Abstract
Background
The mechanisms that could explain the poor sensitivity to 5-FU in certain colorectal cancer (CRC) cells were investigated and whether or not co-treatment with low doses of selenium would offer a therapeutic benefit, was explored.
Materials and Methods
Four CRC cell lines (Caco2, RKO, DLD1 and HT-29), with defined tumor signatures and seven different chemical forms of selenium were tested.
Results
5-FU partially inhibited the HT-29 and RKO cells, but had a weak effect on the DLD1 and almost none on the Caco2 cells. Selenous acid and sodium selenite induced growth inhibition of the DLD1, RKO and HT-29 cells, with a marginal effect on the Caco2 cells. The Caco2 cells with mutant p53, failure to activate caspase-8, −9, −7 and −3 and with hypermethylated caspase-8 were resistant to 5-FU. Conversely, RKO cells expressing wild type p53, proteolytically activated caspase-8, −9, −7 and −3 and unmethylated caspase-8 were more responsive to 5-FU and selenous acid induced apoptosis.
Conclusion
Combination treatment with selenous acid may offer an efficacious strategy to overcome 5-FU resistance in certain CRC cells.
Keywords: Colorectal cancer, caspases, selenium, selenous acid, 5FU, apoptosis
Colorectal cancer (CRC) remains a leading cause of mortality among many racial and ethnic groups throughout the world. In fact, about 782,000 people worldwide are diagnosed with CRC each year. In the United States, CRC is the second most common cause of cancer-related deaths in men and women with an estimated incidence of 153,760 and a mortality of 52,180 in 2007. The incidence of CRC has increased by 6% from 2004 to 2007 (1, 2).
For several years, 5-fluorouracil (5-FU), a pyrimidine anti-metabolite, has been the drug of choice for the treatment of CRC as well as head and neck, pancreatic and breast carcinomas. 5-FU is a pro-drug that requires conversion to 5-fluorodeoxyuridine-monophosphate (5FdUMP) and 5-fluoro-deoxyuridine-triphosphate (5FUTP) in cancer cells. 5-FU, alone or in combination with biomodulators such as leucovorin, levamisol and interferon-α has become an effective systemic treatment in the adjuvant setting and advanced stage of disease (3–5). New drugs such as the topoisomerase I inhibitor, irinotecan (CPT-11) and the platinum derivative oxaliplatin, have shown a significant anti-tumor activity in CRC patients (6). Targeted therapies in the form of the monoclonal antibodies bevacizumab and cetuximab have demonstrated exciting results (7) in controlling CRC metastasis.
Although most CRC patients undergo curative surgery, survival is dependent on several factors, including the pathological stage. Overall survival after adjuvant therapy of 5-FU/leucovorin/oxaliplatin (MOSAIC study) and the oral fluoropyrimidines has improved, but the risk of recurrence is still high (8). 5-FU is known to block DNA synthesis by the inhibition of thymidylate synthase (TS), which is regulated by cell cycle proteins controlled by phosphorylation (9). The anti-tumor effect of 5-FU is due to the induction of apoptosis resulting from the regulation of several molecules such as Bax, relative to bcl-2 or bcl-xL in CRC cells (10). Unfortunately many of the therapies that use 5-FU alone or in combination with other agents are likely to become ineffective due to drug resistance, namely to 5-FU.
The dietary intake of certain nutrients has been associated with a reduction in the risk of CRC. In particular, selenium has been shown to reduce the incidences of several malignancies such as colon, prostate and lung cancer (11). Although, several in vitro studies have suggested strong anti-tumor potential for selenium, the epidemiological reports have been uncertain. In a recent editorial, Duffield-Lillico et al (12) suggested that the association between selenium and CRC has been inconsistent, and only a limited number of studies have been performed. Perhaps the most conclusive evidence of the benefits of selenium for reducing the risk for colorectal cancer has come from a large randomized study on the prevention of melanoma by Clark et al (13). In addition, it is anticipated that the association between selenium and CRC development will be assessed as a pre-specified secondary end-point of the Selenium and Vitamin E Cancer Prevention Trial (SELECT) designed to study prevention of prostate cancer in 35,000 men (14). There are however, no studies that have examined the mechanisms associated with combination treatment of selenium and the chemotherapeutic agent 5-FU in CRC. As a single agent, selenium has been proven to inhibit tumor growth and induce apoptosis in many cancer cells (15). Selenium arrests cells in the G1-phase of the cell cycle by inhibiting cyclins and cyclin-dependent kinases (CDKs), proliferating cell nuclear antigen (PCNA) and transcriptional factor (E2F), while it induces the expression of p53, p19, p21, growth arrest and DNA damage-inducible-153 (Gadd153) and certain caspases (16). Our previous studies have shown that yeast selenium caused cell apoptosis in breast, colon and hepatocellular tumors by tissue specific effects (15). Cancer cells generate increased levels of free radicals and therefore may be more susceptible to attack from anti-oxidants, such as selenium (16). Selenium has been suggested to play a role in building the immune response in patients undergoing chemotherapy (17).
Studies from our laboratory have shown that, among the many available selenium compounds, selenous acid was the most effective selenium source for inhibiting the growth of prostate cancer cells, while yeast selenium was effective for growth inhibition of breast cancer cells (15). In combination with yeast selenium, lower concentrations of both adriamycin and Taxol gave equally effective growth inhibition however CRC cells were relatively unresponsive to growth inhibition by yeast selenium. Yeast selenium has been investigated as a drug of choice in a phase II double-blind, placebo-controlled trial with or without celecoxib for colon polyp recurrence (18). A recent review by Lipinski, however, argued in favor for the use of selenous acid or its sodium salt sodium selenate for clinical studies (19). Physiological levels of selenium compounds in the tissues and serum has been tested for biomarker evaluation in cancer patients (20, 21).
In the present study, several selenium compounds were tested for their anti-cancer properties in four different CRC cell lines. The primary objective was to identify which selenium compound was most effective at a physiologically low and safe dose and if this low dose was efficacious for combination with 5-FU to overcome drug resistance. The mechanisms by which CRC cells undergo drug induced apoptosis, were elucidated.
Materials and Methods
Cell culture and reagents
Four different CRC cell lines, HT-29, Caco2, RKO and DLD1, each with specific pathology and tumor characteristics were tested. The characteristics of each cell line are as follows. The HT-29 cell line was established from a 44 year old female Caucasian with colorectal adenocarcinoma (22). It secretes carcinoembryonic antigen (CEA), transforming growth factor-beta (TGF-beta) binding protein, and mucin. It is positive for the myc, ras, myb, fos, p53, abl- and src oncogenes, overexpresses the p53 antigen and has a G > A mutation in codon 273, resulting in an Arg>His substitution. The Caco2 cell line was established from a 72 year old Caucasian male with colorectal adenocarcinoma. This cell type has a mutated p53 and expresses retinoic acid binding protein I and II (23). Caco2 cells express the mismatch repair (MMR) proteins homologue of the bacterial MutL (hMLH-1), homologue of the postmeiotic segregation (hPMS2), homologues of the bacterial MutS (hMSH-2, hMSH-6) and p27 (confirmed in our laboratory) and are microsatellite stable (MSS). The RKO is a poorly differentiated colon carcinoma cell line (24). RKO cells contain wild-type p53 but lack endogenous human thyroid receptor nuclear receptor (h-TRbeta1). The level of p53 protein is high in the RKO cell line and it expresses the MMR proteins MSH-2 and MSH-6 and nuclear p21 (confirmed in our laboratory). It can be used as the control cell line for investigating the effects of p53 and growth arrest and DNA damage-inducible-45 (gadd45) on cellular parameters. The DLD1 cell line is of epithelial origin and was isolated from an adult male patient with colorectal adenocarcinoma of Dukes’ type C (25). DLD-1 cells are positive for p53 antigen expression (the p53 antigen produced has a C > T mutation resulting in Ser > Phe at position 241). The cells are positive for keratin and for the expression of the c-myc, K-ras, H-ras, N-ras, myb, sis and fos oncogenes. N-myc oncogene expression was not detected. The tumor specific nuclear matrix proteins CC-2, CC-3, CC-4, CC-5 and CC-6 are expressed and these cells are positive for hMLH-1 and hPMS2 (confirmed in our laboratory). MCF7, a human breast cancer cell line that expresses the wild-type p53 was used as a control.
They were all obtained from the American Tissue Culture Collection (ATCC, Manassas, VA, USA) and cultured as described previously (15). In brief, the cells were cultured in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12 (Cellgro, Mediatech, Inc., Herndon, VA, USA), supplemented with 5% (v/v) heat-inactivated fetal bovine serum (FBS) (HyClone, A Perbio Science Co., Logan, Utah, USA)), 2.5 mM L-glutamine, 50 µg/ml penicillin and streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Seven different types of selenium compound either in organic or inorganic form were tested and all the selenium compounds and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reagents were purchased from Sigma (Saint Louis, MO, USA), while yeast selenium was a gift from Viva Life Sciences (Westar, CA, USA) (15). 5-Flourouracil (5-FU) and the caspase inhibitor IV (Z-VAD-FMK) were purchased from EMD Biosciences, Inc. (La Jolla, CA, USA). Antibodies against caspase-9, caspase-8, cleaved caspase-7, cleaved caspase-3, and poly(ADP-ribose)polymerase (PARP) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibody against p53 (DO-1) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA), and p53 (Ab-8) from NeoMarkers, Inc (Fremont, CA, USA). Anti-β-actin was purchased from Sigma. The TUNEL Apoptosis Kit was purchased from Upstate Serologicals Co. (Lake Placid, NY, USA). The mounting medium for immunofluoresence with PI (propidium iodide) was from Vector Laboratories, Inc.(Burlingame, CA, USA).
