Abstract
Proteasome inhibitors (PIs), namely bortezomib, have become a cornerstone therapy for multiple myeloma (MM), potently reducing tumor burden and inhibiting pathologic bone destruction. In clinical trials, carfilzomib, a next generation epoxyketone-based irreversible PI, has exhibited potent anti-myeloma efficacy and decreased side effects compared with bortezomib. Carfilzomib and its orally bioavailable analog oprozomib, effectively decreased MM cell viability following continual or transient treatment mimicking in vivo pharmacokinetics. Interactions between myeloma cells and the bone marrow (BM) microenvironment augment the number and activity of bone-resorbing osteoclasts (OCs) while inhibiting bone-forming osteoblasts (OBs), resulting in increased tumor growth and osteolytic lesions. At clinically relevant concentrations, carfilzomib and oprozomib directly inhibited OC formation and bone resorption in vitro, while enhancing osteogenic differentiation and matrix mineralization. Accordingly, carfilzomib and oprozomib increased trabecular bone volume, decreased bone resorption and enhanced bone formation in non-tumor bearing mice. Finally, in mouse models of disseminated MM, the epoxyketone-based PIs decreased murine 5TGM1 and human RPMI-8226 tumor burden and prevented bone loss. These data demonstrate that, in addition to anti-myeloma properties, carfilzomib and oprozomib effectively shift the bone microenvironment from a catabolic to an anabolic state and, similar to bortezomib, may decrease skeletal complications of MM.
Keywords: proteasome inhibitors, multiple myeloma, osteoblast, osteoclast, bone lesions
INTRODUCTION
Multiple myeloma (MM), a malignancy of plasma cells that reside within the bone marrow (BM), is associated with the development of osteolytic lesions (70–80% of patients) characterized by increased osteoclast (OC) numbers and resorption and suppressed osteoblast (OB) differentiation and bone formation. The interaction of myeloma cells with stromal and osteoprogenitor cells in the BM leads to the overexpression of multiple OC activating factors including RANKL, MIP1-α, interleukin (IL)-3, osteopontin, IL-6 and vascular endothelial growth factor.1,2 In turn, OCs also support myeloma proliferation and survival by production of growth factors such as IL-6, osteopontin, BAFF and APRIL.1 Conversely, OB formation and activity is significantly reduced by tumoral production of OB inhibitory factors such as DKK-1, sFRP-2, sFRP-3, IL-7 and IL-3, and by direct myeloma and OB cell-to-cell interactions.3
The first generation proteasome inhibitor (PI) bortezomib has proven highly efficacious in treating MM, with greatly improved response rates and overall survival in both newly diagnosed and relapsed/refractory myeloma patients.4,5 Bortezomib, a dipeptide boronic acid,6 primarily inhibits the chymotrypsin-like activity of the 20S proteasome with slowly reversible binding kinetics.7 The therapeutic success of bortezomib relies on pleiotropic effects, which decrease both the growth and survival of myeloma cells and the interactions between myeloma cells and the BM microenvironment (reviewed in Hideshima and Anderson8). Bortezomib treatment has been associated with clinically beneficial effects on myeloma bone disease,9 with reports of increase in bone formation markers and decrease in markers of bone resorption (reviewed in Trepos et al.10). This effect of bortezomib on bone remodeling is not only the consequence of reduced tumor burden, but also due to direct effects on bone cells with the promotion of osteoblastogenesis and reduction of OC numbers reported in both myelomatous and non-myelomatous in vivo models11,12 as well as in numerous in vitro studies.13–18
Although results obtained with bortezomib are encouraging, a substantial proportion of myeloma patients are refractory to this agent or develop drug resistance.19 Peripheral neuropathy is a major and dose-limiting adverse effect of bortezomib treatment,19,20 prompting the development of next-generation PIs with safer toxicity profiles, better tissue distribution and/or oral bioavailability. Within these new PIs, carfilzomib and oprozomib (both from Onyx Pharmaceuticals, San Francisco, CA, USA) are peptide epoxyketones6 with selective and irreversible binding to the proteasome chymotrypsin-like subunit.7 In preclinical studies, carfilzomib exhibits anti-myeloma activity with IC50 values and pleiotropic cellular effects comparable to those of bortezomib.21–23 Furthermore, carfilzomib has been reported to overcome acquired resistance to bortezomib, melphalan and dexamethasone.21 Phase II and III studies are ongoing in patients with relapsed or refractory myeloma with no serious adverse reports of peripheral neuropathy.24,25 Oprozomib (formerly known as ONX 0912 and PR-047) is an orally bioavailable analog of carfilzomib, which has been reported to have anti-tumor activity equivalent to carfilzomib in xenograft models of non-Hodgkin’s lymphoma and colorectal cancer,26 and also to exert anti-MM activity in vitro and in myeloma animal models.27 Its favorable pharmacologic profile and tolerability supports its further clinical development and Phase I clinical trials are underway.28
While epoxyketone-based PIs have been shown to stimulate bone formation29 and inhibit osteoclastogenesis,30 the specific bone effects of carfilzomib and oprozomib are unknown. In this report, we show that both compounds promote OB differentiation and function and inhibit OC formation and resorption equivalently to bortezomib in vitro and in vivo. Both drugs were effective at reducing myeloma burden and osteolytic bone destruction in mice bearing BM-disseminated myeloma. Collectively, our data demonstrate that carfilzomib and oprozomib effectively inhibit myeloma growth and shift the bone microenvironment from a catabolic to an anabolic state, with reduced toxicity24,28 and oral administration (in the case of oprozomib)26,27 being significant advantages to patients.
MATERIALS AND METHODS
Animals
C57Bl/6 and NOD.SCID.IL2Rγ−/− mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). C57Bl/KaLwRij mice were obtained from Dr G Mundy (Vanderbilt University, Nashville, TN, USA). All mice were bred in-house under specific pathogen-free conditions according to guidelines of the Washington University Division of Comparative Medicine. The Animal Ethics Committee approved all experiments.
Drugs
Bortezomib was purchased from Selleckem (Houston, TX, USA). Carfilzomib and oprozomib were supplied by Onyx Pharmaceuticals.
Cell lines
Human myeloma cell lines were obtained from the American Type Culture Collection or other origins31 and modified to express firefly luciferase. The 5TGM1-GFP murine myeloma line was obtained from Dr G Mundy.32
Viability assays
A total of 5 × 104 cells/ml were plated and standard MTT assay (Sigma-Aldrich, St Louis, MO, USA) was performed. For transient dosing experiments, cells were washed twice with phosphate-buffered saline and replaced with drug-free media after 1 h (bortezomib, carfilzomib) or 4 h (oprozomib).
MM.1S-luc co-cultures
Primary human CD138-negative BM stromal cells (BMSCs) from MM patients were plated at 1 × 104 cells/well in 96-well plates for 24 h, serum-starved for 12 h and then MM.1 S-luc cells (1 × 105 cells per well) were added and co-cultured for an additional 48 h. Pre-OCs were generated under osteoclastogenic conditions and 4 × 103 MM.1S-luc cells per well were added, with co-cultures maintained in medium supplemented with 0.5% fetal bovine serum for 5 days. MM.1S-luc viability was assessed by luciferase activity.
In vitro OC differentiation and resorption
Peripheral blood mononuclear cells (PBMCs) from healthy donors were differentiated as in Garcia-Gomez et al.33 Briefly, adherent cells were maintained in osteoclastogenic medium (50 ng/ml RANKL and 25 ng/ml M-CSF (Peprotech, London, UK) for 14 days (pre-OCs) or 21 days (mature OCs). TRAP + (Sigma-Aldrich) multinucleated (≥3 nuclei) OCs were enumerated. To measure resorption, PBMCs were seeded on calcium-coated wells (BD Biosciences, Bedford, MA, USA) in osteoclastogenic medium for 17 days (with 1 μM dexamethasone the first week), and resorption pit area was calculated.
Nuclear factor-κB (NF-κB) translocation and actin ring formation
Pre-OCs received a 3 h pulse of PIs followed by stimulation with 50 ng/ml RANKL for 30 min. Cells were fixed in 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and incubated with a mouse anti-p65 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and a secondary rhodamine-conjugated antibody. Pre-OC F-actin microfilaments were stained using rhodamine-conjugated phalloidin (Invitrogen, Carlsbad, CA, USA).
