Abstract
The developing mammalian embryo is entirely dependent on the maternal circulation for its supply of retinoids (vitamin A and its metabolites). The mechanisms through which mammalian developing tissues maintain adequate retinoid levels in the face of suboptimal or excessive maternal dietary vitamin A intake have not been established. We investigated the role of retinyl ester formation catalyzed by lecithin:retinol acyltransferase (LRAT) in regulating retinoid homeostasis during embryogenesis. Dams lacking both LRAT and retinol-binding protein (RBP), the sole specific carrier for retinol in serum, were maintained on diets containing different amounts of vitamin A during pregnancy. We hypothesized that the lack of both proteins would make the embryo more vulnerable to changes in maternal dietary vitamin A intake. Our data demonstrate that maternal dietary vitamin A deprivation during pregnancy generates a severe retinoid-deficient phenotype of the embryo due to the severe retinoid-deficient status of the double mutant dams rather than to the lack of LRAT in the developing tissues. Moreover, in the case of excessive maternal dietary vitamin A intake, LRAT acts together with Cyp26A1, one of the enzymes that catalyze the degradation of retinoic acid, and possibly with STRA6, the recently identified cell surface receptor for retinol-RBP, in maintaining adequate levels of retinoids in embryonic and extraembryonic tissues. In contrast, the pathway of retinoic acid synthesis does not contribute significantly to regulating retinoid homeostasis during mammalian development except under conditions of severe maternal retinoid deficiency.
The crucial role played by vitamin A in embryonic development has long been known (1–5). Both embryonic vitamin A deficiency and excess give rise to fetal death or to a spectrum of congenital defects, in a dose and developmental stage-dependent manner (4). Vitamin A exerts its functions through retinoic acid, a lipid-soluble hormone that regulates the expression of many target genes through receptor-mediated events (6). Retinoic acid is generated from retinol (vitamin A alcohol) through two oxidative enzymatic reactions via retinaldehyde (7). When retinoid signaling needs to be turned off, retinoic acid is enzymatically catabolized into more polar products such as 4-hydroxy retinoic acid or 4-oxo retinoic acid. This oxidation is achieved by the action of several cytochrome P450 enzymes, including Cyp26A1, Cyp26B1, and Cyp26C1 (8–10).
Because there is no de novo fetal synthesis of vitamin A, mammalian developing tissues are entirely dependent on maternal circulating retinoids (vitamin A and its metabolites) that reach the embryo through the maternal-fetal barrier, i.e. the placenta (11). Retinol bound to its specific transport protein, retinol-binding protein (RBP),5 is the major form of vitamin A in the fasting circulation where it is secreted from the liver, the main body store of vitamin A. However, upon dietary vitamin A intake, retinyl ester packaged in lipoprotein particles may account for the majority of circulating vitamin A (7).
Formation of retinyl esters, the storage form of vitamin A, occurs through esterification of retinol primarily by the action of the enzyme lecithin:retinol acyltransferase (LRAT), which is widely expressed in tissues (12–19). Although mice lacking LRAT (LRAT−/−) display only trace amounts of retinyl ester in liver and most of the extrahepatic tissues, both retinol and retinyl ester levels are elevated in adipose tissue (18, 20, 21). These mutant mice are viable and fertile when maintained on a vitamin A-sufficient diet. Indeed, their serum RBP and retinol levels are normal (18, 20,21). However, as a result of only possessing adipose tissue retinyl ester stores and lacking the normally large liver and lung stores of wild-type mice, LRAT−/− mice are highly susceptible to developing adult retinoid deficiency (18, 20, 21). Whether the absence of LRAT in mammalian developing tissues also induces embryonic retinoid deficiency has not been investigated to date. Isken et al. (22) have recently analyzed the expression pattern of two LRAT paralogues from zebrafish and have shown competition among embryonic tissues between retinyl ester- and retinoic acid-synthesizing pathways for their shared common precursor, retinol, that dynamically adjusts retinoic acid levels during early development. Although mammals and zebrafish are very different in the way they provide vitamin A and other nutrients to their developing embryos, these findings raise the question of whether the tight regulation of retinol levels observed for embryonic zebrafish is conserved during mammalian development.
In the present study, we show that LRAT is expressed in developing mouse tissues, even at very early stages of embryogenesis. Furthermore, by analyzing the embryonic development of mice lacking both LRAT and RBP (LRAT−/−RBP−/−) under different dietary conditions, we show that a severe embryonic retinoid deficiency is rapidly achieved in this strain by removing maternal dietary vitamin Aduring pregnancy. The developmental defects we observe are due to the lack of LRAT in maternal tissues that, together with RBP ablation and the dietary vitamin A deprivation, generates a severe retinoid deficiency status in the dams. Our study also reveals that, as in adult mammalian tissues, embryonic and extraembryonic LRAT can divert retinol away from oxidative activation, thus contributing along with Cyp26A1 activity, to maintaining retinoic acid homeostasis in developing tissues. We also provide some evidence for a possible role of STRA6, the recently identified cell surface receptor for retinol-RBP (23), in this process. This homeostatic mechanism may be very important for maintaining normal embryogenesis in the face of excessive maternal dietary vitamin A intake. Finally, we show that, in contrast to zebrafish development, the retinoic acid-synthesizing pathway contributes significantly to maintaining retinoid homeostasis during mammalian development only under conditions of vitamin A deficiency.
EXPERIMENTAL PROCEDURES
Knock-out and Transgenic Mice
RBP−/− and lacZ/RBP−/− mice were previously generated and described (24, 25). Mice lacking RBP and LRAT (LRAT−/−RBP−/−) were generated by crossing RBP−/− (24) and LRAT−/− (18) mice. The resulting double heterozygous mice (LRAT+/−RBP+/−) of the F1 generation were crossed, and the double knock-out animals (LRAT−/− RBP−/−) were obtained in the F2 generation at the expected Mendelian ratio. The RBP genotype was confirmed by Southern blot analysis (24), and the LRAT genotype was confirmed by PCR analysis (18), following published procedures. When maintained on regular chow diet, LRAT−/−RBP−/− mice were viable and fertile and did not show any obvious phenotype. Mice homozygous for the lacZ transgene (lacZ) (26), and lacking both RBP and LRAT (lacZ/LRAT−/−RBP−/−), were generated by crossing LRAT−/−RBP−/− and lacZ/RBP−/− animals. Genotypes were confirmed according to previously published protocols (18,25). All mice employed for this study were from a mixed C57Bl/6 × sv129 genetic background.
Nutritional Manipulation
Female mice were maintained on a standard nutritionally complete vitamin A-sufficient chow diet (25–28 IU of vitamin A/g of diet) up to approximately 3 months of age, when they were mated. At the time of vaginal plug detection (set as 0.5 days post coitum (dpc), the onset of gestation), females were randomly assigned to one of three different diets: 1) vitamin A-sufficient diet (25–28 IU of vitamin A/g of diet), 2) vitamin A-deficient diet (<0.22 IU of vitamin A/g of diet), or 3) vitamin A-excess diet (220 IU of vitamin A/g of diet) until the day of sacrifice (14.5 dpc). Diets were prepared based on the AIN-93 formulation (27) (Test Diet, W. F. Fisher and Son, Inc.). Their nutrient composition was identical except for the concentration of vitamin A. The vitamin A-excess diet contained 10-fold the concentration of retinoids in the vitamin A-sufficient diet. This range was chosen based on guidelines of the World Health Organization for a range of recommended daily allowances for vitamin A during pregnancy that would avoid possible teratogenic effects during embryogenesis. The World Health Organization indeed recommends an intake of 900 IU/day and a maximum of 8000 IU/day (almost a 10-fold range) for pregnant women (28). Diet and water were available to all animals on an ad libitum basis until the time of sacrifice, except as otherwise reported. Mice were maintained on a 12-h dark-light cycle between 7:00 p.m. and 7:00 a.m. All animals were sacrificed by CO2 inhalation between 9:30 and 11:30 a.m. when maternal serum, liver, adipose tissue, placenta, and embryos were collected. All animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (29) and were approved by the Rutgers University Institutional Committee on Animal Care.
HPLC Analysis of Retinoids
Reversed-phase HPLC analysis of retinol and retinyl ester was performed as described (24,30). Mouse serum and tissues were flash-frozen in liquid N2 after collection. Tissues were homogenized in 10 volumes of phosphate-buffered saline using a PRO200 homogenizer (Oxford, CT). Retinoids present in the homogenates were extracted into hexane and separated on a 4.6 × 250 mm Ultrasphere C18 column (Beckman, Fullerton, CA) preceded by a C18 guard column (Supelco Inc., Bellefonte, PA), with 70% acetonitrile, 15% methanol, and 15% methylene chloride used as the running solvent flowing at 1.8 ml/min. Retinol and retinyl esters (retinyl palmitate, oleate, linoleate, and stearate) were separated and identified by comparing retention times and spectral data of experimental compounds with those of authentic standards. Examples of chromatograms are shown in supplemental Fig. S1. Concentrations of retinol and retinyl esters in the tissues were quantified by comparing peak integrated areas for unknowns against those of known amounts of purified standards. Loss during extraction was accounted for by adjusting for the recovery of retinyl acetate, the internal standard added immediately following homogenization of the tissues. Serum and tissue retinoic acid determinations were carried out as previously described (21).