Treatment of CRC cells with 5-FU and selenium compounds
Seventy percent confluent cells were treated with 5 to 25 µM of 5-FU in fresh growth medium for 24 to 96 h. All the selenium compounds selenocystamine; L+selenomethionine; selenous acid; sodium selenate; sodium selenite and selenic acid were tested at the same concentration of 3.88 µM, while the yeast selenium was at 0.5 µg/ml. For the combination treatment, 5 µM of 5-FU with 3.88 µM of selenous acid or 0.5 µg/ml of yeast selenium were used and incubated for 24 to 96 h in DMEM media containing 10% FBS. The cells were incubated with the inhibitors of specific cell signals in growing culture media for 4 h before 5-FU and selenium treatment.
Cell proliferation assay
Cell proliferation was assessed with the MTT assay (15). Briefly, 1× 104 CRC cells/well were plated in a 96 well plate and treated with the range of 5-FU concentrations or the individual selenium compounds, or the combination of 5-FU with selenous acid or yeast selenium for 24 to 72 h. The monolayer cultures were washed 2 times with phosphate buffered saline (PBS) and incubated with MTT reagents for 4 h at 37°C.
Cell morphology analysis
The morphological changes induced by the treatment with 5µM 5-FU were recorded under phase-contrast microscopy.
Western blot analysis
The CRC cells were grown to 70% confluence in 10 cm2 tissue culture dishes and exposed to selenous acid and/or 5-FU. After 48 h of treatment, the cells were lysed using ice-cold cell lysis buffer (Cell Signaling Technology Inc). To study the p53 and PARP expression, the cells were lysed with 1x lysis buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4, 1 µg/ml leupeptin, and 1 mM phenylmethylsulfonyl fluoride). To study caspases-9, −8, −7 and −3, the cells were lysed with 1x Chaps lysis buffer (50 mM Pipes/NaOH, pH 6.5, 2 mM EDTA, 0.1% Chaps, 5 mM Dithiothreitol [DTT], 20 µg/ml leupeptin, 10 µg/ml pepstatin, 10 µg/ml aprotinin, and 1 mM phenyl methyl sulfonyl fluoride [PMSF]) (Cell Signaling Technology Inc.). After sonication and centrifugation (12000 × g at 4°C for 15 min), the supernatant was stored at −80°C for future use. The protein concentrations were determined by the BCA protein assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Equal amounts of the protein samples (50–100 µg) were loaded onto 12–15 % SDS-gel and electrophoresis was performed. The separated proteins were transferred to nitrocellulose membrane and immunoblotted with specific primary antibodies as described in the figure legends. Then the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology Inc.). The proteins were visualized with a chemiluminesence detection system using LumiGLO reagent and peroxidase (Cell Signaling Technology Inc.), and autoradiography was conducted.
Immunohistochemistry
Immunohistochemical (IHC) staining was performed by the standard ABC Immunostaining method. In brief, the cells were fixed with 4% paraformaldehyde in PBS and rinsed with Tris buffered saline (TBS; 3% 5 N NaCl, 1% 1M Tris [pH 8.0] and ddH2O). Next, the cells were blocked at room temperature for 1 h using normal horse serum in PBS (Vectorstain, ABC kit, Vector Laboratories, Inc.) and then incubated with mouse monoclonal anti-p53 primary antibody (#MS-738-R7; Ab-8; NeoMarkers Inc.) for 2 h at room temperature. Controls were incubated with normal serum alone. The cells were washed with TBS to remove residual primary antibody. The secondary antibody and ABC reagents (Vectorstain, ABC kit) were applied for 30 min incubation at room temperature for each. The antigen-antibody complexes were detected by incubation with DAB (DAB substrate kit for peroxidase, Vector Laboratories, Inc.). The cells were counterstained with Harris-Hemotoxylin stain (Sigma-Aldrich, St. Louis, MO, USA) for 1 min before mounting.
TUNEL assay
The CRC cells were treated with 5-FU and/or selenous acid after treatment with specific inhibitors and incubated for 24 h or 96 h. The floating and attached cells were pooled to perform the cytospin and fixed with 4% paraformaldehyde and 100% methanol. The cytoslides were incubated with an enzymatic reaction mixture containing terminal deoxynucleotidyl transferase and FITC-dUTP according to the manufacturer’s instructions (Upstate Co.). The cells were analyzed by fluorescence microscopy and the data were acquired and analyzed by counting the FITC-stained cells against the PI-stained cells.
Methylation specific PCR (MSP)
The DNA was isolated from the RKO and Caco2 cells using a QIAamp DNA mini kit (#51306, Qiagen, Valencia, CA, USA) according to manufacturer’s instructions. Bisulfite conversion of genomic DNA was carried out using a Zymo EZ DNA Methylation-Gold™ kit (D5005, Zymo Research Corp, Orange, CA, USA) according to the manufacturer’s instructions. This process converts unmethylated cytosine residues to uracil and the methylated cytosine residues remain unchanged. Bisulfite modified DNA was used as a template and then MSP was performed to determine the methylation status of caspase-8, and caspase-9. The primer sequences of caspase-8 and caspase-9 for MSP are as follows. caspase-8: 5’-TGTTGTTTGGGTAACGTATCGA-3’ (methylated forward), 5’-CCCTACTTAACTTAACCCTACTCGAC-3’ (methylated reverse), and 5’-TTGTTGTTTGGGTAATGTATTGA-3’ (unmethylated forward), 5’- CAACCCTACTTAACTTAACCCTACTCA-3’ (unmethylated reverse). caspase-9: 5'-GGTTTTGGAGATGCGTTC −3' (methylated forward), 5'-GATCCGCTTCGTCCATAAC-3' (methylated reverse), and 5'-GAGGTTTTGGAGATGTGTTT-3' (unmethylated forward), 5'-CCAATCCACTTCATCCATAAC-3' (unmethylated reverse). The primers were designed using MethPrimer software (26). For caspase-8 the PCR conditions were an initial denaturation at 95°C for 5 min followed by 45 cycles of 94°C for 30 sec, 47°C for 30 sec, and 72°C for 45 sec and a final extension at 72°C for 7 min. For caspase-9 the PCR conditions were an initial denaturation at 94°C for 5 min, followed by 48 cycles of 94°C for 30 sec, 51.5°C for 30 sec and 72°C for 30 sec and a final extension of 7 min at 72°C. The PCR products were electrophoresed through 2% agarose gels, stained with ethidium bromide and visualized on ChemiDox™ XRS system (Bio-Rad Laboratory, Hercules, CA, USA). The primers of caspase-9 for real-time PCR for mRNA expression were designed using Primer 3.0 software and were forward, 5’- CTGAGCATGGAGCCCTGT-3’ and reverse, 5’ CTTCACCTCCACCATGAAATG-3’. The primers of the housekeeping control gene, 18s were 5’-GATCCATTGGAGGGCAAGTC-3’ (forward) and 5’-TCCCAAGATCCAACTACGAG-3’ (reverse). RNA was extracted from cells using the TRIzol method. The real-time PCR was performed with the iCycle iQ real-time PCR detection system (Bio-Rad) using SYBR Green Master Mix (#204143, Qiagen) according to the manufacturer’s instruction. The reactions were characterized at the point during cycling when amplification of the PCR product was first detected (Ct). The relative level of the target messages in the cells was normalized on the basis of their 18s content by taking the difference of threshold cycles between the target gene and 18s. Aza-c (5-Aza-2’-deoxycytidine) (Sigma), an inhibitor of Dnmt1 (DNA methyltransferase) which reverses methylated genes to their unmethylated forms, was used as positive control to confirm demethylation.
