Abstract
Hospital readmissions in the Medicare population may be related to a number of factors, including reoccurrence of illness, failure to understand or follow physician direction, or lack of follow-up care, among others. These readmissions significantly increase cost and utilization in this population, and are expected to increase with the projected growth in Medicare enrollment. The authors examined whether a postdischarge telephonic intervention for patients reduced 30-day hospital readmissions as compared to a matched control population. Postdischarge telephone calls were placed to patients after discharge from a hospital. Readmissions were monitored through health care claims data analysis. Of 48,538 Medicare members who received the intervention, 4504 (9.3%) were readmitted to the hospital within 30 days, as compared to 5598 controls (11.5%, P<0.0001). A direct correlation was observed between the timing of the intervention and the rate of readmission; the closer the intervention to the date of discharge the greater the reduction in number of readmissions. Furthermore, although emergency room visits were reduced in the intervention group as compared to controls (8.1% vs. 9.4%, P<0.0001), physician office visits increased (76.5% vs. 72.3%, P<0.0001), suggesting the intervention may have encouraged members to seek assistance leading to avoidance of readmission. As a group, overall cost savings were $499,458 for members who received the intervention, with $13,964,773 in savings to the health care plan. Support for patients after hospital discharge clearly affected hospital readmission and associated costs and warrants further development. (Population Health Management 2013;16:310–316)
Introduction
As the number of Americans enrolling in Medicare continues to increase each year, the need to evaluate and control the expense of health care rises proportionally. One opportunity for reining in medical costs associated with care of this population lies in reducing the number of Medicare patients returning to the hospital after discharge for preventable complications.
Opportunities for cost reduction in this area appear abundant as the US government reported in 2005 that Medicare expenditures for potentially preventable rehospitalizations may be as high as $12 billion a year.1 The estimated cost to Medicare for unplanned rehospitalizations in 2004 was $17.4 billion,2 and additional reports have stated the current costs for patients rehospitalized within 30 days of discharge may be as high as $44 billion a year for Medicare and other patients' total hospital costs.3 These numbers are significant, not only from a financial standpoint but also from a patient experience perspective, considering that within 30 days of discharge from a hospital, 19.6% of Medicare fee-for-service members are rehospitalized.2
Many hospital readmissions are potentially avoidable and may be considered a reflection of poor care quality and inadequate transitional care.4–8 Readmissions have been linked to systematic discharges because of higher postoperative bed use and the need for hospital bed space,9 the absence of outpatient follow-up care, inadequate understanding of discharge instructions, and the scarcity of help for those transitioning from the hospital to home.10–14 To help address these issues, one of the payment initiatives implemented this year from enactment of the Patient Protection and Affordable Care Act (PPACA) is the Hospital Readmissions Reduction Program.15 This program fines hospitals through a Medicare reimbursement rate cut of 1% in the first year for higher readmission rates for patients with principal diagnoses of pneumonia, heart attack, or congestive heart failure. An additional reduction of 2% by the third year will be implemented if improvements are not evident.
One method for addressing rehospitalization rates beginning to receive a broader and more intense examination is the establishment of outbound telephonic support for patients after they are discharged from the hospital. Several studies examining the influence of this type of program on readmission rates have been conducted for specific conditions, such as congestive heart failure, as well as studies evaluating the effectiveness of transitional care programs, which have demonstrated success in lowering readmission rates.16–20 These programs provided support for patients and their families during transition from the hospital to home, and seem promising for reducing rehospitalization rates.21 However, additional studies are required to establish the impact of transition models on costs and utilization.
To evaluate whether outbound telephonic support for patients and caregivers during transition from hospitalization to home or home health care can reduce readmissions, health care costs, and utilization, the authors evaluated the effectiveness of a telephonic intervention that was designed to support a Medicare population on discharge from a hospital. The authors examined data collected from this population that received a postdischarge telephonic intervention and were monitored for rehospitalization through medical claims data. Health care costs and utilization generated by patients who received postdischarge contact were compared to a matched control population that did not receive the intervention. The resulting data were used to determine whether or not the intervention significantly reduced hospital readmissions among Medicare beneficiaries.