Whole Mount Detection of β-Galactosidase Activity
Embryos were dissected in phosphate-buffered saline to avoid any potential outside source of retinoids. Subsequently, they were pre-fixed in a freshly made solution containing 4% paraformaldehyde in sodium phosphate buffer, pH 7.4, for 60 min on ice. Embryos were rinsed twice with 100 mM phosphate buffer, pH 7.4, 5 mM EGTA, 2 mM MgCl2 for different periods (5 min and 20 min) and then with 100 mM phosphate buffer, pH 7.4, 2 mM MgCl2, 0.01% sodium deoxycholate, 0.02% Nonidet-P40 twice for 5 min and stained at 37 °C overnight in a solution containing this buffer and 1 mg/ml X-gal, 1 mM spermidine HCL, 5 mM K3Fe(CN)6,5 mM K4Fe(CN)6−6H2O. The next day, embryos were post-fixed in 4% paraformaldehyde in phosphate buffer, pH 7.4, for 10–20 min and stored in phosphate-buffered saline at 4 °C.
Total RNA Extraction and RT-PCR
Total RNA from embryos, yolk sac, and placenta was extracted using RNA Bee (Tel-Test, Inc.) according to manufacturer’s instructions. RNA concentrations were measured by spectrophotometer and treated with DNase (Roche Applied Science). Three micrograms of total RNA was used for the synthesis of cDNA using methods and reagents from Stratagene, Brilliant SYBR Green QRT-PCR Master Mix, 2-step (Stratagene). The expression of the LRAT gene in developing tissues during different stages of gestation was assessed by RT-PCR. The primers sequences used for RT-PCR were as follows: LRAT: Fw 5’-ATGAAGAACCCAATGCTGGAA-3’; Rev 5’-CTAATC-CCAAGACAGCCGAAG-3’; β-actin: Fw 5’-CGGAGG-GAAAGATTCCTCTGGC-3’; Rev 5’-AGGGCCGGCACAT-TGAAGGTCT-3’. A final concentration of 200 nM per primer was used in a 25-µl reaction volume. The PCR reaction was performed on a MyCycler Thermal Cycler System (Bio-Rad) under the following conditions: 94 °C for 30 s (1 cycle); 94 °C for 30 s; 60 °C for 30 s; 72 °C for 1 min (35 cycles for LRAT; 30 cycles for β-actin); followed by 72 °C for 7 min (1 cycle). The PCR product was run on a 1.5% agarose gel, and signals were detected by using a Chemidoc XRS Molecular Imager System (Bio-Rad).
Real-time PCR Analysis
For the Real-time PCR, we used a Bio-Rad MyIQ cycler (Bio-Rad) and reagents from Stratagene, Brilliant SYBR Green QRT-PCR Master Mix, 2-step (Stratagene). Primer sequences were as follows: RALDH2: Fw5’-TTGCAGATGCTGACTTGGAC-3’;Rev5’-TCTGA-GGACCCTGCTCAGTT-3’; Cyp26A1: Fw 5’-GAACCTTA-TACACGCGCGCAT-3’; Rev 5’-TCTGAGGACCCTGCTC-AGTT-3’; STRA6: Fw 5’-AGCCAAGTCAGACTCCAAGAG-3’; Rev 5’-CAGAGAGCACACTA-ACTTCTTTCA-3’; β-actin: Fw 5’-CGGAGGGAAAGATTCCTC-TGGC-3’; Rev 5’-AGGGCCGGC-ACATTGAAGGTCT-3’. Primer concentrations, optimal sample dilution concentrations, and relative efficiency tests were previously determined. PCR reaction conditions were as follows: 2× Master Mix, 500 nM Fw primer, 500 nM Rev primer, and 1:500 reference dye. cDNA, non-template controls, and non-reverse transcription preparations were amplified using the following conditions: 95 °C for 10 min, followed by 40 cycles at 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min. Each sample was run in triplicate. Both the gene of interest and the housekeeping gene, β-actin, were run on the same plate for each sample. A dissociation curve was generated to determine the presence of primer dimers. Comparative CT calculations for the expression of RALDH2, Cyp26A1, and STRA6 were all relative to a chosen calibrator (wild type). CT values of β-actin were subtracted from sample CT values to obtain ΔCT values. ΔΔCT values were obtained by subtracting the average ΔCT of the calibrator from each sample ΔCT value. The expression of each gene relative to the calibrator was calculated using the expression 2−ΔΔCT.
Statistical Analyses
The Kolmogorov-Smirnov test was used to test for normality of a particular variable. For HPLC analysis, when HPLC retinol levels were not normally distributed, statistical analysis was performed by Kruskal-Wallis test followed by Mann-Whitney test. When retinyl esters were not normally distributed, values were logarithmically transformed prior to statistical analysis and reported as geometric means, i.e. the antilogarithms of the means of the log transforms. Normally distributed values were statistically analyzed by t test or analysis of variance with correction for multiple comparisons using the Fisher’s least significant difference test. For the real-time PCR analysis, normality of 2−ΔΔCT values for each sample was confirmed by using the Kolmogorov-Smirnov test. For each gene the data were subject to an analysis of variance test followed by Fisher’s least significant difference test to determine significant differences of gene expression from the calibrator. Analyses were performed with SPSS statistical software (SPSS 10, Release 10.0.7a, SPSS Inc.). A p value of <0.05 was used to establish statistical significance.
RESULTS
Temporal Expression of LRAT during Mouse Development
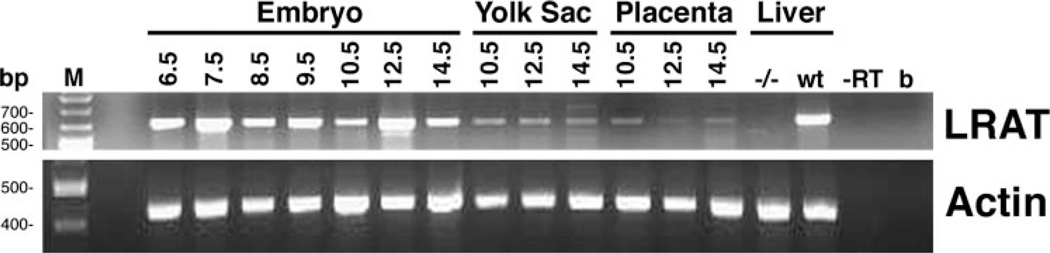
RT-PCR analysis was performed on wildtype embryos, placenta, and yolk sac, collected from 6.5 to 14.5 dpc. Although from 6.5 to 9.5 dpc we analyzed the whole embryo containing maternal, intraembryonic, and extraembryonic tissues, our data indicate that LRAT was expressed at very early stages (by 6.5 dpc) of development (Fig. 1). From 10.5 to 14.5 dpc, LRAT mRNA was detected in embryo, placenta, and yolk sac with varying levels depending on developmental stage (Fig. 1). Because the only known function of LRAT is to synthesize retinyl ester, these data suggest an important role for this retinoid form in mouse embryogenesis.
FIGURE 1. RT-PCR analysis of LRAT expression in wild-type embryos, yolk sacs, and placentas at different stages of development.
Wild-type females were sacrificed at different times during gestation from 6.5 to 14.5 dpc. From 6.5 to 9.5 dpc, the whole embryo, including maternal, intraembryonic, and extraembryonic tissues, was used for RNA extraction. At 10.5, 12.5, and 14.5 dpc, embryo, placenta, and yolk sac were dissected separately, and RNA was extracted from each. Liver from wild-type (wt) and LRAT−/− mice(−/−) were used as positive and negative controls, respectively. M, 100-bp molecular weight marker; b, blank; -RT, sample minus reverse transcription reaction. The molecular weight of the marker bands is indicated on the left. Expected molecular weights of the products: LRAT, 610 bp; actin, 556 bp.