In vitro OB differentiation, alkaline phosphatase (ALP) activity and mineralization
Primary mesenchymal stem cells (MSCs) from BM aspirates of healthy donors (n =6) and MM patients with (n =6) or without osteolytic bone lesions (n =3) were generated and assayed as described.33 The human MSC line (hMSC-TERT) was a generous gift from Dr D Campana (St Jude Children’s Research Hospital, Memphis, TN, USA). Briefly, the hMSC-TERT and primary MSCs (passage 3) were cultured in osteogenic medium (containing 5 mM β-glycerophosphate, 50 μg/ml ascorbic acid and 80 nM dexamethasone) for 11 (early OBs; ALP activity), 14 (pre-OBs) or 21 days (mature OBs; matrix mineralization). ALP activity was quantified by hydrolysis of p-nitrophenylphosphate into p-nitrophenol (Sigma-Aldrich) and mineralization assessed by alizarin red staining.
Real-time reverse transcription-PCR analysis
TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA, USA) were performed according to manufacturer’s instructions. Assay IDs were: Runx2, Hs01047976_m1, Osterix, Hs00541729_m1, Osteopontin, Hs00959010_m1, Osteocalcin, Hs01587814_g1, DKK-1, Hs00183740_m1, Osteoprotegerin, Hs00900358_m1, RANKL, Hs00243522_m1_ and IRE1α, Hs00176385_m1. Relative quantification of the target gene expression was calculated by the comparative threshold cycle method. Samples were performed in duplicate with GAPDH used for normalization.
Reporter Assay
MC3T3-E1 murine pre-OB cells were transfected with Cignal Finder dual-luciferase reporter constructs (SABiosciences, Valencia, CA, USA) using X-tremeGENE HP reagent (Roche, Indianapolis, IN, USA). Twenty-four hours after transfection, cells were drug treated for 24 h in OptiMEM (Invitrogen) containing 1% fetal bovine serum and assayed using the Dual-Glo Luciferase Assay System (Promega, Madison, WI, USA). As the CMV promoter driving the Renilla reporter construct used for normalization was modulated itself by PIs, firefly luciferase activity is presented as a ratio over the average of vehicle group. The assay was repeated twice with six replicates per condition to partially account for the lack of normalization.
Western blot
Protein isolation and western blot analyses were performed as in Garcia-Gomez et al.33 using antibodies against IRE1α (Cell Signaling Technology, Danvers, MA, USA) and α-tubulin (Calbiochem, Damstadt, Germany).
Gene silencing
hMSC-TERT cells were transfected with either ON-TARGETplus SMARTpool siRNA targeting human IRE1 or ON-TARGETplus non-targeting pool as negative control (Dharmacon, Lafayette, CO, USA), using SAFEctin-STEM (Deliverics, Edinburg, UK) following supplierś instructions. Minimal toxicity of transfection reagent allowed repeated siRNA transfections (three times per week for 3 weeks).
In vivo drug treatment
PIs were administered to mice on the following weekly schedules: bortezomib (1 mg/kg intravenously days 1 and 4); carfilzomib (5 mg/kg for C57Bl/6, 3 mg/kg for KaLwRij, intravenously days 1 and 2); oprozomib (30 mg/kg by oral gavage once daily for 5 consecutive days followed by 2 days of rest). Vehicle mice were administered both oral 1% carboxy-methylcellulose (oprozomib schedule) and intravenous 10% Captisol in 10 mM citrate buffer, pH 3.5 (carfilzomib schedule). In Figure 5f, following 14 days of drug treatment, three doses of 1 mg/kg of RANKL were given intraperitoneally at 24 h intervals as described in Tomimori et al.34 Serum was collected 90 min after the final RANKL injection.
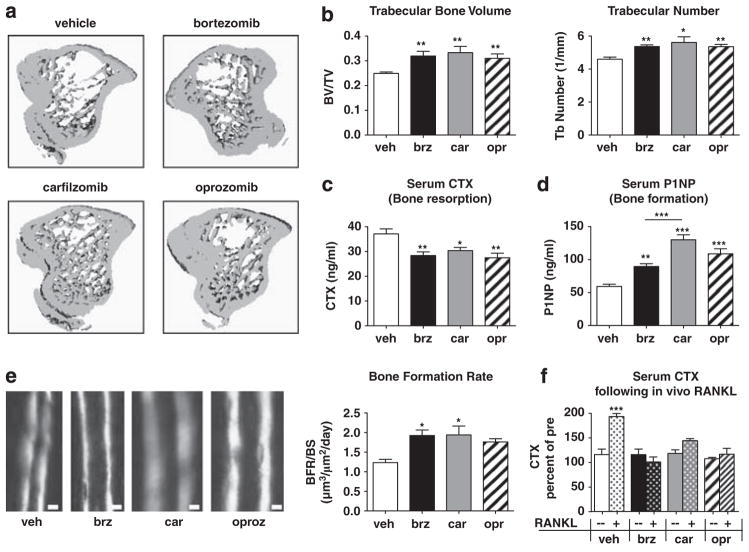
Figure 5.
Epoxyketone-based PIs exert bone anabolic effects on non-tumor bearing mice. C57Bl/6 mice (n =10/group) were treated for 2 weeks with vehicle, bortezomib, carfilzomib or oprozomib on dosing schedules outlined in Materials and Methods. (a, b) MicroCT analyses show that all PIs induced an equivalent increase in trabecular bone volume and number. (c) Bone resorption (serum carboxy-terminal telopeptide collagen crosslinks (serum CTX)) was significantly and equivalently decreased with each PI. (d) OB function (serum N-terminal propeptide of type I procollagen (serum P1NP)) was significantly increased in all PI-treated animals compared with vehicle-treated. Notably, P1NP levels in carfilzomib-treated mice were significantly greater than those of bortezomib-treated mice. (e) Trabecular bone formation rate was measured by double calcein labeling; calcein incorporates into actively mineralizing bone with the distance between labels being proportional to the amount of newly formed bone within the 5-day interlabel period. PI treatment increased the bone formation rate per bone surface (BFR/BS) as assessed by dynamic histomorphometry (Bar =10 μm). (f) PIs inhibited RANKL-induced pathological bone resorption in the absence of tumor. After 2 weeks of PI treatment as above, mice were given three doses of purified RANKL (n =5/drug) or PBS vehicle (n =5) to stimulate OC activity. Serum CTX measured 90 min following the final RANKL dose is expressed as a percent of the same mouse prior to RANKL stimulation. All results are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 versus vehicle or between indicated groups.
Micro-computed tomography (microCT)
Tibial metaphyses were scanned with a microCT-40 system (Scanco Medical, Wayne, PA, USA) as described previously.35 A three-dimensional cubical voxel model of bone was built and calculations were made for relative bone volume per total volume and trabecular number.
Bone turnover markers
Carboxy-terminal telopeptide collagen crosslinks (CTX) and N-terminal propeptide of type I procollagen (P1NP) were measured in fasting serum using ELISA systems (Immunodiagnostic Systems, Scottsdale, AZ, USA).
In vivo bone formation rate
Mice were injected with 20 mg/kg calcein (Sigma-Aldrich) in 2% sodium bicarbonate 7 days and 2 days prior to sacrifice. Bone formation rate (BFR/BS) in femoral trabeculae was calculated as previously described36 using Bioquant Osteo software (Bioquant, Nashville, TN, USA).
Mouse models of BM disseminated MM
A total of 1 × 106 murine 5TGM1-GFP cells were injected intravenously into 8-week-old female KaLwRij mice.32 Clonal tumor expansion was monitored by serum murine IgG2b ELISA (Bethyl Laboratories, Montgomery, TX, USA). Drug treatment was initiated 14 days following tumor injection. At sacrifice, GFP + tumor burden was assessed by flow cytometry. In separate experiments, 2 × 106 human RPMI-8226-luc were injected intravenously into NOD-SCID-IL2Rγ−/− mice and tumor development was monitored by non-invasive bioluminescence imaging with an IVIS 100 system (Caliper, Hopkinton, MA, USA; exposure time 300 s, binning 16, field of view 12, f/stop 1, open filter) following intraperitoneal injection of 150 μg/g D-luciferin (Biosynth, Naperville, IL, USA).37 Serum human Igλ was measured by ELISA (Bethyl Laboratories).
