Abstract
How memory CD4 T cells contribute to protection upon pathogen challenge is not fully understood. Beyond traditional helper functions for CD8 T cell and B cell responses, memory CD4 T cells can have a potent impact on the character and magnitude of inflammatory responses. Here we discuss how memory CD4 T cell control of innate immunity at early time points after pathogen encounter can influence protective responses. We also discuss important aspects of the mechanism whereby memory CD4 T cells directly and indirectly impact the activation status of antigen presenting cells and production of inflammatory cytokines and chemokines from multiple cell types. We suggest that control of innate immune responses by the adaptive immune system is a powerful protective mechanism associated with the memory state and represents an important failsafe in the face of pathogens that fail to trigger robust inflammatory responses through conserved pattern recognition receptors.
Introduction
To successfully combat pathogens, elements of both the innate and adaptive immune system must be brought to bear as quickly as possible upon infection. The recognition of conserved pathogen-associated molecular patterns (PAMP) by germline encoded receptors expressed on the surface of, and within, many different cell types represents a critical pathway for the initiation of inflammatory responses that can act to both limit initial infection and subsequently to enhance the generation of adaptive immune responses 1. A better understanding of the importance of triggering the innate immune system has led to the successful incorporation of PAMP receptor ligands as powerful adjuvants in many vaccine formulations and therapies 2. The ability of the innate immune system to exert a powerful level of control on antigen-specific T and B cell responses is thus well-understood and has sometimes led to the paradigm that triggering of PAMP receptors is an obligate prerequisite for the generation of optimal adaptive immunity 3. Whether the adaptive immune system can influence innate inflammatory responses is less-well studied.
While many important aspects of memory T cell immunobiology have been described, a full understanding of the protective mechanisms employed by these populations during secondary challenges is lacking. This is especially relevant with regards to CD4 T cells, due at least in part to the relative difficulty of their study compared to memory CD8 T cells arising from the often dramatically lower numbers of the former that are maintained long-term in vivo 4,5. A better understanding of how memory CD4 T cells contribute to protective immune responses beyond traditional ‘helper’ functions is critical to the design of vaccine strategies against pathogens where neutralizing antibodies alone are unable to confer long-term protection 6. Here we discuss broad regulation of the innate immune system by memory CD4 T cells. Using influenza virus infection as an example, we discuss elements of the mechanism by which virus-specific memory CD4 T cells directly and indirectly activate cells of the innate immune system and lead to enhanced acute inflammatory responses. We propose that recruitment of the innate immune system represents an underappreciated protective mechanism employed by memory CD4 T cells during the early phases of pathogen challenge.
Pattern recognition, inflammation, and innate control of adaptive immunity
PAMP receptors have evolved to recognize a variety of targets expressed by different microorganisms. While the Toll-like receptors (TLR) constitute the most studied PAMP receptor family, many other distinct classes of PAMP receptors and their signaling pathways have been characterized and recently reviewed 7,8. Generally, these receptors are specific for conserved and unique elements shared by a broad class of potential pathogens such as the constituents of the cell walls of bacteria or fungi, or viral nucleic acids. It is also understood that elements of the innate immune system can be stimulated through the recognition of factors released by stressed, damaged, or dying host cells 9,10. Several of these substances, often termed damage-associated molecular patterns (DAMP), have recently been described, and their roles in the etiology of autoimmunity and potential as therapeutics offer exciting possibilities 10.
The immediate consequences of PAMP or DAMP receptor ligation are numerous leading directly and indirectly to a complex cascade of events that together are rather vaguely termed ‘inflammation’ 11. Briefly, these triggers lead initially to the production of an array of proinflammatory cytokines and chemokines, often including TNF, IL-1, and IL-6 by antigen presenting cells (APC) and other local cellular populations. These factors in turn lead to an increase in permeability of blood vessels and the influx of several classes of leukocytes to the inflamed site while local coagulation acts to constrain the dissemination of potential pathogens.