Results
Effect of 5-FU on growth inhibition of CRC cells
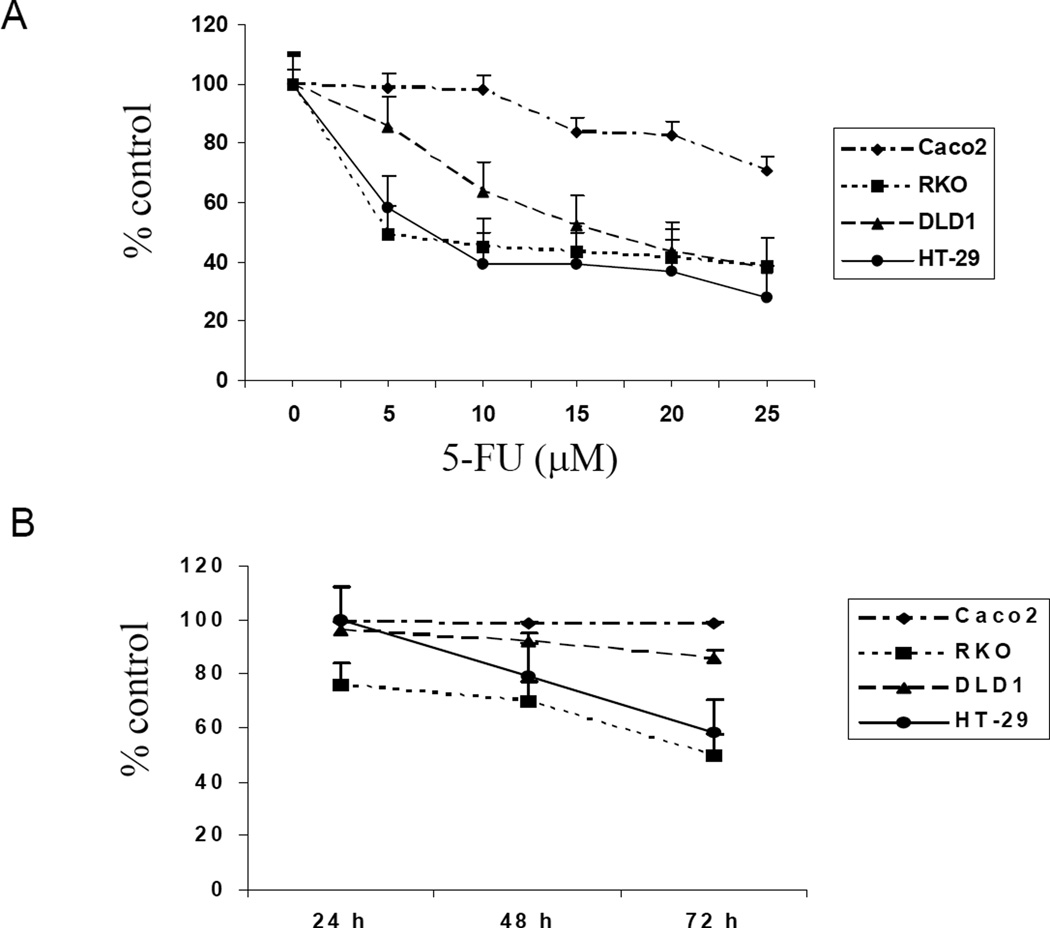
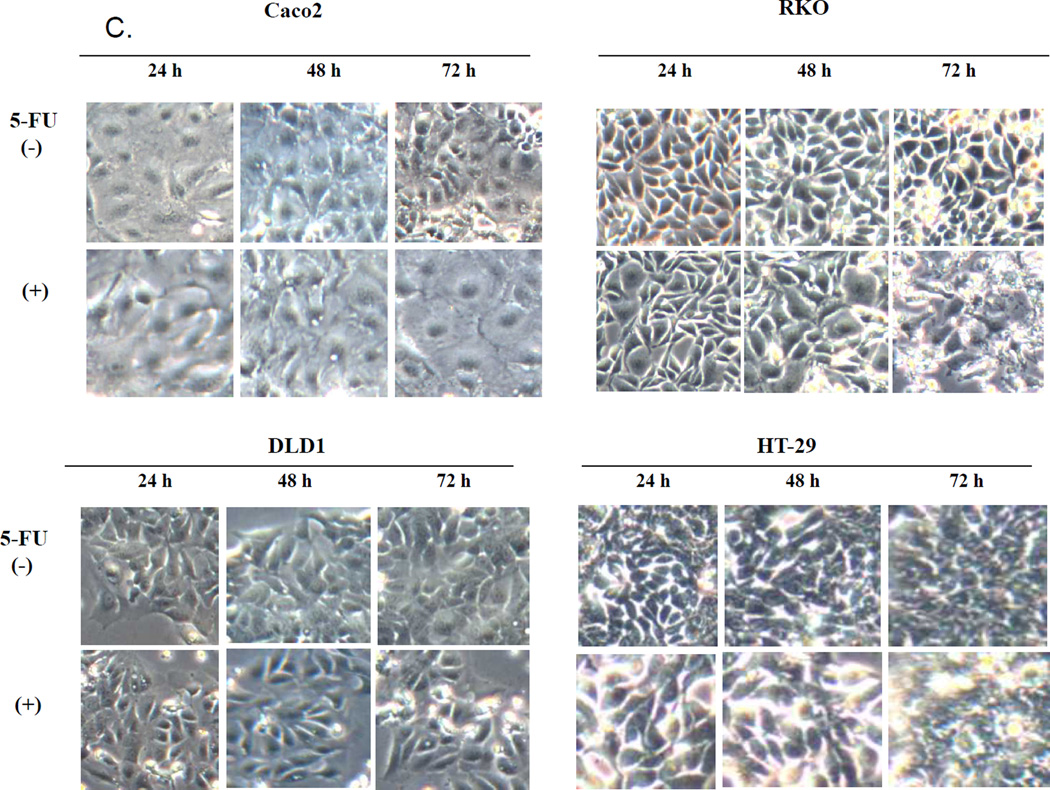
The RKO and HT-29 cells had similar responses to 5-FU inhibition (Figure 1A and B). Both cell types showed approximately 50% inhibition with 5µM 5-FU. These two cell types also showed time dependent inhibition with 5µM 5-FU. In contrast, the DLD1 and Caco2 cells were resistant to 5µM 5-FU, although the DLD1 cells did respond to high concentrations of 5-FU. In contrast, the Caco2 cells showed both dose and time resistance to inhibition by 5-FU. The morphological data confirmed the 5-FU response in these cell types (Figure 1C), essentially, RKO being the most sensitive and Caco2 the most resistant to growth inhibition by 5-FU.
Figure 1.
Effect of 5-fluorouracil (5-FU) on colorectal cancer cell growth. Cells treated with 5, 10, 15, 20 or 25 µM of 5-FU for 72 h (A) or 5 µM of 5-FU for 24 h, 48 h or 72 h (B). Each value was from 6 determinations. Cell growth in RKO and HT-29 decreased in a time-dependent manner (p<0.001=24 h vs 72 h). (C) Phase-contrast microscopy of cells treated with 5 µM of 5-FU.
Effect of different selenium compounds on cell growth of CRC cells
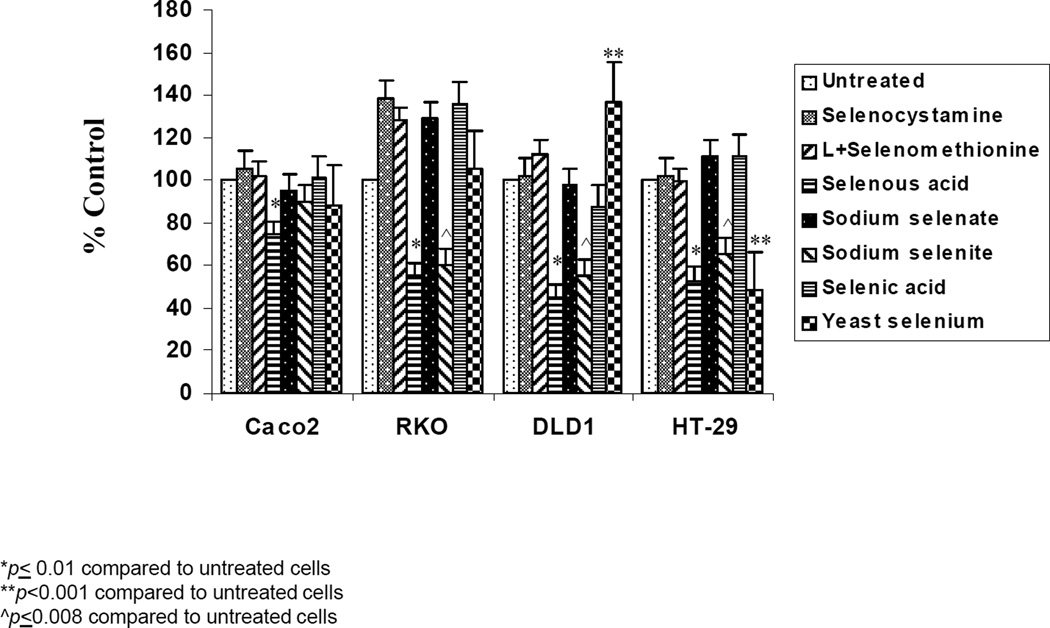
All four CRC cell lines were tested with the seven different selenium compounds for 24, 48 and 72 h of treatment. However, since the responses were marginal at 24 and 48 h of exposure, only the 72 h observations are reported. As shown in Figure 2 the Caco2 cells were resistant to almost all the seven forms of selenium up to 72 h of treatment. Only selenous acid caused a small inhibition (25–30%). In the DLD1 cells only the selenous acid and sodium selenite were able to induce growth inhibition (50–60%) after 72 h of treatment. Interestingly, the treatment with yeast selenium caused growth stimulation however, the error bar is large, suggesting non-specific change. In the RKO cells as with the DLD1 cells, only selenous acid and sodium selenite were able to sustain 30 to 50% growth inhibition after 72 h of treatment. In the case of the RKO cells, selenocystamine, selenomethionine, sodium selenate and selenic acid caused a small but marked increase in growth. In the HT-29 cells selenous acid, sodium selenite and yeast selenium were significantly effective inhibitors (p<0.01).
Figure 2.
Effect of selenium compounds on the growth of Caco2, RKO, DLD1, and HT-29 cells after 72 h. Cells were plated into 96 well-plates and treated with 3.88 µM of selenocystamine, selenomethionine, selenous acid, sodium selenate, sodium selenite, selenic acid or 0.5 µg/ml of yeast selenium for 72 h. Cell proliferation was assessed with the MTT assay. Data are expressed as percent of the untreated control. Each value is a mean of 4 determinations with standard deviation.
Overall, only selenous acid and sodium selenite sustained growth inhibition of the DLD1, RKO and HT-29 CRC cells and selenous acid alone had a marginal effect on the Caco2 cells. Hence, Caco2 cells were selected as 5-FU resistant and RKO as 5-FU sensitive CRC cell models. selenous acid was selected as the most effective source of selenium, to be used in the combination studies. Since yeast selenium is frequently used as a dietary source of selenium and in some cases as a chemopreventive agent (13, 14, 27), yeast selenium was included in the drug combination studies for comparison.
