Abstract
Significance: Angiotensin II (Ang II) influences the function of many cell types and regulates many organ systems, in large part through redox-sensitive processes. In the vascular system, Ang II is a potent vasoconstrictor and also promotes inflammation, hypertrophy, and fibrosis, which are important in vascular damage and remodeling in cardiovascular diseases. The diverse actions of Ang II are mediated via Ang II type 1 and Ang II type 2 receptors, which couple to various signaling molecules, including NADPH oxidase (Nox), which generates reactive oxygen species (ROS). ROS are now recognized as signaling molecules, critically placed in pathways activated by Ang II. Mechanisms linking Nox and Ang II are complex and not fully understood. Recent Advances: Ang II regulates vascular cell production of ROS through various recently characterized Noxs, including Nox1, Nox2, Nox4, and Nox5. Activation of these Noxs leads to ROS generation, which in turn influences many downstream signaling targets of Ang II, including MAP kinases, RhoA/Rho kinase, transcription factors, protein tyrosine phosphatases, and tyrosine kinases. Activation of these redox-sensitive pathways regulates vascular cell growth, inflammation, contraction, and senescence. Critical Issues: Although there is much evidence indicating a role for Nox/ROS in Ang II function, there is still a paucity of information on how Ang II exerts cell-specific effects through ROS and how Nox isoforms are differentially regulated by Ang II. Moreover, exact mechanisms whereby ROS induce oxidative modifications of signaling molecules mediating Ang II actions remain elusive. Future Directions: Future research should elucidate these issues to better understand the significance of Ang II and ROS in vascular (patho) biology. Antioxid. Redox Signal. 19, 1110–1120.
Introduction
Angiotensin II (Ang II), the major bioactive peptide of the renin–angiotensin system (RAS), plays a major role in the regulation of vascular function and structure. It is a multifunctional vasoactive peptide produced systemically and locally within the vascular wall. Ang II is a potent vasoconstrictor that also has mitogenic, proinflammatory, and profibrotic actions. These effects are elicited through myriad-signaling pathways, many of which involve reactive oxygen species (ROS), particularly superoxide anion (O2•−) and hydrogen peroxide (H2O2) (41, 47, 71). Under physiological conditions, ROS production is tightly controlled, and ROS play an important role as a signaling molecule in the control of endothelial function and vascular tone. In pathological conditions, when ROS bioavailability is increased (oxidative stress), Ang II signaling is altered, leading to endothelial dysfunction, vascular remodeling, and inflammation, important processes underlying vascular injury in cardiovascular disease.
Ang II exerts its diverse actions via two G-protein-coupled receptors, Ang II type 1 (AT1R) and type 2 (AT2R) receptors (27, 41, 47, 71). The AT1R mediates most of the known actions of Ang II. The AT2R is associated with antiproliferative, proapoptotic, and vasodilatory actions of Ang II and tends to counteract AT1R effects. Signaling pathways elicited by Ang II/AT1R involve interactions with several heterotrimeric G-proteins coupled to second messengers and cytosolic proteins, including phospholipase C (PLC), phospholipase A2 (PLA2), phospholipase D (PLD), and protein kinase C (PKC) (71). In addition, Ang II/AT1R signals through activation of many receptor and nonreceptor tyrosine kinases and serine threonine kinases, important in cell growth, hypertrophy, and inflammation. Growing evidence indicates that many of the pathways through which Ang II signals involve activation of NADPH oxidase (Nox), which is a major source of ROS in vascular cells.
Nox and Ang II
Griendling and colleagues were among the first to demonstrate that Ang II activates vascular Nox (31, 75). Using rat aortic vascular smooth muscle cells (VSMCs), they showed that treatment of VSMCs with Ang II for 4–6 h caused a nearly threefold increase in intracellular O2•− formation as detected by the lucigenin assay (31). This, derived from activation of both NADPH and NADH oxidases (31, 75) and p22phox, was found to be obligatory in this process (75). The pathophysiological significance of these cell-based findings was confirmed in rat studies where Ang II-induced hypertension was associated with increased vascular generation of Nox-derived O2•− and endothelial dysfunction, effects that were blocked by losartan, an AT1R blocker (59, 62). These early studies paved the way for the field relating to Ang II, oxidative stress, and hypertension.
Mechanisms whereby Ang II regulates Nox are complex and occur at the gene, transcriptional, and post-transcriptional levels and involve numerous intermediate signaling molecules (e.g., c-Src, PKC, receptor tyrosine kinases, protein disulfide isomerase, and polymerase delta-interacting protein 2 [Poldip2]) and scaffolding proteins/platforms (e.g., lipid rafts, caveolae, actin, caveolin, and cortactin) (23, 36, 46). More recently, it has been shown that Nox-derived ROS, in turn, regulate Ang II receptors (58). Hence, there is a feed-forward system where Ang II regulates ROS-generating Nox, which regulates expression and activation of AT1R (Fig. 1).
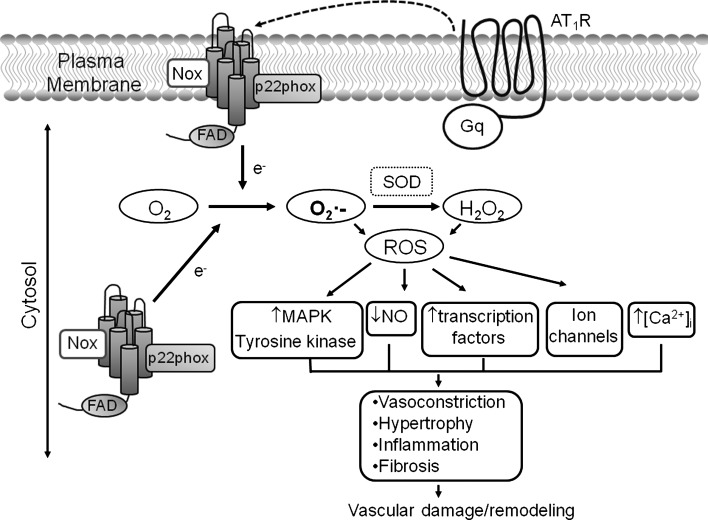
FIG. 1.
Role of Nox-derived ROS in Ang II-mediated effects in vascular cells. Ang II binds to its AT1R, which couples to heterometric Gq proteins, to activate PLC, leading to generation of IP3 and DAG, resulting in increased [Ca2+]i that triggers phosphorylation of MLC20 and stimulation of contraction. Ang II also induces contraction through the RhoA/Rho-kinase pathway that increases Ca2+ sensitivity by inhibiting MLCP. Ang II/AT1R stimulates Nox-derived ROS formation, which regulates MAPKs, tyrosine kinases, PTPs, and transcription factors. Formation of ROS through Ang II/AT1R further regulates AT1R through a feed-forward mechanism (dashed lines). Ang II, angiotensin II; AT1R, Ang II type 1 receptor; [Ca2+]i, intracellular free-calcium concentration; DAG, diacylglycerol; IP3, inositol-3-phosphate; MAPKs, mitogen-activated protein kinases; MLCK, myosin light-chain kinase; MLCP, myosin light-chain phosphatase; Nox, NADPH oxidase; PLC, phospholipase C; PTPs, protein tyrosine phosphatases; ROS, reactive oxygen species.