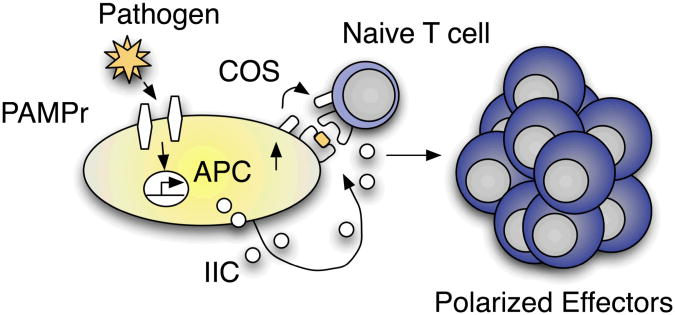
Another critical aspect of the inflammatory response is the activation of APC. Recognition of microbial products or DAMPs can lead to the upregulation of MHC:peptide complexes as well as important costimulatory markers, including CD40, that are critical to the full activation of naïve T cells. This aspect of the inflammatory response is beneficial when activated APC displaying peptides derived from pathogens migrate to draining lymph nodes, but can be potentially harmful if activated APC display immunogenic self-derived peptides. While microbial products have long been understood to enhance the development of antigen-specific immune responses, for example Freund's complete adjuvant and LPS, recent advances have led to the incorporation of other, diverse PAMPs as components of vaccine formulations 2. Such strategies are not only capable of enhancing the kinetics of antigen-specific T and B cell responses, but might also be able to direct the shape of adaptive immunity with regards to Th-polarization and antibody isotype. The paradigm of innate control of adaptive immunity, crystallized by Janeway in 1989 12, highlights the interconnectivity of the innate and adaptive defense mechanisms, but has tended to overlook the ability of elements of the adaptive immune system to shape and regulate innate inflammatory responses (Figure 1).
Figure 1.
Innate control of adaptive immunity. Uptake of pathogens or pathogen products by APC stimulates PAMP receptors (PAMPr) resulting in the upregulation of costimulatory molecules (COS) by APC as well as the production of innate inflammatory cytokines and chemokines (IIC). In addition to recognizing specific antigen presented by APC, COS and IIC are critical in driving naïve CD4 T cells to become properly polarized effector cells.
Functional attributes of memory CD4 T cells
Following the resolution of a primary immune response, the majority of antigen-specific T cell effectors die leaving a small but relatively stable population of memory cells at frequencies higher than found in the naïve state 4. While a quantitative gain in antigen-specific lymphocytes represents an important advantage of the antigen-specific memory state, many studies have demonstrated important qualitative attributes that distinguish naïve from memory CD4 T cells 13. First, and perhaps most importantly, memory CD4 T cells retain the ability to produce Th-associated cytokines associated with the effector cells from which they arise shortly after TCR stimulation – for example IFNγ, IL-4, and IL-17 for Th-1, Th-2, and Th17-polarized populations, respectively 14,15. In contrast, naïve CD4 T cells produce a far more restricted set of cytokines, predominantly IL-2, after TCR stimulation and this only after a substantial lag period. Second, memory cells are less dependent on costimulatory signals for maximal responses than naïve cells 16,17. Third, memory cells respond maximally to substantially lower concentrations of antigen than naïve CD4 T cells bearing the same TCR 14. Finally, while naïve CD4 T cells must be activated in lymphoid tissues by interacting with activated peptide-bearing APC, capable of providing strong costimulation, memory CD4 T cells can be activated in peripheral tissues 15,18.
These properties and others are generally believed to endow memory CD4 T cells with superior helper capabilities as compared to naïve CD4 T cells in activating, and shaping, antigen-specific CD8 T cells and B cell responses leading to secondary responses that are ‘faster, larger, and better’. CD4 T cells are generally referred to as ‘T helper cells’, underscoring their specialized role in orchestrating immune responses, but the term is perhaps sometimes unhelpfully restrictive. We have recently demonstrated that the specialized qualities of memory CD4 T cells discussed above also endow this population with unique abilities to initiate production of broad innate inflammatory responses upon antigen recognition 15. As we will discuss, the elaboration of enhanced innate inflammatory cytokines an chemokines (IIC) and the activation of innate populations at the site of infection mediated by memory CD4 T cells represents a novel protective mechanism operating during the early phases of pathogen challenge.