Effects of Both RBP and LRAT Absence on Mammalian Embryonic Development
To further investigate the role of retinyl ester formation during mammalian embryonic development, we generated mutant mice lacking both LRAT and RBP (LRAT−/−RBP−/−) and analyzed the effects of the absence of these proteins on embryogenesis. Our initial studies suggested that LRAT−/−RBP−/− mice were viable and fertile when maintained on a vitamin A-sufficient regular chow diet, because progeny from the crosses of these mutant animals were obtained. However, to investigate further the viability and fertility of this strain under different maternal dietary regimes of vitamin A intake, groups of LRAT−/−RBP−/− females were maintained on a vitamin A-sufficient diet until approximately 3 months of age, when they were mated with LRAT−/−RBP−/− males. From 0.5 dpc, the females were then maintained either on the same vitamin A-sufficient diet, on a vitamin A-deficient, diet, or on a vitamin A-excess diet throughout gestation. Pregnant age-matched RBP knock-out (RBP−/−) and wild-type females as well as non-pregnant females for each of the three genotypes were maintained on the same dietary regimens and used as controls. Dams were sacrificed at 14.5 dpc, and embryos were dissected, genotyped, and analyzed for external gross morphology.
LRAT−/−RBP−/− embryos developing from LRAT−/− RBP−/− dams maintained on the vitamin A-sufficient diet were grossly normal (Table 1 and Fig. 2G). However, an increased number of resorptions was observed for the double knock-out strain (36%) as compared with wild-type (16%) and RBP−/− (10%) strains (Table 1). A further increase in the percentage of resorptions (60%) occurred when LRAT−/−RBP−/− dams were maintained on the vitamin A-deficient diet. This percentage of resorptions also was elevated compared with those observed in wild-type (17%) and RBP−/− (44%) dams maintained on the same dietary regimen (Table 1). Moreover, LRAT−/−RBP−/− embryos developing from LRAT−/−RBP−/− dams maintained on the vitamin A-deficient diet were malformed (Fig. 2H), and the severity of these malformations was greater than those shown by RBP−/− embryos (Fig. 2E). In addition to malformed eyes and peripheral edema shown by RBP−/−embryos from RBP−/− dams maintained on the vitamin A-deficient diet (Fig. 2E and Ref. 25), LRAT−/−RBP−/− embryos displayed severe malformations of the mid-facial region (cleft face and palate) and forelimbs (Fig. 2H). These are typical signs of mammalian embryonic retinoid deficiency (5, 31–35). Maternal vitamin A supplementation (vitamin A-excess diet) resulted in embryos that were grossly normal for all the three strains analyzed (Table 1 and Fig. 2). Moreover, the percentage of resorptions for LRAT−/−RBP−/− dams on this dietary regimen was in the normal range (Table 1).
TABLE 1. Effects of different regimens of maternal dietary vitamin A on embryonic development.
Three-month-old pregnant wild-type (WT), RBP knock-out (R−/−) and double knock-out LRAT and RBP (L−/−R−/−) female mice were maintained on different regimen of dietary vitamin A intake during pregnancy. WT, R−/−, and L−/−R−/− females were mated with WT, R−/−, and L−/−R−/− males, respectively. WT females maintained on the vitamin A-sufficient diet were also mated with RBP heterozygous (R+/−) males. Dams were sacrificed at 14.5 dpc, and embryos were collected and analyzed for the i external gross morphology. WT embryos from WT dams bred on the vitamin A-sufficient diet were defined as normal. Embryos classified as abnormal displayed reduced size, white appearance, and small or apparent absence of eyes. They also showed one or more of the following features: peripheral edema, abnormal mid-facial region, and/o abnormal limbs. Embryos classified as resorbed were retarded or completely resorbed (no embryo was observed at the time of the dissection). n, number of dams analyzed per group. Statistical analyses were as described under “Experimental Procedures.” A p < 0.05 was considered statistically significant.
| Dams |
Embryos |
Total Implantation |
Resorbeda | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal |
Abnormal |
|||||||||||
| Diet | Genotype | n | WT | R+/− | R−/− | L−/−R−/− | WT | R+/− | R−/− | L−/−R−/− | ||
| % | ||||||||||||
| VitA-suff (25–28 IU/g) | WT | 13 | 60 | 15 | 89 | 16 | ||||||
| R−/− | 7 | 47 | 44 | 10 | ||||||||
| L−/−R−/− | 16 | 87 | 136 | 36b,c | ||||||||
| VitA-def (<0.22 IU/g) | WT | 8 | 44 | 53 | 17 | |||||||
| R−/− | 11 | 43 | 77 | 44b,d | ||||||||
| L−/−R−/− | 15 | 42 | 106 | 60b,c,d | ||||||||
| VitA-exc (220 IU/g) | WT | 13 | 105 | 114 | 8 | |||||||
| R−/− | 13 | 81 | 99 | 18e | ||||||||
| L−/−R−/− | 13 | 76 | 97 | 22e | ||||||||
All different genotypes (when it applies) are included in this category.
p < 0.05 versus WT of the on the same dietary regimen.
p < 0.05 versus R−/− of the on the same dietary regimen.
p < 0.05 versus dams of the same genotype on sufficient diet.
p < 0.05 versus dams of the same genotype on deficient diet.
FIGURE 2. Gross morphology of embryos obtained from dams maintained on different regimens of dietary vitamin A during pregnancy.
The maternal dietary regimen is indicated at the top of each column. Wild-type, RBP knock-out (RBP−/−), and double knock-out embryos (LRAT−/−RBP−/−), from wild-type, RBP−/−, and LRAT−/− RBP−/− dams, respectively, are shown. Embryos were collected at 14.5 dpc. e, abnormal eye (reduced pigmentation in the ventral region);p, peripheral edema; mf, abnormal mid-facial region (snout foreshortened and divided by a sagittal median cleft, prolabium absent, maxillary process bearing whiskers separated by a larger than normal distance); fl, abnormal forelimb. The same magnification was used for all panels.
To discriminate between the contribution of maternal versus fetal LRAT to embryogenesis, we evaluated the development of LRAT+/−RBP−/− embryos from LRAT−/−RBP−/− dams under different regimens of dietary vitamin A intake by crossing LRAT−/− −RBP−/− females with RBP−/− males. Interestingly, LRAT+/− −RBP−/−embryos carried by LRAT−/−RBP−/− dams deprived of dietary vitamin A showed, quantitatively and qualitatively, similar external developmental defects to the LRAT−/−RBP−/− embryos (data not shown). In contrast, LRAT+/−RBP−/− embryos from dams maintained on both the vitamin A-sufficient and -excess diets were normal (data not shown).
Overall these data indicate that embryonic vitamin A deficiency can be achieved rapidly in LRAT−/−RBP−/− mice by removal of maternal dietary vitamin A at the beginning of gestation. The embryonic malformations observed are likely due to lack of maternal LRAT activity, because LRAT+/− RBP−/− and LRAT−/−RBP−/− embryos show similar gross developmental defects. Furthermore, our results suggest that the elevated percentage of early embryonic death, observed when LRAT−/−RBP−/− dams were maintained on either the vitamin A-sufficient or -deficient diet, is also induced by vitamin A deficiency, as it was reverted by maternal dietary vitamin A supplementation.
Maternal Serum and Tissue Retinoid Levels
To better understand the maternal retinoid status under different dietary regimens, we collected maternal serum and tissues. Levels of retinol and retinyl ester in the circulation of pregnant and nonpregnant LRAT−/−RBP−/−, RBP−/−, and wild-type control dams were measured by reversed-phase HPLC (30) (Table 2). As expected due to the lack of RBP (24), LRAT−/−RBP−/− dams maintained on the vitamin A-sufficient diet showed lower serum retinol levels compared with wild-type animals, and these levels were further reduced upon maternal dietary vitamin A deprivation (Table 2). However, when the double knockout dams were fed the vitamin A-excess diet, serum retinol concentration returned to wild-type levels (Table 2), an effect likely due to the greater quantity of free dietary retinol packaged into chylomicrons in the absence of LRAT (21). Indeed, when LRat−/ − RBP−/− females were supplemented with dietary vitamin A for 2 weeks followed by an overnight fast prior to sacrifice, a 44% reduction of their serum retinol levels was observed (retinol: 11.3 ± 1.9 µg/dl; retinyl ester: undetectable; n= 5). Furthermore, owing to the lack of LRAT, serum retinyl ester levels were undetectable when double knock-out females were fed either the vitamin A-sufficient or the vitamin A-deficient diet (Table 2). Serum retinoid levels for wild-type and RBP−/− dams were as previously described (25–37).
TABLE 2. Serum retinol and retinyl ester levels of pregnant and non-pregnant females maintained on different regimens of dietary vitamin A.