Nox Family Oxidases
Nox was originally considered to be expressed only in phagocytic cells involved in host defense and innate immunity. The prototypical reduced nicotinamide adenine dinucleotide phosphate (NAD(P)H) oxidase, Nox2, that is found in phagocytes, comprises five components, (phox for phagocyte oxidase), p47phox, p67phox, p40phox, p22phox, and gp91phox (4), and the small G protein Rac 1/2. It is now clear that there is a family of NAD(P)H oxidases, called Noxs, the primary function of which is to catalyze the transfer of electrons from NADPH to molecular oxygen via the catalytic subunit (Nox). The mammalian Nox family comprises seven members: Nox1, Nox2, Nox3, Nox4, Nox5, Duox1, and Duox2 (28, 67). All are transmembrane proteins that have a core catalytic subunit (Nox) and numerous regulatory subunits. Unlike phagocytic NAD(P)H oxidase, which is activated only upon stimulation and which generates O2•− in a burst-like manner extracellularly, nonphagocytic Noxs are constitutively active, produce O2•− intracellularly in a slow and sustained fashion, and act as intracellular signaling molecules, influencing not only transcription factors but also other molecules involved in inflammation, cell growth, and contraction, such as mitogen-activated protein (MAP) kinases, tyrosine kinases, and protein phosphatases (15, 16).
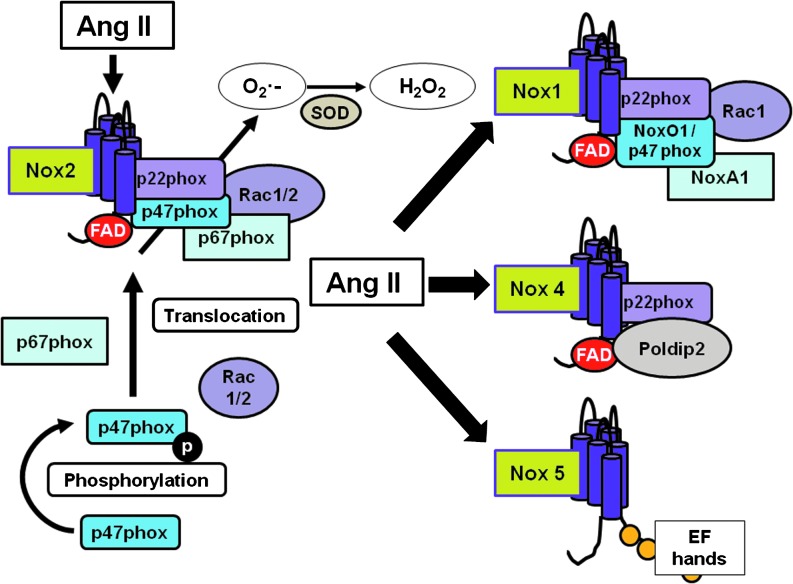
Nox1, Nox2, Nox4, and Nox5 have been identified in cardiovascular and renal tissue (39). Although all Noxs have the same function to generate ROS, mechanisms of activation, subunit requirements, and intracellular distributions vary between isoforms (Fig. 2). Nox1 and Nox2 are constitutively associated with p22phox, and the full activation of Nox1/p22phox and Nox2/p22phox requires interaction with other cytosolic subunits, including p47phox (or its homolog NADPH organizer 1 [NoxO1]), p67phox (or homolog NADPH activator 1 [NoxA1]), and Rac (21, 42, 65). Nox4 is constitutively active and associates with p22phox, but does not require other oxidase subunits for its activation, whereas Nox5 functions independently of any Nox subunits and is activated in a calcium–calmodulin-dependent manner (21, 65).
FIG. 2.
Nox activation and differences between Nox homologs. Nox comprises a complex of membrane and cytosolic subunits. Nox2 is the classical prototype. Membrane proteins are p22phox and the Nox subunit, and form a noncovalent heterodimer. These proteins possess the electron transport apparatus and may act as a physical conduit for the electron transfer that occurs across the membrane. The cytosolic proteins (p47phox, p67phox, NoxO1, NoxA1, and rac 1/2) are cofactors for enzymatic activity and are used to initiate and/or regulate electron transfer. To Nox2 be activated, p47phox is phosphorylated and translocates from the cytosol to the membrane with the other cytosolic subunits (p67phox and rac1/2). Nox1 can also be activated in a similar way to Nox2, but possesses other cytosolic subunits such as NoxO1 and NoxA1. Nox4 does not require any cytosolic subunit to be activated. Nox4 is constitutively active in cells, and its activity is controlled by Poldip2. On the other hand, Nox5 activation is not dependent on any subunit, but because of calcium-binding domains (EF hands), its activity is controlled by calcium and calmodulin. NoxA1, NADPH activator 1; NoxO1, NADPH organizer 1; Poldip2, polymerase (DNA-directed) delta-interacting protein 2.
Hyperactivation of Noxs leads to excessive ROS generation that disrupts redox networks, normally regulated by thiol-dependent antioxidant systems. This results in oxidative stress, triggering molecular processes, which, in the vasculature, contributes to vascular damage (Fig. 3). All vascular cell types, including endothelial cells, VSMCs, and adventitial cells (fibroblasts and adipocytes), are capable of producing ROS through Noxs. Noxs have been extensively reviewed (21, 37, 39, 65), and only an overview of recent developments related to Ang II is discussed here.
FIG. 3.
Ang II-stimulated redox-sensitive pathways that promote vascular remodeling. Generation of ROS by plasma membrane-associated Noxs and cytosolic Noxs in response to Ang II-AT1R signaling leads to activation of multiple pathways that promote vascular injury and remodeling. e−, electron; NO, nitric oxide; SOD, superoxide dismutase.
Regulation of Noxs and Nox Subunits by Ang II
Ang II is functionally associated with Nox1, Nox2, and Nox5 and variably with Nox4 in the vasculature. The biological significance of different Noxs being activated by the same vasoactive agent still awaits clarification, but their differential tissue distribution, cellular localization, and subcellular compartmentalization probably play a major role in Nox-specific actions elicited by Ang II (5). Ang II-activated Nox1 appears to be important in VSMCs from large arteries, but Nox2 may be more important in small-resistance arteries, especially in humans (31, 68, 75). Nox4 abundance is greater in endothelial cells than in VSMCs, at least in basal conditions, although there is probably important cross-talk between Noxs in different cell types (Fig. 4). Regulation also seems to differ in pathological conditions. For example, while Ang II increased expression of all Noxs in VSMCs from normal Wistar Kyoto rats (WKY), only Nox1 was influenced in VSMCs from spontaneously hypertensive rats (SHR) (7, 19, 66). This may be important in vascular dysfunction associated with hypertension. In transgenic mice, in which Nox1 is overexpressed in vascular smooth muscle (SMCnox1), ROS production is enhanced in response to Ang II, causing endothelial nitric oxide synthase (NOS) uncoupling and decreased nitric oxide bioavailability, with resultant impaired vasorelaxation (19).
