Abstract
Significance: Despite recent medical advances, cardiovascular disease and heart failure (HF) continue to be major health concerns, and related mortality remains high. As a result, investigation of the mechanisms involved in the development of HF continues to be an active field of study. Recent Advances: The renin–angiotensin system (RAS) and its effector molecule, angiotensin (Ang) II, affect cardiac function through both systemic and local actions, and have been shown to play a major role in cardiac remodeling and dysfunction in the failing heart. Many of the downstream effects of AngII signaling are mediated by elevated levels of reactive oxygen species (ROS) and oxidative stress, which have also been implicated in the pathology of HF. Critical Issues: Inhibitors of the RAS have proven beneficial in the treatment of patients at risk for and suffering from HF, but remain only partially effective. ROS can be generated from several different sources, and the oxidative state is normally tightly regulated in the heart. How AngII increases ROS levels and causes dysregulation of the cardiac oxidative state has been the subject of considerable interest in recent years. Future Directions: A better understanding of this process and the mechanisms involved should lead to the development of more effective HF therapies and improved outcomes. Antioxid. Redox Signal. 19, 1095–1109.
Introduction
Recent medical advances have led to significant improvements in outcome and quality of life for patients suffering from cardiovascular diseases (CVD). Nevertheless, CVD- and heart failure (HF)-related morbidity and mortality remain high, leading to ongoing interest in the pathophysiological mechanisms involved in and responsible for these conditions. The renin–angiotensin system (RAS) and its primary effector molecule, angiotensin (Ang) II, in particular, have been the focus of much research, and have been found to play a major role in a wide variety of renal and cardiovascular functions, including salt/water retention, vasoconstriction, aldosterone secretion, inflammation, thrombosis, mitochondrial function, and cell growth and death. Once thought to be a fairly simple systemic pathway, the RAS is now known to be much more complex, acting locally, through both intracrine and autocrine/paracrine mechanisms, as well as systemically to exert its effects on target organs, including the heart.
AngII leads to the activation of signaling molecules in multiple downstream pathways, including kinases, transcription factors, cytokines, and growth factors, and much of this activity results from its ability to modulate the generation of unstable oxygen derivatives, known as reactive oxygen species (ROS), and oxidative stress. ROS continuously arise as a result of leakage from the mitochondrial electron transport chain, or may be generated as an enzymatic byproduct of metabolism or the unfolded protein response during endoplasmic reticular stress. In addition, nitric oxide synthase (NOS) uncoupling can lead to production of ROS instead of nitric oxide (NO•), and nicotinamide adenine dinucleotide phosphate (NAD[P]H) oxidases produce ROS as their primary product. Endogenous antioxidants, including superoxide dismutase (SOD), glutathione peroxidase (GPx) and catalase, as well as nonenzymatic scavengers, serve to counteract these sources of ROS, maintaining equilibrium and preventing oxidative stress. Although ROS have been shown to play a role in normal physiological cell signaling, excessive ROS production can be extremely detrimental, through direct damage to cellular components as well as through reduction–oxidation (redox) modification of signaling molecules. Like AngII, ROS and oxidative stress have been shown to play an important role in the progression of CVD and HF. Thus, there is considerable interest in clarifying the relative importance of the various mechanisms of ROS production in the heart, their downstream effects, and the effectiveness of therapeutically targeting them.
Overview of the RAS
In the classical RAS, angiotensinogen (AGT) produced in the liver and renin produced in the kidneys enter the bloodstream, where the renin cleaves the AGT to form the decapeptide, AngI. Angiotensin-converting enzyme (ACE), which is mainly produced in the lung vascular bed, and which is bound to the lumenal side of endothelial cells in the vasculature, then cleaves AngI to produce the biologically active octapeptide, AngII. Finally, AngII binds to either the AngII type 1 receptor (AT1R), a G protein–coupled receptor through which it exerts most of its known functions, or alternatively, to the AngII type 2 receptor (AT2R), another G protein–coupled receptor, whose function was until recently largely unknown, but is thought mainly to oppose AT1R signaling (52, 107, 117). However, it is now known that this linear systemically acting pathway is a greatly oversimplified version of the actual RAS.
Research conducted over the last few decades has shown that AngII is generated locally in some tissues, including the heart (51, 52), as well as systemically, and that there are actually many more components in the RAS than was initially thought. Several other Ang peptides have been discovered, including Ang(1–5), Ang(1–7), Ang(1–9), Ang(2–8) (also known as AngIII), Ang(3–8) (also known as AngIV), and Ang(1–12) (24, 36, 52). Although the functions of these additional Ang peptides have not all been well characterized and some may simply be intermediary or degradation products, there is increasing evidence that most, if not all, of these peptides are biologically active and may be important in modulating RAS function. In particular, Ang(1–7), which is formed by cleavage of either AngII or Ang(1–9), can bind to both the AT1R and AT2R, as well as to the Mas receptor, and plays an important role in opposing the effects of AngII signaling, including cardiac hypertrophy and fibrosis (25, 36, 75, 135). There is some evidence that Ang(1–9) may be an important cardioprotective factor as well (90), and Ang(1–12) serves as an alternate precursor for AngI and AngII formation (100, 130), bypassing the need for AGT in the RAS cascade. It is also now known that renin and ACE are not the only enzymes that take part in the RAS. AGT can be cleaved to form AngI by cathepsin as well as renin, and chymase, rather than ACE, is responsible for cleavage of AngI (18, 51, 79, 136) and possibly Ang(1–12) to AngII (100) under certain circumstances. In addition, an ACE homolog, ACE2, has been identified and shown to be involved in cleaving AngI and AngII to form Ang(1–7) (52). The relative importance of the various RAS components appears to be context- and species-dependent, and continues to be the subject of much research.
In addition, it is now known that the RAS is not the simple linear cascade it was once thought to be. Several endogenous negative regulators and feedback mechanisms exist that prevent excessive AngII/AT1R signaling under normal circumstances. Although its physiological importance requires further clarification, AT1R-associated protein has been shown to bind to the AT1R and promote receptor internalization, thus acting as an endogenous AT1R inhibitor (124, 133). Investigation into AT2R signaling has shown that many, though not all, of its effects oppose those of the AT1R, such that some of the beneficial effects of AT1R blockers (ARBs) in treating CVD may be due to increased AngII signaling through the AT2R (52, 84, 97, 107, 117, 145). However, it appears that the AT2R may also have some functions similar to those of the AT1R, since mice lacking the AT2R do not develop hypertrophy in response to pressure-overload (108), nor do they develop hypertrophy and fibrosis in response to AngII infusion (33). AngII/AT1R signaling can also be self-limiting in that it suppresses AGT and renin expression. Perhaps the most extensively studied negative feedback mechanism, however, is that of the ACE2/Ang(1–7)/Mas axis, which has been shown to play a major role in opposing AngII signaling, both through AngII degradation and the positive and negative actions of Ang(1–7) itself (19, 25, 45, 75, 129, 135, 151). Furthermore, some of the RAS enzymes have additional functions that do not involve Ang peptides. Binding of renin or its precursor, prorenin, to the (pro)renin receptor can activate extracellular signal-regulated kinase in an AngII-independent manner (23). In addition to Ang peptides, ACE also hydrolyzes a variety of other substrates, including the cardioprotective factors bradykinin, providing a link between the RAS and kallikrein/kinin system, and N-acetyl-seryl-aspartyl-lysyl-proline tetrapeptide (52, 95). Likewise, chymase function is not limited to Ang peptide cleavage, playing an important role in tissue remodeling through activation of transforming growth factor-β (TGF-β) and matrix metalloproteases (MMPs), as well as catalyzing degradation of thrombin and plasmin (18, 121).
Thus, the RAS is actually a complex network of intertwining pathways (Fig. 1), and much of the complexity stems from differences between systemic and local tissue AngII generation and signaling. While the question of whether AngII is generated intracellularly as well as extracellularly remains the subject of some debate, there is little doubt that the tissue RAS plays an important role in determining cardiac function. The effects of locally generated AngII may be intracrine or autocrine/paracrine, depending on the cell type and conditions (51, 52, 103).
FIG. 1.
Overview of the RAS as it is currently understood. AngII is considered the main effector molecule of the RAS, and is formed by successive cleavage of AGT and AngI or by cleavage of Ang(1–12). Other Ang peptides, including Ang(1–9), Ang(1–7), Ang(1–5), Ang(2–8), and Ang(3–8), are the products of subsequent or alternative cleavage. Several of the cleavage steps can be mediated by more than one enzyme, and some of these enzymes, including ACE, chymase, and renin, have functions other than cleavage of Ang peptides. Most of the known effects of AngII signaling are mediated by the AT1R, and are, to a large extent, opposed by signaling through the AT2R and by the ACE2/Ang(1–7)/Mas receptor axis. ACE, angiotensin converting enzyme; AGT, angiotensinogen; Ang, angiotensin; APA, aminopeptidase A; APN, aminopeptidase N; Ac-SDKP, N-acetyl-seryl-aspartyl-lysyl-proline; AT1R, AngII type 1 receptor; AT2R, AngII type 2 receptor; CPA, carboxypeptidase A; (P)RR, (pro)renin receptor; RAS, renin-angiotensin system. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
AngII and HF
The first clinical evidence of the benefits of RAS inhibition in treating HF came from the results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS) in 1987, in which treatment with the ACE inhibitor, enalapril, significantly improved mortality and cardiac symptoms in patients with severe congestive HF (1). In the more than two decades since, multiple clinical studies have demonstrated the effectiveness of blocking AngII generation and/or signaling with ACE inhibitors or ARBs in treating hypertension, as well as congestive HF, acute myocardial infarction (MI) and coronary artery disease (47, 114). As a result, current guidelines recommend the use of these drugs as first-line therapy for the treatment of patients at risk for or suffering from HF (38). More recently, there has been some evidence that the use of renin inhibitors may be cardioprotective as well, and additional studies to further clarify their potential benefits are ongoing (34). Nevertheless, therapeutic approaches that inhibit AngII, while beneficial, have not proven to be entirely effective in eradicating CVD, and dual therapies combining different types of RAS inhibitors remain controversial. Two large trials conducted in patients with HF, the Valsartan- HF Trial (ValHeFT) and the Candesartan in HF: Assessment of Reduction in Mortality and Morbidity-Added (CHARM-Added) Trial, showed that combined treatment with ACE inhibitors and ARBs significantly improved morbidity and mortality due to CVD or congestive HF, but not overall mortality (50, 72). In the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), dual therapy with ACE inhibitors and ARBs led to worse renal outcomes than with either ACE inhibitors or ARBs alone, suggesting that there may be significant risks associated with this combined therapy (68). On the other hand, the Aliskiren Observation of HF Treatment (ALOFT) study showed that treatment with the direct renin inhibitor, aliskiren, ameliorated the neurohormonal derangements and reduced left ventricle (LV) filling pressures in patients with congestive HF already under treatment with ACE inhibitors or ARBs (73). Thus, current evidence suggests that more efficient RAS system inhibition may lead to better clinical outcomes in patients with HF, and investigation into AngII function and its inhibition, including endogenous inhibitory mechanisms, continues to be an active area of research (146).
