resulting from MGH being largely an adult hospital, we also found that the average age of onset for CSN is significantly earlier than that for ASTs, even among our predominantly adult population; this is to be expected because CSN are known to occur largely in pediatric populations.24 Although there is clear overlap in age distribution, the disparity suggests that there may be genetic or host factors that influence the expression pattern of these types of lesions. A study of dysplastic nevi demonstrated a similar positive correlation between age and degree of atypia. The mean ages at diagnosis for “mildly” vs “severely” atypical dysplastic nevi were 34.8 and 41.5 years, respectively.25 These time-dependent observations raise 2 speculations: (1) nevi that persist over time sustain more cumulative injury, such as oxidative or UV radiation damage, thereby leading to a higher level of atypia on pathologic evaluation; or (2) nevi that form on aged or photoaged skin may in fact adopt a more atypical appearance.
Putting the clinical and management controversies aside, we believe that the biological implications of a neoplasm with reasonable metastatic but low lethal potential is extraordinary. Molecular analyses of Spitz-type tumors have shown that Spitz tumors harbor HRAS aberrations26 (ie, amplifications and mutations) to a greater extent than BRAF and NRAS abnormalities, which can be recovered from the more common types of moles and melanoma.27 Although oncogenic activation of these 3 genes enhances RAS signaling, there is a clear distinction in clinical behavior. Interestingly, activating germ-line mutations in HRAS, BRAF, and NRAS leads to Costello,28 cardiofaciocutaneous,29 and autoimmune lymphoproliferative30 syndromes, respectively, and yet none of these conditions has been reported to predispose a carrier to melanomas or Spitz-type tumors. Thus, constitutive signaling alone is not sufficient to transform melanocytes in these proliferations. Other specifying features such as cell of origination, timing in development, genetic modifiers, and stromal response may ultimately determine the pathologic phenotype.
One limitation to the study is the identification of Spitz lesions from a single database. Although selection and referral biases may exist, these results would ideally spark additional multicenter studies to better refine prognosis of Spitz-type tumors. Another possible confounder is reclassification based on outcome. For instance, if an AST diagnosis is revised to melanoma after a fatality, the benign nature of ASTs would be preserved. We attempted to minimize this bias by defining the moment of pathologic diagnosis as the point of entry. Patients were then followed up through the MGH system, and all subsequent events were annotated. The apparent association between Spitz tumors and melanomas is also subject to detection bias, since individuals with either diagnosis are monitored more closely; again, additional studies are needed to replicate this finding.
In conclusion, our study suggests that ASTs are associated with low lethal potential, an increased melanoma risk, and a metastatic capability to regional nodes. It is not unreasonable to position Spitz-type tumors in the same general category as another precursor lesion, the dysplastic nevus. Overall, it makes rational clinical sense to minimize aggressive treatment for localized lesions but to offer continued surveillance for rare relapses and subsequent melanomas. Moreover, in the absence of a well-documented prognostic trial specifically addressing survival, the therapeutic role of SNLB sampling in this population is debatable.
Acknowledgments
Funding/Support: This research was supported in part by grant RSG-07-085-01-MGO from the American Cancer Society, grant K24 CA149202-01 from the National Institutes of Health, and by the MGH Millennium Fund for Melanoma Research (Dr Tsao).
Role of the Sponsors: The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Financial Disclosure: Dr Sober is a consultant to MelaFind and MELA Sciences.
Author Contributions: Drs Sepehr and Tsao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Sepehr, Chao, and Tsao. Acquisition of data: Sepehr, Chao, Trefrey, Flotte, Sober, Mihm, and Tsao. Analysis and interpretation of data: Sepehr, Chao, Blackford, Duncan, Flotte, Mihm, and Tsao. Drafting of the manuscript: Sepehr, Chao, Trefrey, and Tsao. Critical revision of the manuscript for important intellectual content: Sepehr, Blackford, Duncan, Flotte, Sober, Mihm, and Tsao. Statistical analysis: Sepehr, Blackford, and Tsao. Obtained funding: Tsao. Administrative, technical, and material support: Chao, Trefrey, Duncan, Sober, and Mihm. Study supervision: Mihm and Tsao.
