Abstract
Angiogenesis, the formation of blood vessels, is necessary for a tumor to grow, but when angiogenesis first appears in the progression of breast ductal carcinomas is unknown. To determine when this occurs, the authors examined microvessel density (MVD) by CD31 and CD105 immunostaining in normal ducts, 32 cases of usual hyperplasia, 19 cases of atypical hyperplasia, and 29 cases of ductal carcinoma in situ (DCIS). Simple hyperplasia had a 22-fold greater MVD than normal ducts (P < .0001). An increase during the progression of ductal changes was highly significant (P < .0001). To determine a possible mechanism, immunohistochemistry for vascular endothelial growth factor (VEGF) was evaluated. VEGF staining intensity of ductal epithelium increased during the progression from normal to hyperplastic to DCIS. This study shows that the first significant increase in angiogenesis occurs very early in the evolution of ductal proliferations as ductal cells become hyperplastic.
Keywords: breast, angiogenesis, hyperplasia, ductal carcinoma in situ, CD31, CD105, vascular endothelial growth factor, microvessel density
Introduction
There is abundant evidence that angiogenesis is a key step in the progression of cancer. New vessels allow nutrients and oxygen to nourish a tumor after it has outgrown its existing blood supply and provide metastatic tumor cells additional access into the vasculature.1-4 Evidence in support of this concept is that breast cancers with greater microvessel density (MVD) are more likely to be metastatic.2,5 At some time in the progression of a tumor, the phenotype will switch from a normal vascular pattern (nonangiogenic) to an angiogenic one. This process of vessel induction is known as the angiogenic switch.1,6 In the progression of ductal carcinomas of the breast, the histological pattern in which angiogenesis is first apparent is unknown. By morphology, the apparent sequence of progression of ductal breast malignancies is normal to usual hyperplasia to atypical ductal hyperplasia (ADH) to low-grade ductal carcinoma in situ (DCIS) to high-grade or comedo DCIS to invasive ductal adenocarcinoma—although molecular studies suggest more complexity in this evolution.7 Many investigators have shown that angiogenesis clearly begins at the DCIS stage8-18 or earlier.9,11,12,19 The presence of a signal in DCIS using contrast-enhanced MRI has been attributed to DCIS-associated angiogenesis.20 Similarly, we had noted increased signal intensity by contrast-enhanced MRI, a radiographic marker of angiogenesis,21 in regions of the breast showing ADH by histological analysis.22
To determine where in the progression sequence of ductal neoplasia angiogenesis first occurs, we examined markers of angiogenesis in normal ducts, usual and atypical hyperplasia, and low- and high-grade DCIS. We examined this question using 2 approaches. First, neovascularization was measured by determining the MVD of these lesions, using sensitive and specific markers of new blood vessels. We measured MVD first with CD31, which is almost specific for blood vessel endothelium and especially sensitive for the detection for new blood vessel growth,23 as would be expected in tumors. We then examined CD105, also known as endoglin, an endothelial protein that is required for tumor angiogenesis and that may thus be a specific marker for neoangiogenic vessels.3,24-26 In addition, increased MVD using CD105 as a marker has been shown to be an independent marker of prognosis in breast cancer.27,28
Second, we studied the expression of vascular endothelial growth factor (VEGF), an inducer of angiogenesis29-31 that is commonly expressed in malignancies undergoing angiogenesis, including breast carcinomas.29,30 Thus, our analysis also included a study of the relationship between VEGF and angiogenesis during the progression of breast epithelial tumorigenesis.
Materials and Methods
Cases of usual ductal hyperplasia, ADH, and DCIS were retrieved from the surgical pathology files of the University of California Irvine Medical Center from 2002 to 2006 using the Natural Language Search function of CoPath Plus (Misys Healthcare Systems, Raleigh, NC). Sequential cases treated at the hospital only were used; no cases referred to Pathology from outside institutions were included. Cases were reviewed independently by one of the authors and assigned a diagnosis using the criteria for hyperplasia and low-grade DCIS of Page and Rogers,32 and grading of DCIS used the Laigos system.33 In cases where the diagnosis disagreed with the initial diagnostic reading, a second pathologist reviewed the slide, and a consensus was reached. For each case, a single slide best representing the diagnosis was chosen for immunohistochemical staining. For each case, only the lesion representing the furthest progression of the disease was studied. In other words, if a case showed both DCIS and hyperplasia, only the DCIS was evaluated for that case. In this way, we prevented misinterpretation of lesions that may have been biologically higher grade than morphology at that particular plane would have indicated.