Methods
Study design
This retrospective study is a descriptive analysis of members of a large national health plan who were enrolled in Medicare Advantage, who had an acute inpatient hospitalization followed by discharge to home, and who chose to participate in a telephonic clinical intervention. Data from members who completed the telephonic intervention were compared to data from those who were unable to be reached by phone, or who were reached but declined to participate. This study evaluated demographic characteristics, health care cost, and utilization during the immediate 30-day postdischarge period.
Patient selection
Fully insured patients ages 18 to 89 years with Medicare Advantage Plan coverage for at least 30 days during the time period of January 1, 2010 to September 30, 2010 were eligible for the study. Patients who died or terminated coverage within 30 days of discharge were excluded. Patients must have been discharged from a hospital with a hospital discharge code in claims data of 01, 06, or 07 (discharges to home, home health, and patients who left against medical advice). The initial hospital admission was considered the index date, and index and subsequent admissions must have been for acute hospital stays to be considered admits for the purpose of this study.
Patients were excluded from this study if they were enrolled in a health plan clinical program other than the intervention evaluated in this study, such as chronic disease management. Additionally, patients were excluded from the study if they had been discharged from a hospital in the 30 days prior to receiving the intervention.
Because outcomes included the 30-day readmission rate, controls were implemented to anticipate a potential bias for members who were readmitted for an inpatient stay prior to completion of the postdischarge intervention, as indicated by the completion of Postdischarge Screening (PDS). This was necessary because of the possibility a patient could have received the intervention at any time during the 30-day window, subsequently leaving less than 30 days for a hospital readmission. In comparison, the control population had a full 30-day window after discharge for potential readmission.
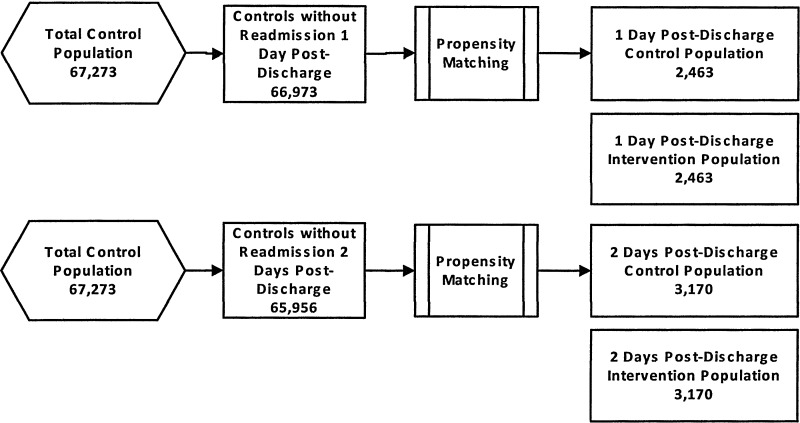
To overcome this potential bias, propensity matching was performed separately for subsets of the test population grouped by days from discharge until PDS (Fig. 1). For each group, the pool of available controls was restricted to members who did not have a readmission prior to when the test group received a PDS. Choosing controls in this manner meant that some control cases could be used more than once. Two advantages to reusing the controls are an improvement in the quality of the match between the tests and matched controls,22 and avoidance of results that are sensitive to the order in which the matching is performed.23
FIG. 1.
Matching of Case and Control Subjects.The pool of controls available for propensity matching remained constant for each day of intervention within the 30-day time frame.
Data source
De-identified administrative health care claims data from a large national health care company were used for this analysis. Data included administrative claims for 104,755 Medicare health plan members, with a total of 115,811 inpatient index admissions. From this pool, health care utilization and cost data were analyzed for the 48,538 controls and 48,538 cases in which the patient received a telephonic intervention after discharge from a hospital. Patient privacy was maintained by masking identification in accordance with the Health Insurance Portability and Accountability Act of 1996.