Memory CD4 T cells enhance innate inflammation upon influenza challenge
When influenza-primed mice are challenged with a heterosubtypic strain of virus (expressing surface proteins not recognized by neutralizing antibody specific for the priming virus), significantly higher levels of many IIC, including TNF, IL-1α, IL-1β, IL-6, IL-12, IFNγ, CXCL9, CXCL10, CCL2, and CXCL1, are observed in the lung and to a lesser extent in the serum at 40 hours post-infection compared to what is observed after challenge of naïve mice with the same dose of virus. The magnitude of the IIC response in primed mice can be significantly decreased by the depletion of CD4 T cells prior to challenge and increased IIC responses are observed in unprimed mice infected with influenza if virus-specific memory CD4 T cells are transferred prior to infection 15. Not unexpectedly, the impact of memory CD4 T cells on the level of IIC requires recognition of specific antigen in an MHC II-restricted manner. Equivalent upregulation of IIC was observed after influenza infection when memory cells were transferred to wildtype mice and mice that only express MHC II on CD11c+ cells. This is somewhat surprising given that influenza infected lung epithelial cells dramatically upregulate expression of MHC II (unpublished observations).
Memory, but not naïve CD4 T cell transfer also dramatically upregulated expression of MHC II and costimulatory molecules on CD11c+ cells present in the lung at 40 hours post-infection and similar effects were observed upon in vitro culture of memory CD4 T cells, specific antigen, and dendritic cells 15. Furthermore, in vitro experiments utilizing both dendritic cells and alveolar macrophages cultured with memory CD4 T cells revealed substantial production of several IIC from both APC populations that required cell to cell contact. Interestingly, while previous studies found that coculture of activated Th1 clones, adjuvant-free soluble antigen and APC in vitro drove IL-12 production in a CD40L-CD40-dependent manner 19, we observed substantial IIC upregulation driven by memory CD4 T cells in the absence of CD40L-, CD28-, ICOS-, and OX-40-, and CD70-dependent signaling (unpublished observations). We also found that IFNγ and TNF signals from memory CD4 T cells were not required to activate APC both in vitro and in vivo (unpublished observations).
In addition to enhanced IIC production from dendritic cells and macrophages, it is likely that several other cell types contribute to the inflammatory response mediated by memory CD4 T cells recognizing antigen in the lung. For example, we observed enhanced IFNγ production from lung-resident γδ T cells and NK cells 15. These findings lead us to propose that the initial encounter between a flu-specific memory CD4 T cell and a peptide-bearing APC results in the activation of the APC, the production of an initial restricted set of IIC, and the induction of a cascade of soluble and perhaps cell-surface signals that eventually recruit other innate populations into the early inflammatory response. Surprisingly, transfer of memory CD4 T cells specific for the protein ovalbumin (OVA) to unprimed mice that were challenged intranasally with LPS-free OVA drove enhanced IIC to similar levels as observed after influenza infection. This suggests that PAMP or DAMP signals are not involved in facilitating the enhanced inflammatory response mediated by memory CD4 T cells upon antigen recognition.
Regulation of inflammation by T cells
To test whether the enhanced inflammatory response mediated by memory CD4 T cells upon antigen recognition represents a general activity of memory cells we transferred either unpolarized (Th0), Th1-, Th2-, or Th17-polarized memory cells specific for influenza to unprimed mice and challenged with virus. Correlating with the ability of Th1- and Th17-polarized CD4 T cell effectors to protect mice against lethal influenza challenge 20, Th1- and Th17-polarized memory cells induced enhanced IIC while Th2 and Th0 populations did not, correlating with the inability of Th2 or Th0 effectors to protect mice against lethal challenge (unpublished observations). These findings demonstrate that differentially polarized memory CD4 T cells specific for the same antigen are capable of influencing acute inflammatory responses in unique ways.
In this respect it is interesting that other recent studies have found an ability of T cells to also suppress innate inflammatory responses. For example, T cell-deficient nude mice infected with mouse hepatitis virus (MHV) demonstrate a high mortality rate compared to infection of WT mice that correlates not with increased viral load, but with much higher levels of IIC 21. Transfer of naïve T cells to nude mice before MHV infection reduces the inflammatory response and rescues nude mice from morbidity 21. Similarly, co-culture of memory phenotype CD4 T cells isolated from the spleen of unimmunized mice with dendritic cells has been reported to dampen production of IL-1β and IL18 22. Thus, it is possible to propose that while naïve T cells, or Treg populations 22, act to temper IIC upon antigen recognition, memory CD4 T cells can act to either enhance or otherwise regulate IIC upon antigen recognition depending on their Th-polarization. It is likely that further variables, such as the site of infection and the route of exposure, might also significantly impact the character and magnitude of the acute inflammatory response.