Statistical analyses
Assays were performed at least three times using cells from at least three different individuals and duplicates (reverse transcription-PCR) or triplicates were measured. Statistical comparisons were performed on in vitro experiments (Figures 1 and 4) using the non-parametric Mann–Whitney U test (2 groups) and Kruskal–Wallis with Mann–Whitney U post-hoc test with Bonferroni’s adjustment (≥3 groups); in vivo studies (Figures 5 and 7) used the Student’s t-test (2 groups) or one-way ANOVA with Tukey’s multiple comparison test (≥3 groups): *P<0.05; **P<0.01; ***P<0.001.
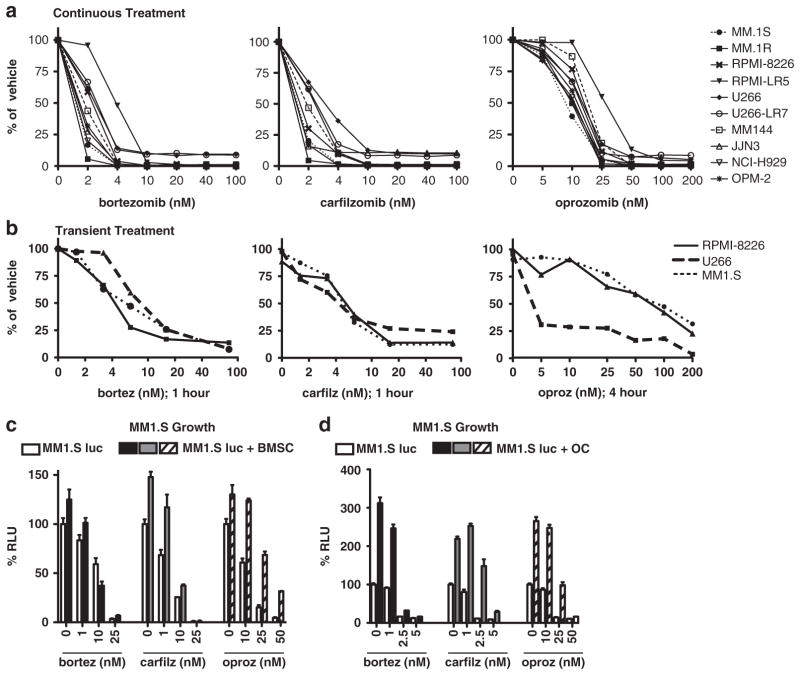
Figure 1.
Continuous or physiologic transient administration of carfilzomib or oprozomib is cytotoxic to human MM cells in vitro. (a) A panel of 10/human MM cell lines were treated with the indicated doses of bortezomib, carfilzomib or oprozomib continuously for 48 h and subjected to MTT assay for viability. (b) MM cell lines were treated with indicated drug doses on day 0 for 1 h (bortezomib, carfilzomib) or 4 h (oprozomib). Cells were then washed and cultured in drug-free media for 48 additional h and viability assessed by MTT assay. (c, d) PIs overcome the proliferative and protective effects of bone microenvironment cells. MM.1S cells labeled with firefly luciferase (MM.1S-luc) were cultured in the presence (filled bars) or absence (open bars) of human (c) CD138− BMSCs or (d) OCs. Cultures were treated with indicated doses of drugs for 48 h (BMSC) or 5 days (OC) and MM cell viability readout by luciferase activity. Results are expressed as mean±s.d. RLU, relative luminescence units.
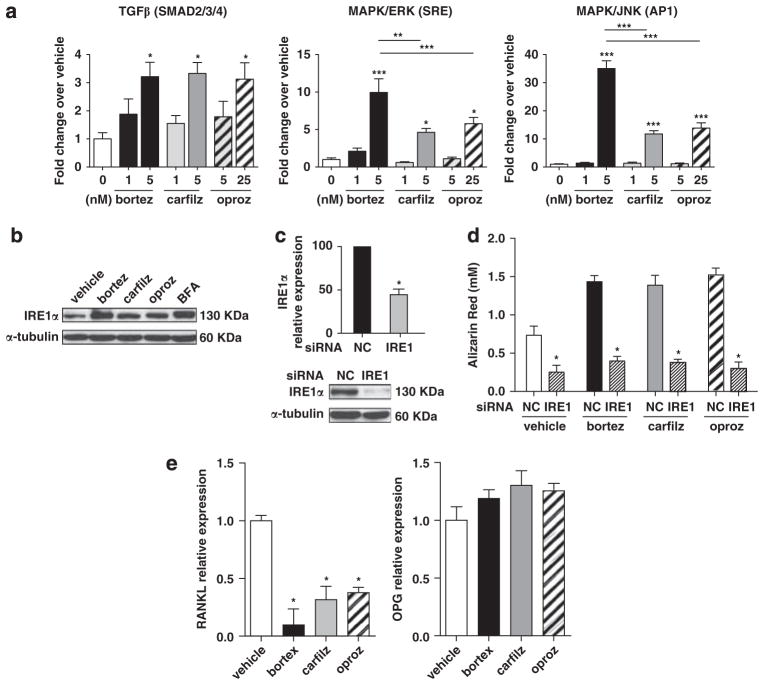
Figure 4.
PI treatment diminishes RANKL expression in OBs and promotes osteogenic differentiation and function through activation of the transforming growth factor β (TGFβ), MAPK and UPR pathways. (a) Reporter assays demonstrated that the activity of the Smad2/3/4, MAPK/ERK (serum response element; SRE) and MAPK/JNK (AP1) pathways were increased in MC3T3-E1 OB-progenitor cells following 24 h of continuous treatment with PIs; results are expressed as mean±s.e.m. (b) Western blot for the IRE1α component of the UPR in hMSC-TERT cells treated with PIs for 24 h (25 nM bortezomib and carfilzomib, 250 nM oprozomib). Brefeldin A (600 ng/ml) was used as a positive control. (c) Expression of IRE1α was reduced at the mRNA and protein levels 48 h after transfection with IRE1 targeting siRNAs. (d) The hMSC-TERT cell line was maintained for 14 days in osteogenic medium with PIs (5 nM bortezomib/carfilzomib or 25 nM oprozomib) and transfected 3 times/week with IRE1 targeting or non-targeting (NC) siRNAs. Mineralization was greatly reduced when IRE1α was silenced even in the presence of PIs. (e) The hMSC-TERT cell line was maintained in osteogenic medium for 21 days in the presence of PIs and expression of RANKL and osteoprotegerin was assessed by real-time reverse transcription-PCR. Maximal effect on the relative expression of RANKL (day 14) or osteoprotegerin (day 7) is shown; results are expressed as mean±s.d. In all panels: *P<0.05, **P<0.01, ***P<0.001 versus vehicle or between indicated groups.
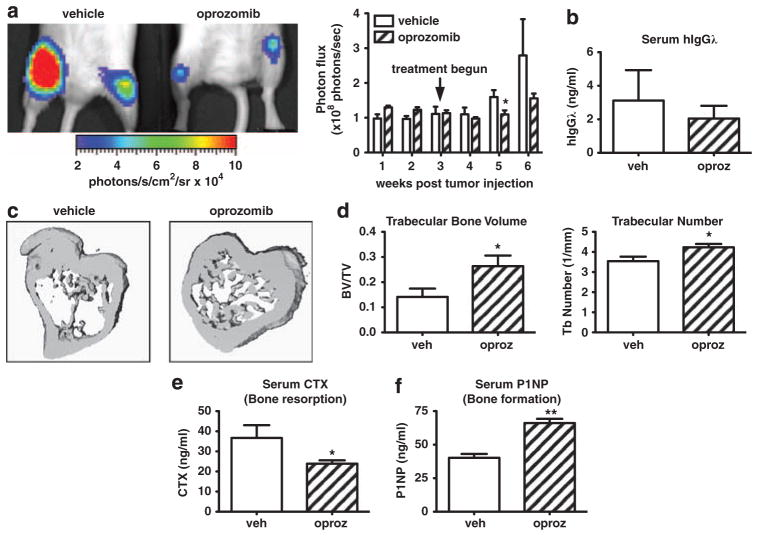
Figure 7.