Intervention
Postdischarge phone contact was implemented to reduce the number of preventable hospital readmissions in the Medicare population. Health plan members were entered into the pool of patients to receive a postdischarge intervention when the health plan was notified of hospital discharge following an inpatient stay. Telephone calls to health plan members were placed by qualified associates, either a licensed nurse (for patients at highest risk of readmission) or a nonlicensed team member with special training (for patients with routine risk of readmission), to complete the PDS. Patients were asked if they received postdischarge instructions, knew the signs and symptoms to be aware of, if they had a follow-up appointment scheduled with their physician, if they were prescribed any medications and whether they had the prescriptions filled, if they needed assistance to perform their activities of daily living, and if they had any health-related questions. Additionally, if home health care services or durable medical equipment had been ordered, confirmation that the services had been implemented and/or delivered was obtained. If gaps in the transition of care were identified, then intervention was performed to close the gap in care or, if needed, patients were referred to a higher level team member to address the gap in care. Nonlicensed team members were trained to address gaps such as delivery of home equipment, but to escalate the patient to a licensed nurse if gaps were identified requiring clinical intervention, such as medication or care instructions.
Data analysis
The Wilcoxon signed rank test was used to assess the difference between the test and the control group with respect to 30-day readmissions, and emergency room (ER) visits and physician visits within 30-days post discharge. The Wilcoxon signed rank test takes into account the matched nature of the sample.24
Propensity score matching was used for the purpose of obtaining a matched control group using multiple independent variables to control for demographic, plan, and clinical characteristics, as well as calendar quarter. Independent variables included age, sex, geographic location, quarter, and plan type (health maintenance organization [HMO], local preferred provider organization [LPPO], regional preferred provider organization [RPPO], and private fee-for-service plan [PFFS]), as well as 2 validated instruments to match clinical characteristics: the Centers for Medicare and Medicaid Services Risk Score and the health plan developed Readmission Predictive Model (RPM) Score. The RPM Score, a continuous variable, is calculated on discharge and incorporates more than 50 data elements, such as length of stay and diagnosis of the index admission as well as historical claims data including prior inpatient utilization, readmissions, pharmacy claims data, and comorbidities.
Results
Within the total population, the average age was 71.6 years, with a slightly higher female than male composition, and an average readmission prediction model score of 172.2 (Table 1). This score is somewhat below the average of 184 observed in other Medicare groups of patients based on internal studies conducted by the health plan largely due to the exclusion of participants enrolled in other disease management programs, inclusion of which would have increased the average score. A majority of patients were enrolled in a Medicare HMO. Although the matched groups were distributed fairly evenly across the mid-west and eastern portion of the United States, a smaller percentage resided in the western region of the country.
Table 1.
Demographics
| PDS patients | Controls | |
|---|---|---|
| Total population | 48,538 | 48,538 |
| Average age | 71.6 | 71.6 |
| Male | 47% | 47% |
| Average RPM Score | 172.2 | 172.1 |
| Average CMS Risk Score | 1.452 | 1.425 |
| Health care plan | ||
| HMO | 39.4% | 39.4% |
| LPPO | 15.6% | 15.6% |
| RPPO | 25.5% | 25.5% |
| PFFS | 19.4% | 19.4% |
| Geography | ||
| Central | 18.1% | 18.1% |
| Eastern (non-Florida) | 26.2% | 26.2% |
| Florida | 21.3% | 21.3% |
| Southern | 21.3% | 21.3% |
| Western | 13.1% | 13.1% |
CMS, Centers for Medicare and Medicaid Services; HMO, health maintenance organization; LPPO, local preferred provider organization; PDS, postdischarge screening; PFFS, private fee for service; RPM, Readmission Prediction Model; RPPO, regional preferred provider organization.
Fewer survey participants (19.5%) than matched controls experienced a readmission in the first 30 days after discharge (Table 2). The greatest impact was observed in the preferred provider subgroups. Overall readmissions within 30 days postdischarge, and in all subgroups, were significantly reduced in the survey patient population. This effect was sustained over the following 60- and 90-day periods for the overall group (reduction rates of 12.9% and 8.8%, respectively, data not shown).
Table 2.