Additional Contributions: Giovanni Parmigiani, PhD, provided guidance during the statistical analysis and Christine D. Witham provided administrative support during this project.
References
- 1.Spitz S. Melanomas of childhood. Am J Pathol. 1948;24(3):591–609. [PMC free article] [PubMed] [Google Scholar]
- 2.Allen AC, Spitz S. Malignant melanoma; a clinicopathological analysis of the criteria for diagnosis and prognosis. Cancer. 1953;6(1):1–45. doi: 10.1002/1097-0142(195301)6:1<1::aid-cncr2820060102>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Ko CB, Walton S, Wyatt EH, Bury HP. Spitz nevus. Int J Dermatol. 1993;32(5):354–357. doi: 10.1111/j.1365-4362.1993.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 4.Weedon D, Little JH. Spindle and epithelioid cell nevi in children and adults: a review of 211 cases of the Spitz nevus. Cancer. 1977;40(1):217–225. doi: 10.1002/1097-0142(197707)40:1<217::aid-cncr2820400134>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Kernen JA, Ackerman LV. Spindle cell nevi and epithelioid cell nevi (so-called juvenile melanomas) in children and adults: a clinicopathological study of 27 cases. Cancer. 1960;13:612–625. doi: 10.1002/1097-0142(196005/06)13:3<612::aid-cncr2820130324>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.Reed RJ, Ichinose H, Clark WH, Jr, Mihm MC., Jr Common and uncommon melanocytic nevi and borderline melanomas. Semin Oncol. 1975;2(2):119–147. [PubMed] [Google Scholar]
- 7.Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol. 1999;30(5):513–520. doi: 10.1016/s0046-8177(99)90193-4. [DOI] [PubMed] [Google Scholar]
- 8.Paniago-Pereira C, Maize JC, Ackerman AB. Nevus of large spindle and/or epithelioid cells (Spitz’s nevus) Arch Dermatol. 1978;114(12):1811–1823. [PubMed] [Google Scholar]
- 9.Spatz A, Calonje E, Handfield-Jones S, Barnhill RL. Spitz tumors in children: a grading system for risk stratification. Arch Dermatol. 1999;135(3):282–285. doi: 10.1001/archderm.135.3.282. [DOI] [PubMed] [Google Scholar]
- 10.Barnhill RL. The Spitzoid lesion: rethinking Spitz tumors, atypical variants, ‘Spitzoid melanoma’ and risk assessment. Mod Pathol. 2006;19(suppl 2):S21–S33. doi: 10.1038/modpathol.3800519. [DOI] [PubMed] [Google Scholar]
- 11.Su LD, Fullen DR, Sondak VK, Johnson TM, Lowe L. Sentinel lymph node biopsy for patients with problematic spitzoid melanocytic lesions: a report on 18 patients. Cancer. 2003;97(2):499–507. doi: 10.1002/cncr.11074. [DOI] [PubMed] [Google Scholar]
- 12.Ludgate MW, Fullen DR, Lee J, et al. The atypical Spitz tumor of uncertain biologic potential: a series of 67 patients from a single institution. Cancer. 2009;115(3):631–641. doi: 10.1002/cncr.24047. [DOI] [PubMed] [Google Scholar]
- 13.Gamblin TC, Edington H, Kirkwood JM, Rao UN. Sentinel lymph node biopsy for atypical melanocytic lesions with spitzoid features. Ann Surg Oncol. 2006;13(12):1664–1670. doi: 10.1245/s10434-006-9142-5. [DOI] [PubMed] [Google Scholar]
- 14.Urso C, Borgognoni L, Saieva C, et al. Sentinel lymph node biopsy in patients with “atypical Spitz tumors”: a report on 12 cases. Hum Pathol. 2006;37(7):816–823. doi: 10.1016/j.humpath.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Roaten JB, Partrick DA, Bensard D, et al. Survival in sentinel lymph node-positive pediatric melanoma. J Pediatr Surg. 2005;40(6):988–992. doi: 10.1016/j.jpedsurg.2005.03.014. discussion 992. [DOI] [PubMed] [Google Scholar]