This mechanism holds two advantages. First, upon initial encounter with a pathogen, the adaptive immune system acts to keep acute inflammation in check that otherwise itself may cause undue immunopathology 22. Second, upon re-infection, memory CD4 T cells specific for the pathogen, that were primed and polarized partly through signals delivered by the initial inflammatory milieu established through PAMP recognition, act to increase the tempo and magnitude of a similar protective inflammatory response. Memory CD4 T cells can remember the inflammatory environment that they were generated in and can play an important role in rapidly re-establishing a similar inflammatory setting.
Protective impact of enhanced inflammation mediated by memory CD4 T cells
To directly test whether the enhanced inflammatory response mediated by memory CD4 T cells can contribute to a protective response against pathogen, we transferred either naïve or memory CD4 T cells recognizing OVA (OT-II) to unprimed mice, infected with A/PR8-OVA virus, which expresses the OVA peptide recognized by OT-II cells, and measured viral titers on days 2-4 post flu-infection. Memory cell transfer resulted in significantly lower virus detected in lungs from mice receiving memory CD4 T cells as early as day 3 post-infection. Importantly, when LPS-free OVA was given together with A/PR8 virus, which does not express the OVA peptide recognized by OT-II cells, to mice that had received OVA-specific memory CD4 T cells, viral titers were similarly reduced when compared to mice that had received naïve OT-II cells. Finally, when adiminstration of OVA protein preceded A/PR8 infection by 7 days, the protective impact of memory OT-II cells was lost 15. This experiment reveals three important points. First, while the initiation of enhanced inflammation by memory CD4 T cells requires cognate antigen recognition, the protective impact of the response does not. Second, as the OVA-specific memory CD4 T cells can not recognize virally infected epithelial cells infected with A/PR8 virus, the protective impact of their response to lower viral titers presumably acts through elements of the enhanced inflammatory response induced and is unlikely to depend on direct antiviral actions of the memory CD4 T cells. This hypothesis is supported by recent studies in which the induction of enhanced inflammatory responses in mice prior to lethal influenza challenge significantly reduced viral titers and enhanced survival 23. Finally, the protection afforded through enhanced inflammatory responses mediated by memory CD4 T cell antigen recognition is transient in nature and does not result in a long-term antiviral state.
How does memory CD4 T cell-mediated enhanced IIC contribute to heterosubtypic protection against influenza?
When mice previously primed with influenza are challenged with a dose of heterosubtypic virus that is supralethal for naïve animals, T cell responses against conserved internal influenza proteins can provide a strong degree of protection. Survival of primed mice is dependent on CD8 T cells, as depletion of this subset prior to heterosubtypic challenge abrogates protection 24. In contrast, depletion of CD4 T cells before challenge results in more severe disease, highlighted by earlier weight loss and significantly delayed recovery. We propose that the acute enhanced inflammatory responses mediated by virus-specific memory CD4 T cells contributes to protection in at least two distinct ways during heterosubtypic challenge.
First, direct control of viral titers through enhanced inflammation during the first few days of infection could provide an important restraint on influenza replication and spread during the development of maximal effector T cell responses that ultimately are responsible for viral clearance. It is likely that this early viral control dependent on memory CD4 T cells is due to elements of the inflammatory response acting through or on various innate populations as well as the lung epithelium 25,26 (Figure 2). The importance of individual components of the enhanced IIC response to viral control remain to be determined, but it is likely that several elements are involved. For example, IL-1, IL-6, and IL-12 have all been shown to influence protective responses against influenza 27-29. Second, the earlier activation of APC populations in the lung mediated through contact-dependent interactions with memory CD4 T cells could contribute to protection indirectly through enhancing the kinetics of and/or magnitude of virus-specific CD4 and CD8 T cell and B cell antibody responses 7,30. It is also possible that chemokine gradients established through enhanced IIC act to facilitate the more rapid influx of cellular populations important for viral control. Such a mode of action has been described by Nakanishi et al. in an herpes simplex virus infection model where CD4 T cells are required for the induction of CD8 T cell-recruiting chemokines in the infected tissue 31.