Cases were excluded if there was a known diagnosis of infiltrating carcinoma in the same breast, if an invasive carcinoma had been previously resected from the same breast, or if invasive carcinoma was identified on an excision performed in response to the biopsy diagnosis. Lobular neoplasia and cases in which there was insufficient material in the block for immunohistochemistry were not studied. The use of these sections for this study was approved by the Human Subjects Committee of the University of California, Irvine.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections of breast specimens were placed on positively charged slides (X-tra Slides, Surgipath Inc, Richmond, IL), deparaffinized with xylene substitute (Clear-Rite 3 Cardinal Health, Dublin, OH), and rehydrated through decreasing concentrations of 90:5:5 ethyl: methyl:isopropyl alcohol. Pretreatment was performed using Dako Target Retrieval Solution, a citrate buffer (pH 6) in a pressure cooker for 5 minutes for CD31 and CD105, and the pH 8.0 EDTA detection system for VEGF. Immunoperoxidase reactions for CD31, CD105, and VEGF were performed using a Dako Autostainer Plus automated immunostainer (Dako Inc, Carpinteria, CA) according to the manufacturer's instructions. Briefly, the automated steps included blockage of endogenous peroxidase with DakoCytomation Dual Endogenous Enzyme Block and reaction with the specific antibody. Antibodies were as follows: monoclonal mouse anti-CD31 (Dako), clone JC70A diluted 1:500; monoclonal mouse anti-CD105 (Dako), clone SN6 diluted 1:3000; and monoclonal mouse anti-VEGF (Neomarkers, Fremont, CA), clone VG1 diluted 1:50. Incubation with DakoCytomation EnVision+ Dual Link System-HRP followed the primary antibody reaction for CD31 and VEGF, and the CSAII detection system followed CD105. The chromogen was diaminobenzidine for all reactions. Negative controls were performed in the same fashion, except that the primary antibody was substituted with mouse immunoglobulin. A section of breast carcinoma provided the positive control.
Evaluation
The slides were scanned at low power; 3 areas of DCIS or hyperplasia were then circled and CD31-stained microvessels were counted to within 100 μm of the lesions. A lumen was not necessary for a structure to be defined as a microvessel, nor was the presence of red blood cells. Where vessels were in clusters, each was counted as separate if it met the criteria. Large vessels with thick muscular walls and vessels within the fibrovascular core of the hyperplasia and neoplasms were not counted. Vessels around lobules were also excluded from this experiment. Reactive areas such as scars, granulation tissues, and foreign body reactions were avoided. Counts were performed at 200 times magnification, and MVD was the number per 200× field on the Olympus BH-2 microscope. MVD was evaluated for 3 normal ducts each from 20 of the cases without DCIS. Normal ducts were defined as those with 2 cell layers and not in continuity with hyperplasia. An identical procedure was used to evaluate MVD on CD105-stained sections, but care was taken to avoid counting nonspecifically stained inflammatory cells. VEGF was analyzed on adjacent sections in the same ducts exhibiting DCIS or hyperplasia that were studied for vascular staining. Manual evaluation of the VEGF staining was scored 0, 1, 2, or 3+ based on the average intensity of staining of 3 separate foci. In the few cases where 3 examples of the lesion were not present, 1 or 2 foci were scored. Cases were separately scored by 2 investigators, including a surgical pathologist with experience in breast pathology.
Statistical Considerations
For each case, the average of 3 numbers of vessels was calculated, and the mean of these values was determined for normal ducts and each type of ductal lesion. To determine significance in differences between the mean values of MVD for each ductal category and the next in the progression, parametric pairwise comparisons and nonparametric Wilcoxon rank sum test and Kruskal-Wallis tests were performed. The Jonckheere-Terpstra trend test was performed to determine whether changes in MVD through the entire progression from normal ducts to DCIS was significant.
Results
Patient Characteristics
Tissue was obtained from 80 patients. There were 32 cases of usual hyperplasia, 19 cases of atypical hyperplasia, and 29 cases of DCIS. Of these, 7 were low-grade DCIS, 8 were intermediate-grade DCIS, and 14 were high-grade DCIS. The average and median age of the patients was 53 years, with an age range of 24 to 90 years. In all, 33 of the specimens were from lumpectomies, 37 were from needle biopsies, 1 was from a reduction mammaplasty and 9 were from mastectomy specimens. The lesion was on the right in 41 cases and on the left in 39 cases.