Mechanisms of action of 5-FU, Selenous acid, and yeast selenium as single agents or with combination treatment
Role of p53
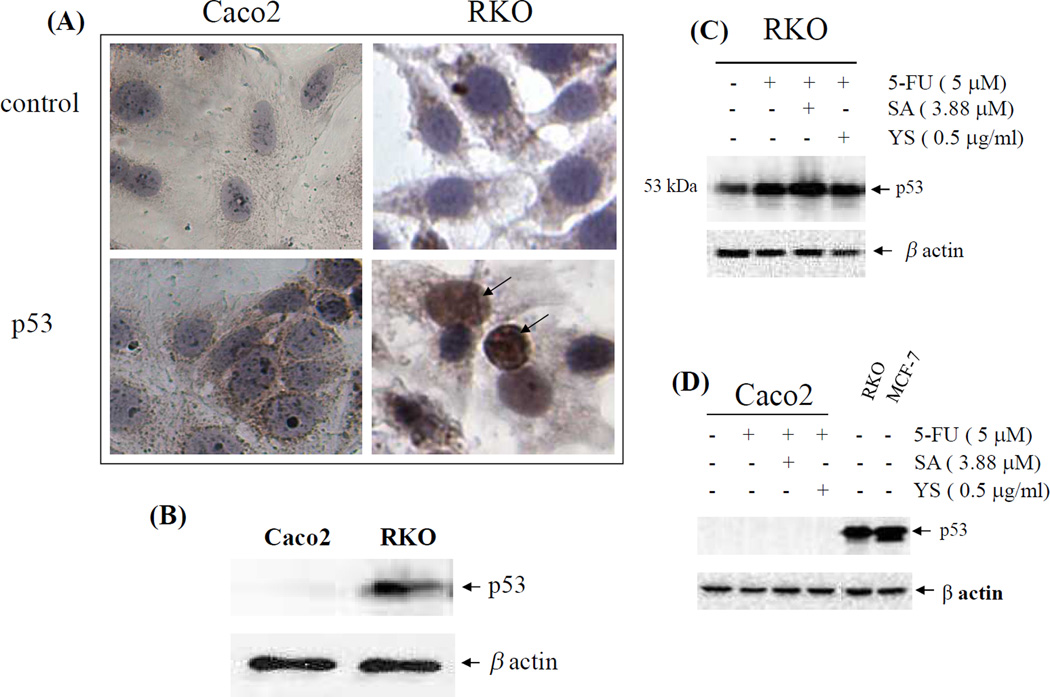
First the expression of normal or wild type p53 was investigated in the Caco2 and RKO cells using IHC and Western blot analysis (Figure 3). The Caco2 cells which were highly resistant to 5-FU and selenium compounds as single agents, lacked normal p53 in their nuclei (Figure 3A). In contrast, the RKO cells which were responsive to both 5-FU and selenous acid, expressed normal p53 in their nuclei. Western blot analysis demonstrated the p53 protein expression pattern in these cells (Figure 3B), the Caco2 cells lacked wild type or normal p53 protein and the RKO cells were positive for normal p53 protein. The β-actin levels remained similar between the two sets, confirming equal protein loading. Subsequently, when the RKO cells were exposed to 5-FU alone or in combination with selenous acid or yeast selenium, there was a noticeable increase in p53 protein, compared to untreated cells (Figure 3C). The greatest increase in p53 expression was in response to 5-FU and selenous acid combination treatment. In contrast, the Caco2 cells showed no change in p53 expression (Figure 3D). Cell lysates from RKO and MCF-7 cells were loaded with the Caco2 samples as positive controls for the expression of normal p53. Again, the levels of β-actin remained constant in each set of analyses.
Figure 3.
p53 expression in Caco2 and RKO CRC cells. (A) Nuclear p53 was detected in RKO, but not in Caco2 cells by immunohistochemistry staining with p53 antibody. Control: normal serum alone.(B) Total cell lysates of Caco2 and RKO cells were subjected to 10% SDS-PAGE and immunoblotted with p53 antibody (DO-1) and the same membranes were re-probed with β-actin antibody to confirm equal sample loading. (C) p53 expression in the RKO cells treated with 5-FU alone or in combination with selenous acid (SA) or yeast selenium (YS). (D) p53 expression in Caco2 cells treated with 5-FU or 5-FU in combination with SA or YS. Cell lysate from MCF-7 cells was loaded in the Caco2 set as a control of normal p53.
Cellular apoptosis
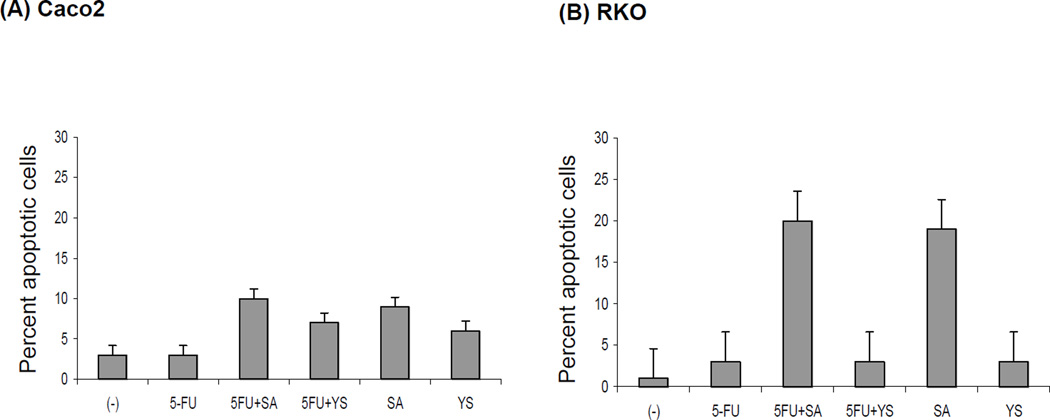
The Tunel assay demonstrated that 5-FU alone had almost no effect on apoptosis of the Caco2 cells and a marginal effect on the RKO cells (Figure 4). However, combination with selenous acid increased the percent of Caco2 apoptotic cells to 12%, and RKO apoptotic cells to 22%. Combination with yeast selenium had a small effect on the Caco2, but not on the RKO.Only selenous acid in combination with 5-FU was able to induce apoptosis in the Caco2 cells as measured by the Tunel assay, although the percent of apoptosis was marginal after 24 h of drug treatment.
Figure 4.
The effect of 5-FU and selenium on the apoptosis of colorectal cancer cells. Caco2 (A) and RKO (B) cells were treated with 5 µM of 5-FU; 3.88 µM of selenous acid (SA) or 0.5 µg/ml of yeast selenium (YS) alone or in combination for 48 h, and then cytospun slides were stained by TUNEL apoptosis reagents and visualized under fluorescence microscopy. FITC-positive cells were counted and shown as percent of apoptosis normalized with control (−) untreated cells. The bars indicate mean percent of the apoptotic cells counted from 5 different areas on the slide. Standard deviation (SD) are indicated.
Role of caspases
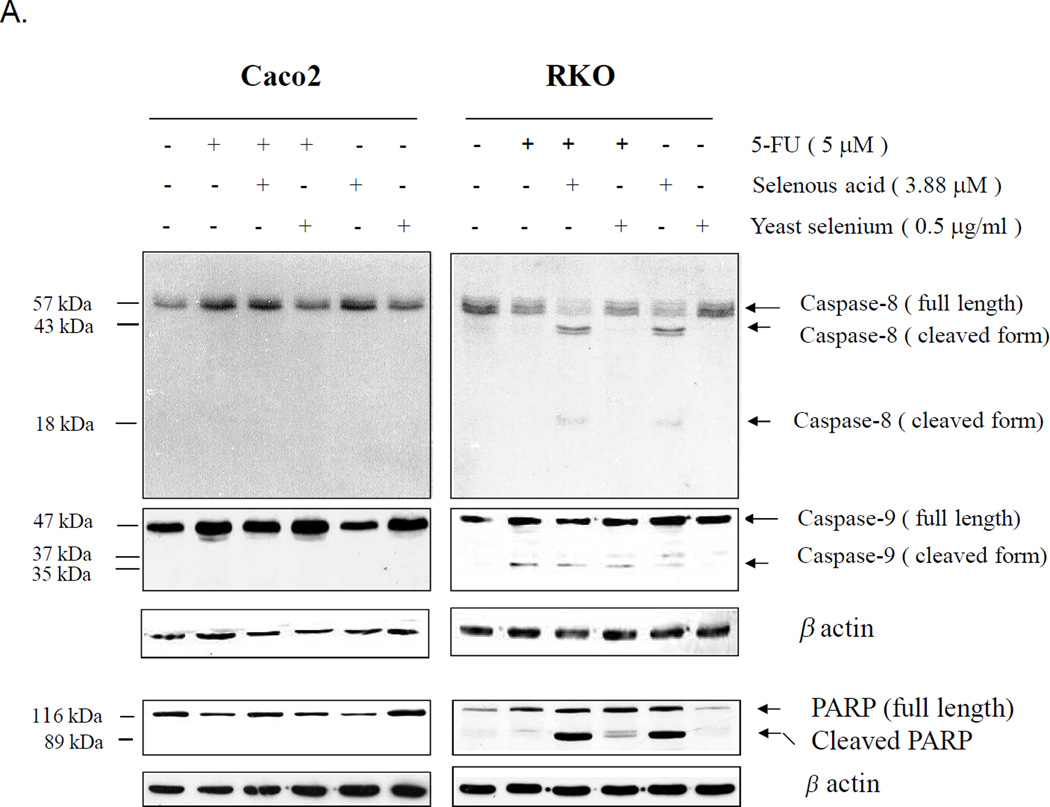
In the Caco2 cells neither caspase-8, −9 nor PARP were activated (or cleaved) in response to 5-FU alone or in combination with selenous acid or yeast selenium (Figure 5A). These observations suggest that the growth inhibition of Caco2 cells to 5-FU in combination with selenous acid was independent of the Caspase-8, −9 pathway and PARP activation. The lack of or very marginal apoptotic response from the Tunel assay (Figure 4) supported the failure of caspase-8, −9 and PARP activation in Caco2 cells. In contrast in the RKO cells caspase-8, −9 and PARP activation with their corresponding cleaved products were demonstrated. Both 5-FU and Selenous acid, as single agents, were able to activate caspase-8, −9 and PARP in the RKO cells. In contrast, yeast selenium which caused poor growth inhibition as single agent or in combination with 5-FU showed no caspase-8, - 9 and PARP activation. Hence, these observations confirm that selenous acid in combination with 5-FU can induce apoptosis of RKO cells by activation of caspase-8, −9 and PARP while 5-FU resistant Caco2 cells do not activate caspase-8, −9 or PARP.
Figure 5.