Among the most well-known effects of AngII are salt/water retention and vasoconstriction, and AngII-induced hypertension plays an important role in the progression of CVD and HF. However, AngII also has blood pressure-dependent and blood pressure-independent effects in the heart that play a critical role in causing cardiac remodeling and dysfunction as well. AngII is an important regulator of cardiac cell growth, causing both cardiac hypertrophy and fibrosis (17, 35, 39, 40, 43, 79, 85, 86, 89, 93, 107, 113, 134, 141, 142, 149). On the other hand, AngII is also involved in cell death, playing a role in cardiomyocyte apoptosis (12, 22, 41, 42, 60, 94, 101, 113, 142, 153) and, as demonstrated in recent studies, in autophagy as well (16, 17, 97, 98). Although generally considered a homeostatic mechanism, autophagy has been shown to be detrimental during reperfusion injury (29, 71), and autophagic cell death has been detected in the cardiac tissue of HF patients (28). AngII signaling activates tumor necrosis factor α (TNFα) and nuclear factor κB (NFκB) (40, 77, 109, 120, 122, 153), and upregulates endothelial chemokines and chemoattractant molecules, thus triggering an inflammatory response in models of diastolic HF and MI, as well as in aging-induced cardiomyopathy (45, 77, 89, 107, 120, 142, 143). In addition, intracellular AngII has been shown to have a negative effect on myocyte junctional conductance and ion channel function (109, 112). However, because it is a more immediate consequence of AngII signaling and acts upstream to promote most of the other effects mentioned above, perhaps the most important effect of AngII is increased production of ROS and oxidative stress.
Overview of ROS
ROS are unstable, reactive oxygen derivatives formed by the reduction of one or more electrons from molecular oxygen, including superoxide (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•), among others. O2•− can be generated by a number of mechanisms in the cell, including leakage from the electron transport chain and as a metabolic byproduct of enzymes such as xanthine oxidase (XO) and cyclooxygenase. ROS can also be produced by oxidoreductases of the endoplasmic reticulum during the unfolded protein response or as a result of NOS uncoupling. In addition, NAD(P)H oxidases (Noxs) produce O2•− as their primary product, using either NADH or NADPH as an electron donor. O2•− is highly reactive but poorly lipid-soluble, such that its direct effects are largely confined to the subcellular compartment in which it is generated except in cases of active transport. However, O2•− is rapidly converted to the less reactive but more highly membrane-permeable H2O2, either spontaneously or by SODs, allowing for a more-widespread effect throughout the cell. Additional highly reactive species are generated when H2O2 is reduced further through the iron-catalyzed Fenton reaction to produce OH•, and when O2•− combines with NO• to form peroxynitrite (ONOO−).
This ongoing ROS production is normally counteracted and an equilibrium oxidative state is maintained by SODs and the reduction of H2O2 to H2O by catalase and peroxidases, as well as the actions of nonenzymatic antioxidants and radical scavengers. Many cellular proteins undergo changes in conformation and activity due to reduction and oxidation of their thiol groups, and ROS have been shown to play a key role in many important physiological processes, including host defense and inflammation, cellular signaling, gene expression and regulation of cell growth and death, and oxygen sensing (7). A recent study demonstrated that ROS play an essential role in starvation-induced autophagy by inhibiting the Atg4 cysteine protease through a redox-dependent mechanism (106). Despite a decrease in oxidative stress, antioxidant treatment exacerbated ischemia/reperfusion (I/R) injury in Dahl salt-sensitive hypertensive rats (69), and antioxidant treatment in conjunction with losartan treatment abolished the protective effect of the ARB against LV remodeling and in cardiomyopathic hamsters, apparently due to the inhibition of redox-dependent activation of NOS (70). Nox4 overexpression in cardiomyocytes and the resultant increase in H2O2 production protected mouse hearts against the effects of chronic pressure overload by increasing angiogenesis, possibly due to enhancement of hypoxia-inducible factor-1 activation and vascular endothelial growth factor release (147). Other examples include the regulation of calcium/calmodulin-dependent protein kinase II (CaMKII), class II histone deacetylases (HDACs), and the Na+-K+ pump in the heart, all of which were recently demonstrated to undergo reversible oxidative modification (3, 22, 26).
Oxidative Stress and HF
Under pathological conditions, however, the equilibrium oxidative state may be disrupted due to either an increase in ROS production or a decrease in antioxidant function, leading to the development of oxidative stress. Excess ROS can cause direct damage to proteins, lipids, and DNA, or can contribute to pathophysiological processes through inappropriate reversible or irreversible redox modification of cellular proteins.
ROS and oxidative stress have been implicated in a number of pathological processes that contribute to HF, including vasoconstriction, cardiac hypertrophy, myocyte apoptosis, fibrosis, inflammation, I/R injury, and myocardial stunning (48, 116). In addition, there is growing evidence that ROS are directly involved in the development of HF. In rats, the progression from hypertension-induced compensated left ventricular hypertrophy to decompensated HF was associated with an increase in myocardial ROS (132). Similarly, a number of studies have shown that O2•− production and oxidative stress are significantly increased in the hearts of patients with dilated cardiomyopathy and HF (11, 30, 37, 66, 87, 104, 131). Thus far, clinical studies have not demonstrated a clear beneficial effect of antioxidant therapy in improving cardiovascular outcomes (46, 62, 128), however, possibly due to inhibition of beneficial ROS signaling mechanisms together with the detrimental ones.
AngII, Oxidative Stress, and HF
Numerous studies have shown that AngII signaling in the failing heart is associated with increased ROS generation and oxidative stress. AngII infusion for 2 weeks, which causes cardiac hypertrophy, doubled OH• production in mouse hearts in an AT1R-dependent manner (43). In rats, increased oxidative stress after MI coincided with an increase in RAS components in infiltrating macrophages, suggesting the presence of local AngII production, and was blocked by treatment with an ARB (65). Similarly, the protective effects of ARB treatment against myocarditis-induced HF and hypertensive diastolic HF in rats and pacing-induced HF in dogs were associated with decreases in ROS production and oxidative stress (27, 80, 119). In addition, oxidative stress markers were shown to be increased and correlated with circulating AngII levels in human HF patients, and were highest in patients with increased AT1R responsiveness to AngII due to homozygocity for the AT1R A1166C gene polymorphism (14).
As mentioned above, many of the downstream effects of AngII signaling are mediated through ROS generation. AngII-induced ROS modulate gene expression through transcription factor activation, upregulate growth factors and cytokines, and activate kinases. Furthermore, AngII-induced oxidative stress can cause mitochondrial damage and dysfunction. Oxidative stress has been shown to be important in mediating AngII-induced cardiac hypertrophy, apoptosis, and fibrosis, as well as playing a role in AngII-dependent inflammation and cardiomyocyte contractile dysfunction. Importantly, cleavage of AGT by renin was recently shown to require a conformational change brought about by formation of a disulfide bridge between two cysteines as a result of oxidation (152), suggesting the presence of a positive feedback mechanism by which AngII-induced ROS further increase AngII formation (Fig. 2). How AngII increases cardiac oxidative stress is still not entirely understood. However, recent studies have pointed to the involvement of several different mechanisms.
FIG. 2.

Redox modulation of AGT cleavage. Oxidation of the sulfhydryl groups of cysteines 18 and 138 in AGT causes formation of a disulfide bridge. The resultant conformational change exposes the cleavage site to the renin active site cleft and leads to preferential binding and activation by the (P)RR, thereby increasing AngI release. AngI, angiotensin I; AGT, angiotensinogen; (P)RR, (pro)renin receptor.
NAD(P)H Oxidase
As mentioned above, Noxs are a family of membrane-bound enzymes that specifically produce O2•− by transferring an electron from NAD(P)H to O2 as their primary function, rather than as a byproduct. The prototype Nox catalytic subunit, gp91phox or Nox2, was first identified as being responsible for the respiratory burst that plays an important part of the killing response in phagocytes. There are now seven known members of the Nox family, Nox1–Nox5 and Duox1–Duox2. Of these, Nox1–Nox4 have highly conserved structures, comprising six transmembrane domains, two hemes, and NAD(P)H- and flavin adenine dinucleotide–binding domains in the C-terminus, and require heterodimerization with a second membrane protein, p22phox, for stabilization and activation (7). In the heart, Nox activity is primarily due to Nox2 and Nox4 (2, 8, 13) (Fig. 3), which, despite their structural similarities, differ in cellular localization and functionality. Nox2 is located primarily on the plasma membrane, and, in addition to binding p22phox, its activation involves binding of cytosolic subunits p47phox and p67phox (and, in some cases, p40phox), and Rac1 GTPase, allowing for post-translational modulation of Nox2 activity through regulation of cytosolic subunit translocation (7). Nox4, on the other hand, is primarily located on perinuclear intracellular membranes, such as those of the mitochondria (2), and does not require cytosolic subunit binding, but rather is regulated primarily at the transcriptional level (7).
FIG. 3.
Structure of Nox2 and Nox4. Nox2 and Nox4 share similar structures, comprising six transmembrane domains, two bound hemes, and a cytosolic domain containing FAD-binding and NAD(P)H-binding domains. Both heterodimerize with p22phox, but Nox2 requires the binding of several cytosolic subunits for activation, while Nox4 does not. FAD, flavin adenine dinucleotide; NAD(P)H, nicotinamide adenine dinucleotide phosphate; Nox, NAD(P)H oxidase.