Thirty-Day Readmissions
| |
|
First readmission within 30 days postdischarge |
Average readmits per person within 30 days postdischarge |
||||
|---|---|---|---|---|---|---|---|
| Total population | PDS patients | Controls | Impact1 | PDS patients | Controls | % Reduction1 | |
| Overall | 48,538 | 4504 (9.3%) | 5598 (11.5%) | −1094 (−2.3%) | 0.097 | 0.124 | 21.80% |
| HMO | 19,138 | 1917 (10.0%) | 2232 (11.7%) | −315 (−1.6%) | 0.104 | 0.124 | 16.10% |
| LPPO | 7575 | 626 (8.3%) | 854 (11.3%) | −228 (−3.0%) | 0.086 | 0.12 | 28.30% |
| RPPO | 12,391 | 1121 (9.1%) | 1507 (12.2%) | −386 (−3.1%) | 0.095 | 0.118 | 19.50% |
| PFFS | 9434 | 840 (8.9%) | 1005 (10.7%) | −165 (−1.7%) | 0.095 | 0.131 | 27.50% |
HMO, health maintenance organization; LPPO, local preferred provider organization; PDS, postdischarge screening; PFFS, private fee for service; RPPO, regional preferred provider organization.
Wilcoxon signed rank test P values<0.0001.
The intervention was most effective for members with the highest RPM scores (Table 3). They received the greatest impact from the intervention, and the RPM was effective at predicting risk of hospital readmission for all members. Notably, the degree of impact increased in parallel with the RPM score.
Table 3.
Readmission Prediction Model
| |
|
|
30-day readmission |
|
|
|---|---|---|---|---|---|
| Control Case count | PDS Case count | Control | PDS | Cases With readmit averted | |
| RPM≤152 | 5228 | 5788 | 146 (2.8%) | 104 (1.8%) | 0.9% |
| 152<RPM≤161 | 9430 | 9033 | 414 (4.4%) | 298 (3.3%) | 1.1% |
| 161<RPM≤170 | 11,028 | 10,646 | 816 (7.4%) | 585 (5.5%) | 1.9% |
| 170<RPM≤179 | 8948 | 8898 | 993 (11.1%) | 756 (8.5%) | 2.6% |
| 179<RPM≤188 | 5880 | 5842 | 935 (15.9%) | 724 (12.4%) | 3.4% |
| 188<RPM | 8024 | 8331 | 2,295 (28.6%) | 2041 (24.5%) | 4.1% |
| Total | 48,538 | 48,538 | 5,582 (11.5%) | 4514 (9.3%) | 2.3% |
PDS, postdischarge screening; RPM, readmission prediction model.
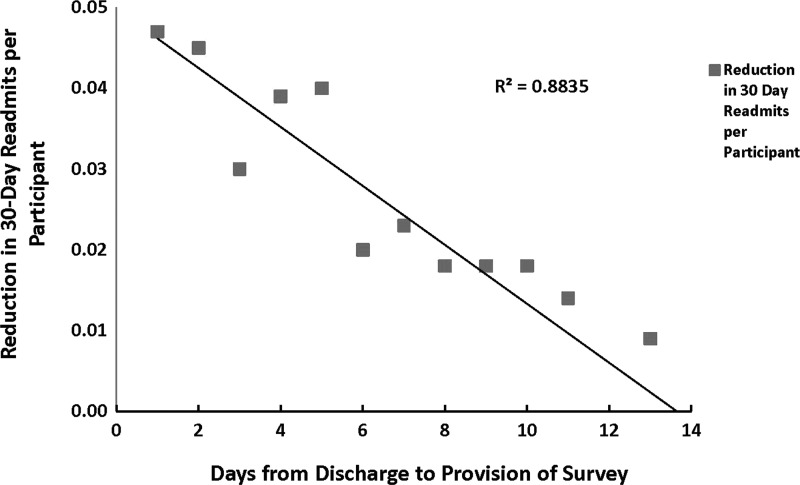
To determine if the impact of the intervention was dependent on the amount of time between discharge and intervention, test cases (and their matched controls) were grouped by days between discharge and intervention. The impact of the intervention was clearly dependent on the relationship to the time of discharge, as demonstrated in Figure 2. The intervention appeared to have the greatest impact when performed as close to the day of discharge as possible. The closer the provision of the intervention to the date of discharge the more likely a readmission was averted (Fig. 2, F-test, P≤0.001).
FIG. 2.
Reduction in Hospital Readmissions. Health plan members were contacted within a 14-day window after hospital discharge and readmissions were tracked through claims analysis for 30 days.