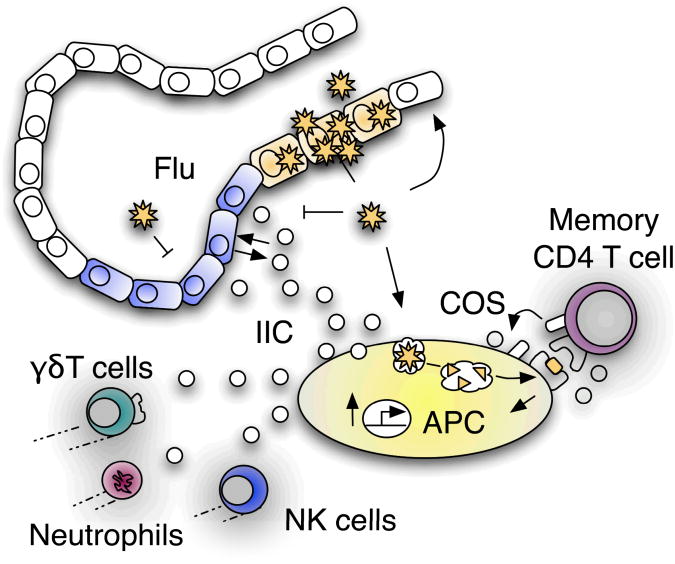
Figure 2.
Memory CD4 T cell control of innate inflammation during influenza infection. Memory CD4 T cells recognizing influenza (flu) presented by APC drive upregulation of COS and IIC independently of PAMP signals. IIC produced by the initial interaction between memory CD4 T cells and APC call in diverse elements of the innate immune system into the infected lung that also produce IIC. IIC and perhaps other aspects of the enhanced innate response mediated by memory CD4 T cells act on lung epithelial cells to drive further IIC production and to control virus during the initial days of infection.
Adaptive Control of Innate Immunity
Memory CD4 T cell control of innate inflammation during pathogen challenge has several teleological benefits. First, this alternative mechanism of eliciting acute inflammation represents a failsafe in situations where pathogens evade PAMP recognition 32. Second, memory CD4 T cells by virtue of the capacity for maximal responses to very low concentrations of antigen and costimulation may initiate innate effector mechanisms before pathogen levels are sufficient to drive maximal inflammatory responses through PAMP receptors. This may be of critical importance in the context of slowly replicating pathogens such as tuberculosis, trypanosomes, and hepatitis B virus 33. Similarly, our results suggest that the ‘stealth’ phase that characterizes infections such as flu, where the pathogen gains an upper hand through several rounds of replication before the initiation of innate or adaptive immunity 34, can be substantially abbreviated, or perhaps eliminated through the action of memory CD4 T cells. It is possible that memory CD4 T cell triggering of earlier inflammation may also be beneficial in the context of vaccine strategies against HIV 35.
Third, regulation of innate effector mechanisms by memory CD4 T cells may counteract direct suppression of inflammation mediated by pathogens such as influenza through the actions of the NS1 protein 36. Fourth, beyond initial control of infection through inflammatory responses, the establishment of Th-polarizing cytokine environments, and the activation of APC populations by memory CD4 T cells could facilitate the development of optimal adaptive immunity at later stages of pathogen-specific responses. Indeed, increased production of TNF, IL-1, and IL-6 driven by memory CD4 T cells may be of particular importance in the context of orchestrating optimal adaptive responses in the elderly 37.
The adjuvant-like properties of CD4 T cells listed above have obvious implications in vaccine design and may help explain observations of strong immune responses to protein antigens in the absence of PAMP signaling 38. However, many of the advantages of memory CD4 T cell control of innate immunity in situations of infectious disease may also be deleterious in situations of autoimmunity. Indeed, memory CD4 T cells are implicated in the etiology of several autoimmune diseases, both through helper functions and other mechanisms 39-42. A recent study found that activated CD4 T cells were able to stimulate production of several IIC from human monocytes in vitro in a contact dependent manner, suggesting that this function of autoreactive CD4 T cells may play an important role in driving inflammation in rheumatoid arthritis 43. A fuller understanding of the signals involved in regulating innate inflammatory responses by memory CD4 T cells may thus not only be harnessed for protective responses against pathogens, or tumors, but could provide an early target in the treatment of autoimmunity.