There is abundant evidence that Nox activity plays an important role in cardiac remodeling and HF (53, 67). Expression of Nox2 and its cytosolic cofactors is increased during the progression of cardiac hypertrophy to HF in guinea pigs subjected to pressure overload and in hypertensive Dahl salt-sensitive rats (56, 122), and Nox activity is increased in end-stage failing human hearts (30, 66, 87). Nox activation plays a critical role in AngII-induced apoptosis. ONOO− formed as a result of AngII-induced Nox activity causes DNA damage and p53 activation, leading to an increased Bax/Bcl-2 ratio, caspase-3 cleavage, and apoptotic cell death in H9c2 cells (60). Since p53 also binds to and activates the promoters of AGT and AT1R (96), this may represent a positive feedback mechanism by which AngII signaling self-amplifies. AngII-induced apoptosis also involves phosphorylation of p38 after activation of CaMKII by ROS generated by Nox (22, 94, 101). ROS lower the CaMKII activation threshold to subdiastolic Ca2+ concentrations (94), and activate the kinase through parallel mechanisms of autophosphorylation at threonine 287, possibly as a result of phosphatase oxidation and inhibition, and oxidation of two redox-sensitive methionine residues (22, 31) (Fig. 4). This ROS-dependent activation of CaMKII may also play a role in the development of cardiac hypertrophy through phosphorylation of class II HDACs. Nox2 and Nox4 have been implicated in AngII- and pressure overload-induced cardiac hypertrophy, respectively (8, 13), and phosphorylation of class II HDACs such as HDAC4 by CaMKII and other HDAC kinases leads to their nuclear export and de-repression of prohypertrophic transcription factors (4, 92). In addition, HDAC4 is subject to reversible redox regulation, with oxidation of conserved cysteine residues and disulfide bond formation leading to nuclear export regardless of its phosphorylation state (3). While a role for Noxs in this mechanism has yet to be established, Nox4 is expressed in the nucleus and is upregulated by hypertrophic stimuli such as AngII and phenylephrine (2), making such a role plausible. Increased Nox-dependent ROS also activate NFκB, TGF-β, MMPs, and growth factors, leading to increased fibrosis, extracellular matrix degradation, and tissue remodeling, and contribute to AngII-dependent cardiac contractile dysfunction (10, 40, 45, 58, 63, 105, 122, 149, 150). The latter may be due in part to a role in the regulation of cardiac ion channels. Nox-dependent O2•− production and oxidative stress due to increased cellular AngII cause a decrease in the K+ currents of ventricular myocytes in diabetic rats (110–112), and AngII inhibits transcription of the cardiac Na+ channel, leading to a decrease in the Na+ current, through activation of NFκB by Nox-dependent ROS (109). Glutathionylation by ONOO− produced as a result of protein kinase C-dependent Nox activity is also responsible for AngII-induced inhibition of the cardiac sarcolemmal Na+-K+ pump, and may contribute to myocardial damage and contractile dysfunction as a result of high Na+ and Ca+ levels (26, 102, 139). The form-specific functions of Nox2 and Nox4 in mediating these effects require further clarification, however. Many of the above studies examined p47phox expression and translocation as an indicator of Nox activity, suggesting the involvement of Nox2, but systemic knockout of p47phox in mice inhibited increases in both Nox2 and Nox4 expression after aortic constriction, and only Nox4 was inhibited in ACE2/p47phox double knockout mice (10). On the other hand, cardiomyocyte-specific deletion of Rac1, which attenuates association of Nox2 with its cytosolic cofactors, also inhibits AngII-induced cardiac hypertrophy and dysfunction (105), and systemic Nox2 knockout protects the heart against AngII-induced hypertrophy, and MI- and doxorubicin-induced adverse remodeling and dysfunction (8, 63, 150). Doxorubicin treatment increases both Nox2 and Nox4 mRNA levels, but neither one is increased in Nox2 knockout mice, suggesting that Nox2 may play a role in regulating Nox4 expression (150). Zhang and colleagues found that Nox4 expression in cardiomyocytes was protective against pressure overload-induced cardiac dysfunction, hypertrophy, and fibrosis due to paracrine activation of angiogenesis (147). However, studies in our laboratory demonstrated that Nox4 expression in cardiomyocytes is detrimental, leading to increased apoptosis and mitochondrial dysfunction in aged mice, and increased cardiac dysfunction, fibrosis, and apoptosis after aortic constriction (2, 54). In both aging and aortic constriction, the cardiomyocyte cross-sectional area was increased, but there was no organ-level hypertrophy (2, 54), suggesting that Nox4-associated hypertrophy may be secondary to its role in apoptosis and dysfunction. Neither cardiac-specific overexpression nor systemic or cardiac-specific knockout of Nox4 resulted in any baseline abnormality in young mice (2, 54, 147), and the discrepancies between these studies may be due to differences in genetic background or experimental conditions.
FIG. 4.
ROS-dependent activation of CaMKII by AngII. AngII signaling leads to NAD(P)H oxidase–dependent ROS generation. Increased ROS levels activate CaMKII via either autophosphorylation of threonine 287, possibly due to oxidation and inhibition of phosphatases, or oxidation of methionines 281 and 282. The resultant calcium/CaM-independent activity of CaMKII leads to p38 phosphorylation and apoptosis, and possibly contributes to hypertrophy through phosphorylation and derepression of prohypertrophic transcription factors. CaM, calmodulin; AngII, angiotensin II; CaMKII, calcium/calmodulin-dependent protein kinase II; HDAC, histone deacetylase; ROS, reactive oxygen species; AT1R, AngII type 1 receptor; NAD(P)H, nicotinamide adenine dinucleotide phosphate.
The vast majority of currently available data point to Nox as the source of AngII-induced ROS. In vitro and in vivo studies in rats and mice have shown that AngII treatment stimulates an increase in ROS production in the heart that is significantly inhibited by diphenylene iodonium (DPI, an inhibitor of flavoproteins such as Nox), apocynin (a Nox inhibitor), or dominant negative Rac, but not by NG-nitro-L-arginine methyl ester (L-NAME, a NOS inhibitor), rotenone (a complex-I mitochondrial electron chain inhibitor), or XO inhibitors, suggesting that Nox is the major source of AngII-induced ROS in cardiomyocytes (13, 27, 88, 89, 101, 118, 134, 138) (This increase in Nox activity and oxidative stress was not observed in female mice, however [21]). Similarly, rats known to have increased AngII levels, such as Ren2 and hypertensive Dahl salt-sensitive rats, displayed an AT1R-dependent increase in ROS generation, which was inhibited by DPI (27, 89, 138). AngII has also been shown to increase translocation of p47phox to the membrane (17, 58, 153), as well as membrane-associated Rac1 GTP-binding activity (86), both of which are involved in Nox2 activation, as mentioned above. In addition, RAS inhibition by ACE inhibitors or ARBs appears to inhibit the increased expression of Nox subunits p22phox, Nox2, p47phox, and p67phox observed in rats and mice with pathological cardiovascular conditions such as spontaneous hypertension, diastolic HF, MI, or cardiomyopathy due to experimental autoimmune myocarditis, doxorubicin treatment, or hyperglycemia (27, 35, 77, 99, 119, 138, 150).
The relative importance of Nox2 and Nox4 in mediating the effects of AngII in the heart remains a subject of some debate, however. Several in vitro and in vivo studies have shown that AngII stimulation increases expression of Nox2, p47phox, and membrane-associated Rac1 (17, 101, 134, 141, 149), and most of the studies investigating the role of AngII-induced Nox activity and expression in pathological cardiovascular conditions have focused on Nox2 and its cytosolic subunits, as noted above. Furthermore, studies with Nox2 knockout mice found that Nox2 deficiency abolished the AngII-induced Nox activity observed in wild-type mice (13, 40). However, these studies do not preclude the possibility that ROS produced by one Nox form might be involved in upregulating the other, with both playing a role in mediating the downstream effects of AngII. Furthermore, these studies used relatively short periods of AngII treatment, leaving untested the possibility that longer chronic exposure to AngII might directly upregulate Nox4. Indeed, our laboratory and others have recently demonstrated that AngII treatment increases Nox4 expression in mouse hearts (2, 17). In addition, Dai and colleagues found that, while AngII increased both Nox2 and Nox4 expression, the increase in Nox2 was inhibited by catalase while the Nox4 increase was not (17), suggesting that the two forms are regulated by different, though possibly interdependent, mechanisms.
Nitric Oxide Synthase
The three NOS isoforms, neuronal NOS, inducible NOS, and endothelial NOS (eNOS), are a family of homodimeric enzymes whose primary function is to produce NO• by transfer of electrons from NADPH to O2 and oxidation of L-arginine to L-citrulline via the stable intermediary, Nω-hydroxy-L-arginine. This process involves shuttling electrons from the C-terminal reductase domain of one monomer to the N-terminal oxidase domain of the other monomer, and requires the presence of several cofactors, including tetrahydrobiopterin (BH4) (57, 81). NO• is a powerful vasodilator, and it and sufficient NOS activity are generally considered to be protective factors in the cardiovascular system. However, NOS can also contribute to ROS production since, as noted above, in the presence of a nitroso-redox imbalance, O2•− can combine with NO• to form ONOO−. Furthermore, oxidation of NOS by ONOO− or a deficiency in either L-arginine or BH4 causes uncoupling of the enzyme, resulting in generation of O2•− instead of NO• (57). BH4 is itself readily oxidized by ROS to the inactive BH2, leading to a self-perpetuating cycle of BH4 deficiency, NOS uncoupling, and ROS generation (81).
ROS generation due to eNOS uncoupling was shown to play a major role in cardiac remodeling brought about by chronic pressure overload in mice (123). However, the role of NOS uncoupling in HF and AngII-induced ROS generation is not well understood. On the one hand, eNOS expression was significantly downregulated in the hearts of spontaneously hypertensive stroke-prone rats, but upregulated to normal levels in the presence of RAS inhibition (125), and NOS inhibition abrogated the protective effect of treatment with the ARB, losartan, against left ventricular remodeling in hamsters with cardiomyopathy, and I/R injury in Dahl salt-sensitive rats (69, 70), suggesting that NOS activity is protective against the effects of AngII. Similarly, adenoviral gene transfer of the human eNOS gene into rat hearts after MI resulted in increased cardiac NO• production and reduced cardiac ROS generation and remodeling (115). More specifically, as mentioned above, AngII-induced ROS generation in the hearts of rats was not inhibited by L-NAME (134). On the other hand, AngII treatment increased eNOS expression while decreasing NO• production, indicating the presence of eNOS uncoupling, in rat aortas (83), and treatment with the ACE inhibitor, captopril, normalized the increased eNOS expression observed in the LVs of hamsters with chronic congestive HF (82). There is some evidence that BH4 supplementation may be effective in counteracting some of the effects of AngII and treating CVD and HF (44, 81). However, BH4 can function as an antioxidant in addition to stabilizing NOS, so its importance in clarifying the role of NOS uncoupling remains to be determined. Losartan treatment increased eNOS expression and NO• production in the cardiomyocytes of obese rats, but had the opposite effect in control animals (32), suggesting that the relationship between AngII signaling and eNOS function may be context-dependent. eNOS expression may be increased by ROS via activation of redox-sensitive transcription factors or mRNA transcript stabilization, but cytokines such as TNFα, which are activated by AngII, may reduce eNOS expression (64). Furthermore, because ROS induce NOS uncoupling, generation of ROS by NOS may be secondary to production of ROS from other sources, so that considerable additional investigation may be required to elucidate its role in mediating the effects of AngII.
Xanthine Oxidase
XO is one of two forms of the metalloflavoprotein, xanthine oxidoreductase, both of which catalyze oxidation of hydroxanthine to xanthine and xanthine to urate, playing a role in purine catabolism. However, whereas xanthine dehydrogenase (XD) uses NAD+ as an electron acceptor and produces urate and NADH as end products, XO transfers electrons to O2, resulting in formation of urate and O2•− instead. XO is derived post-translationally from XD, either by irreversible proteolytic cleavage or by reversible oxidation of sulfhydryl residues (57).
There is a small but growing body of evidence that suggests that XO activity and the resulting generation of ROS play an important role in HF. Protein expression of both XD and XO has been shown to be elevated in failing human myocardium (15), and long-term high-dose treatment with the XO inhibitor, allopurinol, reduced mortality in patients with HF (137). Similarly, chronic allopurinol treatment improved cardiac function in rats with chronic HF, in part through a reduction in oxidative stress (74). In spontaneously hypertensive/HF rats with established dilated cardiomyopathy, although expression of both XO and the Nox subunits, Nox2 and p67phox, was increased, only the XO activity was elevated above normal, while Nox activity remained unchanged (78). Treatment with an XO inhibitor reduced ROS levels and oxidative stress by inhibiting XO activity (but not Nox activity), and improved cardiac function by reversing the existing adverse remodeling in these rats. Similarly, an XO inhibitor reduced left ventricular O2•− levels, prevented the progression of adverse cardiac remodeling, and improved survival in Dahl salt-sensitive rats with established diastolic HF (39, 143).