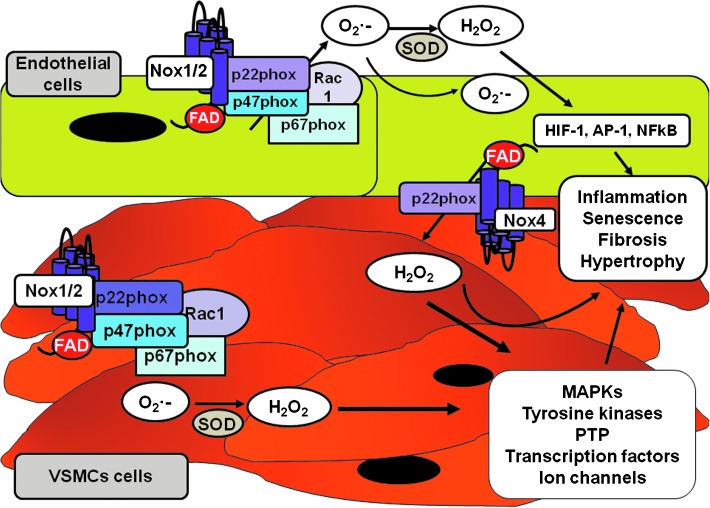
FIG. 4.
Distribution of vascular Noxs in endothelial and VSMCs and downstream signaling pathways regulated by Nox-derived ROS. In basal conditions, Nox4 expression is greater in endothelial cells than in VSMCs. Nox1 and Nox2 are expressed in VSMCs from small arteries, whereas Nox1, but not Nox2, is expressed in VSMCs from large arteries. These Noxs are upregulated in pathological conditions. The exact distribution of vascular Nox isoforms in vivo still awaits confirmation. VSMCs, vascular smooth muscle cells.
In vitro studies have indicated that Ang II is a potent stimulator of vascular Noxs (70). It induces activation (phosphorylation) of the oxidase subunits; it increases expression of Nox isoforms and Nox subunits (p47phox, p67phox, and p22phox); and it stimulates ROS production in cultured VSMCs and in intact arteries (76). Mechanisms linking Ang II to the enzyme and upstream signaling molecules modulating NAD(P)H oxidase in vascular cells have not been fully elucidated, but phospholipases, PKC, Src tyrosine kinases, phosphatidylinositol 3-kinase (PI3K), and Rac may be important (8, 64). Ang II stimulates Nox activity through various phospholipases, including PLC, PLA2, and PLD (8, 64). We showed that c-Src is critically placed between the AT1R and Nox, where it stimulates phosphorylation of p47phox and activation of the oxidase (73). These processes involve cortactin interaction with actin, which may act as a scaffolding network for Nox subunit trafficking to the cell membrane to assemble the functionally active oxidase complex (72). Lipid rafts/caveolae play an important role in agonist-stimulated activation of vascular Noxs and may act as signaling platforms to integrate redox events (13). In pathological conditions, where the RAS is upregulated, for example, hypertension, diabetes, and atherosclerosis, activation of Noxs by Ang II is augmented, leading to increased ROS generation and oxidative stress (11, 45).
Nox-Derived ROS As Signaling Molecules in the Vasculature
The dynamics and chemical properties of ROS dictate the biological role that they will play. The more reactive the species, the shorter is the half-life and the more rapidly it will interact with other molecules. ROS can also be electrically charged or electrically neutral, hydrophobic, or hydrophilic. Such characteristics determine their ability to cross membranes and/or to move in the environment between aqueous and lipophilic environments.
ROS are produced as intermediates in reduction–oxidation (redox) reactions, leading from O2 to H2O. The major mechanism for ROS generation begins with the reduction of O2 by the addition of one electron, to generate O2•−, considered the primary ROS. O2•− interacts with other molecules to produce secondary ROS, directly or through enzyme- or metal-catalyzed reactions (35). Reduction of O2•− leads to formation of H2O2, which is further converted to secondary metabolites such as highly reactive hydroxyl HO•. Although the favored reaction is the generation of H2O2, O2•− also reacts with nitric oxide (NO•) to form peroxinitrite (ONOO−), with transition metals, such as iron found in iron/sulfur center-containing proteins, or it may be protonated to the hydroperoxyl radical (H2O•). H2O• is particularly important in lipid peroxidation and atherogenesis.
Of the ROS generated in vascular cells, O2•− and H2O2 appear to be especially important. In biological systems, O2•− is short-lived owing to its rapid dismutation to H2O2. Dismutation can be spontaneous (rate constant=8×104/mol/s) or enzymatic via superoxide dismutase (SOD) (rate constant=2×109/mol/s) (26). Superoxide reacts with NO• to form ONOO− with a rate constant of 4–16×109/mol/s (3), resulting in NO• quenching and resultant decreased in NO• bioavailability. Redox-regulated uncoupling of NOS also contributes to decreased NO• and increased generation of O2•− (Fig. 5). In addition, oxidants react with FeS4 or with protein thiols such as cysteine residues, an effect that is increased by metabolic stress (10). Although O2•− has the capacity to react with many molecules, the preferred reaction is dismutation to H2O2 because of the fast reaction rate of SOD. Three mammalian SOD isoforms have been identified: copper/zinc SOD (SOD1), manganese-containing mitochondrial SOD (Mn-SOD, SOD2), and extracellular SOD (EC-SOD, SOD3) (48). The major vascular SOD is EC-SOD. The negative charge on O2•− makes it unable to cross cellular membranes, except possibly through ion channels, such as chloride channel 3 (CLC-3). CLC-3 transports O2•− out of endosomes into the cytoplasm in endothelial cells and has been implicated to play an important role in VSMC regulation (51). H2O2 has a longer lifespan than O2•−, is relatively stable, and is easily diffusible within and between cells. The main source of H2O2 in vascular tissue is the dismutation of O2•−: 2O2•−+2H+ → H2O2+O2.
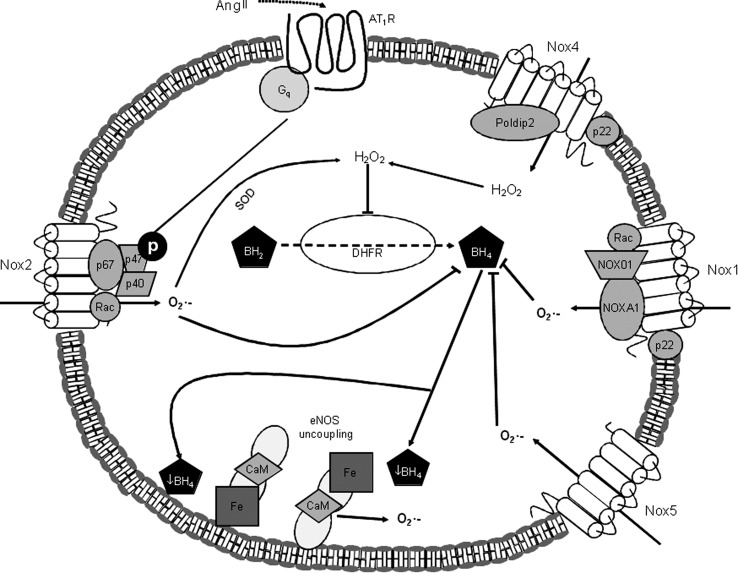
FIG. 5.