CD31 Staining
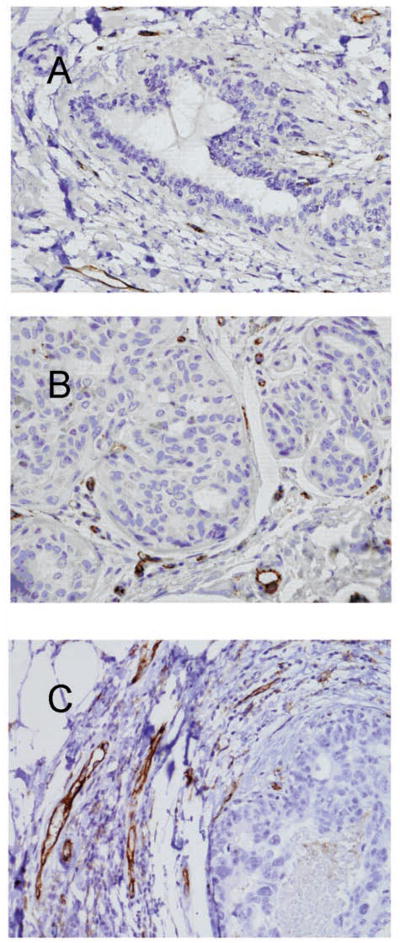
Microvessels were easily visualized on CD31-stained sections of all the breast tissue samples examined, as illustrated in Figure 1. Figure 1 shows representative examples of the increase in MVD as the epithelial proliferations progress from normal breast (Figure 1A) to hyperplasia (Figure 1B) and high-grade DCIS (Figure 1C). Normal ducts showed only a few microvessels surrounding them (mean MVD = 0.8 microvessels per 200× field). The MVD increased as the duct morphology progressed from normal to hyperplastic to DCIS. This increase can be appreciated among the examples in Figure 1 and is graphically represented in the box plot in Figure 2. The greatest increase in MVD was noted in the earliest changes of duct proliferation as the ducts exhibited simple hyperplasia (mean MVD = 18.1 microvessels per 200× field), which had a 22-fold greater MVD than normal ducts. This difference was highly significant by ANOVA and Wilcoxon rank sum tests (P < .0001). A gradual increase in MVD was noted at each step of the progression of ductal changes. The increase in MVD from usual to atypical hyperplasia (mean MVD = 22.4 microvessels per 20× field) was slightly less than significant (P = .067, ANOVA), whereas subsequent differences from one step to the next were not significant. When usual and atypical hyperplasia were compared with all grades of DCIS grouped together, however, the differences were significant (P = .001 by ANOVA and P = .0048 by Kruskal-Wallis test). Furthermore, the progression from usual hyperplasia to atypical hyperplasia to low-, intermediate-, and high-grade DCIS showed a significant increase in MVD along the entire progression using the Jonckheere-Terpstra trend test (P = .0004).
Figure 1.
Examples of angiogenesis in normal, nonproliferative ducts (A), usual ductal hyperplasia (B), and high-grade ductal carcinoma in situ (DCIS) (C) are shown. In normal ducts, only a few vessels are visible in the surrounding stroma (A). In this example, 10 vessels in a surrounding 20× field were counted. Increasing numbers of small vessels are apparent in the stroma surrounding the hyperplasia (B) and the DCIS (C). The vessel counts were 20 and 26 in a 20× field surrounding the hyperplasia and the DCIS, respectively (anti-CD31 with hematoxylin counterstain; original magnification 200×).
Figure 2.