Concluding Remarks
Innate inflammatory responses serve to combat initial infection and to optimize the subsequent generation of antigen-specific immune responses. The importance of early inflammatory responses to host protection is most clearly seen in the multiple, evolutionarily conserved PAMP receptor systems. Control of adaptive immunity through triggering of PAMP receptors occurs both through the activation of APC, which increases the kinetic development of subsequent T cell responses, and through the release of important Th-polarizing cytokines, which help combat infection and also ensure the generation of appropriately polarized T cell responses. It is clear that in a naïve state innate control of adaptive immunity represents a critical mechanism of regulation, without which invading pathogens would more routinely gain an upper hand in what is often a race between microbial replication on the one hand and clonal selection and expansion on the other.
In the memory state, however, the situation is inherently changed. Memory CD4 T cells, that are present in higher numbers than in the naïve state and that exhibit unique functional properties, can dramatically regulate innate inflammatory responses upon antigen recognition. Memory CD4 T cells, in the absence of PAMP receptor recognition, can activate APC, direct the secretion of Th-polarizing cytokines as well as a broad range of IIC that can play a key role in acute control of pathogens. We believe that this represents a critical advantage of the memory state and that this mechanism adds an important branch to the sometimes too prevailing linear view of ‘innate control of adaptive immunity’ (Figure 3). Harnessing the adjuvant qualities of memory CD4 T cells may provide a potent boost to vaccine strategies aimed against many prominent pathogens.
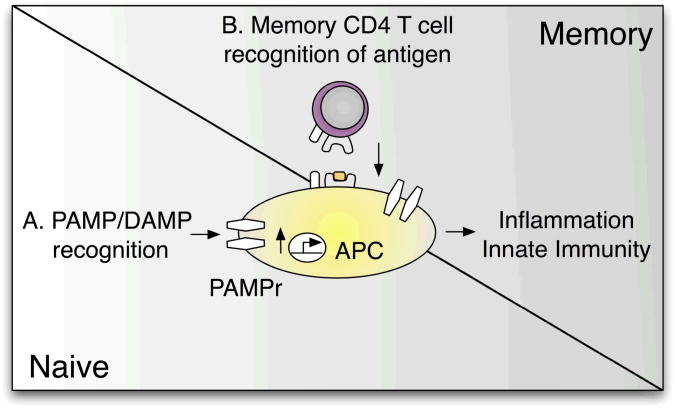
Figure 3.
Memory CD4 T cell control of innate immunity. Recognition of PAMPs and DAMPs (A) is a crucial trigger initiating innate immune responses in the naïve state. In a primed environment, in addition to pattern recognition receptors, antigen-specific memory CD4 T cells can initiate inflammatory responses and APC activation even in the absence of PAMP/DAMP recognition (B).