Nox and eNOS by Ang II in endothelial cells. Ang II stimulates production of O2•− by Nox1, Nox2, and Nox5 through the AT1R. Additionally, Ang II stimulates production of H2O2 directly through Nox4, and indirectly through the SOD-mediated conversion of O2•− produced by Nox1, 2, and 5. The O2•−-mediated oxidation and inactivation of the eNOS cofactor BH4 promotes uncoupling of eNOS, leading to eNOS-mediated production of O2•−. Additionally, H2O2 inhibits DHFR, an enzyme that catalyzes the conversion of BH2 to BH4, which further reduces BH4 bioavailability, leading to eNOS uncoupling. BH2, dihydrobiopterin; BH4, tetrahydrobiopterin; CaM, calmodulin; DHFR, dihydrofolate reductase; eNOS, endothelial nitric oxide synthase; Fe, eNOS heme domain; H2O2, hydrogen peroxide; O2•−, superoxide; p22, p22 phox (Nox subunit); p40, p40 phox (Nox subunit); p47, p47 phox (Nox subunit); p67, p67 phox (Nox subunit); Rac, Rho-like GTPase Rac.
H2O2 is tightly regulated by intracellular and extracellular enzymes, including catalase, glutathione peroxidase, thioredoxin, and other peroxyredoxins, which convert H2O2 to water and O2 and other metabolites. Although both O2•− and H2O2 have been suggested to act as signaling molecules, it is mainly H2O2 that is considered a signaling molecule because of its relative stability, tight regulation, subcellular localization, and ability to react reversibly with cysteine residues (25).
ROS-specific effects are mediated in large part through the oxidative modification of thiols on cysteine residues within redox-sensitive target proteins (24). Of all the unique cysteine residues in the human genome, 20,000–40,000 are highly sensitive to oxidations (1). Oxidation of thiols leads to structural changes in target molecules that result in activation or inactivation of signaling proteins. For example, protein tyrosine phosphatases (PTPs) are inactivated by oxidation, leading to increased phosphorylation of downstream proteins. In addition to protein oxidation, ROS induce oxidative damage through lipid peroxidation of cellular membranes and DNA breaks (1). Through these mechanisms, O2•− and H2O2 influence many signaling molecules important in the regulation of vascular function, including MAP kinases, nonreceptor tyrosine kinases, receptor tyrosine kinases, PTPs, and redox-sensitive transcription factors (17, 40) (Fig. 6). Activation of these molecules participates in cell growth, migration, expression of proinflammatory genes, production of extracellular matrix proteins, and contraction, processes important in the regulation of vascular function and tone.
FIG. 6.
Ang II-mediated redox-sensitive growth signaling in VSMCs. In VSMCs, binding of Ang II to the AT1R leads to increased ROS generation, in part, via activation of c-Src-induced Nox activation. ROS stimulate nonreceptor tyrosine kinases such as FAK, JAK and PI3K, and PLC/PKC as well as receptor tyrosine kinases, such as EGFR, IGFR, and PDGFR. Redox signaling in turn regulates downstream MEK cascades, leading to phosphorylation of MAPKs, which induce growth, apoptosis, differentiation, migration, and inflammation of VSMCs. Ang II/AT1R also induces MMP-mediated extracellular release of HB-EGF, which then stimulates EGFR transactivation and ERK1/2 MAPK activation. EGFR, epidermal growth factor receptor; FAK, focal adhesion kinase; HB-EGF, heparin-binding epidermal growth factor; IGFR, insulin-like growth factor receptor; JAK, Janus-activated kinase; JNK, c-Jun NH2-terminal kinase; MEK, MAPK/ERK kinase; MMP, matrix metalloproteinase; p, phosphorylation; PDGFR, platelet-derived growth factor receptor; PI3K, phosphatidylinositol 3-kinase; PKC, protein kinase C; PLC, phospholipase C; STAT, signal transducers and activators of transcription.
The distinct chemical properties between O2•− and H2O2 and their different sites of distribution mean that different species of ROS activate diverse signaling pathways, which lead to divergent, and potentially opposing, biological responses. For example, in the vasculature, increased O2•− levels inactivate the vasodilator NO•, leading to endothelial dysfunction and vasoconstriction (61), whereas H2O2 acts as a direct vasodilator in some vascular beds, including cerebral, coronary, and mesenteric arteries (54).
The specificity of signaling through ROS is related to many factors, including subcellular localization of Nox isoforms, binding of Noxs to scaffolding proteins, and compartmentalization within intracellular organelles, for example, the mitochondria, the nucleus, and the endoplasmic reticulum (ER). Nox2 is membrane-associated, whereas Nox4 has been identified in the mitochondria, the ER, and the nucleus. Mitochondrial Nox4 expression is increased in kidneys from diabetic rats (29, 30, 78). It has been proposed that localization of Nox4 to the mitochondria creates a short paracrine loop, whereby ROS production by mitochondrial Nox4 regulates or is regulated by ROS generated by the mitochondrial respiratory chain (3). Furthermore, Nox subunits or Nox isoforms may interact with other proteins to facilitate localized ROS generation.
Ang II Signaling and ROS in the Vasculature
Ang II mediates effects via complex intracellular signaling pathways that are stimulated after binding of the peptide to its cell-surface receptors. Both receptors play a role in regulating VSMC function, although they differ in their actions.
Redox-sensitive growth signaling by Ang II in vascular cells
Ang II stimulates cell growth through phosphorylation of tyrosine kinases, activation of MAP kinases, mobilization of intracellular calcium (Ca2+), and production of ROS (77) (Fig. 6). Ang II, via AT1R, induces phosphorylation of multiple tyrosine kinases, including c-Src, Janus family kinases (JAK), focal adhesion kinase (FAK), protein tyrosine kinase 2 (Pyk2), p130Cas, and PI3K (56). c-Src, which regulates Nox, is a critically important kinase involved in trophic and contractile actions of Ang II (12). c-Src is upstream from many growth-signaling molecules, including PLC-γ, Pyk2, FAK, JAK, Shc, MAP kinases, and PI3K (12).
Of the many growth-signaling molecules, the MAP kinase family is best characterized (38). In VSMCs, Ang II activates all four of the major MAP kinases, extracellular signal-regulated kinases (ERK1/2), p38MAP kinase, c-Jun N-terminal kinases (JNK), and ERK5. ERK1/2, phosphorylated by MAP kinase kinase 1/2 (MEK1/2) (MAP/ERK kinase), is a key growth-signaling kinase, whereas JNK and p38MAP kinase, phosphorylated by MEK4/7 and MEK3/6, respectively, influence cell survival, apoptosis, differentiation, and inflammation. ERK5 is involved in protein synthesis, cell cycle progression, and cell growth. Activation of these MAP kinases requires ROS and Nox activation, because Nox inhibitors and various antioxidants attenuate Ang II-induced activation of MAP kinases (22, 63, 69). MAP kinase phosphorylation may not be directly influenced by ROS, but rather by changes in activation of upstream PTPs. PTPs possess highly conserved cysteine residues that are highly sensitive to oxidation (53).