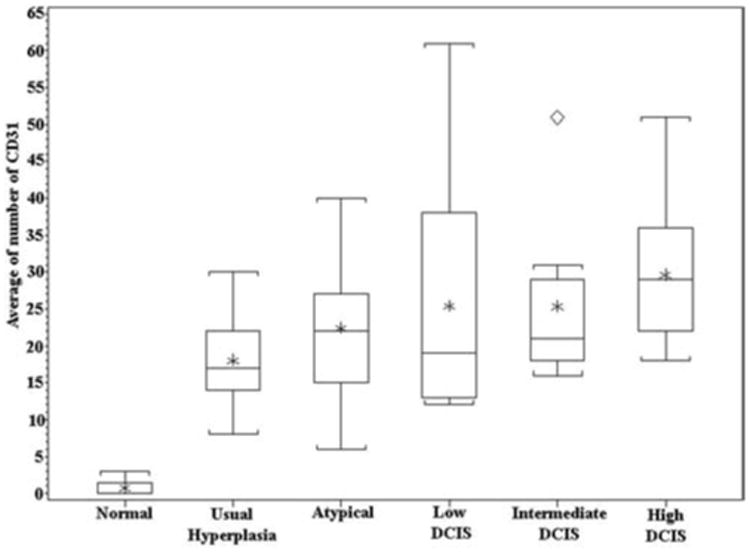
Box plot showing a large increase in CD31-stained vessels surrounding hyperplasia, as compared with normal ducts, and a gradual increase in vessels during the progression of intraductal lesions.
CD105 Staining
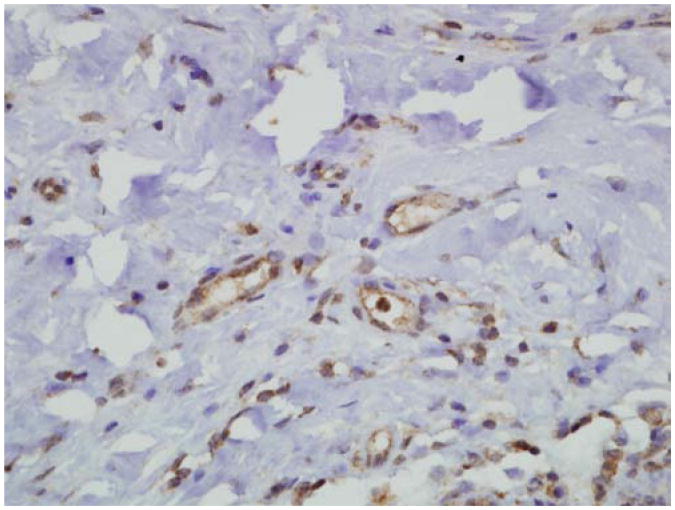
CD105 showed a pattern of microvessel staining similar to that of CD31 for all the specimens in the normal to hyperplastic to DCIS sequence. Staining was also observed in the epithelial cells, making it difficult to observe microvessels immediately adjacent to the epithelium in some cases. CD105 staining was also noted in the endothelium of small arteries and veins distant from the ducts. An example of CD105 staining in the stroma near a duct in a case of hyperplasia is illustrated in Figure 3. The number of microvessels was similar to the numbers observed in the CD31-stained cases. The similarity is readily apparent when we compare the box plot of CD105 expression (Figure 4) with the box plot of CD31 expression. Most notably, the increase in MVD in usual hyperplasia compared with normal is highly significant (P < .0001). As before, the incremental change in MVD was not significant for each type of duct proliferation, but the trend toward increased MVD across the entire spectrum of changes was highly significant by the Jonckheere-Terpstra test (P < .0001).
Figure 3.
CD105 staining in capillaries near a hyperplastic duct (anti-CD105 with hematoxylin counterstain; original magnification 400×).
Figure 4.
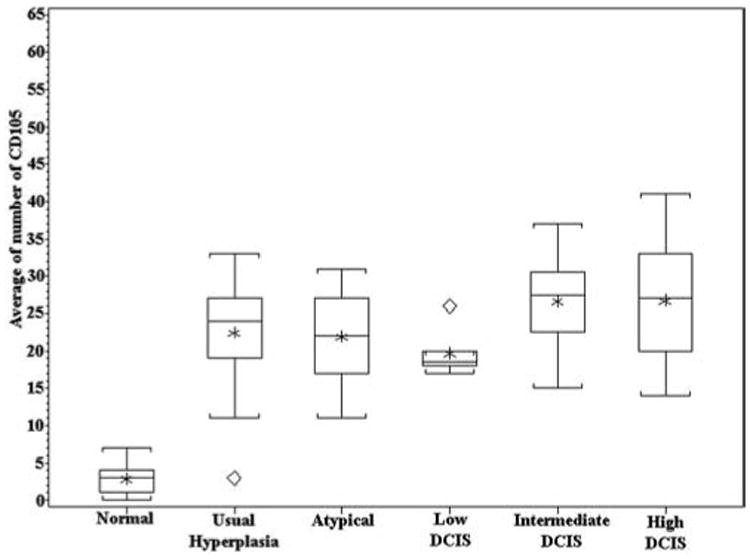
Box plot showing a large increase in CD105-stained vessels surrounding hyperplasia, as compared with normal ducts, and a slightly greater increase in the number of vessels surrounding intermediate- and high-grade DCIS.