Oprozomib decreases human MM tumor burden and protects mice from bone destruction. Immunocompromised NOD-SCID-IL2Rγ−/− mice (n =5/group) were intravenously injected with RMPI-8226 human MM cells stably labeled with firefly luciferase. Tumors were allowed to establish for 3 weeks after which mice were randomized into treatment groups. During weeks 3–6 animals were treated with oprozomib (n =5) or vehicle (n =5). (a) Tumor burden was monitored weekly by in vivo bioluminescence imaging. Mice treated with oprozomib had decreased tumor burden compared with those in the vehicle treated group. Representative image of hind limb tumor burden as visualized on week 6. (b) Serum human Igλ (secreted by RPMI-8226 cells) was decreased in oprozomib-treated mice at the time of killing, indicating decreased tumor burden. (c, d) Although tumor-associated bone loss was evident in vehicle-treated mice, trabecular bone was preserved with oprozomib treatment as measured by micro CT. (c) Representative 3D reconstructions. (d) Oprozomib significantly increased trabecular bone volume and number. Oprozomib treatment decreased serum carboxy-terminal telopeptide collagen crosslinks (serum CTX) (e) and increased serum N-terminal propeptide of type I procollagen (serum P1NP) (f). Results are expressed as mean±s.e.m. *P<0.05, **P<0.01.
RESULTS
Continuous or physiologic transient administration of carfilzomib or oprozomib is cytotoxic to human MM cells in vitro Under 48 h of continual drug incubation, carfilzomib and oprozomib exerted a cytotoxic effect on a panel of 10 human MM cell lines similar to bortezomib. In agreement with previous reports, the IC50 was approximately 2 nM for bortezomib, 3 nM for carfilzomib22 and 25 nM for oprozomib27 (Figure 1a). However, pharmacokinetic data indicate that in vivo exposure to drug is approximately 4 h following oral delivery of oprozomib28 and approximately 1 h with intravenous administration of carfilzomib or bortezomib.22,38 To more accurately replicate this physiological situation in vitro, cells were transiently treated with oprozomib for 4 h and with carfilzomib or bortezomib for 1 h followed by an additional 48 h culture in drug-free media. Myeloma cell lines remained susceptible to proteasome inhibition under short treatment conditions (Figure 1b), although increased doses were required to achieve similar efficacy (8 nM bortezomib, 6 nM carfilzomib and 50 nM oprozomib). Effective transient doses were still well below the maximum serum levels (Cmax) attained in patients (bortezomib: 0.162 μM (1.3 mg/m2 intravenous)39; carfilzomib: 0.95 μM (20 mg/m2 intravenous);40 oprozomib: 3.8 μM (30 mg per os)28). The decrease in MM viability by carfilzomib and oprozomib was attributed to both inhibition of proliferation and apoptosis induction (data not shown), consistent with previous reports examining these PIs.21,27
Cells within the BM microenvironment, specifically BMSCs and OCs, produce factors that support the growth and survival of myeloma cells1 while protecting them from chemotherapy-induced apoptosis.41 Under experimental settings resembling the protective environment of bone, all PIs remained effective at inhibiting MM.1S survival, although co-culture with human MM BMSCs (Figure 1c) or OCs (Figure 1d) required approximately twofold dose increases to reach similar cyto-toxic efficacy (Figure 1c). Notably, the required doses remained well within the range achievable in vivo.28,39,40 In summary, similarly to bortezomib, carfilzomib and oprozomib exert potent cytotoxic effects on myeloma cells under continuous and physiological dosing conditions, even in the presence of protective BMSCs and OCs.
Oprozomib and carfilzomib inhibit OC differentiation and function in vitro
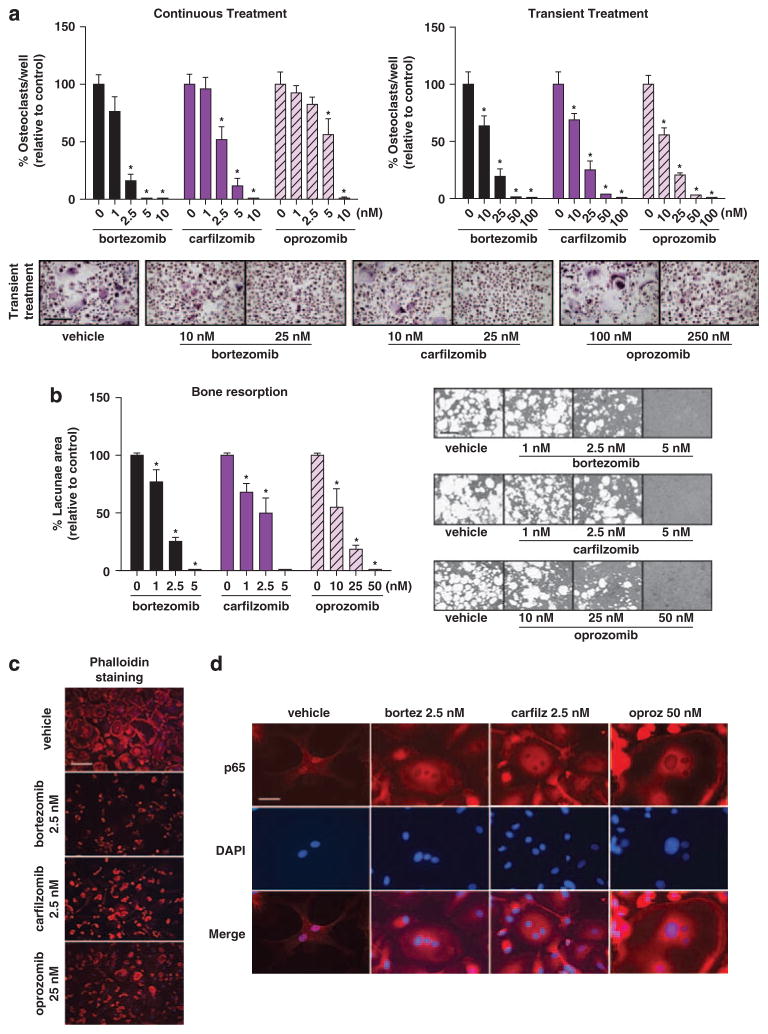
Similar to bortezomib, carfilzomib and oprozomib strongly inhibited the in vitro differentiation and formation of mature, multinucleated OCs of human (Figure 2a) and murine (Supplementary Figure 1A) origin. Under continuous treatment, osteoclastogenesis was abrogated by 50% at concentrations similar to doses exerting myeloma cell cytotoxicity (bortezomib =1.21 nM, carfilzomib = 2.43 nM; oprozomib = 25.88 nM). Importantly, this inhibitory effect was evident under both continuous and physiological transient drug treatment conditions, though transient treatment required approximately fivefold higher doses. Notably, oprozomib and carfilzomib did not exert cytotoxic effects on OCs, as cell densities in cultures for IC50 doses were not markedly reduced (Figure 2a and Supplementary Figure 1A). Murine macrophages (OC progenitors) were also resistant to the cytotoxic effects of PIs, with IC50’s greater than 1 μM, approximately 50–100-fold higher than doses required to kill myeloma cells (Supplementary Figures 1B and C). Thus, under physiological conditions and at concentrations cytotoxic to myeloma cells, carfilzomib and oprozomib inhibited OC differentiation without exerting cytotoxic effects on their precursor cells.
Figure 2.