However, the role of XO in mediating the effects of AngII is less clear. TNFα, which is activated by AngII, can induce proteolytic cleavage of XD, thus contributing to increased expression of XO (57). Alternatively, AngII-induced XO activation could be secondary to ROS generation from other sources through thiol oxidation of the XD sulfhydryl residues. Indeed, increased ROS levels in cultured neonatal rat ventricular myocytes after AngII stimulation were slightly, though not significantly, inhibited by allopurinol, suggesting that XO may contribute to some extent to AngII-induced ROS generation (118). Perhaps more convincing is the fact that allopurinol reduced oxidative stress and improved cardiac fibrosis and function in a blood pressure-independent manner in mice with AngII-induced diastolic dysfunction (39). In addition, although both Nox and XO activities were increased in the hearts of rats with diastolic HF and both were reduced by treatment with the ARB, candesartan, treatment with an XO inhibitor reduced cardiac ROS and closely mimicked the broadly protective effects of candesartan, while apocynin did not (143), suggesting that AngII-induced O2•− formation is XO-dependent rather than Nox-dependent in this model. Accordingly, ACE inhibition significantly reduced XO substrates in hypertensive subjects, suggesting that AngII may regulate the activity of XO by controlling the availability of its substrates (91). In addition, AngII was shown to directly increase endothelial XO activity (55). Nevertheless, studies addressing the relationship between AngII and XO remain scarce, and XO inhibitors can act as direct ROS scavengers in addition to inhibiting XO function, thus complicating interpretation of data obtained from studies using these compounds and making further investigation necessary.
Antioxidant Downregulation
In addition to increasing generation of ROS, AngII could theoretically increase cardiac oxidative stress by downregulating antioxidant enzymes. While there is some evidence to support a role for such a mechanism in mediating the effects of AngII stimulation and in HF, the data are extremely mixed. Several studies have indicated that SOD activity is not significantly affected in the hearts of human HF patients (6, 11, 20). However, one study found that manganese SOD (MnSOD) activity (which comprised 90% of the cardiac SOD activity) and protein expression were significantly decreased in failing human hearts (104). In spontaneously hypertensive stroke-prone rats, expression and activity of MnSOD were unchanged, but those of copper–zinc SOD (Cu/ZnSOD) were decreased, an effect that was attenuated by treatment with an ACE inhibitor and abolished by ARB treatment (125), suggesting that AngII inhibits Cu/ZnSOD, but not MnSOD. However, in AngII-treated rat cardiac fibroblasts, the activity of both MnSOD and Cu/ZnSOD was inhibited, and expression of MnSOD, but not Cu/ZnSOD, was decreased (59). Similarly, in rats subjected to MI, MnSOD expression in the infarct area and activity of both MnSOD and Cu/ZnSOD were reduced, and these decreases were suppressed by treatment with RAS inhibitors (127). In addition, nuclear SIRT1, which is increased in cardiomyocytes in several models of HF, upregulates MnSOD expression, leading to a reduction in ROS levels and apoptosis in AngII-treated C2C12 cells, as well as improved cardiac function and survival in hamsters with chronic HF (126). In rats with MI-induced congestive HF, the activities of SOD, catalase, and GPx were all reduced, but only the GPx activity was improved by losartan treatment (49), suggesting that AngII is involved in downregulating GPx, but not the other antioxidants, in this model. Likewise, treatment with an ACE inhibitor increased GPx activity in rats with chronic MI (127). On the other hand, GPx expression and activity have been shown to be either unchanged or increased in AngII-stimulated rat cardiac fibroblasts and HF patients (6, 11, 20, 59, 104), and GPx mRNA expression is increased rather than decreased during the transition to congestive HF in hypertensive Dahl salt-sensitive rats, an effect that is reversed by treatment with an ARB (122). The data on catalase are even more difficult to interpret, with different studies indicating that catalase expression and activity are alternately upregulated, downregulated, or unchanged in the hearts of patients with end-stage HF (6, 11, 20, 104). Some of this discrepancy may be due to differences in the patient populations, including ischemic versus dilated cardiomyopathy, but further investigation is necessary to clarify this issue. In support of a role for catalase downregulation in mediating the effects of AngII, however, is the fact that catalase expression was shown to be downregulated in the hearts of AGT transgenic mice and in cardiomyocytes treated with AngII (85). Interestingly, although thioredoxin (Trx) can be oxidized (and thereby inhibited) by ROS, it is actually upregulated by AngII and in patients with chronic HF (21, 37, 144). Trx is protective against apoptosis by promoting ubiquitination and degradation of apoptosis signal–regulating kinase 1 (61, 148), prevents oxidation-dependent nuclear export of HDAC4 (3), and inhibits AngII-induced cardiac hypertrophy through upregulation of miR-98/let-7 microRNAs and downregulation of cyclin D2 (140, 144), suggesting that it acts as a negative feedback regulator of AngII (Fig. 5). Thus, while some studies appear to support a role for AngII in the regulation of antioxidants, others seem to negate the existence of such a mechanism or suggest that antioxidant activity may actually be upregulated in order to compensate for increased AngII-induced ROS generation. The solution to this riddle is probably context-dependent, and further investigation is required to fully establish the nature of the relationship between AngII signaling and antioxidant expression and activity.
FIG. 5.
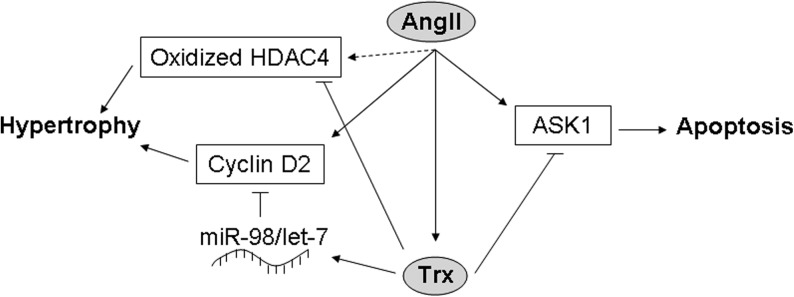
Schematic of the potential mechanism by which Trx acts as a negative feedback regulator of AngII. AngII signaling triggers apoptosis through ASK1, and hypertrophy through cyclin D2 and possibly oxidation of HDAC4. However, it also upregulates Trx, which increases expression of miR-98/let-7 microRNAs, thereby inhibiting cyclin D2, and it directly inhibits HDAC4 oxidation and ASK1. ASK1, apoptosis signal-regulating kinase 1; Trx, thioredoxin.
Mitochondria
Mitochondrial leakage is considered the major source of ROS under normal physiological conditions, but the amount of ROS produced in this way is generally low and is kept in check by antioxidants such as mitochondrial MnSOD and catalase. However, under pathological conditions, mitochondrial damage and dysfunction can lead to blockage of the electron transport chain and increased ROS production, while downregulation of antioxidants may result in decreased scavenging capacity, causing an overall increase in mitochondrial ROS levels. Because of its high energy demand, the heart has a higher mitochondrial volume than many other tissues, and its proper function is especially sensitive to changes in mitochondrial function and maintenance of the energetic balance. Whether or not mitochondrial sources contribute significantly to AngII-induced increases in ROS in the heart has recently come under investigation. Increased ROS generation in cardiomyocytes as a result of AngII treatment was shown to occur primarily in mitochondria, resulting in a decrease in mitochondrial membrane potential (17). Although AngII stimulation of cultured neonatal rat cardiomyocytes did not affect the mitochondrial DNA (mtDNA) copy number (118), AngII-treated mice did exhibit a significantly decreased mtDNA copy number and increased mtDNA deletions, as well as decreased mitochondrial respiratory capacity and increased mitochondrial damage (17). These effects were accompanied by hypertrophy, fibrosis, and diastolic dysfunction, and were inhibited by mitochondrial catalase, suggesting that mitochondrial ROS play a major role in AngII-induced hypertrophy and dysfunction. Studies using metabolomic profiling and electron microscopy have demonstrated that cardiac substrate use and mitochondrial biogenesis and morphology are altered in the presence of increased RAS component expression (17, 76, 138), and mitochondrial autophagy is increased by AngII-induced ROS (17, 103). In addition, AngII can increase the expression of uncoupling proteins and decrease the expression of mitochondrial respiratory chain proteins under certain circumstances (27, 103). How AngII induces mitochondrial ROS generation is not yet known, however. ROS from other sources may cause ROS-induced ROS release (154), through damage to mitochondrial components and oxidation of the membrane permeability transition pore. Indeed, our laboratory has recently shown that overexpression of Nox4 leads to an increase in O2•− production that can be partially inhibited by rotenone as well as DPI, and is accompanied by increased oxidation of mitochondrial proteins, mitochondrial dysfunction, and decreased mitochondrial biogenesis (2). Conversely, cardiac-specific deletion of Nox4 was protective against pressure overload-induced mitochondrial damage (54). Since Nox4 is upregulated by AngII and is expressed in mitochondria (2), it is possible that AngII stimulates mitochondrially localized ROS generation by Nox4, which in turn causes further ROS generation by the mitochondria themselves. Alternatively, mitochondrial ROS generation could be triggered by elevated ROS levels caused by an AngII-induced decrease in mitochondrial antioxidant expression, as suggested by the fact that AngII signaling resulted in decreased MnSOD expression, accompanied by a significant increase in mitochondrial O2•− levels (59). On the other hand, eNOS uncoupling, XO, and p47phox expression and translocation can all be activated by ROS, and AngII-induced Nox2 upregulation is inhibited by catalase (17), suggesting that it, too, is ROS dependent. Thus, it is also possible that ROS originating from the mitochondria could trigger additional ROS generation from these other sources, resulting in the creation of a positive feedback loop. Whether a direct mechanism exists by which AngII signaling leads to mitochondrial ROS generation has yet to be determined.
Conclusions
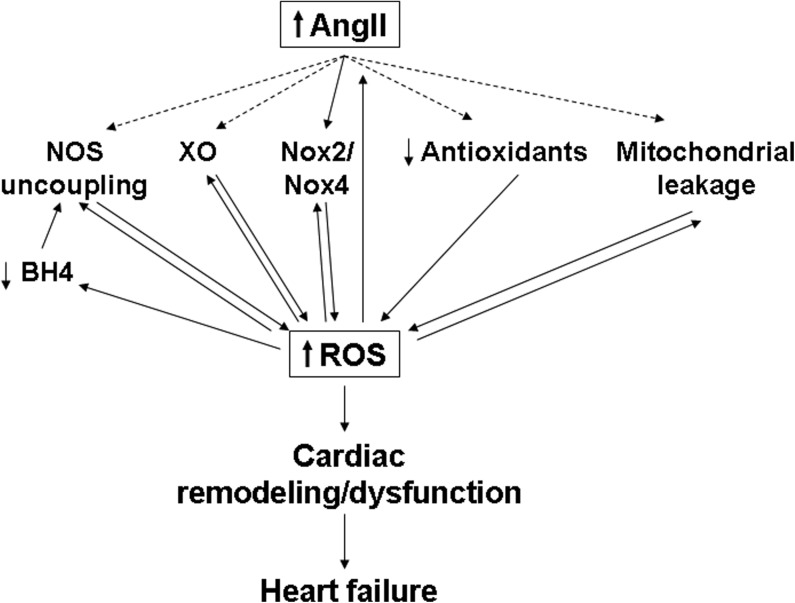
Despite recent medical advances, morbidity and mortality due to CVD and HF remain high. Inhibition of the RAS and AngII signaling has been shown to increase longevity in experimental animal models (5, 9), and RAS inhibitors have been clinically shown to be therapeutically beneficial for patients at risk for and suffering from CVD and HF. Furthermore, ROS generation and oxidative stress are now known to play a critical role in mediating the effects of AngII signaling and in the pathology of the failing heart. However, our current understanding of the signaling pathways and mechanisms connecting AngII signaling, ROS generation, and HF remains incomplete. Several different potential sources of ROS may contribute to the downstream effects of AngII in the heart, and the relative importance of these various sources may vary depending on the cell type and context. In addition, the different ROS pathways may be interdependent, with ROS generated from one source triggering further ROS generation from another source. Such stepwise generation of ROS could theoretically lead to a vicious cycle in which ROS production becomes self-amplifying, and this might contribute to the changes observed in the heart during decompensation and the transition to HF (Fig. 6). Further investigation and a better understanding of how AngII induces ROS generation and how AngII-induced ROS affect cardiac remodeling and function should lead to the development of improved therapies and better outcomes for those suffering from CVD and HF.