VEGF Staining
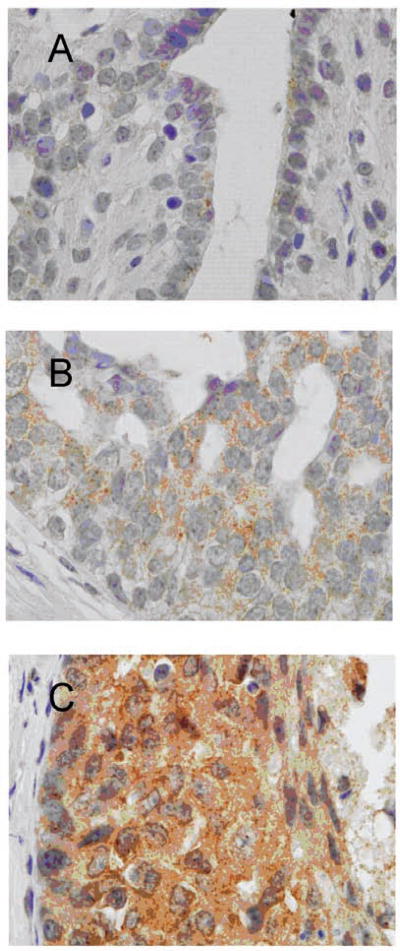
Immunoperoxidase staining for VEGF was evaluated by subjective grading of staining intensity. VEGF stained in a faint to moderate, finely granular cytoplasmic pattern in normal ductal epithelium, hyperplasia, and most cases of DCIS (Figure 5). A few examples of high-grade DCIS stained slightly darker (Figure 5C). When each of the categories of proliferative breast disease was analyzed separately, as was done for the MVD, only the mean increase in staining intensity of hyperplasia compared with normal ductal epithelium was minimally significant (P = .0341 by ANOVA). The differences in staining were best appreciated when normal was compared with hyperplasias grouped together and DCISs grouped together. When this analysis was performed, normal epithelium had a mean intensity value of 1.17, usual and atypical hyperplasia had a mean intensity value of 1.68, and DCIS had a mean intensity value of 1.64. The differences between normal and hyperplastic, and between normal and DCIS were both significant, with P values of .01 and .05, respectively, by Wilcoxon's rank sum test. The change in VEGF staining with the progression from normal to hyperplastic to DCIS was also significant (P = .02) by the Jonckheere-Terpstra trend test.
Figure 5.
Examples of VEGF staining in normal, nonproliferative ducts (A), usual ductal hyperplasia (B), and high-grade ductal carcinoma in situ (DCIS) (C) are shown. In normal ducts, only minimal staining is visible in the ductal cells (A), but the staining is greater in hyperplasia (B) and high-grade DCIS (C).
Discussion
In our study of microvascular density of preinvasive ductal disease, we found an increase in MVD in association with ducts during the progression from normal to hyperplastic to DCIS. Our study measured 2 markers of angiogenesis: CD31 for total vessels and CD105 for newly formed vessels. Similar to our findings, many investigators noted an increase in MVD in intermediate- and high-grade DCIS in comparison with low-grade and/or normal ducts.8-11,14-16,18,29,34,35 Fewer studies have examined the entire progression of preneoplastic ductal disease. Ottinetti and Sapino19 noted an increase in vessel size but not vessel density as lesions progressed from normal to hyperplastic to DCIS. In that study, only a small number of cases, including only 2 cases of atypical hyperplasia, were examined. Heffelfinger et al11 noted that atypical hyperplasia, lobular carcinoma in situ, and micropapillary DCIS had significantly more basement membrane vessels than usual proliferative breast disease. They examined benign proliferative disease coexistent with DCIS, in contrast to our study where we only examined the highest grade lesion of each case. In this way, we could rule out potential influences of a higher grade lesion on an otherwise lower grade one in the same specimen and could confidently rule out any misinterpretation of potential overlap between benign proliferative disease and DCIS in any given specimen. Finally, they used factor VIII to label endothelial cells, but this marker tends to stain endothelium of larger vessels and, thus, is less sensitive for tumor microvasculature than CD31 or CD105.23 In 2 recent studies, MVD was significantly higher in intermediate- and high-grade DCIS when compared with hyperplasia, as in our study, but increases in MVD when compared with normal ducts were only observed in DCIS in 1 study17 and in DCIS and ADH in the other.18 In contrast, we observed that MVD is significantly greater in hyperplastic as compared with normal ducts and that there is a significant increase in angiogenesis through the entire progression of preinvasive ductal proliferations.