Wild-type (WT), RBP knock-out (R−/−) and double knock-out females for LRAT and RBP (L−/−R−/−) were maintained on different regimens of dietary vitamin A during pregnancy (see text and Table 1). Age-matched non-pregnant females maintained on identical dietary regimens for similar periods of time served as controls. Pregnant females were sacrificed at 14.5 dpc. Retinol and retinyl ester levels were determined by reversed-phase HPLC. Retinol levels are expressed as mean ± S.D. Retinyl ester levels are expressed as geometric mean (range of absolute values). Statistical analyses as described under “Experimental Procedures.” Ap< 0.05 was considered statistically significant. n, number of mice analyzed per group.
| Maternal genotype |
Serum retinol and retinyl ester levels |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| VitA-suff |
VitA-def |
VitA-exc |
|||||||
| Retinol mean ± S.D. |
n | Retinyl Ester geometric mean (range) |
Retinol mean ± S.D. |
n | Retinyl Ester geometric mean (range) |
Retinol mean ± S.D. |
n | Retinyl Ester geometric mean (range) |
|
| µg/dl | µg/dl | µg/dl | |||||||
| WT | |||||||||
| Non-pregnant | 20.5 ± 3.2 | 6 | 3.6 (1.4–9.3) | 32.1 ± 3.7a | 4 | NDb | 29.1 ± 6.0a | 5 | 44.9 (20.7–74.0) |
| Pregnant | 8.9 ± 2.9c | 4 | 4.4 (3.7–5.3) | 5.7 ± 1.1a,c | 5 | ND | 16.0 ± 2.7a,c | 6 | 24.0 (9.4–53.6) |
| R−/− | |||||||||
| Non-pregnant | 2.5 ±0.9d | 6 | 3.6 (1.5–10.0) | 1.5 ±0.4 d | 5 | 4.9 (4.1–5.8) | 6.7 ± 3.3a,d | 6 | 10.4 (7.2–15.1)d |
| Pregnant | 2.6 ±03d | 4 | 2.3 (2.3–2.4) | 1.3 ± 0.4a,d | 6 | ND | 5.7 ± 3.0c | 6 | 6.2 (3.0–19.5) |
| L−/−R−/− | |||||||||
| Non-pregnant | 5.2 ± 2.0d,e | 10 | ND | 0.6 ± 0.2a,d,e | 11 | ND | 20.3 ± 3.2a,d,e | 6 | 4.1 (1.3–8.4)e |
| Pregnant | 5.0 ± 1.4d,e | 10 | ND | 0.4 ± 0.2a,c,d,e | 9 | ND | 13.2 ± 4.0a,c,e | 6 | 3.8 (2.6–5.6)e |
p < 0.05 versus sufficient diet of same genotype.
ND, not detectable (<0.01 µg/dl).
p < 0.05 versus non-pregnant of same genotype.
p < 0.05 versus WT of same dietary regimen.
p < 0.05 versus RBP−/− of same dietary regimen.
Taken together, these data indicate that a severe decline of circulating retinoid levels occurs when LRAT−/−RBP−/− dams are deprived of dietary vitamin A during pregnancy. These retinoid levels are reduced compared with those of RBP−/− dams bred under a similar dietary regimen, thereby explaining the most severe retinoid-deficient phenotype of both LRAT−/− RBP − /− and LRAT+/−RBP −/− embryos compared with RBP −/− embryos. With sufficient or excessive dietary intake of vitamin A, serum retinoid levels in LRAT−/− −RBP−/− dams are sufficient to support normal development of the embryos. Furthermore, increased serum retinoid levels in the double knock-out dams supplemented with vitamin A likely account for the lower percentage of embryonic resorptions observed.
To establish the effects of different regimens of dietary vitamin A intake on liver and adipose retinoid stores in LRAT−/−RBP−/− dams, we measured the concentration of retinol and retinyl ester in these tissues from pregnant and non-pregnant females. Because in LRAT−/− mice liver stores of retinyl ester are completely absent, whereas adipose retinoid concentrations are markedly elevated (18,20,21), LRAT−/−RBP−/− females maintained on the vitamin A-sufficient diet also showed very limited liver retinoid stores, represented exclusively by retinol, and significant adipose retinoid levels (supplemental Table S1). Interestingly, both hepatic and adipose retinoid levels varied according to the dietary intake of vitamin A, directly reflecting the overall retinoid status of the animals.
Retinoid Status of LRAT−/−RBP−/− Embryos
To evaluate fetal retinoid status under various maternal dietary conditions, we measured vitamin A levels in 14.5 dpc wild-type, RBP−/−, and LRAT−/−RBP−/− embryos developing from wild-type, RBP−/−, and LRAT−/−RBP−/− dams, respectively, when these dams were maintained on the vitamin A-sufficient, -deficient, or -excess diet during pregnancy (Table 3). In agreement with the severity of their developmental defects, LRAT−/−RBP−/− embryos from double knockout dams on the vitamin A-deficient diet displayed the lowest retinoid concentrations. Furthermore, LRAT−/−RBP−/− embryos always showed reduced total retinol levels compared with wild-type and RBP−/− embryos, regardless of the maternal dietary vitamin A intake. Specifically, their retinyl ester concentrations were always very low due to the lack of LRAT (18, 20, 21). In contrast, except when developing from dams deprived of dietary vitamin A, their retinol levels were similar to those of wild-type embryos. Finally, total retinol levels of RBP−/− embryos were always lower than wild-type and higher than LRAT−/−RBP−/− embryos. Moreover, RBP−/− embryos showed nearly normal retinyl ester levels and reduced retinol levels as compared with wild-type embryos, consistent with the lack of RBP (24).
TABLE 3. Retinol and retinyl ester levels of embryos from dams maintained on different dietary regimens of vitamin A.
Retinol and retinyl ester levels in embryos collected at 14.5 dpc were determined by reversed-phase HPLC. Embryonic genotype are indicated as follows: WT, wild-type; R−/−, RBP knockout; L−/−R−/−, double knock-out for LRAT and RBP. Three different regimens of maternal dietary vitamin A intake are indicated (see text and Table 1). Retinol, retinyl ester (sum of retinyl linoleate, oleate, palmitate, and stearate), and total retinoid (sum of retinol and retinyl ester) concentrations are expressed as mean ± S.D. Statistical analyses were performed for retinol and retinyl ester levels only, as described under “Experimental Procedures.” n = 3–4 embryos/group. A p < 0.05 was considered statistically significant.
| Genotype | Embryonic retinol and retinyl ester levels |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| VitA-suff |
VitA-def |
VitA-exc |
|||||||
| Retinol | Retinyl ester |
Total retinoid |
Retinol | Retinyl ester |
Total retinoid |
Retinol |
Retinyl ester |
Total retinoid |
|
| µg/g | µg/g | µg/g | |||||||
| WT | 0.12 + 0.03 | 0.32 + 0.04 | 0.44 + 0.06 | 0.09 + 0.01 | 0.19 + 0.01a | 0.29 + 0.01 | 0.29 + 0.03a | 1.03 + 0.08a | 1.25 + 0.11 |
| R−/− | 0.05 + 0.01b | 0.22 + 0.01b | 0.27 + 0.03 | 0.04 + 0.01b | 0.09 + 0.03a,b | 0.13 + 0.02 | 0.12 + 0.03a,b | 0.81 + 0.36a | 0.93 + 0.39 |
| L −/− R−/− | 0.11 ± 0.01c | 0.03 ± 0.01b,c | 0.14 ± 0.03 | 0.01 ± 0.01a,b,c | 0.03 ± 0.02b,c | 0.05 ± 0.02 | 0.26 ± 0.02a,c | 0.03 ± 0.01b,c | 0.29 ± 0.03 |
p < 0.05 versus sufficient diet group of the same genotype.
p < 0.05 versus WT on the same dietary regimen.
p < 0.05 versus R−/− on the same dietary regimen.