FIG. 6.
The vicious cycle of AngII signaling and ROS generation. AngII signaling triggers an increase in ROS levels through activation of NAD(P)H oxidases (Nox2/Nox4), and possibly through NOS uncoupling, XO, increased mitochondrial leakage, and antioxidant downregulation. The increased ROS and oxidative stress can increase NOS uncoupling (either through direct oxidation of NOS or decreased BH4 availability) and Nox and XO activation, and cause mitochondrial damage, as well as upregulating expression of AngII itself. The result is a self-amplifying cycle of AngII signaling and increased ROS generation, leading to decompensatory cardiac remodeling and dysfunction, and, eventually, heart failure. BH4, tetrahydrobiopterin; NOS, nitric oxide synthase; XO, xanthine oxidase.
Abbreviations Used
- ACE
angiotensin converting enzyme
- Ac-SDKP
N-acetyl-seryl-aspartyl-lysyl-proline
- AGT
angiotensinogen
- Ang
angiotensin
- APA
aminopeptidase A
- APN
aminopeptidase N
- ARB
AT1R blocker
- ASK1
apoptosis signal-regulating kinase 1
- AT1R
AngII type 1 receptor
- AT2R
AngII type 2 receptor
- BH4
tetrahydrobiopterin
- CaM
calmodulin
- CaMKII
calcium/calmodulin-dependent protein kinase II
- CPA
carboxypeptidase A
- Cu/ZnSOD
copper–zinc SOD
- CVD
cardiovascular disease
- DPI
diphenylene iodonium
- eNOS
endothelial NOS
- FAD
flavin adenine dinucleotide
- GPx
glutathione peroxidase
- H2O2
hydrogen peroxide
- HDAC
histone deacetylase
- HF
heart failure
- I/R
ischemia/reperfusion
- L-NAME
NG-nitro-L-arginine methyl ester
- LV
left ventricle
- MI
myocardial infarction
- MMP
matrix metalloprotease
- MnSOD
manganese SOD
- mtDNA
mitochondrial DNA
- NAD(P)H
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor κB
- NO•
nitric oxide
- NOS
nitric oxide synthase
- Nox
NAD(P)H oxidase
- O2•−
superoxide
- OH•
hydroxyl radical
- ONOO−
peroxynitrite
- (P)RR
(pro)renin receptor
- RAS
renin–angiotensin system
- redox
reduction–oxidation
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TGF-β
transforming growth factor-β
- TNFα
tumor necrosis factor α
- Trx
thioredoxin
- XD
xanthine dehydrogenase
- XO
xanthine oxidase
Acknowledgments
We thank Dr. Sebastiano Sciarretta for critical reading of the article. This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, and AG27211 and the Foundation of Leducq Transatlantic Network of Excellence.
References
- 1.Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med. 1987;316:1429–1435. doi: 10.1056/NEJM198706043162301. [DOI] [PubMed] [Google Scholar]
- 2.Ago T. Kuroda J. Pain J. Fu C. Li H. Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T. Liu T. Zhai P. Chen W. Li H. Molkentin JD. Vatner SF. Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 4.Backs J. Song K. Bezprozvannaya S. Chang S. Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–1864. doi: 10.1172/JCI27438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basso N. Cini R. Pietrelli A. Ferder L. Terragno NA. Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–H1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 6.Baumer AT. Flesch M. Wang X. Shen Q. Feuerstein GZ. Bohm M. Antioxidative enzymes in human hearts with idiopathic dilated cardiomyopathy. J Mol Cell Cardiol. 2000;32:121–130. doi: 10.1006/jmcc.1999.1061. [DOI] [PubMed] [Google Scholar]
- 7.Bedard K. Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 8.Bendall JK. Cave AC. Heymes C. Gall N. Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 9.Benigni A. Corna D. Zoja C. Sonzogni A. Latini R. Salio M. Conti S. Rottoli D. Longaretti L. Cassis P. Morigi M. Coffman TM. Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodiga S. Zhong JC. Wang W. Basu R. Lo J. Liu GC. Guo D. Holland SM. Scholey JW. Penninger JM. Kassiri Z. Oudit GY. Enhanced susceptibility to biomechanical stress in ACE2 null mice is prevented by loss of the p47phox NADPH oxidase subunit. Cardiovasc Res. 2011;91:151–161. doi: 10.1093/cvr/cvr036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borchi E. Bargelli V. Stillitano F. Giordano C. Sebastiani M. Nassi PA. d'Amati G. Cerbai E. Nediani C. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta. 2010;1802:331–338. doi: 10.1016/j.bbadis.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Burniston JG. Saini A. Tan LB. Goldspink DF. Angiotensin II induces apoptosis in vivo in skeletal, as well as cardiac, muscle of the rat. Exp Physiol. 2005;90:755–761. doi: 10.1113/expphysiol.2005.030908. [DOI] [PubMed] [Google Scholar]
- 13.Byrne JA. Grieve DJ. Bendall JK. Li JM. Gove C. Lambeth JD. Cave AC. Shah AM. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 14.Cameron VA. Mocatta TJ. Pilbrow AP. Frampton CM. Troughton RW. Richards AM. Winterbourn CC. Angiotensin type-1 receptor A1166C gene polymorphism correlates with oxidative stress levels in human heart failure. Hypertension. 2006;47:1155–1161. doi: 10.1161/01.HYP.0000222893.85662.cd. [DOI] [PubMed] [Google Scholar]
- 15.Cappola TP. Kass DA. Nelson GS. Berger RD. Rosas GO. Kobeissi ZA. Marban E. Hare JM. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation. 2001;104:2407–2411. doi: 10.1161/hc4501.098928. [DOI] [PubMed] [Google Scholar]
- 16.Dai DF. Rabinovitch P. Mitochondrial oxidative stress mediates induction of autophagy and hypertrophy in angiotensin-II treated mouse hearts. Autophagy. 2011;7:917–918. doi: 10.4161/auto.7.8.15813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai DF. Johnson SC. Villarin JJ. Chin MT. Nieves-Cintron M. Chen T. Marcinek DJ. Dorn GW., 2nd Kang YJ. Prolla TA. Santana LF. Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dell'Italia LJ. Husain A. Dissecting the role of chymase in angiotensin II formation and heart and blood vessel diseases. Curr Opin Cardiol. 2002;17:374–379. doi: 10.1097/00001573-200207000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Der Sarkissian S. Grobe JL. Yuan L. Narielwala DR. Walter GA. Katovich MJ. Raizada MK. Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension. 2008;51:712–718. doi: 10.1161/HYPERTENSIONAHA.107.100693. [DOI] [PubMed] [Google Scholar]
- 20.Dieterich S. Bieligk U. Beulich K. Hasenfuss G. Prestle J. Gene expression of antioxidative enzymes in the human heart: increased expression of catalase in the end-stage failing heart. Circulation. 2000;101:33–39. doi: 10.1161/01.cir.101.1.33. [DOI] [PubMed] [Google Scholar]
- 21.Ebrahimian T. He Y. Schiffrin EL. Touyz RM. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J Hypertens. 2007;25:1263–1271. doi: 10.1097/HJH.0b013e3280acac60. [DOI] [PubMed] [Google Scholar]
- 22.Erickson JR. Joiner ML. Guan X. Kutschke W. Yang J. Oddis CV. Bartlett RK. Lowe JS. O'Donnell SE. Aykin-Burns N. Zimmerman MC. Zimmerman K. Ham AJ. Weiss RM. Spitz DR. Shea MA. Colbran RJ. Mohler PJ. Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feldt S. Batenburg WW. Mazak I. Maschke U. Wellner M. Kvakan H. Dechend R. Fiebeler A. Burckle C. Contrepas A. Jan Danser AH. Bader M. Nguyen G. Luft FC. Muller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension. 2008;51:682–688. doi: 10.1161/HYPERTENSIONAHA.107.101444. [DOI] [PubMed] [Google Scholar]
- 24.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira AJ. Santos RA. Bradford CN. Mecca AP. Sumners C. Katovich MJ. Raizada MK. Therapeutic implications of the vasoprotective axis of the renin-angiotensin system in cardiovascular diseases. Hypertension. 2010;55:207–213. doi: 10.1161/HYPERTENSIONAHA.109.140145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figtree GA. Liu CC. Bibert S. Hamilton EJ. Garcia A. White CN. Chia KK. Cornelius F. Geering K. Rasmussen HH. Reversible oxidative modification: a key mechanism of Na+-K+ pump regulation. Circ Res. 2009;105:185–193. doi: 10.1161/CIRCRESAHA.109.199547. [DOI] [PubMed] [Google Scholar]
- 27.Guo P. Nishiyama A. Rahman M. Nagai Y. Noma T. Namba T. Ishizawa M. Murakami K. Miyatake A. Kimura S. Mizushige K. Abe Y. Ohmori K. Kohno M. Contribution of reactive oxygen species to the pathogenesis of left ventricular failure in Dahl salt-sensitive hypertensive rats: effects of angiotensin II blockade. J Hypertens. 2006;24:1097–1104. doi: 10.1097/01.hjh.0000226200.73065.5d. [DOI] [PubMed] [Google Scholar]
- 28.Gurusamy N. Das DK. Autophagy, redox signaling, and ventricular remodeling. Antioxid Redox Signal. 2009;11:1975–1988. doi: 10.1089/ars.2009.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hariharan N. Zhai P. Sadoshima J. Oxidative stress stimulates autophagic flux during ischemia/reperfusion. Antioxid Redox Signal. 2011;14:2179–2190. doi: 10.1089/ars.2010.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heymes C. Bendall JK. Ratajczak P. Cave AC. Samuel JL. Hasenfuss G. Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 31.Howe CJ. Lahair MM. McCubrey JA. Franklin RA. Redox regulation of the calcium/calmodulin-dependent protein kinases. J Biol Chem. 2004;279:44573–44581. doi: 10.1074/jbc.M404175200. [DOI] [PubMed] [Google Scholar]
- 32.Huisamen B. Perel SJ. Friedrich SO. Salie R. Strijdom H. Lochner A. ANG II type I receptor antagonism improved nitric oxide production and enhanced eNOS and PKB/Akt expression in hearts from a rat model of insulin resistance. Mol Cell Biochem. 2011;349:21–31. doi: 10.1007/s11010-010-0656-6. [DOI] [PubMed] [Google Scholar]
- 33.Ichihara S. Senbonmatsu T. Price E., Jr. Ichiki T. Gaffney FA. Inagami T. Angiotensin II type 2 receptor is essential for left ventricular hypertrophy and cardiac fibrosis in chronic angiotensin II-induced hypertension. Circulation. 2001;104:346–351. doi: 10.1161/01.cir.104.3.346. [DOI] [PubMed] [Google Scholar]
- 34.Israili ZH. Velasco M. Bermudez V. Direct renin inhibitors as antihypertensive agents. Am J Ther. 2010;17:237–254. doi: 10.1097/MJT.0b013e3181c08096. [DOI] [PubMed] [Google Scholar]