It is unclear why, in contrast to our results, these 2 studies showed little difference between the vascularity of normal ducts and usual hyperplasia. To further investigate the vascularity of normal and hyperplastic ducts, we used a second angiogenic marker, CD105. CD105 was chosen for use in addition to CD31 because of reports of its specificity for neoangiogenesis compared with established vessels,3,23-25 although we noted staining in the endothelium of small arteries and veins, and epithelium also. Even so, our study indicated that CD105 is a marker of angiogenesis not only in invasive breast cancer, as noted in previous studies,27,28,34-37 but also in breast DCIS and hyperplasia. Furthermore, the similarity in the number of vessels staining for CD105 and CD31 provides confirmation of the CD31 MVD results and provides additional evidence of the initiation of angiogenesis early in the sequence of breast duct proliferation.
Finally, our study provides evidence that for hyperplasia, as well as for DCIS,8,12,13,16-18,36-38 angiogenic factors exert influence beyond the basement membrane into the stroma. Immunohistochemistry for VEGF provides a mechanism for angiogenesis in proliferative ductal breast lesions. Although many angiogenic factors have been identified, VEGF is one of the most widely implicated in tumor angiogenesis, particularly in invasive breast cancer,1,29 and thus, it was chosen for this investigation. Gradually increasing VEGF staining intensity was noted as the lesions progressed, but demonstration of significant increases required the grouping of usual and atypical hyperplasias together and grouping of all grades of DCISs together. In other investigations, high levels of VEGF mRNA have been noted in approximately half of DCIS cases but with little or no expression in nearby normal or preneoplastic ducts.36-37 High VEGF expression by immunohisto chemistry in more than half of DCIS cases was noted by other investigators,8,38 and this correlated with periductal cuffing of new vessels8 or MVD.38 No study of normal or hyperplastic lesions was done by these investigators. Closer to our observations, Wulfing et al13 noted that normal ducts had little or no VEGF staining, with only faint or moderate staining of VEGF in DCIS. In a study that investigated VEGF in hyperplastic ducts, only slightly stronger staining was noted in DCIS in comparison with normal and hyperplastic ducts, with only the difference between normal and CIS showing significance.12 Thus, our investigation builds on prior studies by showing a significant increase in VEGF expression in ductal hyperplasia when compared with normal ducts. As noted, this increase in VEGF staining occurs along with increased vascularity in ductal hyperplasia. Our findings are in contrast, however, to a previous study that found no difference in VEGF between the various types of intraductal proliferations.17
In summary, this study shows that there is a significant increase in angiogenesis in mammary ductal proliferations as they progress and a significant increase in VEGF expression during progression. This association suggests that VEGF expression in proliferating ducts provides the signal to endothelium in the stroma to proliferate. Other factors from both the epithelium and stroma may play a role. Some that have been previously investigated include insulin-like growth factor,12 platelet-derived growth factor,12 thymidine phosphorylase,12,39 fibroblast growth factor,13 and VEGF-C.13
This study shows that the first significant increase in angiogenesis occurs very early in the evolution of ductal proliferations, as ductal cells become hyperplastic. This is of interest because such early lesions are not generally considered neoplastic, yet angiogenesis is required for tumors to expand in size. This implies that at least in the breast, angiogenesis is one of the earliest changes required for cancer formation and likely precludes most of the genetic abnormalities associated with malignancy. From a diagnostic standpoint, this study provides a possible explanation of why hyperplasia may produce a signal on MRI. From a therapeutic standpoint, these findings raise the possibility that antiangiogenic interventions may have a role in cancer chemoprevention in high-risk individuals.