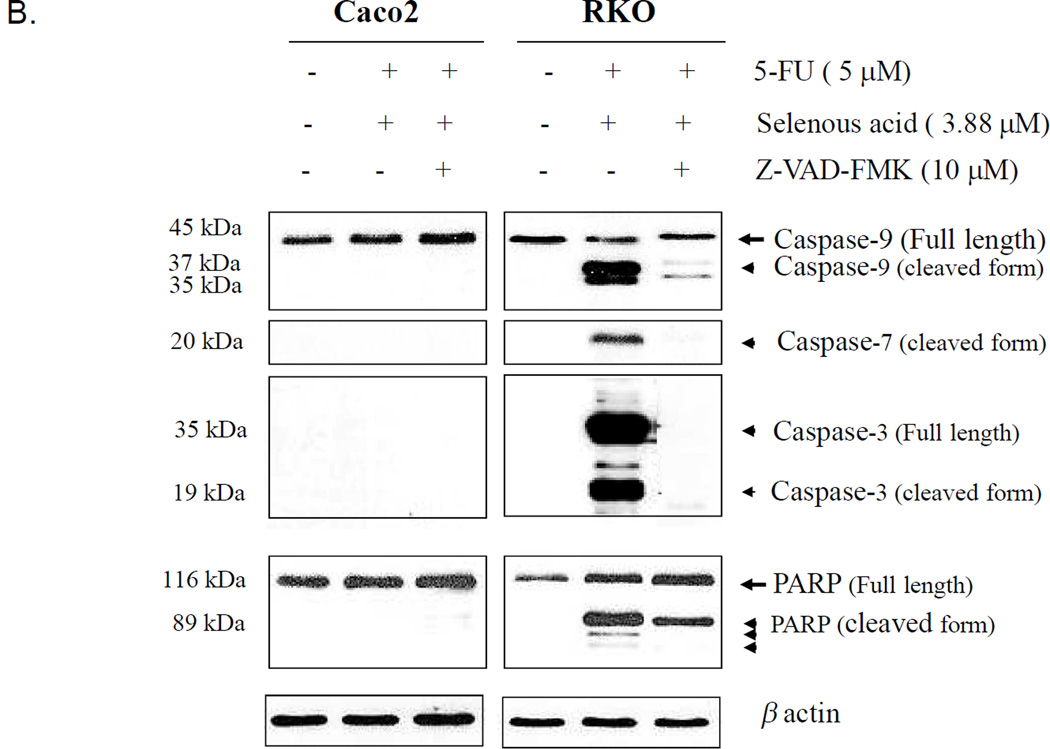
Activation of caspase-8, caspase-9, caspase-7, caspase-3 and PARP in Caco2 and RKO cells treated with 5-FU and or selenous acid. (A) Cell lysates were prepared after 48 h of treatment and analyzed by Western blotting with anti-caspase-8 or caspase-9 antibody or antibody against PARP which recognized both full length (116 kDa) and cleaved product (89 kDa). Same membranes were re-probed with anti-β-actin antibody to confirm equal sample loading (lower panel). (B). Activation of caspases-9, -7 and -3 by combination treatment with 5-FU and selenous acid and the effect of Caspase inhibitor IV (Z-VAD-FMK). Cell lysates were immunoblotted with specific caspase antibody or PARP antibody.
Figure 5B demonstrates the effect of 5-FU in combination with selenous acid in the presence or absence of a general caspase inhibitor Z-VAD-FMK. In the Caco2 in addition to a lack of caspase 9 and PARP activation, a complete absence of the activated or cleaved form of caspases-7 and −3 was shown. In contrast, the RKO cells demonstrating activation of caspase-9 and PARP also showed increased caspase-7 and −3 activation. The caspase inhibitor Z-VAD-FMK almost completely blocked the activation of all three caspases. These observations confirm that Selenous acid in combination with 5-FU induced apoptosis of RKO CRC cells is caspase dependent. Although the caspase inhibitor Z-VAD-FMK decreased PARP activation, it did not block it completely (Figure 5B). These observations suggest that the PARP activation or cleavage in response to selenous acid and 5-FU is not totally caspase dependent, at least on caspase-9, −7, and −3.
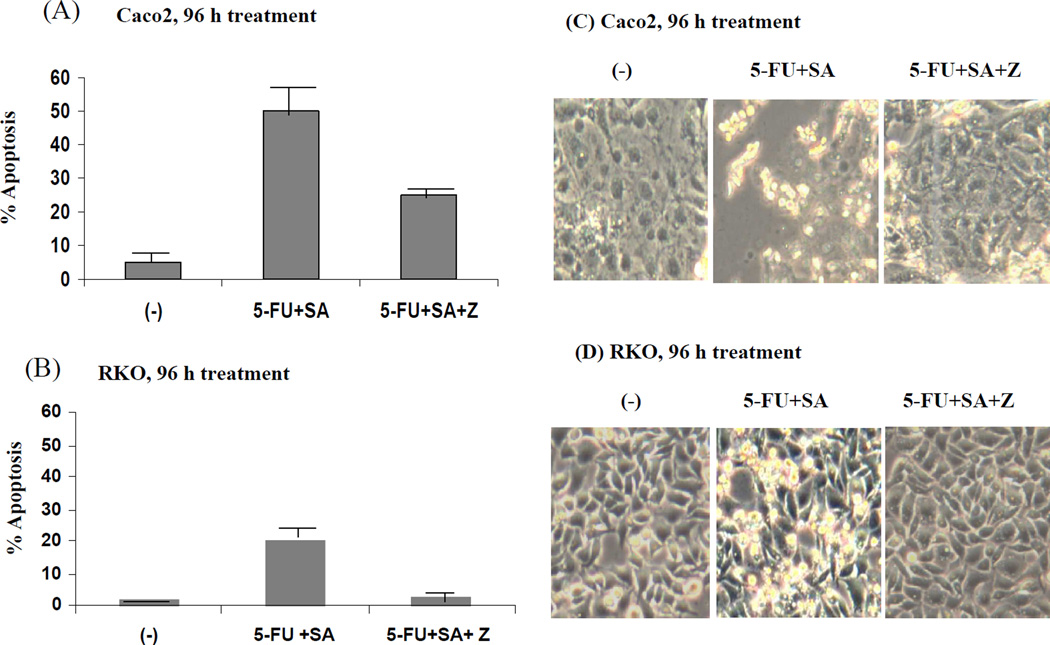
Whether or not the inhibition of caspases could reverse the apoptotic changes observed in response to selenous acid and 5-FU treatment was next determined. Figure 6 shows the percentage of apoptotic Caco2 and RKO cells (left panel, Figure 6A and 6B, respectively) and the corresponding morphological changes (right panel, Figure 6C and 6D, respectively) in response to 5-FU plus selenous acid, and in the presence of the caspase inhibitor Z-VAD-FMK. The Caspase inhibitor had a limited effect on reversing the percentage of apoptotic Caco2 cells in response to 5-FU plus selenous acid (Figure 6A and C). However, treatment with the caspase inhibitor almost completely inhibited the 5-FU plus selenous acid induced apoptosis of the RKO cells (Figure 6B). The morphological data in the right panel complemented these findings (Figure 6C and D).
Figure 6.
The effect of caspase inhibitor IV (Z-VAD-FMK) (Z) on 5-FU and selenous acid (SA) induced apoptosis. After 96 h treatment, cells were collected and cytospun onto slides and TUNEL assay was performed. FITC-positive cells were counted and percent of apoptosis is shown in Caco2 (A) and RKO (B) cells. The corresponding morphological changes are shown for Caco2 (C), and RKO (D) cells.
The lack of response to caspase inhibitor toward apoptosis or growth inhibition of the Caco2 cells was not surprising, since these cells did not demonstrate caspase activation upon 5-FU plus selenous acid treatment (Figure. 5A and 5B).
Effect of Caspase methylation
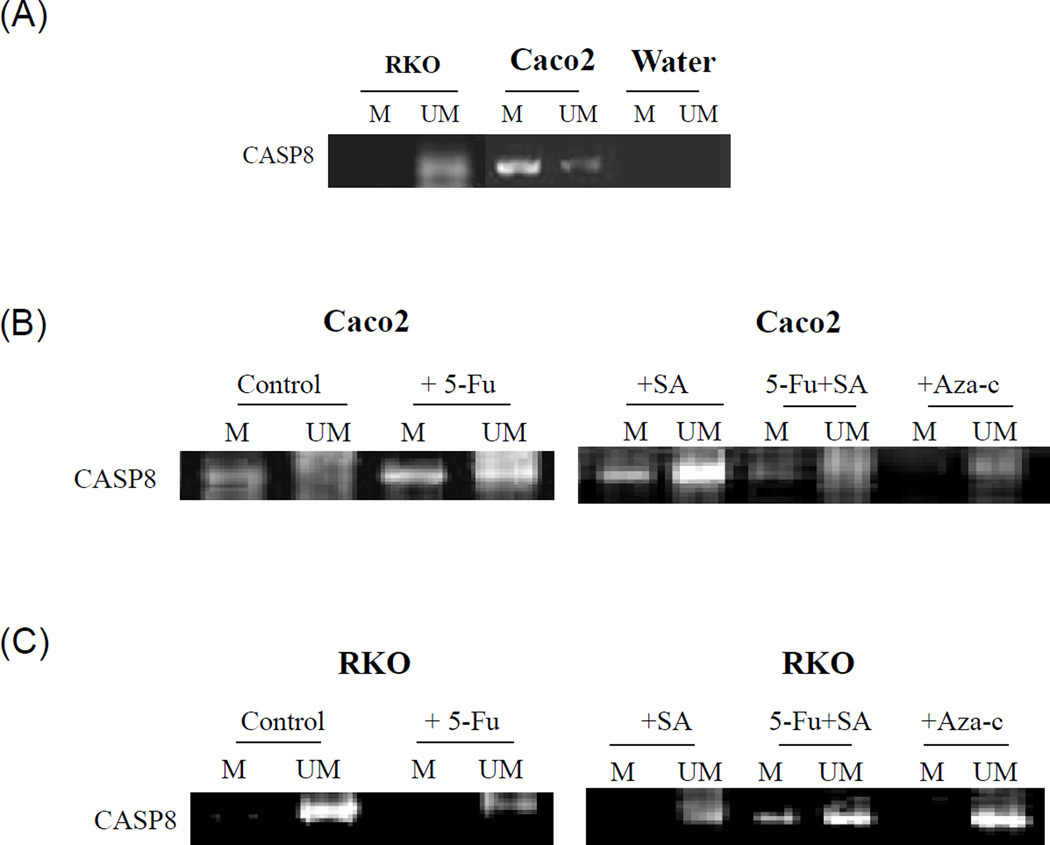
The methylation status of caspase-8 and caspase-9 was examined in the 5-FU resistant Caco2 and sensitive RKO cells (Figure 7A). Caspase-8 was highly methylated in the Caco2 cells which also expressed small levels of unmethylated caspase-8. In contrast, the RKO cells expressed predominantly unmethylated caspase-8 (Figure 7A). As shown in Figure 7B both 5-FU and selenous acid were able to convert a large portion of the methylated caspase-8 to its unmethylated form although a large portion of caspase-8 remained methylated. However, when 5-FU and selenous acid were used in combination, most of the caspase-8 became unmethylated, Aza-c (methylation reversal) treatment converted almost all of the methylated caspase-8 to its unmethylated form. These observations confirmed that 5-FU in combination with selenous acid was an effective treatment for demethylating caspase-8 in the Caco2 cells, and thereby sensitizing Caco2 cells to apoptosis. In contrast to Caco2, the RKO cells expressed constitutively unmethylated caspase 8 (Figure 7C). These cells were able to undergo 5-FU and selenous acid induced apoptosis.