Oprozomib and carfilzomib inhibit OC differentiation and function in vitro. (a) Human OCs were generated from PBMCs cultured in osteoclastogenic medium for 21 days in the presence or absence of indicated concentrations of the PIs (continuous treatment, left panel); alternatively, PIs were present only for the initial 4 h of differentiation (transient treatment, right panel). OCs were identified as multinucleated (≥3 nuclei) TRAP + cells. Representative micrographs of differentiated OCs after transient treatment are shown. (b) To assess inhibition of mineralized matrix resorption, PBMCs were seeded on calcium-coated slides and maintained in osteoclastogenic medium for 17 days with or without indicated concentrations of the drugs. Graphs represent mean values of samples from OCs derived from three healthy donors±s.d. *P<0.05 between treated cultures and vehicle control. Bar =50 μm. (c) PIs disrupted the integrity of the actin ring in multinucleated pre-OCs (14 days in osteoclastogenic medium under continuous PI-treatment). Actin =phalloidin–rhodamine, DAPI =nuclei; representative micrographs are reported. Bar =50 μm. (d) Pre-OCs were treated with indicated concentrations of PIs for 3 h prior to stimulation with RANKL for 30 min. In absence of PIs (vehicle), RANKL induces p65 translocation to the nucleus, whereas in PI-treated cells the p65 subunit of NF-κB is retained in the cytoplasm. p65 subunit =visualized in red, DAPI =nuclei; representative micrographs for each PI are shown (Bar =12.5 μm).
To test the ability of the new PIs to inhibit osteoclastic bone resorption, OC cultures from human PBMCs were established on calcium substrate-coated slides. Similarly to bortezomib,17,18,42 a dose-dependent reduction in resorption pit area was observed following continuous incubation with carfilzomib or oprozomib (Figure 2b). The concentration of each drug required to inhibit resorption was less than that required to inhibit OC differentiation, most notably for oprozomib (Figures 2a and b, left), suggesting that these PIs may independently affect OC resorptive function. Both preservation of the F-actin ring and expression of the αVβ3 integrin are necessary for maintenance of OC structural polarization, adhesion to bone matrix and formation of a sealing zone for effective bone resorption.43,44 Treatment with all PIs resulted in a partial or complete disruption of the F-actin ring (Figure 2c) and reduced expression of αVβ3 integrin (Supplementary Table 1). OC-mediated resorption also requires functional signaling through a complex pathway involving mitogen-activated protein kinase (MAPK) and NF-κB45. PI treatment of human pre-OCs prevented RANKL-induced NF-κB activation, with the p65 subunit being retained in the cytoplasm (Figure 2d). This effect is consistent with impaired proteasomal degradation of I-κB, suggesting that, similar to bortezomib,17,18 carfilzomib and oprozomib-mediated inhibition of ex vivo OC activity may partially act through disruption of RANKL-induced NF-κB signaling. Together, these data demonstrate that epoxyketone-based PIs are capable of inhibiting OC resorptive function through multiple mechanisms.
Carfilzomib and oprozomib promote osteogenic differentiation and mineralization in vitro
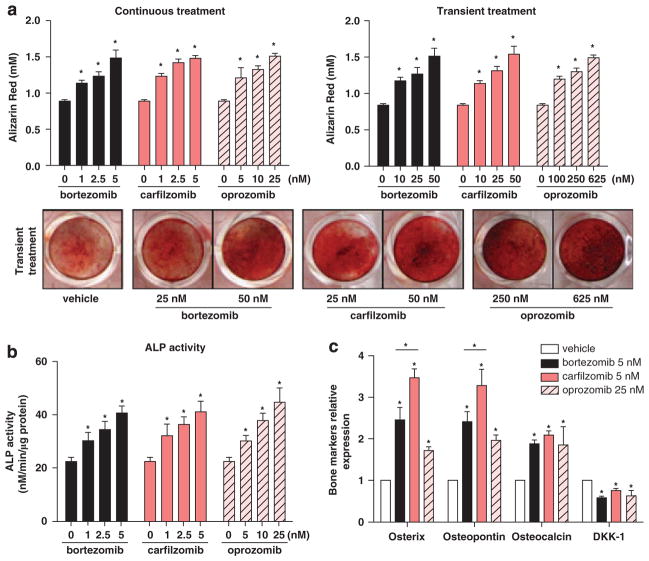
While the inhibition of pathological bone resorption through anti-catabolic agents is inarguably crucial for the control of myeloma bone disease, anabolic treatments capable of stimulating new bone formation are important for reversing damage. As several PIs including bortezomib13–16 and epoxomicin29 are recognized to enhance OB formation and function, we tested whether the same held true for carfilzomib and oprozomib. Murine mesenchymal stem cells (MSC), the OB progenitors, were resistant to cytotoxic effects (Supplementary Figure 2A) and alterations in proliferation (Supplementary Figure 2B) at clinically relevant doses of PIs. When differentiating primary human MM patient MSCs (Figure 3a) or murine MSCs (Supplementary Figure 2C) into OBs in vitro, carfilzomib and oprozomib increased matrix mineralization and calcium deposition under both continuous (days 0–21) and transient (1–4 h dose on day 0) dosing conditions. Furthermore, both drugs dose-dependently increased ALP activity, a surrogate marker of early osteoblastic activation, in human myeloma patient OBs (Figure 3b). Increased ALP activity and mineralization were also observed when treating MSCs from healthy donors (data not shown), suggesting that such effects are not isolated to myelomatous stroma. Likewise, markers of OB differentiation were significantly elevated in PI-treated OBs derived from the hMSC-TERT cell line compared with vehicle-treated controls (Figure 3c). Of note, at equimolar concentrations, carfilzomib induced a significantly higher expression of Osterix and osteo-pontin as compared with bortezomib. In addition, all PIs induced modest but significant reductions in mRNA levels of the OB inhibitory protein Dkk-1 in pre-OBs (Figure 3c).
Figure 3.
PIs promote osteogenic differentiation and mineralization in vitro. (a) Primary MSCs from MM patients (6/9 with osteolytic lesions) were cultured in osteogenic medium either in the continuous presence of PIs (left panel) or transiently treated on day 0 for 4 h (right panel). Mineralization was analyzed by alizarin red staining and subsequent dye quantification in OBs differentiated for 21 days; representative images of alizarin red staining following transient treatment are shown. Results are expressed as the mean±s.d. (b) In the presence of PIs and at day 11 of osteogenic differentiation, ALP activity was measured in OBs derived from MSCs from five MM patients (3/5 with osteolytic lesions). Graphs illustrate mean values±s.e.m. (c) Total RNA from the hMSC-TERT cell line was isolated at day 14 of differentiation in the presence of PIs at indicated doses, and expression of osteogenic-related markers (Osterix, osteopontin, osteocalcin) and DKK-1 were evaluated using real-time reverse transcription-PCR. Expression levels for each gene were normalized with respect to GAPDH expression and referred to vehicle control. Data are represented as the mean±s.d. from three different experiments. In all panels: *P<0.05, versus vehicle control or between indicated groups.
In reporter assay systems, 24 h treatment of MC3T3-E1 osteoprogenitor cells with PIs enhanced the activity of Smad2/3/4, serum response element (SRE) and AP1 transcription factors (Figure 4a). Although transforming growth factor β signaling exerts inhibitory effects in mature OBs,46 activity of Smad2/3 together with Smad4 promotes early osteoprogenitor commitment and differentiation by inducing OB-specific gene transcription.47 Likewise, MAPK signaling cascades through ERK (extracellular-signal-regulated kinase) (SRE) and JNK (c-Jun N-terminal kinase) (AP1) have been reported to upregulate Runx2 and Osterix, supporting osteogenic differentiation.48,49 Conversely, the activation of the unfolded protein response (UPR) is of particular importance in cells specialized to secrete proteins, such as plasma cells, endocrine cells and OBs. The IRE1-XBP1 pathway has been recently shown to promote OB differentiation by driving transcription of Osterix.50 Treatment of hMSC-TERT cells with carfilzomib or oprozomib resulted in upregulation of the IRE1α component of the UPR (Figure 4b). IRE1α inhibition by siRNA significantly diminished PI-enhanced mineralization (Figures 4c and d), underscoring the crucial role of IRE1α in the promotion of OB activity by PIs. In MM, osteoclastogenesis and OC activity is partially modulated by OB expression of membrane-bound RANKL and secreted osteoprote-gerin.1 The presence of PIs during OB differentiation inhibited RANKL expression, yet only a modest trend toward increase of osteoprotegerin mRNA levels was observed (Figure 4e). In summary, PIs directly stimulated the transforming growth factor β and MAPK pathways and increased the activity of the UPR resulting in enhanced OB differentiation and matrix mineralization, while indirectly hindering OC stimulation through decreased OB expression of RANKL.