- 35.Ito N. Ohishi M. Yamamoto K. Tatara Y. Shiota A. Hayashi N. Komai N. Yanagitani Y. Rakugi H. Ogihara T. Renin-angiotensin inhibition reverses advanced cardiac remodeling in aging spontaneously hypertensive rats. Am J Hypertens. 2007;20:792–799. doi: 10.1016/j.amjhyper.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Iusuf D. Henning RH. van Gilst WH. Roks AJ. Angiotensin-(1–7): pharmacological properties and pharmacotherapeutic perspectives. Eur J Pharmacol. 2008;585:303–312. doi: 10.1016/j.ejphar.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 37.Jekell A. Hossain A. Alehagen U. Dahlstrom U. Rosen A. Elevated circulating levels of thioredoxin and stress in chronic heart failure. Eur J Heart Fail. 2004;6:883–890. doi: 10.1016/j.ejheart.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Jessup M. Abraham WT. Casey DE. Feldman AM. Francis GS. Ganiats TG. Konstam MA. Mancini DM. Rahko PS. Silver MA. Stevenson LW. Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 39.Jia N. Dong P. Ye Y. Qian C. Dai Q. Allopurinol Attenuates Oxidative Stress and Cardiac Fibrosis in Angiotensin II-Induced Cardiac Diastolic Dysfunction. Cardiovasc Ther. 2012;30:117–123. doi: 10.1111/j.1755-5922.2010.00243.x. [DOI] [PubMed] [Google Scholar]
- 40.Johar S. Cave AC. Narayanapanicker A. Grieve DJ. Shah AM. Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase. FASEB J. 2006;20:1546–1548. doi: 10.1096/fj.05-4642fje. [DOI] [PubMed] [Google Scholar]
- 41.Kajstura J. Bolli R. Sonnenblick EH. Anversa P. Leri A. Cause of death: suicide. J Mol Cell Cardiol. 2006;40:425–437. doi: 10.1016/j.yjmcc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 42.Kajstura J. Cigola E. Malhotra A. Li P. Cheng W. Meggs LG. Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 43.Kakishita M. Nakamura K. Asanuma M. Morita H. Saito H. Kusano K. Nakamura Y. Emori T. Matsubara H. Sugaya T. Ogawa N. Ohe T. Direct evidence for increased hydroxyl radicals in angiotensin II-induced cardiac hypertrophy through angiotensin II type 1a receptor. J Cardiovasc Pharmacol. 2003;42(Suppl 1):S67–S70. doi: 10.1097/00005344-200312001-00015. [DOI] [PubMed] [Google Scholar]
- 44.Kase H. Hashikabe Y. Uchida K. Nakanishi N. Hattori Y. Supplementation with tetrahydrobiopterin prevents the cardiovascular effects of angiotensin II-induced oxidative and nitrosative stress. J Hypertens. 2005;23:1375–1382. doi: 10.1097/01.hjh.0000173520.13976.7d. [DOI] [PubMed] [Google Scholar]
- 45.Kassiri Z. Zhong J. Guo D. Basu R. Wang X. Liu PP. Scholey JW. Penninger JM. Oudit GY. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 46.Kataja-Tuomola MK. Kontto JP. Mannisto S. Albanes D. Virtamo JR. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: results of the ATBC Study. Ann Med. 2010;42:178–186. doi: 10.3109/07853890903508887. [DOI] [PubMed] [Google Scholar]
- 47.Katragadda S. Arora RR. Role of angiotensin-converting enzyme inhibitors in vascular modulation: beyond the hypertensive effects. Am J Ther. 2010;17:e11–e23. doi: 10.1097/MJT.0b013e31815addd9. [DOI] [PubMed] [Google Scholar]
- 48.Kawaguchi M. Takahashi M. Hata T. Kashima Y. Usui F. Morimoto H. Izawa A. Takahashi Y. Masumoto J. Koyama J. Hongo M. Noda T. Nakayama J. Sagara J. Taniguchi S. Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 49.Khaper N. Singal PK. Modulation of oxidative stress by a selective inhibition of angiotensin II type 1 receptors in MI rats. J Am Coll Cardiol. 2001;37:1461–1466. doi: 10.1016/s0735-1097(01)01126-3. [DOI] [PubMed] [Google Scholar]
- 50.Krum H. Carson P. Farsang C. Maggioni AP. Glazer RD. Aknay N. Chiang YT. Cohn JN. Effect of valsartan added to background ACE inhibitor therapy in patients with heart failure: results from Val-HeFT. Eur J Heart Fail. 2004;6:937–945. doi: 10.1016/j.ejheart.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Kumar R. Singh VP. Baker KM. The intracellular renin-angiotensin system in the heart. Curr Hypertens Rep. 2009;11:104–110. doi: 10.1007/s11906-009-0020-y. [DOI] [PubMed] [Google Scholar]
- 52.Kurdi M. De Mello WC. Booz GW. Working outside the system: an update on the unconventional behavior of the renin-angiotensin system components. Int J Biochem Cell Biol. 2005;37:1357–1367. doi: 10.1016/j.biocel.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 53.Kuroda J. Sadoshima J. NADPH oxidase and cardiac failure. J Cardiovasc Transl Res. 2010;3:314–320. doi: 10.1007/s12265-010-9184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuroda J. Ago T. Matsushima S. Zhai P. Schneider MD. Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landmesser U. Spiekermann S. Preuss C. Sorrentino S. Fischer D. Manes C. Mueller M. Drexler H. Angiotensin II induces endothelial xanthine oxidase activation: role for endothelial dysfunction in patients with coronary disease. Arterioscler Thromb Vasc Biol. 2007;27:943–948. doi: 10.1161/01.ATV.0000258415.32883.bf. [DOI] [PubMed] [Google Scholar]
- 56.Li JM. Gall NP. Grieve DJ. Chen M. Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 57.Li JM. Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 58.Li SY. Yang X. Ceylan-Isik AF. Du M. Sreejayan N. Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 59.Lijnen PJ. van Pelt JF. Fagard RH. Downregulation of manganese superoxide dismutase by angiotensin II in cardiac fibroblasts of rats: Association with oxidative stress in myocardium. Am J Hypertens. 2010;23:1128–1135. doi: 10.1038/ajh.2010.128. [DOI] [PubMed] [Google Scholar]
- 60.Liu Q. Wang G. Zhou G. Tan Y. Wang X. Wei W. Liu L. Xue W. Feng W. Cai L. Angiotensin II-induced p53-dependent cardiac apoptotic cell death: its prevention by metallothionein. Toxicol Lett. 2009;191:314–320. doi: 10.1016/j.toxlet.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y. Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–1266. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- 62.Lonn E. Yusuf S. Hoogwerf B. Pogue J. Yi Q. Zinman B. Bosch J. Dagenais G. Mann JF. Gerstein HC. Effects of vitamin E on cardiovascular and microvascular outcomes in high-risk patients with diabetes: results of the HOPE study and MICRO-HOPE substudy. Diabetes Care. 2002;25:1919–1927. doi: 10.2337/diacare.25.11.1919. [DOI] [PubMed] [Google Scholar]
- 63.Looi YH. Grieve DJ. Siva A. Walker SJ. Anilkumar N. Cave AC. Marber M. Monaghan MJ. Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension. 2008;51:319–325. doi: 10.1161/HYPERTENSIONAHA.107.101980. [DOI] [PubMed] [Google Scholar]
- 64.Lopez Farre A. Casado S. Heart failure, redox alterations, and endothelial dysfunction. Hypertension. 2001;38:1400–1405. doi: 10.1161/hy1201.099612. [DOI] [PubMed] [Google Scholar]
- 65.Lu L. Quinn MT. Sun Y. Oxidative stress in the infarcted heart: role of de novo angiotensin II production. Biochem Biophys Res Commun. 2004;325:943–951. doi: 10.1016/j.bbrc.2004.10.106. [DOI] [PubMed] [Google Scholar]
- 66.Maack C. Kartes T. Kilter H. Schafers HJ. Nickenig G. Bohm M. Laufs U. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation. 2003;108:1567–1574. doi: 10.1161/01.CIR.0000091084.46500.BB. [DOI] [PubMed] [Google Scholar]
- 67.Maejima Y. Kuroda J. Matsushima S. Ago T. Sadoshima J. Regulation of myocardial growth and death by NADPH oxidase. J Mol Cell Cardiol. 2011;50:408–416. doi: 10.1016/j.yjmcc.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mann JF. Schmieder RE. McQueen M. Dyal L. Schumacher H. Pogue J. Wang X. Maggioni A. Budaj A. Chaithiraphan S. Dickstein K. Keltai M. Metsarinne K. Oto A. Parkhomenko A. Piegas LS. Svendsen TL. Teo KK. Yusuf S. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372:547–553. doi: 10.1016/S0140-6736(08)61236-2. [DOI] [PubMed] [Google Scholar]
- 69.Matsuhisa S. Otani H. Okazaki T. Yamashita K. Akita Y. Sato D. Moriguchi A. Imamura H. Iwasaka T. Angiotensin II type 1 receptor blocker preserves tolerance to ischemia-reperfusion injury in Dahl salt-sensitive rat heart. Am J Physiol Heart Circ Physiol. 2008;294:H2473–H2479. doi: 10.1152/ajpheart.91533.2007. [DOI] [PubMed] [Google Scholar]
- 70.Matsuhisa S. Otani H. Okazaki T. Yamashita K. Akita Y. Sato D. Moriguchi A. Iwasaka T. N-acetylcysteine abolishes the protective effect of losartan against left ventricular remodeling in cardiomyopathy hamster. Antioxid Redox Signal. 2008;10:1999–2008. doi: 10.1089/ars.2008.2069. [DOI] [PubMed] [Google Scholar]
- 71.Matsui Y. Takagi H. Qu X. Abdellatif M. Sakoda H. Asano T. Levine B. Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 72.McMurray JJ. Ostergren J. Swedberg K. Granger CB. Held P. Michelson EL. Olofsson B. Yusuf S. Pfeffer MA. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 73.McMurray JJ. Pitt B. Latini R. Maggioni AP. Solomon SD. Keefe DL. Ford J. Verma A. Lewsey J. Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail. 2008;1:17–24. doi: 10.1161/CIRCHEARTFAILURE.107.740704. [DOI] [PubMed] [Google Scholar]
- 74.Mellin V. Isabelle M. Oudot A. Vergely-Vandriesse C. Monteil C. Di Meglio B. Henry JP. Dautreaux B. Rochette L. Thuillez C. Mulder P. Transient reduction in myocardial free oxygen radical levels is involved in the improved cardiac function and structure after long-term allopurinol treatment initiated in established chronic heart failure. Eur Heart J. 2005;26:1544–1550. doi: 10.1093/eurheartj/ehi305. [DOI] [PubMed] [Google Scholar]
- 75.Mercure C. Yogi A. Callera GE. Aranha AB. Bader M. Ferreira AJ. Santos RA. Walther T. Touyz RM. Reudelhuber TL. Angiotensin(1–7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ Res. 2008;103:1319–1326. doi: 10.1161/CIRCRESAHA.108.184911. [DOI] [PubMed] [Google Scholar]
- 76.Mervaala E. Biala A. Merasto S. Lempiainen J. Mattila I. Martonen E. Eriksson O. Louhelainen M. Finckenberg P. Kaheinen P. Muller DN. Luft FC. Lapatto R. Oresic M. Metabolomics in angiotensin II-induced cardiac hypertrophy. Hypertension. 2010;55:508–515. doi: 10.1161/HYPERTENSIONAHA.109.145490. [DOI] [PubMed] [Google Scholar]
- 77.Miguel-Carrasco JL. Zambrano S. Blanca AJ. Mate A. Vazquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of NF-kB. J Inflamm (Lond) 2010;7:21. doi: 10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minhas KM. Saraiva RM. Schuleri KH. Lehrke S. Zheng M. Saliaris AP. Berry CE. Barouch LA. Vandegaer KM. Li D. Hare JM. Xanthine oxidoreductase inhibition causes reverse remodeling in rats with dilated cardiomyopathy. Circ Res. 2006;98:271–279. doi: 10.1161/01.RES.0000200181.59551.71. [DOI] [PubMed] [Google Scholar]