- 16.Lohmann CM, Coit DG, Brady MS, Berwick M, Busam KJ. Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. Am J Surg Pathol. 2002;26(1):47–55. doi: 10.1097/00000478-200201000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Gurbuz Y, Apaydin R, Muezzinoğlu B, Buyukbabani N. A current dilemma in histopathology: atypical Spitz tumor or Spitzoid melanoma? Pediatr Dermatol. 2002;19(2):99–102. doi: 10.1046/j.1525-1470.2002.00056.x. [DOI] [PubMed] [Google Scholar]
- 18.McArthur GJ, Banwell ME, Cook MG, Powell BW. The role of sentinel node biopsy in the management of melanocytic lesions of uncertain malignant potential (MUMP) J Plast Reconstr Aesthet Surg. 2007;60(8):952–954. doi: 10.1016/j.bjps.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Murakami M, Wada T, Kashiwagi T, Ishida-Yamamoto A, Iizuka H. Nodular malignant melanoma with Spitz nevus-like pathological features finally confirmed by the pathological feature of the sentinel lymph node. J Dermatol. 2007;34 (12):821–828. doi: 10.1111/j.1346-8138.2007.00393.x. [DOI] [PubMed] [Google Scholar]
- 20.Murali R, Sharma RN, Thompson JF, et al. Sentinel lymph node biopsy in histologically ambiguous melanocytic tumors with spitzoid features (so-called atypical spitzoid tumors) Ann Surg Oncol. 2008;15(1):302–309. doi: 10.1245/s10434-007-9577-3. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerman R, Maier JP, Guiney WB, Jr, Huntsman WT, Mooney EK. Pediatric melanoma: confirming the diagnosis with sentinel node biopsy. Ann Plast Surg. 2001;46(4):394–399. doi: 10.1097/00000637-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma: I, common and atypical naevi. Eur J Cancer. 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Magro CM, Yaniv S, Mihm MC. The superficial atypical Spitz tumor and malignant melanoma of superficial spreading type arising in association with the superficial atypical Spitz tumor: a distinct form of dysplastic Spitzoid nevomelanocytic proliferation. J Am Acad Dermatol. 2009;60(5):814–823. doi: 10.1016/j.jaad.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 24.Lyon VB. The Spitz nevus: review and update. Clin Plast Surg. 2010;37(1):21–33. doi: 10.1016/j.cps.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Sagebiel RW, Banda PW, Schneider JS, Crutcher WA. Age distribution and histologic patterns of dysplastic nevi. J Am Acad Dermatol. 1985;13(6):975–982. doi: 10.1016/s0190-9622(85)70248-4. [DOI] [PubMed] [Google Scholar]
- 26.Bastian BC, LeBoit PE, Pinkel D. Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol. 2000;157(3):967–972. doi: 10.1016/S0002-9440(10)64609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dijk MC, Bernsen MR, Ruiter DJ. Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and spitzoid melanoma. Am J Surg Pathol. 2005;29(9):1145–1151. doi: 10.1097/01.pas.0000157749.18591.9e. [DOI] [PubMed] [Google Scholar]
- 28.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37(10):1038–1040. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 29.Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardiofaciocutaneous syndrome. Nat Genet. 2006;38(3):294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira JB, Bidére N, Niemela JE, et al. NRAS mutation causes a human auto-immune lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 2007;104 (21):8953–8958. doi: 10.1073/pnas.0702975104. [DOI] [PMC free article] [PubMed] [Google Scholar]