Ang II transactivation of receptor tyrosine kinases: role of ROS
Ang II also activates receptor tyrosine kinases, classically linked to growth-signaling pathways, even though it may not bind directly to these receptors. This process of transactivation has been demonstrated for epidermal growth factor (EGF) receptor, platelet-derived growth factor receptor, subtype β, and insulin-like 1 growth factor receptor (16) (Fig. 6). Mechanisms whereby Ang II-induces receptor tyrosine kinase transactivation include activation of Pyk2 and Src, metalloprotease-dependent shedding of heparin-binding EGF-like growth factor and ROS (50). In rat VSMCs, Ang II, through increased oxidative stress and activation of c-Src, transactivates EGF-R, which leads to MAP kinase activation and cell growth, an effect that is augmented in SHR (43).
Ang II signaling, RhoA/Rho kinase, and ROS
Activation of RhoA and its downstream target Rho-kinase is increasingly being recognized as an important mechanism of vasoconstriction by Ang II and accordingly has been implicated in the pathophysiology of hypertension and other vascular diseases (44, 57). RhoA, a member of the Rho family of small GTPase-binding proteins, is abundantly expressed in VSMCs and participates in vasoconstriction via phosphorylation of myosin light chain and sensitization of contractile proteins to Ca2+. RhoA/Rho-kinase also influences VSMC migration, a process that is redox sensitive, since apocynin, which inhibits Nox activity and ROS generation, blocked Ang II-stimulated effects (55). In the renal vasculature, alpha(2)-adrenoceptors potentiate renal vascular responses to Ang II. This interaction, which influences vascular resistance, involves RhoA/Rho-kinase, ROS, and Nox2, since Ang II effects were attenuated by Y27632 (Rho-kinase inhibitor), tempol (superoxide dismutase mimetic), and gp91ds-tat (Nox2 inhibitor) (34).
RhoA and Nox also regulate microparticle release from Ang II-stimulated endothelial cells (9). Microparticles are submicron fragments that arise from plasma membrane blebbing and subsequently shed from activated or apoptotic cells. They impair angiogenesis, promote oxidative stress, and influence vasodilation, and they themselves produce ROS (49). Studies in cultured endothelial cells and in Ang II-infused mice demonstrated that fasudil and apocynin, inhibitors of Rho kinase and Nox, respectively, blunted microparticle formation (6). These processes have been implicated in Ang II-mediated oxidative damage of the endothelium.
Ang II, transcription factors, inflammation, and ROS
Important downstream targets of signaling pathways are transcription factors, which are highly redox sensitive. Many transcription factors important in inflammation, cell growth, and fibrosis are activated by Ang II through mechanisms that involve Nox and ROS. Among transcription factors that are activated and sensitive to both Ang II and ROS, there is the activator protein 1 (AP-1), nuclear factor kappa B, cyclic AMP response element-binding protein (CREB), and hypoxia-inducible factor 1 (HIF-1) (6, 74). Mitochondrial-generated ROS have also been shown to be essential intermediates for HIF-1 activation in VSMCs (18, 60).
Transcription factors act as signal integrators regulating processes related to vascular inflammation. Responses to proinflammatory stimuli, such as Ang II and ROS, lead to activation of transcription factors important in the production of proinflammatory cytokines and interleukins. These processes in turn influence leukocyte adherence, chemotaxis, and the inflammatory response, which underlie vascular damage in cardiovascular disease.
Ang II signaling, ROS, and vascular cell senescence
In addition to regulating cell growth, Ang II influences other components of the life cycle of cells, including senescence. Senescence is defined as a form of irreversible arrest in cell proliferation and is associated with a characteristic gene and protein phenotype different to that of proliferating cells. Senescent cells characteristically express senescence-associated β- galactosidase and pShc-66 (1, 15). As cells reach the end of their lifespan, they undergo replicative senescence, driven by telomere dysfunction, a process that occurs with normal aging. In pathological conditions, such as hypertension and atherosclerosis, vascular cells undergo premature senescence, which can be triggered by Ang II and oxidative stress (52). Such processes contribute to vascular remodeling and early vascular aging. Clinical studies using angiotensin receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEis) demonstrated protective effects against age-related cardiovascular changes (52). In vitro studies supported these findings showing a link between Ang II stimulation and vascular cell senescence, an effect blocked by ARBs and ACEis (14). In AT1R knockout mice, lifespan was prolonged by almost 30% compared to genetically matched wild-type controls (2, 20). This enhanced longevity was associated with improved cardiovascular morphology, reduced ROS production, attenuated mitochondrial loss, and enhanced expression of survival genes (nicotinamide phosphoribosyltransferase [Nampt] and sirtuin-3 [Sirt3]) (2).
Molecular mechanisms through which Ang II regulates vascular cell senescence seem to involve signaling pathways similar to those that control cell growth. In addition, ROS are emerging as important modulators of senescence (32). This is evidenced by studies demonstrating that H2O2 induces vascular cell senescence, that Nox and ROS production are increased in senescent cells, and that antioxidants inhibit Ang II-induced senescence (32, 33). ROS induce DNA damage, which is critically involved in Ang II-mediated redox-triggered cell senescence. Both telomere-dependent and telomere-independent mechanisms have been linked to Ang II-mediated senescence (32). Telomere-related effects influence replicative senescence, whereas telomere-independent processes may contribute to premature senescence. The net effect of Ang II may relate to the concentration and chronicity of Ang II stimulation.
Conclusions
Over the recent past, our views of Ang II have changed from being a simple vasoconstrictor to that of a complex pleiotropic factor, involved in vascular hypertrophy, fibrosis, inflammation, and aging. These effects are mediated through diverse signaling pathways involving PLC/PKC/Ca2+ mobilization, PLA2, PLD, MAP kinases, tyrosine kinases, proto-oncogene expression, RhoA/Rho-kinase, inflammation, and cell cycle modulation. Common to these pathways are ROS, derived in large part from vascular Noxs. Through increased generation of ROS and activation of redox-sensitive transcription factors, Ang II promotes expression of cell adhesion molecules and induces synthesis of proinflammatory mediators and growth factors. These molecular and cellular processes facilitate increased vascular permeability, leukocyte recruitment, calcification, and vascular fibrosis, leading to vascular injury, structural remodeling, and premature aging. Targeting some of these molecular events with novel therapeutic strategies, possibly at the level of Nox-derived ROS, may regress or prevent arterial remodeling and aging and thereby provide important vascular protection in cardiovascular and age-related diseases.