- 79.Miyazaki M. Takai S. Jin D. Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. doi: 10.1016/j.pharmthera.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 80.Moe G. Konig A. Liu P. Jugdutt BI. Selective type 1 angiotensin II receptor blockade attenuates oxidative stress and regulates angiotensin II receptors in the canine failing heart. Mol Cell Biochem. 2008;317:97–104. doi: 10.1007/s11010-008-9835-0. [DOI] [PubMed] [Google Scholar]
- 81.Moens AL. Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol. 2007;50:238–246. doi: 10.1097/FJC.0b013e318123f854. [DOI] [PubMed] [Google Scholar]
- 82.Mollnau H. Oelze M. August M. Wendt M. Daiber A. Schulz E. Baldus S. Kleschyov AL. Materne A. Wenzel P. Hink U. Nickenig G. Fleming I. Munzel T. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler Thromb Vasc Biol. 2005;25:2554–2559. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 83.Mollnau H. Wendt M. Szocs K. Lassegue B. Schulz E. Oelze M. Li H. Bodenschatz M. August M. Kleschyov AL. Tsilimingas N. Walter U. Forstermann U. Meinertz T. Griendling K. Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 84.Moltzer E. Verkuil AV. van Veghel R. Danser AH. van Esch JH. Effects of angiotensin metabolites in the coronary vascular bed of the spontaneously hypertensive rat: loss of angiotensin II type 2 receptor-mediated vasodilation. Hypertension. 2010;55:516–522. doi: 10.1161/HYPERTENSIONAHA.109.145037. [DOI] [PubMed] [Google Scholar]
- 85.Murtaza I. Wang HX. Feng X. Alenina N. Bader M. Prabhakar BS. Li PF. Down-regulation of catalase and oxidative modification of protein kinase CK2 lead to the failure of apoptosis repressor with caspase recruitment domain to inhibit cardiomyocyte hypertrophy. J Biol Chem. 2008;283:5996–6004. doi: 10.1074/jbc.M706466200. [DOI] [PubMed] [Google Scholar]
- 86.Nakagami H. Takemoto M. Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- 87.Nediani C. Borchi E. Giordano C. Baruzzo S. Ponziani V. Sebastiani M. Nassi P. Mugelli A. d'Amati G. Cerbai E. NADPH oxidase-dependent redox signaling in human heart failure: relationship between the left and right ventricle. J Mol Cell Cardiol. 2007;42:826–834. doi: 10.1016/j.yjmcc.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Nishida M. Tanabe S. Maruyama Y. Mangmool S. Urayama K. Nagamatsu Y. Takagahara S. Turner JH. Kozasa T. Kobayashi H. Sato Y. Kawanishi T. Inoue R. Nagao T. Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 89.Nishio M. Sakata Y. Mano T. Yoshida J. Ohtani T. Takeda Y. Miwa T. Masuyama T. Yamamoto K. Hori M. Therapeutic effects of angiotensin II type 1 receptor blocker at an advanced stage of hypertensive diastolic heart failure. J Hypertens. 2007;25:455–461. doi: 10.1097/HJH.0b013e328010d635. [DOI] [PubMed] [Google Scholar]
- 90.Ocaranza MP. Lavandero S. Jalil JE. Moya J. Pinto M. Novoa U. Apablaza F. Gonzalez L. Hernandez C. Varas M. Lopez R. Godoy I. Verdejo H. Chiong M. Angiotensin-(1–9) regulates cardiac hypertrophy in vivo and in vitro. J Hypertens. 2010;28:1054–1064. doi: 10.1097/hjh.0b013e328335d291. [DOI] [PubMed] [Google Scholar]
- 91.Ohtahara A. Hisatome I. Yamamoto Y. Furuse M. Sonoyama K. Furuse Y. Hamada T. Katoh M. Watanabe M. Kinugawa T. Ogino K. Igawa O. Shimomura T. Murakami F. Yamamoto T. Shigemasa C. The release of the substrate for xanthine oxidase in hypertensive patients was suppressed by angiotensin converting enzyme inhibitors and alpha1-blockers. J Hypertens. 2001;19:575–582. doi: 10.1097/00004872-200103001-00009. [DOI] [PubMed] [Google Scholar]
- 92.Oka S. Ago T. Kitazono T. Zablocki D. Sadoshima J. The role of redox modulation of class II histone deacetylases in mediating pathological cardiac hypertrophy. J Mol Med (Berl) 2009;87:785–791. doi: 10.1007/s00109-009-0471-2. [DOI] [PubMed] [Google Scholar]
- 93.Oudit GY. Kassiri Z. Patel MP. Chappell M. Butany J. Backx PH. Tsushima RG. Scholey JW. Khokha R. Penninger JM. Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc Res. 2007;75:29–39. doi: 10.1016/j.cardiores.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 94.Palomeque J. Rueda OV. Sapia L. Valverde CA. Salas M. Petroff MV. Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+- calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 95.Peng H. Carretero OA. Liao TD. Peterson EL. Rhaleb NE. Role of N-acetyl-seryl-aspartyl-lysyl-proline in the antifibrotic and anti-inflammatory effects of the angiotensin-converting enzyme inhibitor captopril in hypertension. Hypertension. 2007;49:695–703. doi: 10.1161/01.HYP.0000258406.66954.4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pierzchalski P. Reiss K. Cheng W. Cirielli C. Kajstura J. Nitahara JA. Rizk M. Capogrossi MC. Anversa P. p53 Induces myocyte apoptosis via the activation of the renin-angiotensin system. Exp Cell Res. 1997;234:57–65. doi: 10.1006/excr.1997.3604. [DOI] [PubMed] [Google Scholar]
- 97.Porrello ER. D'Amore A. Curl CL. Allen AM. Harrap SB. Thomas WG. Delbridge LM. Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension. 2009;53:1032–1040. doi: 10.1161/HYPERTENSIONAHA.108.128488. [DOI] [PubMed] [Google Scholar]
- 98.Porrello ER. Delbridge LM. Cardiomyocyte autophagy is regulated by angiotensin II type 1 and type 2 receptors. Autophagy. 2009;5:1215–1216. doi: 10.4161/auto.5.8.10153. [DOI] [PubMed] [Google Scholar]
- 99.Privratsky JR. Wold LE. Sowers JR. Quinn MT. Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- 100.Prosser HC. Forster ME. Richards AM. Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. doi: 10.1093/cvr/cvp003. [DOI] [PubMed] [Google Scholar]
- 101.Qin F. Patel R. Yan C. Liu W. NADPH oxidase is involved in angiotensin II-induced apoptosis in H9C2 cardiac muscle cells: effects of apocynin. Free Radic Biol Med. 2006;40:236–246. doi: 10.1016/j.freeradbiomed.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 102.Rasmussen HH. Hamilton EJ. Liu CC. Figtree GA. Reversible oxidative modification: implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc Med. 2010;20:85–90. doi: 10.1016/j.tcm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 103.Re RN. Cook JL. The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol. 2010;299:H577–H583. doi: 10.1152/ajpheart.00421.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sam F. Kerstetter DL. Pimental DR. Mulukutla S. Tabaee A. Bristow MR. Colucci WS. Sawyer DB. Increased reactive oxygen species production and functional alterations in antioxidant enzymes in human failing myocardium. J Card Fail. 2005;11:473–480. doi: 10.1016/j.cardfail.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 105.Satoh M. Ogita H. Takeshita K. Mukai Y. Kwiatkowski DJ. Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci U S A. 2006;103:7432–7437. doi: 10.1073/pnas.0510444103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scherz-Shouval R. Shvets E. Fass E. Shorer H. Gil L. Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schluter KD. Wenzel S. Angiotensin II: a hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacol Ther. 2008;119:311–325. doi: 10.1016/j.pharmthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Senbonmatsu T. Ichihara S. Price E., Jr. Gaffney FA. Inagami T. Evidence for angiotensin II type 2 receptor-mediated cardiac myocyte enlargement during in vivo pressure overload. J Clin Invest. 2000;106:R25–R29. doi: 10.1172/JCI10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shang LL. Sanyal S. Pfahnl AE. Jiao Z. Allen J. Liu H. Dudley SC., Jr. NF-kappaB-dependent transcriptional regulation of the cardiac scn5a sodium channel by angiotensin II. Am J Physiol Cell Physiol. 2008;294:C372–C379. doi: 10.1152/ajpcell.00186.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shimoni Y. Chen K. Emmett T. Kargacin G. Aldosterone and the autocrine modulation of potassium currents and oxidative stress in the diabetic rat heart. Br J Pharmacol. 2008;154:675–687. doi: 10.1038/bjp.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shimoni Y. Hunt D. Chen K. Emmett T. Kargacin G. Differential autocrine modulation of atrial and ventricular potassium currents and of oxidative stress in diabetic rats. Am J Physiol Heart Circ Physiol. 2006;290:H1879–H1888. doi: 10.1152/ajpheart.01045.2005. [DOI] [PubMed] [Google Scholar]
- 112.Shimoni Y. Hunt D. Chuang M. Chen KY. Kargacin G. Severson DL. Modulation of potassium currents by angiotensin and oxidative stress in cardiac cells from the diabetic rat. J Physiol. 2005;567:177–190. doi: 10.1113/jphysiol.2005.090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Singh VP. Le B. Khode R. Baker KM. Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siragy HM. Comparing angiotensin II receptor blockers on benefits beyond blood pressure. Adv Ther. 2010;27:257–284. doi: 10.1007/s12325-010-0028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith RS., Jr. Agata J. Xia CF. Chao L. Chao J. Human endothelial nitric oxide synthase gene delivery protects against cardiac remodeling and reduces oxidative stress after myocardial infarction. Life Sci. 2005;76:2457–2471. doi: 10.1016/j.lfs.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 116.Sorescu D. Griendling KK. Reactive oxygen species, mitochondria, and NAD(P)H oxidases in the development and progression of heart failure. Congest Heart Fail. 2002;8:132–140. doi: 10.1111/j.1527-5299.2002.00717.x. [DOI] [PubMed] [Google Scholar]
- 117.Steckelings UM. Kaschina E. Unger T. The AT2 receptor—a matter of love and hate. Peptides. 2005;26:1401–1409. doi: 10.1016/j.peptides.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 118.Suematsu N. Tsutsui H. Wen J. Kang D. Ikeuchi M. Ide T. Hayashidani S. Shiomi T. Kubota T. Hamasaki N. Takeshita A. Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation. 2003;107:1418–1423. doi: 10.1161/01.cir.0000055318.09997.1f. [DOI] [PubMed] [Google Scholar]