References
- 1.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 2.Weidner N. Angiogenesis as a predictor of clinical outcome in cancer patients. Hum Pathol. 2000;31:403–405. doi: 10.1053/hp.2000.6724. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Sharma MC, Sarkar C. Morphology of angiogenesis in human cancer: a conceptual overview, histoprognostic perspective and significance of neoangiogenesis. Histopathology. 2005;46:481–489. doi: 10.1111/j.1365-2559.2005.02142.x. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 5.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 7.Simpson PT, Reis-Filho JS, Gale T, Lakhani SR. Molecular evolution of breast cancer. J Pathol. 2005;205:248–254. doi: 10.1002/path.1691. [DOI] [PubMed] [Google Scholar]
- 8.Vogl G, Dietze O, Hauser-Kronberger C. Angiogenic potential of ductal carcinoma in situ (DCIS) of human breast. Histopathology. 2005;47:617–624. doi: 10.1111/j.1365-2559.2005.02299.x. [DOI] [PubMed] [Google Scholar]
- 9.Guidi AJ, Fischer L, Harris JR, Schnitt SJ. Microvessel density and distribution in ductal carcinoma in situ of the breast. J Natl Cancer Inst. 1994;86:614–619. doi: 10.1093/jnci/86.8.614. [DOI] [PubMed] [Google Scholar]
- 10.Warnberg F, Nordgren H, Bergkvist L, Holmberg L. Tumour markers in breast carcinoma correlate with grade rather than with invasiveness. Br J Cancer. 2001;85:869–874. doi: 10.1054/bjoc.2001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heffelfinger SC, Yassin R, Miller MA, Lower E. Vascularity of proliferative breast disease and carcinoma in situ correlates with histological features. Clin Cancer Res. 1996;2:1873–1878. [PubMed] [Google Scholar]
- 12.Heffelfinger SC, Miller MA, Yassin R, Gear R. Angiogenic growth factors in preinvasive breast disease. Clin Cancer Res. 1999;5:2867–2876. [PubMed] [Google Scholar]
- 13.Wulfing P, Kersting C, Buerger H, et al. Expression patterns of angiogenic and lymphangiogenic factors in ductal breast carcinoma in situ. Br J Cancer. 2005;92:1720–1728. doi: 10.1038/sj.bjc.6602567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Paner GP, Kahn LB, Rajan PB. Noninvasive carcinoma of the breast: angiogenesis and cell proliferation. Arch Pathol Lab Med. 2004;128:893–896. doi: 10.5858/2004-128-893-NCOTBA. [DOI] [PubMed] [Google Scholar]
- 15.Teo NB, Shoker BS, Jarvis C, Martin L, Sloane JP, Holcombe C. Vascular density and phenotype around ductal carcinoma in situ (DCIS) of the breast. Br J Cancer. 2002;86:905–911. doi: 10.1038/sj.bjc.6600053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL. Distinct angiogenic patterns are associated with high-grade in situ ductal carcinomas of the breast. J Pathol. 1997;181:207–212. doi: 10.1002/(SICI)1096-9896(199702)181:2<207::AID-PATH758>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Viacava P, Naccarato AG, Bocci G, et al. Angiogenesis and VEGF expression in pre-invasive lesions of the human breast. J Pathol. 2004;204:140–146. doi: 10.1002/path.1626. [DOI] [PubMed] [Google Scholar]
- 18.Pavlakis K, Messini I, Vrekoussis T, et al. The assessment of angiogenesis and fibroblastic stromagenesis in hyperplastic and pre-invasive breast lesions. BMC Cancer. 2008;8:88. doi: 10.1186/1471-2407-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ottinetti A, Sapino A. Morphometric evaluation of microvessels surrounding hyperplastic and neoplastic mammary lesions. Breast Cancer Res Treat. 1988;11:241–248. doi: 10.1007/BF01807282. [DOI] [PubMed] [Google Scholar]
- 20.Gilles R, Zafrani B, Guinebretiere JM, et al. Ductal carcinoma in situ: MR imaging-histopathologic correlation. Radiology. 1995;196:415–419. doi: 10.1148/radiology.196.2.7617854. [DOI] [PubMed] [Google Scholar]
- 21.Teifke A, Behr O, Schmidt M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology. 2006;239:351–360. doi: 10.1148/radiol.2392050205. [DOI] [PubMed] [Google Scholar]
- 22.Su MY, Yu HJ, Carpenter PM, McLaren CE, Nalcioglu O. Pharmacokinetic parameters analyzed from MR contrast enhancement kinetics of multiple malignant and benign breast lesions detected in the same patients. Technol Cancer Res Treat. 2005;4:255–263. doi: 10.1177/153303460500400305. [DOI] [PubMed] [Google Scholar]