Abbreviations Used
- ACEi
angiotensin-converting enzyme inhibitor
- Ang II
angiotensin II
- AP-1
activator protein 1
- ARB
angiotensin receptor blocker
- AT1R
Ang II type 1 receptors
- AT2R
Ang II type 2 receptors
- BH2
dihydrobiopterin
- BH4
tetrahydrobiopterin
- [Ca2+]i
intracellular free-calcium concentration
- CaM
calmodulin
- CIHR
Canadian Institute of Health Research
- CLC-3
chloride channel 3
- DAG
diacylglycerol
- DHFR
dihydrofolate reductase
- EC-SOD (SOD3)
extracellular superoxide dismutase
- EGF
epidermal growth factor
- eNOS
endothelial nitric oxide synthase
- ER
endoplasmic reticulum
- ERK1/2
extracellular signal-regulated kinase1/2
- FAK
focal adhesion kinase
- H2O•
hydroperoxyl radical
- H2O2
hydrogen peroxide
- HB-EGF
heparin-binding EGF-like growth factor
- HIF-1
hypoxia-inducible factor 1
- IGF-1
insulin-like 1 growth factor
- IP3
inositol-3-phosphate
- JAK
Janus family kinases
- JNK
c-Jun N-terminal kinases
- MAP
mitogen-activated protein
- MAPK
mitogen-activated protein kinase
- MEK1/2
MAP kinase kinase 1/2
- MLC
myosin light chain
- MLCP
myosin light-chain phosphatase
- MLCK
myosin light-chain kinase
- MMP
matrix metalloproteinase
- Mn SOD (SOD2)
manganese-containing mitochondrial SOD
- NAD(P)H
reduced nicotinamide adenine dinucleotide phosphate
- Nampt
nicotinamide phosphoribosyltransferase
- NFκB
nuclear factor kappa B
- NO•
nitric oxide
- Nox
NADPH oxidase
- NoxA1
NADPH activator 1
- NoxO1
NADPH organizer 1
- O2•−
superoxide anion
- ONOO−
peroxinitrite
- PDGFR
platelet-derived growth factor receptor
- Phox
phagocyte oxidase
- PI3K
phosphatidylinositol 3-kinase
- PKC
protein kinase C
- PLA2
phospholipase A2
- PLC
phospholipase C
- PLD
phospholipase D
- Poldip2
polymerase delta-interacting protein 2
- PTP
protein tyrosine phosphatases
- Pyk2
protein tyrosine kinase 2
- RAS
renin–angiotensin system
- ROS
reactive oxygen species
- SHR
spontaneously hypertensive rats
- Sirt3
sirtuin-3
- SMCnox1
smooth muscle cell Nox1
- SOD
superoxide dismutase
- SOD1
copper/zinc superoxide dismutase 1
- STAT
signal transducers and activators of transcription
- VSMCs
vascular smooth muscle cells
- WKY
Wistar Kyoto rats
Acknowledgments
Studies performed by the author were supported by Grants 57786 and 44018 from the Canadian Institutes of Health Research (CIHR). R.M.T. was supported through a Canada Research Chair/Canadian Foundation for the Innovation award. A.C.M. was supported by a fellowship from the CIHR. D.B. was supported through a KRESCENT fellowship from the Kidney Foundation of Canada.
References
- 1.Al Ghouleh I. Khoo NK. Knaus UG. Griendling KK. Touyz RM. Thannickal VJ. Barchowsky A. Nauseef WM. Kelley EE. Bauer PM. Darley-Usmar V. Shiva S. Cifuentes-Pagano E. Freeman BA. Gladwin MT. Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med. 2011;51:1271–1288. doi: 10.1016/j.freeradbiomed.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benigni A. Corna D. Zoja C. Sonzogni A. Latini R. Salio M. Conti S. Rottoli D. Longaretti L. Cassis P. Morigi M. Coffman TM. Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block K. Gorin Y. Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokoch GM. Zhao T. Regulation of the phagocyte NAD(P)H oxidase by Rac GTPase. Antioxid Redox Signal. 2006;8:1533–1548. doi: 10.1089/ars.2006.8.1533. [DOI] [PubMed] [Google Scholar]
- 5.Brandes RP. Schröder K. Differential vascular functions of Nox family NAD(P)H oxidases. Curr Opin Lipidol. 2008;19:513–518. doi: 10.1097/MOL.0b013e32830c91e3. [DOI] [PubMed] [Google Scholar]
- 6.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briones AM. Tabet F. Callera GE. Montezano AC. Yogi A. He Y. Quinn MT. Salaices M. Touyz RM. Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR. J Am Soc Hypertens. 2011;5:137–153. doi: 10.1016/j.jash.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Brown DI. Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger D. Montezano AC. Nishigaki N. He Y. Carter A. Touyz RM. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH 18 oxidase/Rho kinase pathways targeted to lipid rafts. Arterioscler Thromb Vasc Biol. 2011;31:1898–1899. doi: 10.1161/ATVBAHA.110.222703. [DOI] [PubMed] [Google Scholar]
- 10.Burgoyne JR. Haeussler DJ. Kumar V. Ji Y. Pimental DR. Zee RS. Costello CE. Lin C. McComb ME. Cohen RA. Bachschmid MM. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J. 2012;26:832–841. doi: 10.1096/fj.11-189415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callera GE. Montezano AC. Yogi A. Tostes RC. Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 12.Callera GE. Yogi A. Briones AM. Montezano AC. He Y. Tostes RC. Schiffrin EL. Touyz RM. Vascular proinflammatory responses by aldosterone are mediated via c-Src trafficking to cholesterol-rich microdomains: role of PDGFR. Cardiovasc Res. 2011;91:720–731. doi: 10.1093/cvr/cvr131. [DOI] [PubMed] [Google Scholar]
- 13.Camici GG. Cosentino F. Tanner FC. Lüscher TF. The role of p66Shc deletion in ageassociated arterial dysfunction and disease states. J Appl Physiol. 2008;105:1628–1631. doi: 10.1152/japplphysiol.90579.2008. [DOI] [PubMed] [Google Scholar]
- 14.Capettini LS. Montecucco F. Mach F. Stergiopulos N. Santos RA. da Silva RF. Role of renin-angiotensin system in inflammation, immunity and aging. Curr Pharm Des. 2012;18:963–970. doi: 10.2174/138161212799436593. [DOI] [PubMed] [Google Scholar]
- 15.Cosentino F. Francia P. Camici GG. Pelicci PG. Lüscher TF. Volpe M. Final common molecular pathways of aging and cardiovascular disease: role of the p66Shc protein. Arterioscler Thromb Vasc Biol. 2008;28:622–628. doi: 10.1161/ATVBAHA.107.156059. [DOI] [PubMed] [Google Scholar]
- 16.Cruzado MC. Risler NR. Miatello RM. Yao G. Schiffrin EL. Touyz RM. Vascular smooth muscle cell NAD(P)H oxidase activity during the development of hypertension: Effect of angiotensin II and role of insulinlike growth factor-1 receptor transactivation. Am J Hypertens. 2005;18:81–87. doi: 10.1016/j.amjhyper.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Dikalov SI. Dikalova AE. Bikineyeva AT. Schmidt HH. Harrison DG. Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dikalova AE. Bikineyeva AT. Budzyn K. Nazarewicz RR. McCann L. Lewis W. Harrison DG. Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikalova AE. Góngora MC. Harrison DG. Lambeth JD. Dikalov S. Griendling KK. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnini S. Terzuoli E. Ziche M. Morbidelli L. Sulfhydryl angiotensin-converting enzyme inhibitor promotes endothelial cell survival through nitric-oxide synthase, fibroblast growth factor-2, and telomerase cross-talk. J Pharmacol Exp Ther. 2010;332:776–784. doi: 10.1124/jpet.109.159178. [DOI] [PubMed] [Google Scholar]