In addition to the reduction in hospital readmissions, patients who received the intervention had significantly fewer ER visits as compared to controls (Table 4). Interestingly, physician office visits were higher in the survey group than for patients in the control group. As physician office visits cannot be reliably counted in claims data for members with HMO coverage, these results excluded Medicare HMO members for both the test and control groups.
Table 4.
Utilization 30 Days Postdischarge
| PDS patients | Controls | Difference1 | |
|---|---|---|---|
| At least 1 readmission | 4,504 (9.3%) | 5,598 (11.5%) | −1,094 (−2.3%) |
| At least 1 ER visit | 3,922 (8.1%) | 4,504 (9.4%) | −617 (−1.3%) |
| At least 1 physician office visit2 | 22,492 (76.5%) | 21,268 (72.3%) | 1,224 (4.2%) |
Wilcoxson signed-rank test P values<0.0001.
Excluding health maintenance organizations (to avoid issues with missing encounter data).
ER, emergency room; PDS, postdischarge screening.
Total group savings for the Medicare members who received the intervention are shown in Table 5. In parallel to the observed increase in physician office visits, the cost for physician office visits was higher for the intervention group as compared to controls. Although physician office visit costs were higher, the increase is more than offset by the savings in total costs for hospital readmissions for the overall group.
Table 5.
Financial Impact of Intervention
| 30-Day readmissions1 | Controls | PDS patients | Total savings | Saving per PDS |
|---|---|---|---|---|
| Member out of pocket spend | $2,333,856 | $1,834,398 | $499,458 | $10 |
| Plan spend | $54,447,704 | $40,482,931 | $13,964,773 | $288 |
| Total (Plan + Member) spend | $56,781,559 | $42,317,329 | $14,464,231 | $298 |
| Emergency Room visits1 | Controls | PDS patients | Total savings | Saving per PDS |
|---|---|---|---|---|
| Member out of pocket spend | $294,835 | $247,934 | $46,901 | $1 |
| Plan spend | $5,081,466 | $4,174,452 | $907,013 | $19 |
| Total (Plan + Member) spend | $5,376,301 | $4,422,387 | $953,914 | $20 |
| Physician visits1,2 | Controls | PDS patients | Total savings | Saving per PDS |
|---|---|---|---|---|
| Member out of pocket spend | $716,150 | $779,177 | −$63,027 | −$2 |
| Plan spend | $14,205,872 | $15,255,473 | −$1,049,601 | −$36 |
| Total (Plan + Member) spend | $14,922,022 | $16,034,650 | −$1,112,628 | −$38 |
Spend includes multiple readmits/visits within 30 days of discharge where present.
Physician visit spend excludes HMO members to avoid missing data related to risk-sharing arrangements.
PDS, postdischarge screening.
Discussion
The health reform act PPACA, which introduced the Hospital Readmissions Reduction Program in October of 2012, has spurred examination of ways to reduce hospital readmissions across the health care industry.4,6,7,21 Readmissions are costly, preventable, and risky for patients; many readmissions are related to fragmentation of care between outpatient, inpatient, and transitional care settings.25 Although a number of approaches have been developed to address this issue, determining what support will help patients remain healthy once they leave the hospital, as well as support their caregivers, has garnered much attention as a viable approach to rein in readmissions.14,26,27 However, few studies have addressed the financial and utilization aspect of this approach. In this vein, the authors evaluated the impact of a postdischarge telephonic intervention by a large national health insurance provider on health care cost and utilization.
The results of the intervention evaluated in this study clearly indicate that the postdischarge intervention reduced the likelihood of a hospital readmission. The data visibly demonstrated a relationship between the timing and influence of the intervention on hospital readmission rates in a large Medicare population. Specifically, the data showed that the shorter the time frame between discharge of a member from the hospital and administration of the intervention, the greater the influence of the contact on decreasing readmission. Moreover, provision of the intervention correlated to a persistent reduction in hospital readmissions for patients over a 90-day time period.