Epoxyketone-based PIs exert bone anabolic effects on non-tumor bearing mice
In vitro evidence suggests that PIs exert cell-autonomous effects on both OCs and OBs. To examine their effects on non-myelomatous bone, PIs were administered to non-tumor bearing immunocompetent C57Bl/6 mice for two weeks. Similar to bortezomib, treatment with carfilzomib or oprozomib increased trabecular bone parameters (Figures 5a and b). All three PIs comparably inhibited OC function as measured by decreased serum levels of collagen breakdown products (carboxy-terminal telopeptide collagen crosslinks) resulting from bone resorption (Figure 5c). Furthermore, all drugs significantly increased OB activity as measured by increased serum levels of N-terminal propeptide of type I procollagen, a marker of bone formation, compared with controls (Figure 5d). Notably, carfilzomib exerted an increase in N-terminal propeptide of type I procollagen that was significantly greater than that obtained with bortezomib. In agreement, double calcein labeling demonstrated that PIs increased bone formation rate (Figure 5e). These data demonstrate that the epoxyketone-based PIs carfilzomib and oprozomib enhance bone volume in healthy mice through both anabolic and anti-catabolic properties that are equipotent to or even superior to that of bortezomib.
Following treatment with anti-cancer agents, it is difficult to discern whether protection from tumor-associated bone loss is due to direct effects on bone cells or indirectly to a decrease in overall tumor burden. To examine the efficacy of PIs in decreasing pathological OC activation without the confounding factor of tumor burden, in vivo injection of RANKL (three doses over 50 h34) was used to mimic OC stimulation by myeloma cell-derived RANKL. We found that all PIs prevented a RANKL-induced increase in carboxy-terminal telopeptide collagen crosslinks (Figure 5f), demonstrating that this class of compounds exerts direct effects on the activity of pathologically activated OCs.
Carfilzomib and oprozomib decrease MM tumor burden and protect mice from bone destruction
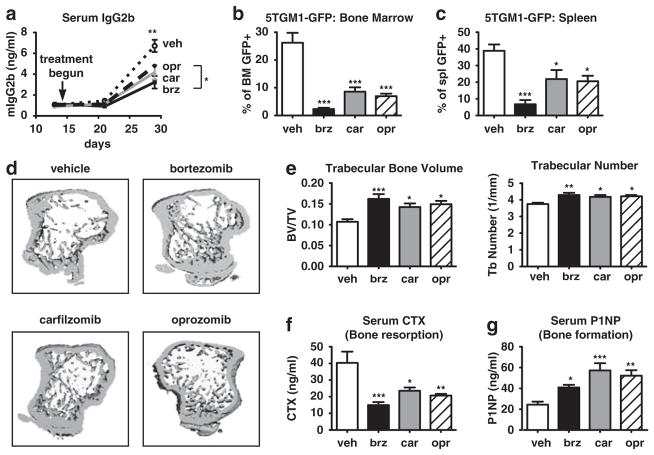
To examine the combined anti-tumor and bone-preserving effects of carfilzomib and oprozomib for therapeutic treatment of established myeloma, we utilized two in vivo mouse models. Intravenous injection of 5TGM1-GFP murine myeloma cells into immunocompetent, syngeneic C57Bl/KaLwRij mice yields disseminated tumors with significant bone destruction within 28 days.51,52 5TGM1 tumors were established for 14 days after which bortezomib, carfilzomib, or oprozomib were administered on schedules correlating with each drug’s clinical dosing (see Materials and Methods). All PIs significantly decreased tumor burden as measured by serum levels of the clonotypic antibody IgG2b (Figure 6a) or by percentage of BM or spleen comprised of GFP-expressing tumor cells (Figures 6b and c). Protection from tumor-induced bone loss was evident by microCT in all PI-treated groups (Figures 6d and e), with serum markers of bone turnover showing significant anti-resorptive (Figure 6f) and bone anabolic (Figure 6g) effects. Notably, although differences within PIs were not statistically significant, a trend toward increased N-terminal propeptide of type-I procollagen activity with carfilzomib and oprozomib versus bortezomib was observed.
Figure 6.
PIs decrease myeloma burden and associated bone destruction in an immunocompetent murine model. 5TGM1-GFP murine myeloma cells were injected into syngeneic C57Bl/KaLwRij mice. After 14 days, mice were randomized into PI treatment groups (n≥7/group) and dosed for an additional 2 weeks. All PIs decreased tumor burden as measured by (a) levels of serum IgG2b (clonotype of 5TGM1 cells) and (b, c) percent of GFP + tumor cells within the BM or spleen upon sacrifice at day 28. (d, e) MicroCT analysis demonstrated that treatment with PIs protected animals from myeloma-induced loss of trabecular bone volume and number. (f) Serum carboxy-terminal telopeptide collagen crosslinks (serum CTX) was significantly decreased and (g) serum N-terminal propeptide of type I procollagen (serum P1NP) was significantly increased in all PI-treated animals compared with vehicle-treated animals. All results are expressed as mean±s.e.m. *P<0.05, **P<0.01, ***P<0.001 versus vehicle or between indicated groups.
Finally, the efficacy of oprozomib was examined in NOD-SCID-IL2Rγ−/− mice bearing established human RPMI-8226-luc myeloma cells. Oprozomib treatment decreased tumor burden as measured by bioluminescent imaging (Figure 7a) and serum levels of human Igλ secreted by RPMI-8226-luc cells (Figure 7b). MicroCT analysis demonstrated marked tumor-associated bone loss in vehicle-treated mice. By contrast, oprozomib-treated mice presented significant increases in trabecular bone parameters (Figures 7c and d). Serum markers of bone turnover showed that oprozomib inhibited bone resorption (Figure 7e) while enhancing bone formation (Figure 7f). In summary, these data demonstrate that orally administered oprozomib exerts in vivo anti-myeloma activity along with bone anti-catabolic and anabolic effects in mice bearing human MM.
DISCUSSION
In this report, we have demonstrated that the next generation epoxyketone-based PIs carfilzomib and oprozomib exerted potent anti-myeloma growth effects, inhibited osteoclastogenesis, and resorption, and enhanced OB formation and function in vitro under clinically relevant doses and exposure periods. Consistently, these PIs decreased bone resorption and increased bone formation in non-tumor bearing mice while decreasing tumor growth and pathologic bone loss in models of BM-disseminated myeloma. Notably, in both in vitro and in vivo models, carfilzomib consistently appeared to enhance OB activity to a greater extent than bortezomib (Figures 3c, 5d and 6f), suggesting that this compound may offer an additional benefit to patients by enhancing the extent to which lost bone can be rebuilt.