- 21.Dutta S. Rittinger K. Regulation of NOXO1 activity through reversible interactions with p22 and NOXA1. PLoS One. 5:e10478. doi: 10.1371/journal.pone.0010478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimian T. Li MW. Lemarié CA. Simeone SM. Pagano PJ. Gaestel M. Paradis P. Wassmann S. Schiffrin EL. Mitogen-activated protein kinase-activated protein kinase 2 in angiotensin II-induced inflammation and hypertension: regulation of oxidative stress. Hypertension. 2011;57:245–254. doi: 10.1161/HYPERTENSIONAHA.110.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandes DC. Manoel AH. Wosniak J., Jr Laurindo FR. Protein disulfide isomerase overexpression in vascular smooth muscle cells induces spontaneous preemptive NADPH oxidase activation and Nox1 mRNA expression: effects of nitrosothiol exposure. Arch Biochem Biophys. 2009;484:197–204. doi: 10.1016/j.abb.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 24.Flohé L. Toppo S. Cozza G. Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 25.Freinbichler W. Colivicchi MA. Stefanini C. Bianchi L. Ballini C. Misini B. Weinberger P. Linert W. Varešlija D. Tipton KF. Della Corte L. Highly reactive oxygen species: detection, formation, and possible functions. Cell Mol Life Sci. 2011;68:2067–2079. doi: 10.1007/s00018-011-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fridovich I. Superoxide anion radical (O2-.), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 27.Garrido AM. Griendling KK. NADPH oxidases, angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geiszt M. NAD(P)H oxidases: New kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Gordillo G. Fang H. Park H. Roy S. Nox-4-dependent nuclear H2O2 drives DNA oxidation resulting in 8-OHdG as urinary biomarker and hemangioendothelioma formation. Antioxid Redox Signal. 2010;12:933–943. doi: 10.1089/ars.2009.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham KA. Kulawiec M. Owens KM. Li X. Desouki MM. Chandra D. Singh KK. NADPH oxidase 4 is an oncoprotein localized to mitochondria. Cancer Biol Ther. 2010;10:223–231. doi: 10.4161/cbt.10.3.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Griendling KK. Minieri CA. Ollerenshaw JD. Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res. 1994;74:1141–1148. doi: 10.1161/01.res.74.6.1141. [DOI] [PubMed] [Google Scholar]
- 32.Herbert KE. Mistry Y. Hastings R. Poolman T. Niklason L. Williams B. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res. 2008;102:201–208. doi: 10.1161/CIRCRESAHA.107.158626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imanishi I. Hano T. Nishio I. Angiotensin II accelerates endothelial progenitor cell senescence through induction of oxidative stress. J Hypertens. 2005;23:97–104. doi: 10.1097/00004872-200501000-00018. [DOI] [PubMed] [Google Scholar]
- 34.Jackson EK. Gillespie DG. Zhu C. Ren J. Zacharia LC. Mi Z. Alpha2-adrenoceptors enhance angiotensin II-induced renal vasoconstriction: role for NADPH oxidase and RhoA. Hypertension. 2008;51:719–726. doi: 10.1161/HYPERTENSIONAHA.107.096297. [DOI] [PubMed] [Google Scholar]
- 35.Jacob C. Jamier V. Ba LA. Redox active secondary metabolites. Curr Opin Chem Biol. 2011;15:149–155. doi: 10.1016/j.cbpa.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Jagadeesha DK. Takapoo M. Banfi B. Bhalla RC. Miller FJ., Jr Nox1 transactivation of epidermal growth factor receptor promotes N-cadherin shedding and smooth muscle cell migration. Cardiovasc Res. 2012;93:406–413. doi: 10.1093/cvr/cvr308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katsuyama M. Matsuno K. Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. Clin Biochem Nutr. 2012;50:9–22. doi: 10.3164/jcbn.11-06SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knock GA. Ward JP. Redox regulation of protein kinases as a modulator of vascular function. Antioxid Redox Signal. 2011;15:1531–1547. doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- 39.Lassegue B. Clempus RE. Vascular NAD(P)H oxidases: specific features, expression, and regulation. Am J Physiol Regul Integr Comp Physiol. 2003;285:R277–R297. doi: 10.1152/ajpregu.00758.2002. [DOI] [PubMed] [Google Scholar]
- 40.Lassègue B. Sorescu D. Szöcs K. Yin Q. Akers M. Zhang Y. Grant SL. Lambeth JD. Griendling KK. Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ Res. 2010;88:888–894. doi: 10.1161/hh0901.090299. [DOI] [PubMed] [Google Scholar]
- 41.Lemarié CA. Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2010;11:19–31. doi: 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- 42.Leto TL. Morand S. Hurt D. Ueyama T. Targeting and regulation of reactive oxygen species generation by Nox family NADPH oxidases. Antioxid Redox Signal. 2009;11:260. doi: 10.1089/ars.2009.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li Y. Lévesque LO. Anand-Srivastava MB. Epidermal growth factor receptor transactivation by endogenous vasoactive peptides contributes to hyperproliferation of vascular smooth muscle cells of SHR. Am J Physiol Heart Circ Physiol. 2010;299:H1959–H1967. doi: 10.1152/ajpheart.00526.2010. [DOI] [PubMed] [Google Scholar]
- 44.Loirand G. Pacaud P. The role of Rho protein signaling in hypertension. Nat Rev Cardiol. 2010;7:637–647. doi: 10.1038/nrcardio.2010.136. [DOI] [PubMed] [Google Scholar]
- 45.Lu J. Mitra S. Wang X. Khaidakov M. Mehta JL. Oxidative stress and lectin-like ox-LDLreceptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 46.Lyle AN. Deshpande NN. Taniyama Y. Seidel-Rogol B. Pounkova L. Du P. Papaharalambus C. Lassègue B. Griendling KK Poldip2, a novel regulator of Nox4, cytoskeletal integrity in vascular smooth muscle cells. Circ Res. 2009;105:249–259. doi: 10.1161/CIRCRESAHA.109.193722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mehta PK. Griendling KK. Angiotensin II cell signalling: physiological, pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 48.Mendez JI. Nicholson WJ. Taylor WR. SOD isoforms and signaling in blood vessels: evidence for the importance of ROS compartmentalization. Arterioscler Thromb Vasc Biol. 2005;25:887–888. doi: 10.1161/01.ATV.0000164043.24549.50. [DOI] [PubMed] [Google Scholar]
- 49.Meziani F. Tesse A. Andriantsitohaina R. Microparticles are vectors of paradoxical information in vascular cells including the endothelium: role in health and diseases. Pharmacol Rep. 2008;60:75–84. [PubMed] [Google Scholar]