Transition support provided by the intervention may have reduced readmissions by identifying and addressing potential gaps in the transition of care, such as: helping patients and caregivers understand discharge instructions, performing medication reconciliation, reminding patients of their follow-up visit to the doctor, assuring that any planned home care interventions were implemented, and checking that adequate support for activities of daily living and meals was in place. Similar types of outreach have been shown to reduce ER visits.28 Interestingly, the fact that ER visits were reduced in the group that received the intervention in this study, while physician office visits increased, suggests the intervention may encourage patients to seek guidance from their providers. The additional care this enables may have led to avoidance of rehospitalization. Supporting this idea, a study by Jencks et al2 showed that over half of patients who were rehospitalized within 30 days failed to visit a physician's office between admissions.
The financial benefit of the intervention was evident in the reduction of overall costs associated with hospital readmissions. While the dollar amount for physician office visits increased in the intervention group, this was mostly offset by the savings from decreased ER visits. Consistently contacting patients closer to the date of discharge may further increase the financial benefits observed in this study.
This study was limited by the variability in the timing of postdischarge contact, as well as common limitations related to the use of administrative claims data. This includes the potential lack of information in the database and errors in claims coding. The impact of the intervention could have been reduced by the exclusion of members participating in other health management programs to avoid bias. The test and control group readmission rate is less than the general rate for all Medicare members,2 consistent with the exclusion of those members presumed to be at greater risk, who were engaged in other health plan programs also. This study used data from one national health plan and, as such, may not be generalizable to the US population. However, the health plan includes members who reside in a broad range of the country.
Further limitations of this study include the self-selection of participation, as well as inclusion of those who were unable to be reached phone in the control group, as both constitute a potential bias and will be addressed in future studies. Additionally, unobservable variables may have impacted the propensity score approach used in this study, as only observable variables were included in the model. Bias from unobservable variables, such as a patient's willingness to change and the type of support the member may have been receiving in the home, could have been introduced into the study and impacted the propensity scores through unmeasured confounding.29,30 In future studies, a sensitivity analysis could address the degree to which a possible unmeasured confounder influenced the study results.
The development of a true predictive model employing a robust number of variables, such as the RPM, should allow for risk stratification and prioritization of patients resulting in optimization of future postdischarge interventions. Patients rated as being at higher risk might receive varied forms of intervention, such as a home visit, or a more intense intervention, such as follow-up over a longer period of time. Intervention for lower risk patients could start with a simpler approach, such as an automated call. Wide use of such a program would maximize impact while providing cost-effective outreach for all postdischarge patients. Furthermore, the development of a prospective intent-to-treat study to confirm these results would help to strengthen the support for establishing the approach to reduce hospital readmissions.
This study patently indicates the reduction of the burden of cost and hospitalization for the patient, and subsequently their caregiver if present, due to the implementation of a telephonic intervention. However, execution of a successful program across the majority of the population of the United States likely would require normalization of a postdischarge process across the health care industry. Subsequent studies would ideally address a standardization of interventions across health care,14 and the possibility of a uniform process to aid in reduction of hospital readmissions.
Author Disclosure Statement
Drs.Constantino, Hall, and Painter, and Ms. Frey declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: all authors are employed by Humana, Inc., which funded this study.