References
- 1.Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–775. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 3.Joffre O, Nolte MA, Sporri R, Reis e Sousa C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol Rev. 2009;227:234–247. doi: 10.1111/j.1600-065X.2008.00718.x. [DOI] [PubMed] [Google Scholar]
- 4.McKinstry KK, Strutt TM, Swain SL. The effector to memory transition of CD4 T cells. Immunol Res. 2008;40:114–127. doi: 10.1007/s12026-007-8004-y. [DOI] [PubMed] [Google Scholar]
- 5.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 6.Strutt TM, McKinstry KK, Swain SL. Functionally diverse subsets in CD4 T cell responses against influenza. J Clin Immunol. 2009;29:145–150. doi: 10.1007/s10875-008-9266-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 140:805–820. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 10.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rivas F. In this Issue: Inflammation. Cell. 140(755):757. [PubMed] [Google Scholar]
- 12.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 13.McKinstry KK, Strutt TM, Swain SL. The potential of CD4(+) T-cell memory. Immunology. doi: 10.1111/j.1365-2567.2010.03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinstry KK, et al. Rapid default transition of CD4 T cell effectors to functional memory cells. J Exp Med. 2007;204:2199–2211. doi: 10.1084/jem.20070041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strutt TM, et al. Memory CD4(+) T cells induce innate responses independently of pathogen. Nat Med. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–2685. [PubMed] [Google Scholar]
- 17.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–272. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 18.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 19.Shu U, et al. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 20.McKinstry KK, et al. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guarda G, et al. T cells dampen innate immune responses through inhibition of NLRP1 and NLRP3 inflammasomes. Nature. 2009;460:269–273. doi: 10.1038/nature08100. [DOI] [PubMed] [Google Scholar]
- 22.Kim KD, et al. Adaptive immune cells temper initial innate responses. Nat Med. 2007;13:1248–1252. doi: 10.1038/nm1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tuvim MJ, Evans SE, Clement CG, Dickey BF, Gilbert BE. Augmented lung inflammation protects against influenza A pneumonia. PLoS One. 2009;4:e4176. doi: 10.1371/journal.pone.0004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell TJ, et al. Priming with cold-adapted influenza A does not prevent infection but elicits long-lived protection against supralethal challenge with heterosubtypic virus. J Immunol. 2007;178:1030–1038. doi: 10.4049/jimmunol.178.2.1030. [DOI] [PubMed] [Google Scholar]
- 25.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- 27.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SW, Youn JW, Seong BL, Sung YC. IL-6 induces long-term protective immunity against a lethal challenge of influenza virus. Vaccine. 1999;17:490–496. doi: 10.1016/s0264-410x(98)00223-0. [DOI] [PubMed] [Google Scholar]
- 29.Hama Y, et al. Interleukin 12 is a primary cytokine responding to influenza virus infection in the respiratory tract of mice. Acta Virol. 2009;53:233–240. doi: 10.4149/av_2009_04_233. [DOI] [PubMed] [Google Scholar]
- 30.Riberdy JM, Christensen JP, Branum K, Doherty PC. Diminished primary and secondary influenza virus-specific CD8(+) T-cell responses in CD4-depleted Ig(-/-) mice. J Virol. 2000;74:9762–9765. doi: 10.1128/jvi.74.20.9762-9765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinski P, Moser M. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat Rev Immunol. 2005;5:251–260. doi: 10.1038/nri1569. [DOI] [PubMed] [Google Scholar]
- 33.Davenport MP, Belz GT, Ribeiro RM. The race between infection and immunity: how do pathogens set the pace? Trends Immunol. 2009;30:61–66. doi: 10.1016/j.it.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Moltedo B, et al. Cutting edge: stealth influenza virus replication precedes the initiation of adaptive immunity. J Immunol. 2009;183:3569–3573. doi: 10.4049/jimmunol.0900091. [DOI] [PubMed] [Google Scholar]
- 35.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hale BG, Randall RE, Ortin J, Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 37.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Saout C, Mennechet S, Taylor N, Hernandez J. Memory-like CD8+ and CD4+ T cells cooperate to break peripheral tolerance under lymphopenic conditions. Proc Natl Acad Sci U S A. 2008;105:19414–19419. doi: 10.1073/pnas.0807743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elyaman W, et al. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am J Pathol. 2008;173:411–422. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latham KA, Whittington KB, Zhou R, Qian Z, Rosloniec EF. Ex vivo characterization of the autoimmune T cell response in the HLA-DR1 mouse model of collagen-induced arthritis reveals long-term activation of type II collagen-specific cells and their presence in arthritic joints. J Immunol. 2005;174:3978–3985. doi: 10.4049/jimmunol.174.7.3978. [DOI] [PubMed] [Google Scholar]
- 42.Davis LS, Schulze-Koops H, Lipsky PE. Human CD4+ T cell differentiation and effector function: implications for autoimmunity. Immunol Res. 1999;19:25–34. doi: 10.1007/BF02786474. [DOI] [PubMed] [Google Scholar]
- 43.Beech JT, et al. T-cell contact-dependent regulation of CC and CXC chemokine production in monocytes through differential involvement of NFkappaB: implications for rheumatoid arthritis. Arthritis Res Ther. 2006;8:R168. doi: 10.1186/ar2077. [DOI] [PMC free article] [PubMed] [Google Scholar]