- 50.Mifune M. Ohtsu H. Suzuki H. Nakashima H. Brailoiu E. Dun NJ. Frank GD. Inagami T. Higashiyama S. Thomas WG. Eckhart AD. Dempsey PJ. Eguchi S. G protein coupling and second messenger generation are indispensable for metalloprotease-dependent, heparin binding epidermal growth factor shedding through angiotensin II type-1 receptor. J Biol Chem. 2005;280:26592–26599. doi: 10.1074/jbc.M502906200. [DOI] [PubMed] [Google Scholar]
- 51.Miller FJ., Jr Filali M. Huss GJ. Stanic B. Chamseddine A. Barna TJ. Lamb FS. Cytokine activation of nuclear factor kappa B in vascular smooth muscle cells requires signaling endosomes containing Nox1 and ClC-3. Circ Res. 2007;101:663–671. doi: 10.1161/CIRCRESAHA.107.151076. [DOI] [PubMed] [Google Scholar]
- 52.Min LJ. Mogi M. Iwai M. Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 53.Monteiro HP. Arai RJ. Travassos LR. Protein tyrosine phosphorylation and protein tyrosine nitration in redox signaling. Antioxid Redox Signal. 2008;10:843–889. doi: 10.1089/ars.2007.1853. [DOI] [PubMed] [Google Scholar]
- 54.Montezano AC. Burger D. Paravicini TM. Chignalia AZ. Yusuf H. Almasri M. He Y. Callera GE. He G. Krause KH. Lambeth D. Quinn MT. Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Montezano AC. Callera GE. Yogi A. He Y. Tostes RC. He G. Schiffrin EL. Touyz RM. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler Thromb Vasc Biol. 2008;28:1511–1518. doi: 10.1161/ATVBAHA.108.168021. [DOI] [PubMed] [Google Scholar]
- 56.Montezano AC. Touyz RM. Reactive oxygen species and endothelial function—role of nitric oxide synthase uncoupling and Nox family nicotinamide adenine dinucleotide phosphate oxidases. Basic Clin Pharmacol Toxicol. 2012;110:87–94. doi: 10.1111/j.1742-7843.2011.00785.x. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen Dinh Cat A. Touyz RM. Cell signaling of angiotensin II on vascular tone: novel mechanisms. Curr Hypertens Rep. 2011;13:122–128. doi: 10.1007/s11906-011-0187-x. [DOI] [PubMed] [Google Scholar]
- 58.Nishida M. Kitajima N. Saiki S. Nakaya M. Kurose H. Regulation of Angiotensin II receptor signaling by cysteine modification of NF-κB. Nitric Oxide. 2011;25:112–117. doi: 10.1016/j.niox.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 59.Paravicini TM. Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 60.Patten DA. Lafleur VN. Robitaille GA. Chan DA. Giaccia AJ. Richard DE. Hypoxiainducible factor-1 activation in nonhypoxic conditions: the essential role of mitochondrialderived reactive oxygen species. Mol Biol Cell. 2010;21:3247–3257. doi: 10.1091/mbc.E10-01-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Queisser N. Fazeli G. Schupp N. Superoxide anion and hydrogen peroxide-induced signaling and damage in angiotensin II and aldosterone action. Biol Chem. 2010;391:1265–1279. doi: 10.1515/BC.2010.136. [DOI] [PubMed] [Google Scholar]
- 62.Rajagopalan S. Kurz S. Münzel T. Tarpey M. Freeman BA. Griendling KK. Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rasmussen HH. Hamilton EJ. Liu CC. Figtree GA. Reversible oxidative modification: implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc Med. 2010;20:85–90. doi: 10.1016/j.tcm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 64.Seshiah PN. Weber DS. Rocic P. Valppu L. Taniyama Y. Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 65.Streeter J. Thiel W. Brieger K. Miller FJ., Jr Opportunity Nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther. 2012 doi: 10.1111/j.1755-5922.2011.00310.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Tabet F. Schiffrin EL. Callera GE. He Y. Yao G. Ostman A. Kappert K. Tonks NK. Touyz RM. Redox-sensitive signaling by angiotensin II involves oxidative inactivation and blunted phosphorylation of protein tyrosine phosphatase SHP-2 in vascular smooth muscle cells from SHR. Circ Res. 2008;103:149–154. doi: 10.1161/CIRCRESAHA.108.178608. [DOI] [PubMed] [Google Scholar]
- 67.Touyz RM. Briones AM. Sedeek M. Burger D. Montezano AC. NOX Isoforms and Reactive Oxygen Species in Vascular Health. Mol Interv. 2011;11:27–35. doi: 10.1124/mi.11.1.5. [DOI] [PubMed] [Google Scholar]
- 68.Touyz RM. Chen X. Tabet F. Yao G. He G. Quinn MT. Pagano PJ. Schiffrin EL. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res. 2002;90:1205–1213. doi: 10.1161/01.res.0000020404.01971.2f. [DOI] [PubMed] [Google Scholar]
- 69.Touyz RM. Cruzado M. Tabet F. Yao G. Salomon S. Schiffrin EL. Redox-dependent MAP kinase signaling by Ang II in vascular smooth muscle cells: role of receptor tyrosine kinase transactivation. Can J Physiol Pharmacol. 2003;81:159–167. doi: 10.1139/y02-164. [DOI] [PubMed] [Google Scholar]
- 70.Touyz RM. Schiffrin EL. Ang II-stimulated superoxide production is mediated via phospholipase D in human vascular smooth muscle cells. Hypertension. 1999;34:976–982. doi: 10.1161/01.hyp.34.4.976. [DOI] [PubMed] [Google Scholar]
- 71.Touyz RM. Schiffrin EL. Signal transduction mechanisms mediating the physiological, pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev. 2000;52:639–672. [PubMed] [Google Scholar]
- 72.Touyz RM. Yao G. Quinn MT. Pagano PJ. Schiffrin EL. p47p hox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 73.Touyz RM. Yao G. Schiffrin EL. c-Src induces phosphorylation and translocation of p47phox: role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2003;23:981–987. doi: 10.1161/01.ATV.0000069236.27911.68. [DOI] [PubMed] [Google Scholar]
- 74.Ungvari Z. Bailey-Downs L. Gautam T. Sosnowska D. Wang M. Monticone RE. Telljohann R. Pinto JT. de Cabo R. Sonntag WE. Lakatta EG. Csiszar A. Age-associated vascular oxidative stress, Nrf2 dysfunction, and NF-{kappa}B activation in the nonhuman primate Macaca mulatta. J Gerontol A Biol Sci Med Sci. 2011;66:866–875. doi: 10.1093/gerona/glr092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ushio-Fukai M. Zafari AM. Fukui T. Ishizaka N. Griendling KK. p22phox is a critical component of the superoxide-generating NADH/NADPH oxidase system and regulates angiotensin II-induced hypertrophy in vascular smooth muscle cells. J Biol Chem. 1996;271:23317–23321. doi: 10.1074/jbc.271.38.23317. [DOI] [PubMed] [Google Scholar]
- 76.Virdis A. Neves MF. Amiri F. Touyz RM. Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 77.Wassmann S. Nickenig G. Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006;24:S15–S21. doi: 10.1097/01.hjh.0000220402.53869.72. [DOI] [PubMed] [Google Scholar]
- 78.Zhang L. Nguyen MV. Lardy B. Jesaitis AJ. Grichine A. Rousset F. Talbot M. Paclet MH. Qian G. Morel F. New insight into the Nox4 subcellular localization in HEK293 cells: first monoclonal antibodies against Nox4. Biochimie. 2011;93:457–468. doi: 10.1016/j.biochi.2010.11.001. [DOI] [PubMed] [Google Scholar]