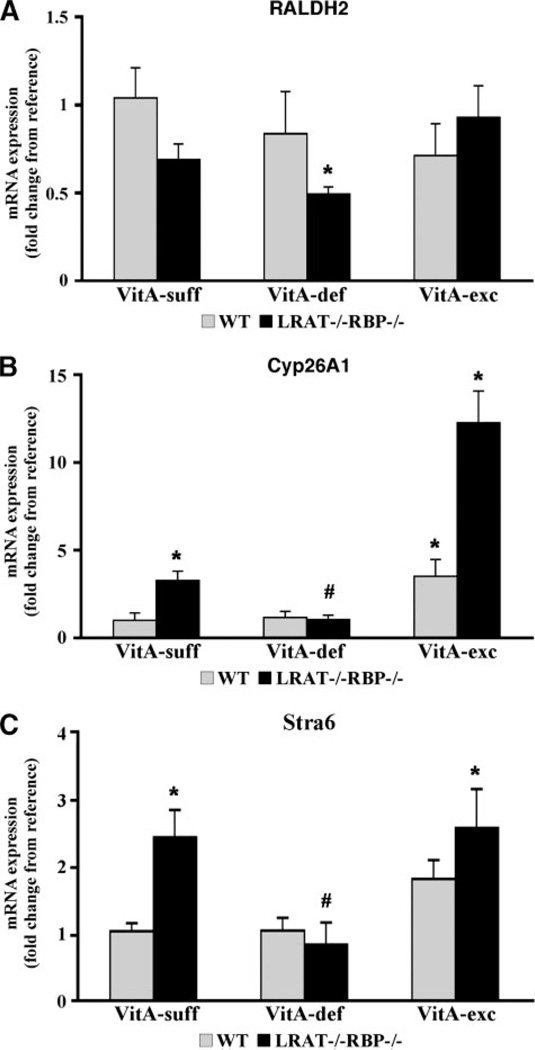
Retinol is the precursor of retinoic acid, the active form of vitamin A (7). Retinoic acid is synthesized from retinol via retinaldehyde, by a family of retinal dehydrogenases (RALDHs) that irreversibly oxidize retinaldehyde to retinoic acid (38). In the embryo, RALDH2 is the most abundant and widely expressed member of this family (39,40). Cyp26A1 is one of the cytochrome P450 family members that catalyze the oxidation of retinoic acid to polar retinoids. These retinoid forms are believed to be transcriptionally inactive and consequently unable to support embryogenesis (8–10). Cyp26A1 is also widely expressed in mouse embryonic tissues (10), and its expression, as well as that of RALDH2, appears both to be regulated by retinoic acid and to correlate with tissue levels of vitamin A (20, 39, 41, 42). Hence, we used RALDH2 and Cyp26A1 as molecular markers to monitor the retinoid status of LRAT−/−RBP−/− embryos. Expression of both genes was examined by real-time PCR in 14.5 dpc LRAT−/−RBP−/− embryos from LRAT−/−RBP−/− dams fed the three different diets. Wild-type embryos from wild-type dams maintained under identical dietary regimens served as controls. As shown in Fig. 3A, no changes were observed in RALDH2 mRNA levels in wild-type embryos, regardless of the maternal dietary vitamin A regimen. In contrast, LRAT−/−RBP−/− embryos showed increased levels of RALDH2mRNA only when LRAT−/− −RBP−/− dams were maintained on the vitamin A-deficient diet. On the other hand, Cyp26A1 expression was up-regulated in LRAT−/−RBP−/− embryos from dams maintained on the vitamin A-sufficient or -excess diets as compared with wildtype embryos from vitamin A-sufficient diet fed dams (Fig. 3B). However, when the double knock-out embryos developed from dams deprived of dietary vitamin A, Cyp26A1 levels were similar to those of controls, but lower than those of LRAT−/−RBP−/− embryos from dams on either the vitamin A-sufficient or -excess diet (Fig. 3B). Interestingly, Cyp26A1 expression was also up-regulated in wild-type embryos from dams bred on the vitamin A-excess diet (Fig. 3B).
FIGURE 3. Retinoic acid distribution and expression levels of RALDH2, Cyp26A1, and STRA6 in embryos from dams maintained on different dietary vitamin A regimens during pregnancy.
Expression levels of RALDH2 (A), Cyp26A1 (B), and STRA6 (C ) mRNA measured by real-time PCR in embryos from wild-type and LRAT−/−RBP−/− dams maintained on vitamin A-sufficient (VitA-suff), vitamin A-deficient (VitA-def), or vitamin A-excess (VitA-exc) diets during pregnancy. Values are expressed as mean ± S.E. using 2−ΔΔCT. Statistical analysis was performed as described under “Experimental Procedures.” *, p< 0.05 versus the calibrator (WT on VitA-suff diet);# p <0.05 versus the calibrator and LRAT−/−RBP−/−on the VitA-suff and VitA-excdiets. n= 4/each group. D, whole mount X-gal staining of embryos developed from dams maintained on different regimens of dietary vitamin A. The maternal dietary regimen is indicated at the top of each panel. The embryonic genotype is indicated at the bottom of each panel. Embryos were collected at 14.5 dpc from wild-type females mated with lacZ/wild-type males or from LRAT−/−RBP−/− females mated with lacZ/LRAT−/−RBP−/− males. Both lacZ/wild-type and lacZ/LRAT−/−RBP−/− males carried two copies of the transgene. Thus, all embryos from these crosses carried one copy of the lacZ transgene. The same magnification was used for all panels.
The embryonic distribution of retinoic acid in superficial tissues such as eye, limb, skin, and spinal cord was also determined by means of a retinoic acid reporter mouse strain. In these animals, the expression of a lacZ transgene is controlled by the retinoic acid response element of the RARβ2 promoter (26) and thus depends on the local availability of retinoic acid. We generated mice lacking LRAT and RBP and also carrying two copies of the lacZ transgene (lacZ/LRAT−/−RBP−/−). Males lacZ/ LRAT−/−RBP−/− were crossed with LRAT−/−RBP−/− females fed one of the different experimental diets, and whole mount X-gal staining was performed on the resulting embryos at 14.5 dpc. LacZ/wild-type embryos, from wild-type dams fed the vitamin A-sufficient diet, were used as controls (Fig. 3D, panel a). Consistent with data presented earlier, a severe and general down-regulation of transgene activity (trunk, limb, craniofacial region, and forebrain areas) was observed in lacZ/ LRAT−/−RBP−/− embryos from LRAT−/−RBP−/− dams deprived of dietary vitamin A during pregnancy (Fig. 3D, panel c) as compared with control embryos (Fig. 3D, panel a). Note, for example, the total lack of staining in the intradigital areas and eyes. In contrast, the transgene activity in lacZ/LRAT−/− RBP−/− embryos from dams maintained on the vitamin A-sufficient or -excess diet was similar to that displayed by control embryos (Fig. 3D, panels b and d).
Collectively, these data consistently confirm the severity of the retinoid deficiency experienced by the LRAT−/−RBP−/− embryos from dams deprived of dietary vitamin A. This conclusion is supported by low retinoid levels, up-regulation of RALDH2 levels, down-regulation of Cyp26A1 expression, and reduced retinoic acid distribution in superficial embryonic tissues. The data also suggest that activation of Cyp26A1 compensates for the lack of LRAT in maintaining normal distribution of retinoic acid in embryos from dams maintained on the vitamin A-sufficient diet. Interestingly, when dams are supplemented with vitamin A, embryonic up-regulation of Cyp26A1 takes place regardless of the lack of LRAT activity. This result is in contrast to RALDH2 embryonic levels that, except for LRAT−/−RBP−/− embryos from dams on the vitamin A-deficient diet, are not affected by changes in the maternal dietary vitamin A intake, regardless of the genotype of the embryos.
Role of Retinyl Ester Formation in Placenta
The placenta has been suggested to serve as a site of vitamin A storage until the embryonic liver becomes functional (11, 36,43). It also has been proposed that the placenta may release retinol to the fetus when maternal vitamin A intake is deficient and store retinol to protect the embryo from potential harmful effects of excessive maternal vitamin A intake (11, 36). Both hydrolysis and esterification of retinyl ester have been demonstrated to occur in human placenta (11). As shown in Fig. 1, LRAT is expressed in mouse placenta from 10.5 to 14.5 dpc, albeit at lower levels than in the embryonic tissues. To investigate how the placenta responds to different regimens of maternal dietary vitamin A intake and whether LRAT influences this response, we examined the expression of both RALDH2 and Cyp26A1 by real-time PCR in placentas collected at 14.5 dpc from wild-type and LRAT−/−RBP−/− dams fed the different diets. As shown in Fig. 4A, no changes were observed in RALDH2 mRNA levels in wild-type placentas, regardless of the maternal dietary vitamin A regimen. In contrast, maternal dietary vitamin A deprivation resulted in a down-regulation of RALDH2 expression in LRAT−/−RBP−/− placentas. On the other hand, Cyp26A1 expression was up-regulated in LRAT−/−RBP−/− placentas from dams on vitamin A-sufficient or -excess diets, when compared with wild-type placentas of dams bred on the vitamin A-sufficient diet (Fig. 4B). However, when double knock-out dams were maintained on the vitamin A-deficient diet, placental Cyp26A1 levels were similar to those of controls, but reduced compared with those of LRAT−/−RBP−/− placenta of dams on either the vitamin A-sufficient or -excess diet (Fig. 4B). Interestingly, Cyp26A1 expression was also up-regulated in wild-type placenta from dams maintained on the vitamin A-excess diet.
FIGURE 4. Expression levels of RALDH2, RALDH2, Cyp26A1, and STRA6 in dietary vitamin A during pregnancy.
Expression levels of RALDH2 (A), Cyp26A1 (B), and STRA6 (C ) mRNA were measured by real-time PCR in placentas from wild-type and LRAT−/−RBP−/− dams maintained on vitamin A-sufficient (VitA-suff), vitamin A-deficient (VitA-def), or vitamin A-excess (VitA-exc) diets during pregnancy. Values are expressed as mean ± S.E. using the 2−ΔΔCT. Statistical analyses were performed as described under “Experimental Procedures.” *, p< 0.05 versus the calibrator (WT, VitA-suff diet); #, p <0.05 versus the calibrator and LRAT−/−RBP−/− on the VitA-suff and VitA-exc diets. n= 4/each group.