Figure 7.
Promoter methylation status of caspase-8 (CASP8). DNA was extracted from Caco2 or RKO cells and modified by bisulfite treatment and subjected to MSP (methylation specific PCR) using appropriate methylated and unmethylated CASP8 PCR primers. M represents methylated CASP8 and UM represents unmethylated products. (A) Constitutive methylated and unmethylated CASP8 in Caco2 and RKO cells. (B) methylated and unmethylated pattern in Caco2 cells upon 5-FU, selenous acid (SA) treatment. (C) Similar analysis of RKO cells. DNA from Aza-c treated cells is included to demonstrate reversal of methylation.
Effect on Caspase 9
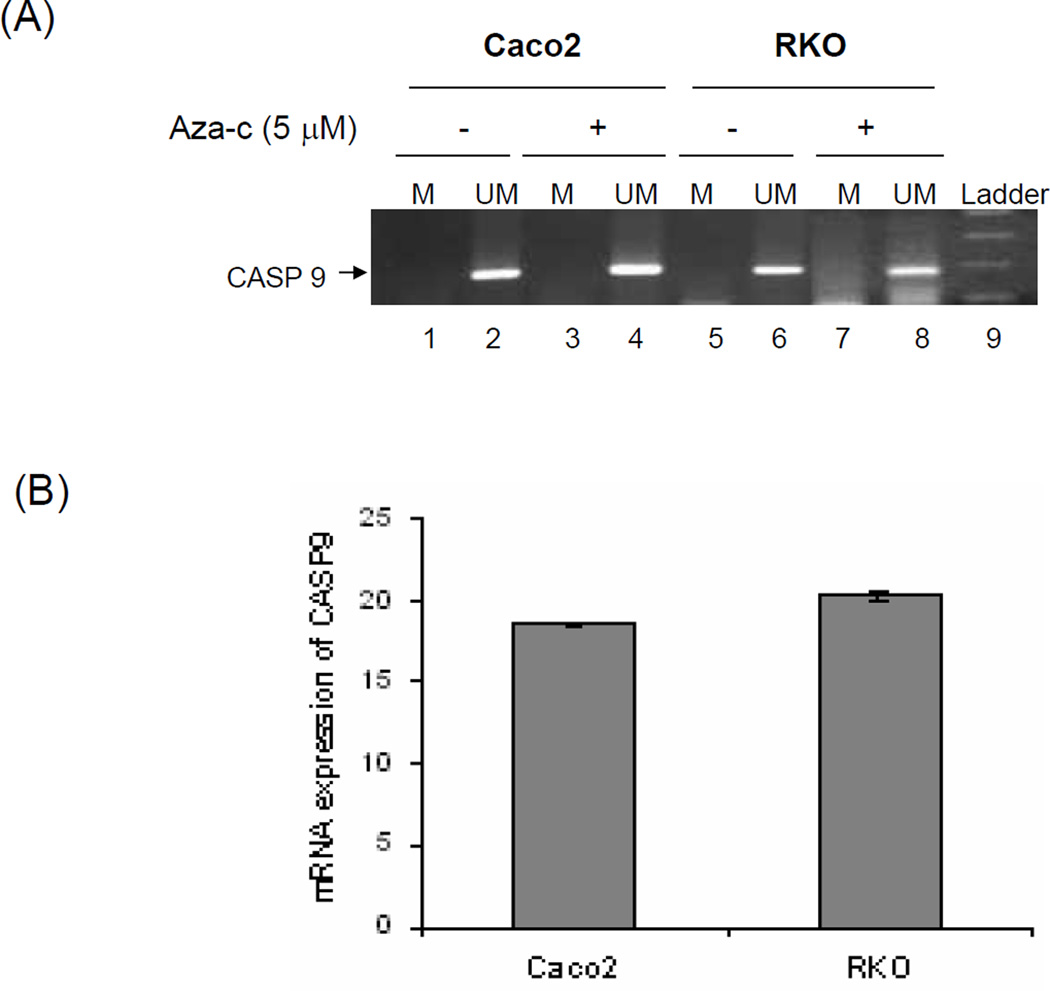
Figure 8A shows that caspase-9 was unmethylated in both the RKO (lane 6), and in Caco2 cells (lane 2). Upon treatment with aza-c, there was no further change in unmethylated form of caspase-9 in both RKO and Caco2 cells.
Figure 8.
(A) Methylation specific PCR (MSP) of caspase-9 (CASP9) promoter in untreated and Aza-c treated Caco2 and RKO cells. DNA from these cells was bisulfite modified and then MSP was performed using methylated (M) and unmethylated (UM) specific primers. Lane 9 represents the 100bp molecular weight marker. (B) Relative mRNA expression of CASP9 in Caco2 and RKO cell lines. Real-time PCR was conducted with iCycle iQ detection system (Bio-Rad). Each bar represents the mean ± SD of triplicate Ct values. Expression of CASP9 in each cell line was calculated by subtracting the Ct of 18s from Ct of CASP9 (CtCASP9-Ct18s).
Figure 8B demonstrates the presence of caspase-9 mRNA in both the Caco2 and RKO cells and further demonstrates the lack of caspase-9 methylation in these cells. No significant difference in mRNA expression of the caspase-9 gene was observed between the Caco2 and RKO cells which supported the methylation specific PCR (MSP) results showing that caspase-9 was not methylated in both cell lines.
In summary, the RKO cells expressed unmethylated forms of caspase-8 and −9 and their corresponding proteins were able to undergo cleavage or activation upon 5-FU and selenous acid treatment, and causing the RKO cells to undergo apoptosis. In contrast, the Caco2 cells, express predominantly methylated form of caspase-8, which upon 5-FU, selenous acid, and aza-c treatment, was converted to unmethylated caspase-8.
Discussion
The present study demonstrated that a large part of CRC cell resistance to 5-FU may be due to the failure in expression or activation of key caspases that are known to be essential in the apoptotic process by leading to PARP cleavage which commits the cell to apoptosis (28, 29)
Several studies have suggested that the presence or absence of wild type p53 may influence the drug induced apoptotic response in tumor cells (30). It is possible that p53 mutations in both Caco2 and DLD1 made these cells more resistant to chemotherapy. Several preclinical studies have demonstrated that the loss of p53 function reduced cellular sensitivity to 5-FU (30, 31). Furthermore, a number of clinical studies have found that p53 mutations correlated with resistance to 5-FU, although other studies found no such association (32). Longley et al (33) assessed the effect of p53 inactivation on drug-induced Fas-mediated apoptosis in two p53 wild-type and p53-null isogenic cell line pairs, the MCF-7-derived M7TS90 and M7TS90-E6 cell lines and the HCT116 p53 +/+ and HCT116 p53 −/− cell lines. p53 inactivation attenuated Fas up-regulation in response to both 5-FU and raltitrexed (RTX) in both the MCF-7 and HCT116 cell lines and inhibited the activation of apoptosis by CH-11 (Fas antibody) in 5-FU- and antifolate-treated cells, indicating that p53 is an important determinant of Fas-mediated apoptosis in response to these agents. The resistance of tumor cells against the induction of apoptosis leads to the failure of antitumor therapy by cytotoxic drugs or irradiation and a worse prognosis for the patients (34).
The presence of MMR proteins in particular hMLH1 and PMS2 in both the Caco2 and DLD1 cells appear to offer no advantage toward lack of sensitivity to 5-FU. Perhaps, a high expression of MMR proteins together with mutant p53 and MSI (microsatellite instability) or microsatellite-abundance) may in fact render CRC cells more resistant to chemotherapy drugs such as 5-FU. In contrast, the RKO cells which express wild type p53 and are deficient in the MMR protein hMLH1, were sensitive to 5-FU induced growth inhibition. Only selenous acid and sodium selenite induced growth inhibition while selenocystamine, selenomethionine and sodium selenate caused a small but marked increase in growth of the RKO cells. This differed from the observation by Goel et al (35), who showed that selenomethionine inhibited the growth of RKO and HCT116 CRC cells by inducing G2/M arrest. However, these authors employed very high concentrations of selenomethionine up to 100 µM. In our study, we employed relatively low concentration of selenous acid (and other selenium compounds) at 3.88 µM. This concentration was chosen from our dose response studies with eight different forms of selenium, and was found to most efficacious at ID50 and without non-specific cell death.