- 23.Wang JM, Kumar S, Pye D, Haboubi N, Al-Nakib L. Breast carcinoma: comparative study of tumor vasculature using two endothelial cell markers. J Natl Cancer Inst. 1994;86:386–388. doi: 10.1093/jnci/86.5.386. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Guo B, Bernabeu C, Kumar S. Angiogenesis in breast cancer: the role of transforming growth factor beta and CD105. Microsc Res Tech. 2001;52:437–449. doi: 10.1002/1097-0029(20010215)52:4<437::AID-JEMT1029>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Duff SE, Li C, Garland JM, Kumar S. CD105 is important for angiogenesis: evidence and potential applications. FASEB J. 2003;17:984–992. doi: 10.1096/fj.02-0634rev. [DOI] [PubMed] [Google Scholar]
- 26.Balza E, Castellani P, Zijlstra A, Neri D, Zardi L, Siri A. Lack of specificity of endoglin expression for tumor blood vessels. Int J Cancer. 2001;94:579–585. doi: 10.1002/ijc.1505. [DOI] [PubMed] [Google Scholar]
- 27.Dales JP, Garcia S, Carpentier S, et al. Long-term prognostic significance of neoangiogenesis in breast carcinomas: comparison of Tie-2/Tek, CD105, and CD31 immunocytochemical expression. Hum Pathol. 2004;35:176–183. doi: 10.1016/j.humpath.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Kumar S, Ghellal A, Li C, et al. Breast carcinoma: vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999;59:856–861. [PubMed] [Google Scholar]
- 29.Rice A, Quinn CM. Angiogenesis, thrombospondin, and ductal carcinoma in situ of the breast. J Clin Pathol. 2002;55:569–574. doi: 10.1136/jcp.55.8.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fox SB, Generali DG, Harris AL. Breast tumour angiogenesis. Breast Cancer Res. 2007;9:216–226. doi: 10.1186/bcr1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veikkola T, Karkkainen M, Claesson-Welsh L, Alitalo K. Regulation of angiogenesis via vascular endothelial growth factor receptors. Cancer Res. 2000;60:203–212. [PubMed] [Google Scholar]
- 32.Page DL, Rogers LW. Combined histologic and cytologic criteria for the diagnosis of mammary atypical ductal hyperplasia. Hum Pathol. 1992;23:1095–1097. doi: 10.1016/0046-8177(92)90026-y. [DOI] [PubMed] [Google Scholar]
- 33.Lagios MD. Duct carcinoma in situ: pathology and treatment. Surg Clin North Am. 1990;70:853–871. doi: 10.1016/s0039-6109(16)45185-6. [DOI] [PubMed] [Google Scholar]
- 34.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Over-expression of endoglin (CD105): a marker of breast carcinoma-induced neo-vascularization. Anticancer Res. 1998;18:3621–3628. [PubMed] [Google Scholar]
- 35.Gómez-Esquer F, Agudo D, Martínez-Arribas F, Nuñez-Villar MJ, Schneider J. mRNA expression of the angiogenesis markers VEGF and CD105 (endoglin) in human breast cancer. Anticancer Res. 2004;24:1581–1585. [PubMed] [Google Scholar]
- 36.Brown LF, Guidi AJ, Schnitt SJ, et al. Vascular stroma formation in carcinoma in situ, invasive carcinoma, and metastatic carcinoma of the breast. Clin Cancer Res. 1999;5:1041–1056. [PubMed] [Google Scholar]
- 37.Guidi AJ, Schnitt SJ, Fischer L, et al. Vascular permeability factor (vascular endothelial growth factor) expression and angiogenesis in patients with ductal carcinoma in situ of the breast. Cancer. 1997;80:1945–1953. doi: 10.1002/(sici)1097-0142(19971115)80:10<1945::aid-cncr11>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 38.Hieken TJ, Farolan M, D'Alessandro S, Velasco JM. Predicting the biologic behavior of ductal carcinoma in situ: an analysis of molecular markers. Surgery. 2001;130:593–600. doi: 10.1067/msy.2001.116921. [DOI] [PubMed] [Google Scholar]
- 39.Engels K, Fox SB, Whitehouse RM, Gatter KC, Harris AL. Up-regulation of thymidine phosphorylase expression is associated with a discrete pattern of angiogenesis in ductal carcinomas in situ of the breast. J Pathol. 1997;182:414–420. doi: 10.1002/(SICI)1096-9896(199708)182:4<414::AID-PATH897>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]