Our data indicate that up-regulation of Cyp26A1 expression acts as a compensatory mechanism that helps to overcome the lack of LRAT and maintains normal retinoic acid homeostasis also in extraembryonic tissues. Moreover, as is the case for the embryo, maternal vitamin A supplementation induces up-regulation of Cyp26A1 in placenta, regardless of the presence or lack of LRAT activity. Except when LRAT−/−RBP−/− dams are maintained on the vitamin A-deficient diet, placenta RALDH2 levels are not affected by changes in the maternal dietary vitamin A intake, regardless of the genotype of the embryos.
STRA6 Contributes to Retinoid Homeostasis during Embryogenesis
The majority of vitamin A is acquired by tissues from retinol bound to RBP, by a mechanism that was long hypothesized to be a facilitated, protein-mediated process (44, 45). Only recently, the work of Kawaguchi et al. (23) provided evidence that Stra6 acts as a cell surface receptor for retinol-RBP. Interestingly, their work also showed that STRA6-mediated retinol uptake is enhanced in the presence of LRAT (23), leading to the hypothesis that STRA6 and LRAT act together in facilitating vitamin A uptake into tissues. Even very early in development STRA6 is widely expressed in the mouse embryo, as well as in placenta (46,47). Furthermore, STRA6 expression is up-regulated by retinoic acid (48). To investigate whether retinoid homeostasis during embryogenesis could also be maintained by regulating retinol transport across the plasma membrane, we examined the expression of STRA6 by real-time PCR in embryos and placenta collected at 14.5 dpc from wildtype and LRAT−/−RBP−/− dams fed the different diets. As shown in Fig. 3C, STRA6 expression was up-regulated in LRAT−/−RBP−/− embryos from dams maintained on the vitamin A-sufficient or -excess diets, as compared with wild-type embryos. Interestingly, STRA6 expression was also up-regulated in wild-type embryos from dams bred on the vitamin A-excess diet. However, when the double knock-out embryos developed from dams deprived of dietary vitamin A, STRA6 levels were similar to those of controls, despite being lower than those of LRAT−/−RBP−/− embryos from dams on either the vitamin A-sufficient or -excess diets (Fig. 3C). Expression levels of STRA6 in placenta of LRAT−/−RBP−/− mothers maintained on the diets with different vitamin A content followed the same pattern observed in the embryo (Fig. 4C). However, no statistically significant changes were observed in STRA6 mRNA levels in wild-type placenta, regardless of the maternal dietary vitamin A regimen (Fig. 4C).
Our data suggest that up-regulation of STRA6 expression may be a compensatory mechanism that helps overcome the lack of LRAT and maintains normal retinoid homeostasis in embryonic and extraembryonic tissues when the LRAT−/− RBP−/− mothers are maintained on the vitamin A-sufficient or -excess diets. In addition, vitamin A supplementation of wildtype dams up-regulates STRA6 expression in the embryo but not the placenta. Finally, maternal vitamin A deprivation during pregnancy does not affect expression levels of STRA6, even when it induces a severe vitamin A deficient status in the mothers, as in the LRAT−/−RBP−/− females.
DISCUSSION
Mammalian embryogenesis must be supported by retinoids acquired from the maternal circulation. These circulating retinoids consist mainly of retinol bound to RBP but also of retinyl ester incorporated in lipoprotein particles (11, 25, 37). Levels of circulating maternal retinoids, in turn, reflect maternal retinoid status, i.e. the concentration of tissue retinoid stores as well as recent dietary vitamin A. The mechanisms through which mammalian embryonic and maternal tissues maintain normal retinoid homeostasis in the face of fluctuating maternal retinoid status have not been established. Our goal for the present study was to evaluate the importance of LRAT in synthesizing retinyl ester for use in maintaining retinoid homeostasis during normal embryogenesis. To our knowledge, this function of LRAT has never been investigated.
Our data demonstrate that LRAT is expressed in both embryonic and extraembryonic tissues (placenta and yolk sac) at different stages of murine development (Fig. 1). Although we could not distinguish between the contributions of embryonic versus extraembryonic tissues to the levels of LRAT mRNA detected at very early stages of mouse development (Fig. 1), our data indicate that LRAT and retinyl ester formation may have important roles in maintaining normal embryogenesis.
To further investigate this possibility, we generated mice lacking both LRAT and RBP (LRAT−/−RBP−/− mice). In this strain, the retinol-RBP pathway, that normally ensures delivery of an adequate steady flow of retinoid to the maternal-fetal interface, is abrogated (25, 37). Furthermore, lack of LRAT markedly reduces circulating retinyl ester levels, even on a vitamin A-sufficient diet (18, 20, 21). The presence of these two mutations provides a mouse model that is extremely vulnerable to the development of vitamin A deficiency and one that could be very useful to study retinoid actions during embryonic development. Using the double knock-out mice, we showed that embryonic vitamin A deficiency can be achieved in LRAT−/− RBP−/− mice simply by removing dietary retinoid from the dams at the beginning of gestation (Table 1 and Fig. 2). The external gross malformations of these embryos were identical to those reported by other investigators employing either nutritional models of retinoid deficiency (5, 31–33) or mice bearing retinoid receptor gene disruptions (34, 35). Notably, 14.5-dpc LRAT−/−RBP−/− embryos from LRAT−/−RBP−/− dams maintained on the vitamin A-deficient diet displayed more severe malformations than 14.5−dpc RBP−/− embryos from RBP−/− dams on the same dietary regimen (Fig. 2 and Ref. 25). Malformations observed for LRAT−/−RBP−/− mice resemble those of 14.5-dpc RBP−/− embryos from RBP−/− dams deprived of dietary retinoid for more than 5 weeks prior to fertilization (25). Hence, severe embryonic vitamin A deficiency can be achieved more rapidly than in any other previously reported mammalian model of vitamin A deficiency. The ease of generating retinoid-deficient embryos as well as the paucity of reports of other models of embryonic vitamin A deficiency for which the malformed embryos survived after midgestation make the LRAT−/−RBP−/− mice a very practical model for studying retinoid-dependent organogenesis throughout gestation, especially at later developmental stages (14.5 dpc and later).
The most severe retinoid-deficient embryonic phenotypes observed in LRAT−/−RBP−/− mice were associated with a dramatic reduction of serum retinoid levels in LRAT−/−RBP−/− dams fed the vitamin A-deficient diet during pregnancy (Table 2). Moreover, LRAT+/−RBP−/− embryos developing from LRAT−/−RBP−/− dams deprived of dietary vitamin A showed the same developmental defects as LRAT−/−RBP−/− embryos, confirming that it is the maternal retinoid status rather than the lack of LRAT in embryonic tissues that ultimately causes the malformations. We speculate that the retinoid-deficient status of the double knock-out dams also caused the large percentage of resorptions observed for this strain, because the resorptions could be rescued by maternal dietary vitamin A supplementation (Table 1). Note that circulating levels of all-trans-retinoic acid (atRA), measured by normal phase HPLC (21), were not differentinLRAT−/−RBP−/− dams deprived of dietary vitamin A compared with LRAT−/−RBP−/− or wild-type dams maintained on a vitamin A-sufficient diet (wild-type dams on Vit A suff (n = 3): 2.5 ± 0.5 ng atRA/ml; LRAT−/−RBP−/− dams on VitA-suff (n = 2): 2.0 ng atRA/ml; and LRAT−/−RBP−/− dams on VitA-def (n = 3): 1.6 ± 0.5 ng atRA/ml). Neither 9-cis- and 13-cis-retinoic acids were detected in the serum of any of the mice. These results confirm that the main role of maternally circulating retinol and retinyl ester, but not circulating retinoic acid, is in providing the embryo with adequate amounts of vitamin A sufficient to support normal embryonic development.