These data also demonstrate that proteasome inhibition with the orally bioavailable compound, oprozomib, has similar efficacy to intravenously delivered PIs. Although effects were achieved at higher concentrations, pharmacokinetic data demonstrate that concentrations well surpassing these doses are readily obtained following oral dosing of oprozomib.28,40 Importantly, peptide epoxyketones such as carfilzomib and oprozomib, specifically inhibit N-terminal threonine active proteasome subunits in contrast to dipeptide boronates that can also inhibit serine proteases. This difference may account for the favorable toxicity profiles and relatively low rates of peripheral neuropathy associated with epoxyketone PIs.24,53 This could permit more prolonged and intense dosing regimens, potentially increasing the efficacy of the drug. Bortezomib is dosed on a day 1, day 4 schedule, allowing for full recovery of proteasome activity between doses.38 In ongoing clinical trials, carfilzomib is dosed intravenously on two subsequent days; experimental evidence suggests that daily dosing is similarly well tolerated, although the necessity for intravenous delivery limits this use in practice.22,24,25 In current trials, oprozomib is dosed orally on five continuous days. While we demonstrate that single in vitro pulse treatments of oprozomib effectively exert anti-tumor, anti-OC, and pro-OB effects, the QDx5 repeated dosing schedule increases the overall period during which proteasome activity is inhibited. Therefore, the continuous treatment of cell cultures in vitro may more closely mimic the in vivo activity of oprozomib under this regimen. Furthermore, we have observed these effects below the maximal tolerated dose of oprozomib, reported to exceed 50 mg/kg.27
The BM microenvironment supports MM cell growth and certain drugs are unable to overcome this protection.54 In addition to their direct effects on tumor cell survival, PIs also exert indirect anti-tumor effects by rendering the host microenvironment less hospitable. In agreement with data in Waldenstrom’s macroglobulinemia,55 we found that both carfilzomib and oprozomib remained cytotoxic to MM cells co-cultured with BMSCs or OCs. Furthermore, tumor-produced factors can unbalance normal bone turnover resulting in pathological osteolysis, which in turn further stimulates tumor growth.45 Shifting the bone microenvironment to an anabolic or bone-building state, would then negatively impact myeloma progression.3 Comparably to bortezomib and other PIs, our data demonstrate that oprozomib and carfilzomib exert direct effects on OCs in part through disruption of RANKL-induced NF-κB signaling,17,18,30,56 together with reduced expression of integrin αVβ3 and F-actin ring disruption.30,42 Carfilzomib and oprozomib also modulate OB differentiation and function in vitro similarly to bortezomib,14–16 augmenting bone formation marker expression and increasing ALP activity and bone nodule formation. We have identified the UPR as a novel pathway impacted by PIs that results in enhanced osteoblastogenesis. This is of particular interest as induction of a pro-apoptotic UPR has been shown to be a mechanism by which PIs induce cytoxicity in myeloma cells.57,58 Thus, agents inducing the UPR may prove beneficial in myeloma owing to both direct anti-tumor59 and OB-stimulatory effects, similar to PIs. In differentiating OBs, bortezomib,42 carfilzomib and oprozomib also reduced RANKL expression, therefore diminishing their OC stimulating ability. Other groups have reported that oprozomib may block migration of MM to the bone and decrease angiogenesis.27 Of note, epoxyketone-based PIs also modulated OC and OB activity in non-tumor bearing mice, suggesting that they may be equally effective as an adjuvant therapy in other pathologic bone diseases, including rheumatoid arthritis and osteoporosis.
In summary, the next generation PIs carfilzomib and oprozomib are effective at decreasing both myeloma growth and myeloma-associated bone disease by: (i) direct killing of myeloma cells; (ii) inhibition of OC differentiation and resorption; and (iii) enhancement of OB formation and function. As these drugs progress through clinical trials, prospective studies of bone turnover markers, bone mineral density and documentation of skeletal events would be of particular value to determine whether these new PIs obtain meaningful combined benefits on myeloma and associated bone disease.
Supplementary Material
Acknowledgments
We are grateful to the Washington University MM/MGUS Research Program and Tissue Bank and to Lindsay Goddard, Montserrat Martín, Isabel Isidro, Teresa Prieto and Almudena Martín for their excellent technical work. This research was supported by grants from the National Institutes of Health (T32CA113275:MAH; P01CA100730:KNW; P50CA94056:DP-W), the St Louis Men’s Group Against Cancer (KNW), the Holway Myeloma Fund (KNW), the Spanish MICINN-ISCIII (PI081825), the Fundación de Investigación Médica Mutua Madrileña (AP27262008), the Centro en Red de Medicina Regenerativa y Terapia Celular de Castilla y León, the Spanish Myeloma Network Program (RD06/0020/0006 and RD06/0020/0041) and Spanish FIS (PS09/01897). MicroCT services were provided by the WU musculoskeletal core (P30AR057235).
Footnotes
CONFLICT OF INTEREST
CJK is an employee of Onyx Pharmaceuticals. All other authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Leukemia website (http://www.nature.com/leu)
References
- 1.Basak GW, Srivastava AS, Malhotra R, Carrier E. Multiple myeloma bone marrow niche. Curr Pharm Biotechnol. 2009;10:345–346. doi: 10.2174/138920109787847493. [DOI] [PubMed] [Google Scholar]
- 2.Esteve FR, Roodman GD. Pathophysiology of myeloma bone disease. Best Pract Res Clin Haematol. 2007;20:613–624. doi: 10.1016/j.beha.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Yaccoby S. Osteoblastogenesis and tumor growth in myeloma. Leuk Lymphoma. 2010;51:213–220. doi: 10.3109/10428190903503438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle RA, Rajkumar SV. Multiple myeloma. Blood. 2008;111:2962–2972. doi: 10.1182/blood-2007-10-078022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bettignies G, Coux O. Proteasome inhibitors: Dozens of molecules and still counting. Biochimie. 2010;92:1530–1545. doi: 10.1016/j.biochi.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Dick LR, Fleming PE. Building on bortezomib: s-generation proteasome inhibitors as anti-cancer therapy. Drug Discov Today. 2010;15:243–249. doi: 10.1016/j.drudis.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Hideshima T, Anderson KC. Preclinical studies of novel targeted therapies. Hematol Oncol Clin North Am. 2007;21:1071–1091. viii–ix. doi: 10.1016/j.hoc.2007.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terpos E, Sezer O, Croucher P, Dimopoulos MA. Myeloma bone disease and proteasome inhibition therapies. Blood. 2007;110:1098–1104. doi: 10.1182/blood-2007-03-067710. [DOI] [PubMed] [Google Scholar]
- 10.Terpos E, Dimopoulos MA, Sezer O, Roodman D, Abildgaard N, Vescio R, et al. The use of biochemical markers of bone remodeling in multiple myeloma: a report of the International Myeloma Working Group. Leukemia. 2010;24:1700–1712. doi: 10.1038/leu.2010.173. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee S, Raje N, Schoonmaker JA, Liu JC, Hideshima T, Wein MN, et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J Clin Invest. 2008;118:491–504. doi: 10.1172/JCI33102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pennisi A, Li X, Ling W, Khan S, Zangari M, Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am J Hematol. 2009;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oyajobi BO, Garrett IR, Gupta A, Flores A, Esparza J, Munoz S, et al. Stimulation of new bone formation by the proteasome inhibitor, bortezomib: implications for myeloma bone disease. Br J Haematol. 2007;139:434–438. doi: 10.1111/j.1365-2141.2007.06829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuliani N, Morandi F, Tagliaferri S, Lazzaretti M, Bonomini S, Crugnola M, et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 15.Qiang YW, Hu B, Chen Y, Zhong Y, Shi B, Barlogie B, et al. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Matteo M, Brunetti AE, Maiorano E, Cafforio P, Dammacco F, Silvestris F. Constitutive down-regulation of Osterix in osteoblasts from myeloma patients: in vitro effect of Bortezomib and Lenalidomide. Leuk Res. 2010;34:243–249. doi: 10.1016/j.leukres.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 17.von Metzler I, Krebbel H, Hecht M, Manz RA, Fleissner C, Mieth M, et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 18.Boissy P, Andersen TL, Lund T, Kupisiewicz K, Plesner T, Delaisse JM. Pulse treatment with the proteasome inhibitor bortezomib inhibits osteoclast resorptive activity in clinically relevant conditions. Leuk Res. 2008;32:1661–1668. doi: 10.1016/j.leukres.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14:1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 21.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood. 2007;110:3281–3290. doi: 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demo SD, Kirk CJ, Aujay MA, Buchholz TJ, Dajee M, Ho MN, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. doi: 10.1158/0008-5472.CAN-06-4086. [DOI] [PubMed] [Google Scholar]