HCT116 and RKO cells are DNA MMR deficient and demonstrate MSI. hMLH1 is lost in HCT116 cells due to homozygous mutation and in RKO cells, due to transcriptional silencing resulting from hypermethylation of its promoter region (36). HCT116 as well as RKO are wild type for p53. It is therefore probable that CRC cells that express mutant p53, normal or high levels of MMR proteins and in the presence of MSS are more likely to become resistant to chemotherapeutic agent 5-FU. Conversely, CRC cells that express wild type (normal) p53, have low or absent MMR protein hMLH1, and with MSI are more likely to respond to growth inhibition by 5-FU. Clinical observations that might support the above statement have been reported in which patients with CRC tumors that expressed MMR proteins and had MSS status, were more likely to fail 5-FU therapy (37). The present studies also suggested selenous acid induced growth inhibition of CRC cells may be partially dependent on DNA MMR and MSS status. Unlike selenomethionine, selenous acid might be able to overcome 5-FU resistance caused by mutant p53.
The role of caspases-8 and −9 in contributing to resistance to apoptosis in CRC cells has not previously been investigated. It needs to be determined if Caspase-9 could be a p53 target gene and that it can be regulated by 5-FU in CRC cells such as Caco2 and RKO. In addition, it would be interesting to investigate if treatment with Selenous acid alone or in combination with 5-FU can overcome resistance to induction of apoptosis by activating caspase-9. The present data suggested that although, the combination treatment did induce strong apoptosis in the Caco2 cells, this induction did not require the activation of caspase-9. In addition to caspase-9, the down-regulation of caspase-8 is frequently seen in tumor cells, leading to resistance against the induction of apoptosis and an unfavorable prognosis for the patients (38). The down-regulation of caspase-8 is associated with increased caspase-8 promoter hypermethylation. Recently, Ehrhardt et al (39) demonstrated that the cytotoxic drugs methotrexate (MTX) and 5-FU were both able to sensitize resistant tumor cells for the induction of apoptosis by p53-mediated up-regulation of caspase-8. An increase in caspase-8 messenger RNA and protein expression disabled tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced proliferation and restored sensitivity toward TRAIL-induced apoptosis. These authors showed that caspase-8 is an important p53 target gene regulated by cytotoxic drugs. These observations were made using primary leukemia cells and tumor cell lines.
It is possible that certain caspases are down-regulated or inactivated due to hypermethylation in their gene promoter region which could lead to resistance to drug induced apoptosis. In the present study, the Caco2 cells expressed both methylated (predominant) and unmethylated forms of caspase-8 while the RKO cells express predominantly unmethylated caspase-8. As single agents, both 5-FU and selenous acid were able to convert a large portion of methylated caspase-8 to its unmethylated form. However, a substantial component remained methylated. Interestingly, when 5-FU and selenous acid were used in combination, most of the caspase-8 became unmethylated. Treatment with Aza-c resulted in complete conversion to the unmethylated form of caspase-8. These observations suggested an important mechanism that could overcome resistance to 5-FU in the combination treatment with selenous acid induce apoptosis in CRC cells, by a process requiring demethylation of caspase-8. In contrast to Caco2, the RKO cells expressed constitutively unmethylated caspase-8 and as expected, were able to undergo apoptosis induced by 5-FU and selenous acid. The expression of the unmethylated form of caspase-8 did not change, but the combination treatment led to a small increase in methylated caspase-8 while the aza-c treatment maintained caspase-8 in the unmethylated form.
The mRNA analysis of caspase-8 by real-time PCR, showed an increase in total levels of caspase-8 mRNA when corrected with corresponding levels of 18s mRNA (data not shown). The physiological significance of the presence of both methylated and unmethylated forms of caspase-8, and their subsequent activation or inactivation in response to treatment, needs further study. The present data suggested that hypermethylation of certain promoter regions of caspase-8 may not result in the complete loss or down-regulation of mRNA levels, but may affect caspase-8 protein function. Additional studies of the caspase-8 gene are needed to confirm the number and location of promoter methylation sites and their effect on caspase-8 protein activation in CRC cells.
Recent studies in other cancer cells have suggested that down-regulation of caspase-8 may be due to epigenetic regulatory mechanisms such as hypermethylation, mutations or loss of the caspase-8 gene. Demethylation by Aza-c or activation of the signal transducer and activator of transcription-1/interferon response factor-1 (STAT-1/IRF-1) pathway by gamma-interferon induced re-expression of caspase-8 (40, 41). Ehrhardt et al (39) showed a new mechanism for the re-expression of caspase-8 drug-induced accumulation of p53. In contrast to Aza-c and gamma-interferon, p53-activating cytotoxic drugs such as MTX or 5-FU are broadly used in anticancer therapy. The authors suggest that these cytotoxic drugs might enable apoptosis in tumor cells by the up-regulation of caspase-8 and this mechanism might represent an important new explanation for the successful use of these well-proven drugs in anticancer therapy. Therefore, p53-mediated up-regulation of caspase-8 might represent a widespread general proapoptotic mechanism used by cytotoxic drugs like MTX or 5-FU to sensitize tumor cells for the induction of apoptosis. Cytotoxic drugs are able to induce the p53-dependent up-regulation of caspase-8 and increase sensitivity to apoptosis in tumor cells with wild-type p53 or mutant forms of p53 with intact transcriptional activity. A p53-inducible element downstream of the transcriptional start site of the caspase-8 promoter was identified by Liedtke et al (42), which was responsible for inducible, but not for baseline activity of the promoter. Uchida et al (43) have shown that adenovirus-mediated transfer of caspase-8 induced apoptosis in glioma and in CRC DLD1 cells. In the present studies, the DLD1 cells were partially resistant to 5-FU but treatment with selenous acid as a single agent or in combination with 5-FU resulted in significant inhibition of growth (data not shown). It needs to be determined, if caspase-8 plays a role in these cells, in response to selenous acid alone or in combination with 5-FU.
Other possible mechanisms of action may involve TS. The mechanism of cytotoxicity of 5-FU has been ascribed to the mis-incorporation of fluoronucleotides into RNA and DNA and to the inhibition of the nucleotide synthetic enzyme thymidylate synthase [44]. TS catalyzes the conversion of dUMP to dTMP with 5, 10-methylene tetrahydrofolate as the methyl donor. This reaction provides the sole intracellular source of thymidylate, which is essential for DNA synthesis and repair. The 5-FU metabolite fluorodeoxyuridine monophosphate forms a stable complex with TS and 5, 10-methylene tetrahydrofolate, resulting in enzyme inhibition (45). TS inhibition caused nucleotide pool imbalances that resulted in S-phase cell cycle arrest and apoptosis (44, 46). Treatment with TS inhibitors has been shown to acutely induce TS expression in cell lines and tumors (47). Furthermore, preclinical and clinical studies have found that TS is a key determinant of sensitivity to 5-FU, with high TS expression correlating with increased resistance (44). TS induction abrogated caspase-8 activation in response to co-treatment with both antifolates and CH-11, but had no effect on caspase-8 activation in response to co-treatment with 5-FU and CH-11. The lack of caspase-8 activation in response to treatment with 5-FU and the antifolates suggested that Fas-mediated apoptosis may be inhibited in MCF-7, HCT116, and RKO cancer cells. However, co-treatment with CH-11 was sufficient to overcome this resistance and activate Fas-mediated apoptosis. Similarly, TS induction abrogated the processing of PARP in the cells co-treated with the antifolates and CH-11, but not in cells co-treated with 5-FU and CH-11. It would be interesting to determine if treatment with selenous acid augments the caspase-8 activation through Fas ligand activation pathway, and if this pathway regulates TS expression. The effect of selenium (if any) on TS is unknown.
In summary, apoptotic changes in CRC cells were more potent with combination treatment of 5-FU and selenous acid compared to yeast selenium. Together with morphological changes, such as cell swelling, nuclear condensation and vacuole formation, cellular and biochemical changes that were selective in cells with specific tumor signatures were observed. For instance, combination treatment with 5-FU and selenous acid in RKO cells resulted in activation of caspase-9, −8, −7 and −3 and PARP activation. These cells expressed wild type or normal p53, loss of MMR protein hMlH1 due to hypermethylation of its promoter region, and were MSI. In addition, the RKO cells expressed predominantly unmethylated caspase-8. In contrast, Caco2 cells were resistant to 5-FU and showed a weak response to selenous acid as a single agent. Caco2 are DNA MMR proficient and demonstrate MSS, but contain deleted p53 alleles. Caspase-8 was highly methylated, while caspases- 9, −7 and −3 were not activated upon 5-FU and/or selenous acid treatment. Co-treatment with low and safe doses of 5-FU and selenous acid, but not yeast selenium offers an effective strategy to overcome resistance to 5-FU and assist these resistant cells to undergo apoptosis. Epigenetic changes in caspase-8 (and perhaps others) resulting from hypermethylation of its promoter region, may contribute to resistance to 5-FU treatment in CRC cells. Interestingly combination treatment with selenous acid and 5-FU can overcome this resistance, by partially restoring the unmethylated form of caspase-8. Hence, combination treatment with low doses of cytotoxic agents and selenous acid may be an important direction for clinical treatment of 5-FU resistant CRC.