It has been hypothesized that, by catalyzing the formation of retinoid stores, LRAT plays a crucial role in maintaining retinoic acid homeostasis by diverting retinol away from its oxidative activation to retinoic acid in adult mammalian tissues (42). This action of LRAT could be very important especially under conditions of excessive retinoid intake (42). The combined action of LRAT and Cyp26A1, one of the three cytochrome P450 enzymes that oxidize retinoic acid to more polar metabolites (8,9,49), is proposed to maintain adequate levels of tissue retinoic acid in the face of changes in dietary retinoid intake or local availability of retinoid stores in adult animals. Indeed, LRAT actions reduce the amount of retinol available for oxidation to retinoic acid, whereas Cyp26A1 actions increase the catabolism of excess retinoic acid. This hypothesis is based on several studies conducted in adult rats (42) as well as in LRAT−/− mice (20). Both LRAT and Cyp26A1 genes have been shown to be responsive to changes in local retinol levels (42). In this report, we provide evidence that LRAT contributes to retinoic acid homeostasis in mouse embryonic tissues as well. Our data demonstrate that expression of Cyp26A1 mRNA is higher in LRAT−/−RBP−/− embryos compared with wild-type embryos from dams fed the vitamin A-sufficient diet (Fig. 3B). This result suggests that LRAT and Cyp26A1 actions are coordinated in the embryo to maintain adequate levels of retinoic acid needed to support normal development. Interestingly, when dams were fed the retinoid-excess diet, embryonic Cyp26A1 mRNA expression was up-regulated, not only in the absence of LRAT but also in wild-type mice (Fig. 3B). This result suggests that there is a limit to the retinoid storage capability of the embryo, at least at 14.5 dpc. We observed no effects on embryonic Cyp26A1 expression for wild-type mice fed the vitamin A-deficient diet (Fig. 3B), possibly because this dietary regimen does not induce a retinoid-deficient status in the embryos as judged by their retinoid levels (Table 3). In contrast, LRAT−/−RBP−/− embryos from dams deprived of dietary vitamin A (Fig. 2), showed down-regulation of Cyp26A1 as well as up-regulation of RALDH2, a retinoic acid synthesizing enzyme widely expressed in embryonic tissues (39,40) (Fig. 3,B and A). These findings agree with previous reports about the expression levels of Cyp26A1 in adult tissues of LRAT−/− mice fed different vitamin A-containing diets (20) and the regulation of the RALDH2 enzyme by retinoid levels in embryonic tissues (39, 50). However, except under conditions of severe retinoid deficiency, the pathway of retinoic acid synthesis does not seem to play a crucial role in regulating retinoic acid homeostasis in developing mammalian tissue, as shown by the relatively constant expression levels of RALDH2 in the absence of LRAT or in wild-type animals maintained on different regimens of dietary vitamin A intake (Fig. 3). Our data confirm previous reports of limited or no effects of retinoic acid on embryonic RALDH2 expression both in vivo and in vitro (39, 51–53).
STRA6 was originally identified as a member of a large group of genes “stimulated by retinoic acid” in P19 embryonic carcinoma cells (48) that encoded for a protein with eleven transmembrane domains (45). The function of STRA6 remained unknown until recently, when Kawaguchi et al. (23) provided evidence that it is the cell surface receptor for retinol bound to RBP. The authors of this work also demonstrated that the tissue uptake of retinol is more efficient in the presence of LRAT, leading to the hypothesis that STRA6 and LRAT act together in facilitating this process (23). Here we demonstrate up-regulation of STRA6 mRNA embryonic levels in the absence of LRAT as well as upon excessive maternal dietary vitamin A intake (Fig. 3C). No changes in STRA6 mRNA levels were observed in embryos from dams deprived of dietary vitamin A, regardless of the presence or absence of LRAT (Fig. 3C). These findings, together with the notion that STRA6 expression is positively regulated by retinoic acid (48), led us to speculate that STRA6 may act in vivo as a bidirectional transporter for retinol. This hypothesis would fit with the need to up-regulate Stra6 expression (and thus its activity) to eliminate excessive intracellular retinol resulting from the lack of LRAT or from the maternal vitamin A supplementation. Bidirectionality of Stra6-mediated vitamin A transport has been recently shown to occur in vitro.6 Our results suggest a coordinated action of LRAT, Cyp26A1, and STRA6 to maintain normal retinoid homeostasis in the embryo. This homeostatic mechanism appears to be effective for protecting developing tissues from potentially toxic retinoic acid doses. Indeed, whole mount X-gal staining showed that retinoic acid distribution in superficial tissues of LRAT−/− RBP−/− embryos supported on either the vitamin A-sufficient or -excess diets did not differ from control mice (Fig. 3C and data not shown). Moreover, embryonic levels of atRA, assessed by normal phase HPLC (21), were not different among these groups (wild-type embryos on VitA-suff: 4.7 ± 3.6 ng atRA/g; LRAT−/−RBP−/− embryos on VitA-suff: 4.1 ± 0.5 ng atRA/g; and LRAT−/−RBP−/− embryos on VitA-exc: 6.5 ± 2.6 ng atRA/g; n = 3/group). Both 9-cis- and 13-cis-retinoic acids were not detected in any of the embryos by our HPLC assay.
The exchange of molecules and nutrients between embryonic and maternal circulations takes place in the yolk sac from 7.5 dpc and in the labyrinthine zone of the placenta as early as 12.5 dpc (54). It has been proposed that the placenta serves as a site of retinoid stores that are used to maintain steady retinol delivery in the face of fluctuations in the maternal intake of vitamin A (11). In this report, we show that, similar to embryonic tissues, an up-regulation of retinoic acid catabolism is a compensatory mechanism that maintains retinoid homeostasis in the extraembryonic tissues in the absence of LRAT for dams fed either the vitamin A-sufficient or -excess diet (Fig. 4B). We also showed that STRA6 is up-regulated in the placenta of LRAT−/−RBP−/− mice maintained on the vitamin A-sufficient or -excess diets (Fig. 4C), suggesting that, in the absence of LRAT, both Cyp26 and STRA6 contribute to retinoid homeostasis in extraembryonic tissues. However, in contrast to the embryo, vitamin A supplementation of wild-type dams only up-regulates the expression levels of placental Cyp26A1 and not STRA6 (Fig. 4,B and C), suggesting that the placenta might have a larger storage capacity compared with the embryo. Furthermore, the lack of an RALDH2 expression response to variations in maternal retinoid intake in wild-type placenta confirms that regulation of retinoic acid homeostasis does not normally involve modulating the synthesis of retinoic acid but rather its degradation even in extraembryonic tissues. Only when severe maternal-fetal retinoid deficiency occurs, as observed for LRAT−/−RBP−/− dams maintained on the vitamin A-deficient diet, will placental expression levels of RALDH2 change (Fig. 4A). However, in contrast to previous reports (39, 50), we observed a down-regulation of RALDH2 associated with low levels of maternal circulating retinoids (Fig. 4A). Possibly there are tissue-specific differences in the response of RALDH2 to retinoic acid. We speculate that the placenta responds to severe maternal vitamin A deficiency by reducing both local synthesis and degradation of retinoic acid so as to maximize the amount of retinoic acid precursors delivered to the embryo.
It has long been well documented that retinoids are indispensable for maintaining normal embryonic development (1–5). We have been interested in understanding the metabolic mechanisms through which embryonic and extraembryonic tissues respond to changes in maternal dietary retinoid intake to maintain essential retinoid-dependent functions during embryogenesis. Overall, our data demonstrate that LRAT and retinyl esters synthesized via LRAT action play a crucial role in maintaining a tight regulation of retinoid levels during embryonic development and that this regulation is achieved by striking a balance between retinyl ester synthesis, retinoic acid degradation, and possibly elimination of excess of retinol via its specific receptor. In our model system, we found little evidence to support the notion that retinoic acid synthesis is regulated in a manner that buffers against changes in maternal dietary retinoid intake.
Supplementary Material
Acknowledgments
We thank Dr. Johannes von Lintig and Dr. Filippo Mancia for helpful comments on the manuscript.
Footnotes
This work was supported in part by National Research Initiative Grant 2006-35200-16580 from the U. S. Department of Agriculture Cooperative State Research, Education, and Extension Service, Bioactive Food Components for Optimal Health (31.0) Program (to L. Q.).
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Fig. S1.
Supported by Grant EY09339 from the National Institutes of Health (NIH).
Supported by NIH Grants DK061310, DK068437, and DK079221.
The abbreviations used are: RBP, retinol-binding protein (also known as RBP4); RBP−/−, mice lacking RBP; LRAT, lecithin:retinol acyltransferase; LRAT−/−RBP−/−, mice lacking both LRAT and RBP; RALDH2, retinaldehyde dehydrogenase 2; Cyp26A1, cytochrome P450 isoform 26A1; STRA6, gene stimulated by retinoic acid 6; dpc, days post coitum; HPLC, high-performance liquid chromatography; X-gal, 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside; RT, reverse transcription; atRA, all-trans-retinoic acid; VitA-suff, vitamin A-sufficient diet; VitA-def, vitamin A-deficient diet; VitAexc, vitamin A-excess diet.