- 119.Sukumaran V. Watanabe K. Veeraveedu PT. Gurusamy N. Ma M. Thandavarayan RA. Lakshmanan AP. Yamaguchi K. Suzuki K. Kodama M. Olmesartan, an AT1 antagonist, attenuates oxidative stress, endoplasmic reticulum stress and cardiac inflammatory mediators in rats with heart failure induced by experimental autoimmune myocarditis. Int J Biol Sci. 2011;7:154–167. doi: 10.7150/ijbs.7.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun Y. Myocardial repair/remodelling following infarction: roles of local factors. Cardiovasc Res. 2009;81:482–490. doi: 10.1093/cvr/cvn333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Takai S. Jin D. Miyazaki M. New approaches to blockade of the renin-angiotensin-aldosterone system: chymase as an important target to prevent organ damage. J Pharmacol Sci. 2010;113:301–309. doi: 10.1254/jphs.10r05fm. [DOI] [PubMed] [Google Scholar]
- 122.Takenaka H. Kihara Y. Iwanaga Y. Onozawa Y. Toyokuni S. Kita T. Angiotensin II, oxidative stress, and extracellular matrix degradation during transition to LV failure in rats with hypertension. J Mol Cell Cardiol. 2006;41:989–997. doi: 10.1016/j.yjmcc.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 123.Takimoto E. Champion HC. Li M. Ren S. Rodriguez ER. Tavazzi B. Lazzarino G. Paolocci N. Gabrielson KL. Wang Y. Kass DA. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest. 2005;115:1221–1231. doi: 10.1172/JCI21968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tamura K. Tanaka Y. Tsurumi Y. Azuma K. Shigenaga A. Wakui H. Masuda S. Matsuda M. The role of angiotensin AT1 receptor-associated protein in renin-angiotensin system regulation and function. Curr Hypertens Rep. 2007;9:121–127. doi: 10.1007/s11906-007-0022-6. [DOI] [PubMed] [Google Scholar]
- 125.Tanaka M. Umemoto S. Kawahara S. Kubo M. Itoh S. Umeji K. Matsuzaki M. Angiotensin II type 1 receptor antagonist and angiotensin-converting enzyme inhibitor altered the activation of Cu/Zn-containing superoxide dismutase in the heart of stroke-prone spontaneously hypertensive rats. Hypertens Res. 2005;28:67–77. doi: 10.1291/hypres.28.67. [DOI] [PubMed] [Google Scholar]
- 126.Tanno M. Kuno A. Yano T. Miura T. Hisahara S. Ishikawa S. Shimamoto K. Horio Y. Induction of manganese superoxide dismutase by nuclear translocation and activation of SIRT1 promotes cell survival in chronic heart failure. J Biol Chem. 2010;285:8375–8382. doi: 10.1074/jbc.M109.090266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theres H. Wagner KD. Schulz S. Strube S. Leiterer KP. Romberg D. Gunther J. Scholz H. Baumann G. Schimke I. Oxygen radical system in chronic infarcted rat heart: the effect of combined beta blockade and ACE inhibition. J Cardiovasc Pharmacol. 2000;35:708–715. doi: 10.1097/00005344-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 128.Thiele H. Hildebrand L. Schirdewahn C. Eitel I. Adams V. Fuernau G. Erbs S. Linke A. Diederich KW. Nowak M. Desch S. Gutberlet M. Schuler G. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J Am Coll Cardiol. 2010;55:2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 129.Trask AJ. Groban L. Westwood BM. Varagic J. Ganten D. Gallagher PE. Chappell MC. Ferrario CM. Inhibition of angiotensin-converting enzyme 2 exacerbates cardiac hypertrophy and fibrosis in Ren-2 hypertensive rats. Am J Hypertens. 2010;23:687–693. doi: 10.1038/ajh.2010.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Trask AJ. Jessup JA. Chappell MC. Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol. 2008;294:H2242–H2247. doi: 10.1152/ajpheart.00175.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsutamoto T. Wada A. Matsumoto T. Maeda K. Mabuchi N. Hayashi M. Tsutsui T. Ohnishi M. Sawaki M. Fujii M. Yamamoto T. Horie H. Sugimoto Y. Kinoshita M. Relationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:2086–2092. doi: 10.1016/s0735-1097(01)01299-2. [DOI] [PubMed] [Google Scholar]
- 132.Tsutsui H. Ide T. Hayashidani S. Kinugawa S. Suematsu N. Utsumi H. Takeshita A. Effects of ACE inhibition on left ventricular failure and oxidative stress in Dahl salt-sensitive rats. J Cardiovasc Pharmacol. 2001;37:725–733. doi: 10.1097/00005344-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 133.Wakui H. Tamura K. Tanaka Y. Matsuda M. Bai Y. Dejima T. Masuda S. Shigenaga A. Maeda A. Mogi M. Ichihara N. Kobayashi Y. Hirawa N. Ishigami T. Toya Y. Yabana M. Horiuchi M. Minamisawa S. Umemura S. Cardiac-specific activation of angiotensin II type 1 receptor-associated protein completely suppresses cardiac hypertrophy in chronic angiotensin II-infused mice. Hypertension. 2010;55:1157–1164. doi: 10.1161/HYPERTENSIONAHA.109.147207. [DOI] [PubMed] [Google Scholar]
- 134.Wang M. Zhang J. Walker SJ. Dworakowski R. Lakatta EG. Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 2010;48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang Y. Qian C. Roks AJ. Westermann D. Schumacher SM. Escher F. Schoemaker RG. Reudelhuber TL. van Gilst WH. Schultheiss HP. Tschope C. Walther T. Circulating rather than cardiac angiotensin-(1–7) stimulates cardioprotection after myocardial infarction. Circ Heart Fail. 2010;3:286–293. doi: 10.1161/CIRCHEARTFAILURE.109.905968. [DOI] [PubMed] [Google Scholar]
- 136.Wei CC. Hase N. Inoue Y. Bradley EW. Yahiro E. Li M. Naqvi N. Powell PC. Shi K. Takahashi Y. Saku K. Urata H. Dell'italia LJ. Husain A. Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents. J Clin Invest. 2010;120:1229–1239. doi: 10.1172/JCI39345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wei L. Fahey T. Struthers AD. MacDonald TM. Association between allopurinol and mortality in heart failure patients: a long-term follow-up study. Int J Clin Pract. 2009;63:1327–1333. doi: 10.1111/j.1742-1241.2009.02118.x. [DOI] [PubMed] [Google Scholar]
- 138.Whaley-Connell A. Govindarajan G. Habibi J. Hayden MR. Cooper SA. Wei Y. Ma L. Qazi M. Link D. Karuparthi PR. Stump C. Ferrario C. Sowers JR. Angiotensin II-mediated oxidative stress promotes myocardial tissue remodeling in the transgenic (mRen2) 27 Ren2 rat. Am J Physiol Endocrinol Metab. 2007;293:E355–E363. doi: 10.1152/ajpendo.00632.2006. [DOI] [PubMed] [Google Scholar]
- 139.White CN. Figtree GA. Liu CC. Garcia A. Hamilton EJ. Chia KK. Rasmussen HH. Angiotensin II inhibits the Na+-K+ pump via PKC-dependent activation of NADPH oxidase. Am J Physiol Cell Physiol. 2009;296:C693–C700. doi: 10.1152/ajpcell.00648.2008. [DOI] [PubMed] [Google Scholar]
- 140.Widder JD. Fraccarollo D. Galuppo P. Hansen JM. Jones DP. Ertl G. Bauersachs J. Attenuation of angiotensin II-induced vascular dysfunction and hypertension by overexpression of Thioredoxin 2. Hypertension. 2009;54:338–344. doi: 10.1161/HYPERTENSIONAHA.108.127928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu R. Laplante MA. de Champlain J. Cyclooxygenase-2 inhibitors attenuate angiotensin II-induced oxidative stress, hypertension, and cardiac hypertrophy in rats. Hypertension. 2005;45:1139–1144. doi: 10.1161/01.HYP.0000164572.92049.29. [DOI] [PubMed] [Google Scholar]
- 142.Xu J. Carretero OA. Liao TD. Peng H. Shesely EG. Liu TS. Yang JJ. Reudelhuber TL. Yang XP. Local angiotensin II aggravates cardiac remodeling in hypertension. Am J Physiol Heart Circ Physiol. 2010;299:H1328–H1338. doi: 10.1152/ajpheart.00538.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamamoto E. Kataoka K. Yamashita T. Tokutomi Y. Dong YF. Matsuba S. Ogawa H. Kim-Mitsuyama S. Role of xanthine oxidoreductase in the reversal of diastolic heart failure by candesartan in the salt-sensitive hypertensive rat. Hypertension. 2007;50:657–662. doi: 10.1161/HYPERTENSIONAHA.107.095315. [DOI] [PubMed] [Google Scholar]
- 144.Yang Y. Ago T. Zhai P. Abdellatif M. Sadoshima J. Thioredoxin 1 negatively regulates angiotensin II-induced cardiac hypertrophy through upregulation of miR-98/let-7. Circ Res. 2011;108:305–313. doi: 10.1161/CIRCRESAHA.110.228437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yayama K. Okamoto H. Angiotensin II-induced vasodilation via type 2 receptor: role of bradykinin and nitric oxide. Int Immunopharmacol. 2008;8:312–318. doi: 10.1016/j.intimp.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 146.Zablocki D. Sadoshima J. Knocking out angiotensin II in the heart. Curr Hypertens Rep. 2011;13:129–135. doi: 10.1007/s11906-011-0180-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang M. Brewer AC. Schroder K. Santos CX. Grieve DJ. Wang M. Anilkumar N. Yu B. Dong X. Walker SJ. Brandes RP. Shah AM. NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 2010;107:18121–18126. doi: 10.1073/pnas.1009700107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang R. Al-Lamki R. Bai L. Streb JW. Miano JM. Bradley J. Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 149.Zhao W. Zhao T. Chen Y. Ahokas RA. Sun Y. Oxidative stress mediates cardiac fibrosis by enhancing transforming growth factor-beta1 in hypertensive rats. Mol Cell Biochem. 2008;317:43–50. doi: 10.1007/s11010-008-9803-8. [DOI] [PubMed] [Google Scholar]
- 150.Zhao Y. McLaughlin D. Robinson E. Harvey AP. Hookham MB. Shah AM. McDermott BJ. Grieve DJ. Nox2 NADPH oxidase promotes pathologic cardiac remodeling associated with Doxorubicin chemotherapy. Cancer Res. 2010;70:9287–9297. doi: 10.1158/0008-5472.CAN-10-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhong J. Basu R. Guo D. Chow FL. Byrns S. Schuster M. Loibner H. Wang XH. Penninger JM. Kassiri Z. Oudit GY. Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation. 2010;122:717–728. doi: 10.1161/CIRCULATIONAHA.110.955369. 18 p following 728. [DOI] [PubMed] [Google Scholar]
- 152.Zhou A. Carrell RW. Murphy MP. Wei Z. Yan Y. Stanley PL. Stein PE. Broughton Pipkin F. Read RJ. A redox switch in angiotensinogen modulates angiotensin release. Nature. 2010;468:108–111. doi: 10.1038/nature09505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou G. Li X. Hein DW. Xiang X. Marshall JP. Prabhu SD. Cai L. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–666. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 154.Zorov DB. Filburn CR. Klotz LO. Zweier JL. Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]