Acknowledgments
Grant Support: NIH/NCI U56 CA101599-01 (J.V.V); CA15083-25S3 (J.V.V); NIH/NIDDK R25 DK067015-01 (J.V.V); Department of Defense (BCRP) BC043180 (J.V.V); MBRS, NIH SO6 GM0685-10-01 (Y.Wu). We thank Mercedes Guzman and Nicole Ujadughele for their technical assistance.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ries LA, Eisner MP, Kosary CL. SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2004. Nov, SEER data submission. [Google Scholar]
- 3.Grem JL. 5-Fluoropyrimidines. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy and Biotherapy: Principles and Practice. 2nd Ed. Philadelphia: Lippincot William and Wilkins; 1996. pp. 149–163. [Google Scholar]
- 4.Francini G, Petrioli R, Lorenzini L, Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G, Civitelli S. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterol. 1994;106:899–906. doi: 10.1016/0016-5085(94)90748-x. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD. Promising new agents for treatment of patients with colorectal cancer. Semin Oncol. 1998;5:47–52. [PubMed] [Google Scholar]
- 6.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first line treatment for metastatic colorectal cancer a multicentre randomized trial. Lancet. 2000;355:1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 7.Martin MJ. Current stage-specific chemotherapeutic options in colon cancer. Expert Rev Anticancer Ther. 2005;5(4):695–704. doi: 10.1586/14737140.5.4.695. [DOI] [PubMed] [Google Scholar]
- 8.Bleiberg H. Adjuvant treatment of colon cancer. Curr Opin Oncol. 2005;17(4):381–385. doi: 10.1097/01.cco.0000166648.92674.4c. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn JG. Fluorouracil and the new oral fluorinated pyrimidines. Ann Pharmacother. 2001;35:217–227. doi: 10.1345/aph.10096. [DOI] [PubMed] [Google Scholar]
- 10.Nita ME, Nagawa H, Tominaga O, Tsuno N, Fujii S, Sasaki S, Fu CG, Takenoue T, Tsuruo T, Muto T. 5-Fluorouracil induces apoptosis in human colon cancer cell lines with modulation of Bcl2-family proteins. Br J Cancer. 1998;78:986–992. doi: 10.1038/bjc.1998.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyn P. Selenium biochemistry and cancer. A review of the literature. Alternative Med Rev. 2004;9(3):239–258. [PubMed] [Google Scholar]
- 12.Duffield-Lillico AJ, Shureiqi I, Lippman SM. Can selenium prevent colorectal cancer? A signpost from epidemiology. J Natl Cancer Inst. 2004;96(22):1645–1647. doi: 10.1093/jnci/djh332. [DOI] [PubMed] [Google Scholar]
- 13.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sanders BB, Jr, Smith CL, Taylor JR. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276(24):1957–1963. Erratum in JAMA 277(19):1520, 1996. [PubMed] [Google Scholar]
- 14.Lippman SM, Goodman PJ, Klein EA, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongrad A, Lesher JL, Jr, Park HK, Sander BB, Jr, Smith CL, Taylor JR. Designing the selenium and Vitamin E cancer prevention trial (SELECT) J Natl Cancer Inst. 2005;97(2):94–102. doi: 10.1093/jnci/dji009. [DOI] [PubMed] [Google Scholar]
- 15.Vadgama JV, Wu Y, Shen D, Hsia S, Block J. Effect of selenium in combination with adriamycin or taxol on several different cancer cells. Anticancer Res. 2000;20:1391–1414. [PubMed] [Google Scholar]
- 16.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2001;591(1–2):224–236. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Combs GF., Jr Impact of selenium and cancer prevention findings on the nutrition-health paradigm. Nutr Cancer. 2001;40:6–11. doi: 10.1207/S15327914NC401_4. [DOI] [PubMed] [Google Scholar]
- 18.Frank DH, Roe DJ, Chow HH, Guillen JM, Choquette K, Gracie D, Francis J, Fish A, Alberts DS. Effects of a high-selenium yeast supplement on celecoxib plasma levels a randomized phase II trial. Cancer Epidemiol Biomarkers Prev. 2004;13:299–303. doi: 10.1158/1055-9965.epi-03-0163. [DOI] [PubMed] [Google Scholar]
- 19.Lipinski B. Rational for the treatment of cancer with sodium selenite. Med Hypotheses. 2005;64(4):806–810. doi: 10.1016/j.mehy.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–41. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 21.Broghamer WL, McConnell KP, Blotcky AL. Relationship between serum selenium levels and patients with carcinoma. Cancer. 1976;37:1384–1388. doi: 10.1002/1097-0142(197603)37:3<1384::aid-cncr2820370319>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 22.Fogh J, Trempe G. New human tumor cell lines. In: Fogh J, editor. Human Tumor Cells “in Vitro”. New York: Plenum Publishing Corp; 1975. pp. 115–141. [Google Scholar]
- 23.Fogh J, Fogh J, Orfeo T. One hundred and twenty seven cultured human tumor cell lines producing tumors in nude mice. J Natl Cancer Inst. 1977;59:221–226. doi: 10.1093/jnci/59.1.221. [DOI] [PubMed] [Google Scholar]
- 24.Boyd D, Florent G, Kim P, Brattain M. Determination of the levels of urokinase and its receptor in human colon carcinoma cell lines. Cancer Res. 1988;48:3112–3116. [PubMed] [Google Scholar]
- 25. http://www.atcc.org/catalogs.html.
- 26.Li LC, Dahiya R. MethPrimer: designing primers for methylation PCRs. Bioinformatics. 2002;18(11):1427–1431. doi: 10.1093/bioinformatics/18.11.1427. [DOI] [PubMed] [Google Scholar]
- 27.Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56(1):11–21. doi: 10.1207/s15327914nc5601_3. [DOI] [PubMed] [Google Scholar]
- 28.Gartel AL, Serfas MS, Gartel M, Goufman E, Wu GS, el Deiry WS, Tyner AL. P21 (WAF1/C1P1) expressión is induced in newly nondividing cells in diverse epithelia and during differentiation of Caco-2 intestinal cell line. Exp Cell Res. 1996;227:171–181. doi: 10.1006/excr.1996.0264. [DOI] [PubMed] [Google Scholar]
- 29.Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 30.Bunz F, Hwang PM, Torrance C, Waldman T, Zhang Y, Dillehay L, Willams J, Lengauer C, Kinzler KW, Vogelstein B. Disruption of p53 in human cancer cells alters the responses to therapeutic agents. J Clin Invest. 1999;104:263–269. doi: 10.1172/JCI6863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Longley DB, Boyer J, Allen WL, Latif T, Ferguson PR, Maxwell PJ, McDermott U, Lynch M, Harkin DP, Johnston PG. The role of thymidylate synthase induction in modulating p53-regulated gene expression in response to 5-fluorouracil and antifolates. Cancer Res. 2002;62:2644–2649. [PubMed] [Google Scholar]
- 32.Longley DB, McDermott U, Johnston PG. Clinical significance of prognostic and predictive markers in colorectal cancer. Pharmacogenomics J. 2002;2:209–216. doi: 10.1038/sj.tpj.6500124. [DOI] [PubMed] [Google Scholar]
- 33.Longley DB, Allen WL, McDermott U, Wilson TR, Latif T, Boyer J, Lynch M, Johnston PG. The roles of thymidylate synthase and p53 in regulating Fas-mediatedapoptosis in response to antimetabolites. Clin Cancer Res. 2004;10:3562–3571. doi: 10.1158/1078-0432.CCR-03-0532. [DOI] [PubMed] [Google Scholar]
- 34.Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- 35.Goel A, Fuerst F, Hotchkiss E, Boland CR. Selenomethionine induces p53 mediated cell cycle arrest and apoptosis in human colon cancer cells. Cancer Biol Ther. 2006;5(5):529–535. doi: 10.4161/cbt.5.5.2654. [DOI] [PubMed] [Google Scholar]
- 36.Deng G, Chen A, Hong J. Methylation of CpG in a small region of the hMLH1 promoter invariably correlates with the absence of gene expression. Cancer Res. 1999;59:2029–2033. [PubMed] [Google Scholar]
- 37.Ribic CM, Sargent DJ, Moore MJ, Thibodeau SN, Laurent-Puig P, Gryfe R, Shepherd LE, Tu D, Redston M, Gallinger S. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pingoud-Meier C, Lang D, Janss AJ, Rorke LB, Phillips PC, Shalaby T, Grotzer MA. Downregulation of caspase-8 protein expression correlates with unfavorable survival outcome in childhood medulloblastoma. Clin Cancer Res. 2003;9:6401–6409. [PubMed] [Google Scholar]
- 39.Ehrhardt H, Hacker S, Wittmann S, Maurer M, Borkhardt A, Toloczko A, Debatin KM, Fulda S, Jeremias I. Cytotoxic drug-induced, p53-mediated upregulation of caspase-8 in tumor cells. Oncogene. 2008;27(6):783–793. doi: 10.1038/sj.onc.1210666. [DOI] [PubMed] [Google Scholar]
- 40.Teitz T, Wei T, Valentine MB, Vanin EF, Grenet J, Valentine VA, Behm FG, Look AT, Lahti JM, Kidd VJ. Caspase-8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6:529–535. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- 41.Fulda S, Debatin KM. IFNgamma sensitizes for apoptosis by upregulating caspase-8 expression through the Stat1 pathway. Oncogene. 2002;21:2295–2308. doi: 10.1038/sj.onc.1205255. [DOI] [PubMed] [Google Scholar]
- 42.Liedtke C, Groger N, Manns MP, Trautwein C. The human caspase-8 promoter sustains basal activity through SP1 and ETS-like transcription factors and can be up-regulated by a p53-dependent mechanism. J Biol Chem. 2003;278:27593–27604. doi: 10.1074/jbc.M304077200. [DOI] [PubMed] [Google Scholar]
- 43.Uchida H, Shinoura N, Kitayama J, Watanabe T, Nagawa H, Hamada H. 5-Fluorouracil efficiently enhanced apoptosis induced by adenovirus-mediated transfer of caspase-8 in DLD1 colon cancer cells. J Gene Med. 2003;5:287–299. doi: 10.1002/jgm.339. [DOI] [PubMed] [Google Scholar]
- 44.Longley DB, Harkin DP, Johnston PG. 5-Fluorouracil mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 45.Longley DB, Ferguson PR, Boyer J, Latif T, Lynch M, Maxwell P, Harkin DP, Johnston PG. Characterization of a thymidylate synthase (TS)-inducible cell line a model system for studying sensitivity to TS- and non-TS-targeted chemotherapies. Clin Cancer Res. 2001;7:3533–3539. [PubMed] [Google Scholar]
- 46.Chu E, Koeller DM, Johnston PG, Zinn S, Allegra CJ. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-gamma. Mol Pharmacol. 1993;43:527–533. [PubMed] [Google Scholar]
- 47.Swain SM, Lippman ME, Egan EF, Drake JC, Steinberg SM, Allegra CJ. Fluorouracil and high dose leucovorin in previously treated patients with metastatic breast cancer. J Clin Oncol. 1989;7:890–899. doi: 10.1200/JCO.1989.7.7.890. [DOI] [PubMed] [Google Scholar]