J. von Lintig and K. Palczewski, personal communication.
REFERENCES
- 1.Chambon P. Semin. Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 2.Zile MH. J. Nutr. 2001;131:705–708. doi: 10.1093/jn/131.3.705. [DOI] [PubMed] [Google Scholar]
- 3.Ross SA, McCaffery PJ, Drager UC, De Luca LM. Physiol. Rev. 2000;80:1021–1054. doi: 10.1152/physrev.2000.80.3.1021. [DOI] [PubMed] [Google Scholar]
- 4.Morriss-Kay GM, Ward SJ. Int. Rev. Cytol. 1999;188:73–131. doi: 10.1016/s0074-7696(08)61566-1. [DOI] [PubMed] [Google Scholar]
- 5.Clagett-Dame M, DeLuca HF. Annu. Rev. Nutr. 2002;22:347–381. doi: 10.1146/annurev.nutr.22.010402.102745E. [DOI] [PubMed] [Google Scholar]
- 6.Balmer JE, Blomhoff R. J. Lipid Res. 2002;43:1773–1808. doi: 10.1194/jlr.r100015-jlr200. [DOI] [PubMed] [Google Scholar]
- 7.Vogel S, Gamble MV, Blaner WS. In: in Handbook of Experimental Pharmacology. Nau H, Blaner WS, editors. Vol. 139. Heidelberg, Germany: Springer Verlag Publishing; 1999. pp. 31–96. [Google Scholar]
- 8.White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M. J. Biol. Chem. 1996;271:29922–29927. doi: 10.1074/jbc.271.47.29922. [DOI] [PubMed] [Google Scholar]
- 9.Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. J. Biol. Chem. 2004;279:77–85. doi: 10.1074/jbc.M308337200. [DOI] [PubMed] [Google Scholar]
- 10.MacLean G, Abu-Abed S, Dolle P, Tahayato A, Chambon P, Petkovich M. Mech. Dev. 2001;107:195–201. doi: 10.1016/s0925-4773(01)00463-4. [DOI] [PubMed] [Google Scholar]
- 11.Marceau G, Gallot D, Lemery D, Sapin V. Vitam. Horm. 2007;75:97–115. doi: 10.1016/S0083-6729(06)75004-X. [DOI] [PubMed] [Google Scholar]
- 12.Zolfaghari R, Ross AC. J. Lipid Res. 2000;41:2024–2034. [PubMed] [Google Scholar]
- 13.Ruiz A, Winston A, Lim YH, Gilbert BA, Rando RR, Bok D. J. Biol. Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- 14.Herr FM, Ong DE. Biochemistry. 1992;31:6748–6755. doi: 10.1021/bi00144a014. [DOI] [PubMed] [Google Scholar]
- 15.Saari JC, Bredberg DL. J. Biol. Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 16.Yost RW, Harrison EH, Ross AC. J. Biol. Chem. 1988;263:18693–18701. [PubMed] [Google Scholar]
- 17.Kurlandsky SB, Duell EA, Kang S, Voorhees JJ, Fisher GJ. J. Biol. Chem. 1996;271:15346–15352. doi: 10.1074/jbc.271.26.15346. [DOI] [PubMed] [Google Scholar]
- 18.Batten ML, Imanishi Y, Maeda T, Tu DC, Moise AR, Bronson D, Possin D, Van Gelder RN, Baehr W, Palczewski K. J. Biol. Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo X, Knudsen BS, Peehl DM, Ruiz A, Bok D, Rando RR, Rhim JS, Nanus DM, Gudas LJ. Cancer Res. 2002;62:1654–1661. [PubMed] [Google Scholar]
- 20.Liu L, Gudas LJ. J. Biol. Chem. 2005;280:40226–40234. doi: 10.1074/jbc.M509643200. [DOI] [PubMed] [Google Scholar]
- 21.O’Byrne SM, Wongsiriroj N, Libien J, Vogel S, Goldberg IJ, Baehr W, Palczewski K, Blaner WS. J. Biol. Chem. 2005;280:35647–35657. doi: 10.1074/jbc.M507924200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isken A, Holzschuh J, Lampert JM, Fischer L, Oberhauser V, Palczewski K, von Lintig J. J. Biol. Chem. 2007;282:1144–1151. doi: 10.1074/jbc.M609109200. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi R, Yu J, Honda J, Hu J, Whitelegge J, Ping P, Wiita P, Bok D, Sun H. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- 24.Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. EMBO J. 1999;17:4633–4644. doi: 10.1093/emboj/18.17.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, Mendelsohn CL. Endocrinology. 2005;146:4479–4490. doi: 10.1210/en.2005-0158. [DOI] [PubMed] [Google Scholar]
- 26.Mendelsohn C, Ruberte E, LeMeur M, Morriss-Kay G, Chambon P. Development. 1991;113:723–734. doi: 10.1242/dev.113.3.723. [DOI] [PubMed] [Google Scholar]
- 27.Reeves PG, Nielsen FH, Fahey JGC. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization. Micronutrient Series. Geneva: World Health Organization; 1998. Safe Vitamin A Dosage During Pregnancy and Lactation. Recommendations and Report from a Consultation. [Google Scholar]
- 29.National Research Council. Guide for the Care and Use of Laboratory Animals. 7th Ed. Washington, DC: National Academy Press; 1996. [Google Scholar]
- 30.Blaner WS, Obunike JC, Kurlandsky SB, al-Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. J. Biol. Chem. 1994;269:16559–16565. [PubMed] [Google Scholar]
- 31.Wolbach B. J. Bone Joint Surg. Br. 1947;29:171–192. [PubMed] [Google Scholar]
- 32.Warkany J, Schraffenberger E. Arch. Ophthalmol. 1946;35:150–169. doi: 10.1001/archopht.1946.00890200155008. [DOI] [PubMed] [Google Scholar]
- 33.Wilson JG, Roth CB, Warkany J. Am J. Anat. 1953;92:189–217. doi: 10.1002/aja.1000920202. [DOI] [PubMed] [Google Scholar]
- 34.Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- 35.Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch JL, Dolle P, Chambon P. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- 36.Satre MA, Ugen KE, Kochhar DM. Biol. Reprod. 1992;46:802–810. doi: 10.1095/biolreprod46.5.802. [DOI] [PubMed] [Google Scholar]
- 37.Quadro L, Hamberger L, Gottesman ME, Colantuoni V, Ramakrishnan R, Blaner WS. Am. J. Physiol. Endocrinol. Metab. 2004;286:E844–E851. doi: 10.1152/ajpendo.00556.2003. [DOI] [PubMed] [Google Scholar]
- 38.Duester G. Eur. J. Biochem. 2000;267:4315–4324. doi: 10.1046/j.1432-1327.2000.01497.x. [DOI] [PubMed] [Google Scholar]
- 39.Niederreither K, McCaffery P, Drager UC, Chambon P, Dolle P. Mech. Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 40.Niederreither K, Fraulob V, Garnier JM, Chambon P, Dolle P. Mech. Dev. 2002;110:165–171. doi: 10.1016/s0925-4773(01)00561-5. [DOI] [PubMed] [Google Scholar]
- 41.Loudig O, Maclean GA, Dore NL, Luu L, Petkovich M. Biochem. J. 2005;392:241–248. doi: 10.1042/BJ20050874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross AC. J. Nutr. 2003;133:291S–296S. doi: 10.1093/jn/133.1.291S. [DOI] [PubMed] [Google Scholar]
- 43.Deligdish L. In: Hormones and Fetal Pathophysiology. 5th Ed. Pasqulini JR, Sholler R, editors. New York: Marcel Dekker Inc; 1992. [Google Scholar]
- 44.Blaner WS. Cell Metabol. 2007;5:164–166. doi: 10.1016/j.cmet.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Wolf G. Nutr. Rev. 2007;65:385–388. doi: 10.1301/nr.2007.aug.385-388. [DOI] [PubMed] [Google Scholar]
- 46.Bouillet P, Sapin V, Chazaud C, Messaddeq N, Decimo D, Dolle P, Chambon P. Mech. Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 47.Sapin V, Bouillet P, Oulad-Abdelghani M, Dastugue B, Chambon P, Dolle P. Mech. Dev. 2000;92:295–299. doi: 10.1016/s0925-4773(00)00241-0. [DOI] [PubMed] [Google Scholar]
- 48.Taneja R, Bouillet P, Boylan JF, Gaub MP, Roy B, Gudas LJ, Chambon P. Proc. Natl. Acad. Sci. U. S. A. 1995;92:7854–7858. doi: 10.1073/pnas.92.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White JA, Ramshaw H, Taimi M, Stangle W, Zhang A, Everingham S, Creighton S, Tam SP, Jones G, Petkovich M. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6403–6408. doi: 10.1073/pnas.120161397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobbs-McAuliffe B, Zhao Q, Linney E. Mech. Dev. 2004;121:339–350. doi: 10.1016/j.mod.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 51.Osmond MK, Butler AJ, Voon FC, Bellairs R. Development. 1991;113:1405–1417. doi: 10.1242/dev.113.4.1405. [DOI] [PubMed] [Google Scholar]
- 52.Reijntjes S, Blentic A, Gale E, Maden M. Dev. Biol. 2005;285:224–237. doi: 10.1016/j.ydbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Sperkova Z, Napoli JL. Genomics. 2001;74:245–250. doi: 10.1006/geno.2001.6546. [DOI] [PubMed] [Google Scholar]
- 54.Cross JC. Reprod. Fertil. 2006;18:71–76. doi: 10.1071/rd05121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.