- 23.Parlati F, Lee SJ, Aujay M, Suzuki E, Levitsky K, Lorens JB, et al. Carfilzomib can induce tumor cell death through selective inhibition of the chymotrypsin-like activity of the proteasome. Blood. 2009;114:3439–3447. doi: 10.1182/blood-2009-05-223677. [DOI] [PubMed] [Google Scholar]
- 24.Singhal SB, Siegel DSd, Martin T, Vij R, Wang M, Jakubowiak AJ, et al. Pooled safety analysis from phase (Ph) 1 and 2 studies of carfilzomib (CFZ) in patients with relapsed and/or refractory multiple myeloma (MM) Blood. 2010;116 abstract 1954. [Google Scholar]
- 25.Vij R, Wang M, Kaufman JL, Lonial S, Jakubowiak AJ, Stewart AK, et al. An open-label, single-arm, phase 2 (PX-171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. Blood. 2012 May; doi: 10.1182/blood-2012-03-414359. e-pub ahead of print 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou HJ, Aujay MA, Bennett MK, Dajee M, Demo SD, Fang Y, et al. Design and synthesis of an orally bioavailable and selective peptide epoxyketone proteasome inhibitor (PR-047) J Med Chem. 2009;52:3028–3038. doi: 10.1021/jm801329v. [DOI] [PubMed] [Google Scholar]
- 27.Chauhan D, Singh AV, Aujay M, Kirk CJ, Bandi M, Ciccarelli B, et al. A novel orally active proteasome inhibitor ONX 0912 triggers in vitro and in vivo cytotoxicity in multiple myeloma. Blood. 2011;116:4906–4915. doi: 10.1182/blood-2010-04-276626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos KP, Mendelson DS, Tolcher AW, Patnaik A, Burris HA, Rasco DW, et al. A phase I, open-label, dose-escalation study of the novel oral proteasome inhibitor (PI) ONX 0912 in patients with advanced refractory or recurrent solid tumors. J Clin Oncol. 2011;29 abstract 3075. [Google Scholar]
- 29.Garrett IR, Chen D, Gutierrez G, Zhao M, Escobedo A, Rossini G, et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J Clin Invest. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ang E, Pavlos NJ, Rea SL, Qi M, Chai T, Walsh JP, et al. Proteasome inhibitors impair RANKL-induced NF-kappaB activity in osteoclast-like cells via disruption of p62, TRAF6, CYLD, and IkappaBalpha signaling cascades. J Cell Physiol. 2009;220:450–459. doi: 10.1002/jcp.21787. [DOI] [PubMed] [Google Scholar]
- 31.Carvajal-Vergara X, Tabera S, Montero JC, Esparis-Ogando A, Lopez-Perez R, Mateo G, et al. Multifunctional role of Erk5 in multiple myeloma. Blood. 2005;105:4492–4499. doi: 10.1182/blood-2004-08-2985. [DOI] [PubMed] [Google Scholar]
- 32.Garrett IR, Dallas S, Radl J, Mundy GR. A murine model of human myeloma bone disease. Bone. 1997;20:515–520. doi: 10.1016/s8756-3282(97)00056-2. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Gomez A, Ocio EM, Crusoe E, Santamaria C, Hernandez-Campo P, Blanco JF, et al. Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One. 2012;7:e34914. doi: 10.1371/journal.pone.0034914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomimori Y, Mori K, Koide M, Nakamichi Y, Ninomiya T, Udagawa N, et al. Evaluation of pharmaceuticals with a novel 50-hour animal model of bone loss. J Bone Miner Res. 2009;24:1194–1205. doi: 10.1359/jbmr.090217. [DOI] [PubMed] [Google Scholar]
- 35.Lane NE, Yao W, Nakamua MC, Humphrey MB, Kimmel D, Huang X, et al. Mice lacking the integrin beta5 subunit have accelerated osteoclast maturation and increased activity in the estrogen-deficient state. J Bone Miner Res. 2005;20:58–66. doi: 10.1359/JBMR.041017. [DOI] [PubMed] [Google Scholar]
- 36.Hapidin H, Othman F, Soelaiman IN, Shuid AN, Luke DA, Mohamed N. Negative effects of nicotine on bone-resorbing cytokines and bone histomorphometric parameters in male rats. J Bone Miner Metab. 2007;25:93–98. doi: 10.1007/s00774-006-0733-9. [DOI] [PubMed] [Google Scholar]
- 37.Gross S, Piwnica-Worms D. Real-time imaging of ligand-induced IKK activation in intact cells and in living mice. Nat Methods. 2005;2:607–614. doi: 10.1038/nmeth779. [DOI] [PubMed] [Google Scholar]
- 38.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22:2108–2121. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 39.Moreau P, Coiteux V, Hulin C, Leleu X, van de Velde H, Acharya M, et al. Prospective comparison of subcutaneous versus intravenous administration of bortezomib in patients with multiple myeloma. Haematologica. 2008;93:1908–1911. doi: 10.3324/haematol.13285. [DOI] [PubMed] [Google Scholar]
- 40.O’Connor OA, Stewart AK, Vallone M, Molineaux CJ, Kunkel LA, Gerecitano JF, et al. A phase 1 dose escalation study of the safety and pharmacokinetics of the novel proteasome inhibitor carfilzomib (PR-171) in patients with hematologic malignancies. Clin Cancer Res. 2009;15:7085–7091. doi: 10.1158/1078-0432.CCR-09-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, et al. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–2491. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 42.Breitkreutz I, Raab MS, Vallet S, Hideshima T, Raje N, Mitsiades C, et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia. 2008;22:1925–1932. doi: 10.1038/leu.2008.174. [DOI] [PubMed] [Google Scholar]
- 43.Vaananen HK, Laitala-Leinonen T. Osteoclast lineage and function. Arch Biochem Biophys. 2008;473:132–138. doi: 10.1016/j.abb.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura I, Duong le T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25:337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 45.Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11:411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto T, Abe M. TGF-beta-related mechanisms of bone destruction in multiple myeloma. Bone. 2011;48:129–134. doi: 10.1016/j.bone.2010.05.036. [DOI] [PubMed] [Google Scholar]
- 47.Chen G, Deng C, LiLi YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8:272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi YH, Gu YM, Oh JW, Lee KY. Osterix is regulated by Erk1/2 during osteoblast differentiation. Biochem Biophys Res Commun. 2011;415:472–478. doi: 10.1016/j.bbrc.2011.10.097. [DOI] [PubMed] [Google Scholar]
- 49.Lee KS, Hong SH, Bae SC. Both the Smad and p38 MAPK pathways play a crucial role in Runx2 expression following induction by transforming growth factor-beta and bone morphogenetic protein. Oncogene. 2002;21:7156–7163. doi: 10.1038/sj.onc.1205937. [DOI] [PubMed] [Google Scholar]
- 50.Tohmonda T, Miyauchi Y, Ghosh R, Yoda M, Uchikawa S, Takito J, et al. The IRE1alpha-XBP1 pathway is essential for osteoblast differentiation through promoting transcription of Osterix. EMBO Rep. 2011;12:451–457. doi: 10.1038/embor.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dallas SL, Garrett IR, Oyajobi BO, Dallas MR, Boyce BF, Bauss F, et al. Ibandronate reduces osteolytic lesions but not tumor burden in a murine model of myeloma bone disease. Blood. 1999;93:1697–1706. [PubMed] [Google Scholar]
- 52.Edwards CM, Lwin ST, Fowler JA, Oyajobi BO, Zhuang J, Bates AL, et al. Myeloma cells exhibit an increase in proteasome activity and an enhanced response to proteasome inhibition in the bone marrow microenvironment in vivo. Am J Hematol. 2009;84:268–272. doi: 10.1002/ajh.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, et al. Non-proteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clin Cancer Res. 2011;17:2734–2743. doi: 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]
- 54.Chauhan D, Auclair D, Robinson EK, Hideshima T, Li G, Podar K, et al. Identification of genes regulated by dexamethasone in multiple myeloma cells using oligonucleotide arrays. Oncogene. 2002;21:1346–1358. doi: 10.1038/sj.onc.1205205. [DOI] [PubMed] [Google Scholar]
- 55.Roccaro AM, Sacco A, Aujay M, Ngo HT, Azab AK, Azab F, et al. Selective inhibition of chymotrypsin-like activity of the immunoproteasome and constitutive proteasome in Waldenstrom macroglobulinemia. Blood. 2010;115:4051–4060. doi: 10.1182/blood-2009-09-243402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zavrski I, Krebbel H, Wildemann B, Heider U, Kaiser M, Possinger K, et al. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem Biophys Res Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 57.Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci USA. 2003;100:9946–9951. doi: 10.1073/pnas.1334037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Obeng EA, Carlson LM, Gutman DM, Harrington WJ, Jr, Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood. 2006;107:4907–4916. doi: 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davenport EL, Moore HE, Dunlop AS, Sharp SY, Workman P, Morgan GJ, et al. Heat shock protein inhibition is associated with activation of the unfolded protein response pathway in myeloma plasma cells. Blood. 2007;110:2641–2649. doi: 10.1182/blood-2006-11-053728. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.