References
- 1.Medicare Payment Advisory Commission. Washington, DC: Medicare Payment Advisory Commission; 2005. Report to the Congress: Reforming the Delivery System; pp. 83–103. [Google Scholar]
- 2.Jencks SF. Williams MV. Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 3.Jencks SF. Defragmenting care. Ann Intern Med. 2010;153:757–758. doi: 10.7326/0003-4819-153-11-201012070-00010. [DOI] [PubMed] [Google Scholar]
- 4.Grimmer KA. Moss JR. Gill TK. Discharge planning quality from the carer perspective. Qual Life Res. 2000;9:1005–1013. doi: 10.1023/a:1016693825758. [DOI] [PubMed] [Google Scholar]
- 5.Coleman EA. Boult C. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51:556–557. doi: 10.1046/j.1532-5415.2003.51186.x. [DOI] [PubMed] [Google Scholar]
- 6.Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc. 2003;51:549–555. doi: 10.1046/j.1532-5415.2003.51185.x. [DOI] [PubMed] [Google Scholar]
- 7.Coleman EA. Parry C. Chalmers S. Min SJ. The care transitions intervention: Results of a randomized controlled trial. Arch Intern Med. 2006;166:1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 8.Goldfield NI. McCullough EC. Hughes JS, et al. Identifying potentially preventable readmissions. Health Care Financ Rev. 2008;30:75–91. [PMC free article] [PubMed] [Google Scholar]
- 9.Anderson C. Deepak BV. Amoateng-Adjepong Y. Zarich S. Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11:315–321. doi: 10.1111/j.1527-5299.2005.04458.x. [DOI] [PubMed] [Google Scholar]
- 10.Chugh A. Williams MV. Grigsby J. Coleman EA. Better transitions: Improving comprehension of discharge instructions. Front Health Serv Manage. 2009;25:11–32. [PubMed] [Google Scholar]
- 11.Kripalani S. Jackson AT. Schnipper JL. Coleman EA. Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists. J Hosp Med. 2007;2:314–323. doi: 10.1002/jhm.228. [DOI] [PubMed] [Google Scholar]
- 12.Misky GJ. Wald HL. Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5:392–397. doi: 10.1002/jhm.666. [DOI] [PubMed] [Google Scholar]
- 13.Arora VM. Prochaska ML. Farnan JM, et al. Problems after discharge and understanding of communication with their primary care physicians among hospitalized seniors: A mixed methods study. J Hosp Med. 2010;5:385–391. doi: 10.1002/jhm.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen LO. Young RS. Hinami K. Leung A. Williams MV. Interventions to reduce 30-day rehospitalization: A systematic review. Ann Intern Med. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Medicare & Medicaid Services. Readmission Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. [Jul 3;2005 ]. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html
- 16.Rich MW. Beckham V. Wittenberg C. Leven CL. Freedland KE. Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 17.Stewart S. Pearson S. Luke CG. Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. J Am Geriatr Soc. 1998;46:174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald JL. Jack BW. Preventing the preventable: Reducing rehospitalizations through coordinated, patient-centered discharge processes. Prof Case Manag. 2009;14:135–140. doi: 10.1097/NCM.0b013e318198d4e1. quiz 141–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed OI. Rak DJ. Hospital readmission among participants in a transitional case management program. Am J Manag Care. 2010;16:778–783. [PubMed] [Google Scholar]
- 20.Harrison PL. Hara PA. Pope JE. Young MC. Rula EY. The impact of postdischarge telephonic follow-up on hospital readmissions. Popul Health Manag. 2011;14:27–32. doi: 10.1089/pop.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman EA. Smith JD. Frank JC. Min SJ. Parry C. Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: The Care Transitions Intervention. J Am Geriatr Soc. 2004;52:1817–1825. doi: 10.1111/j.1532-5415.2004.52504.x. [DOI] [PubMed] [Google Scholar]
- 22.Dehejia HR. Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84:151–161. [Google Scholar]
- 23.Rosenbaum P. Observational Studies. New York: Springer Verlag; 1995. [Google Scholar]
- 24.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011:1292–1301. doi: 10.1002/sim.4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cykert S. Improving care transitions means more than reducing hospital readmissions. NC Med J. 2012;73:31–33. [PubMed] [Google Scholar]
- 26.Braun E. Baidusi A. Alroy G. Azzam ZS. Telephone follow-up improves patients satisfaction following hospital discharge. Eur J Intern Med. 2009;20:221–225. doi: 10.1016/j.ejim.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Watkins L. An evidence-based strategy for transitioning patients from the hospital to the community. NC Med J. 2012;73:48–50. [PubMed] [Google Scholar]
- 28.Dudas V. Bookwalter T. Kerr KM. Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Dis Mon. 2002;48:239–248. doi: 10.1016/s0011-5029(02)90031-3. [DOI] [PubMed] [Google Scholar]
- 29.Duncan I. Managing and Evaluating Healthcare Intervention Programs. Winsted, CT: ACTEX Publications, Inc.; 2008. [Google Scholar]
- 30.Arbogast PG. Seeger JD DEcIDE Methods Center Summary Variable Working Group. Summary Variables in Observational Research: Propensity Scores and Disease Risk Scores. Effective Health Care Program Research Report No. 33. Rockville, MD: Agency for Healthcare Research and Quality; May, 2012. [Google Scholar]