Abstract
Purpose.
Laser-induced choroidal neovascularization (CNV) is a widely used model to mimic many features of CNV resulting from wet AMD. Macrophages have been implicated in the pathogenesis of AMD. Class A scavenger receptors, scavenger receptor-A (SR-A) and macrophage receptor with collagenous domain (MARCO), are expressed on macrophages and are associated with macrophage function. The goal of this study is to examine the role of macrophage scavenger receptors in immune cell recruitment and the formation of CNV.
Methods.
Laser photocoagulation was performed in wild-type and knockout mice with deletion of SR-A (SR-A−/−), MARCO (MARCO−/−), or both SR-A and MARCO double knockout (DKO). Immune cell recruitment at different time points and CNV lesions at 14 days after laser treatment were evaluated through immunostaining and confocal microscopy. Microarray analysis was performed in eyes 1 day after laser injury.
Results.
Wild-type eyes showed higher chemokine/receptor expression compared with knockout eyes after laser injury. Scavenger receptor deficiency markedly impaired the recruitment of neutrophils and macrophages to CNV lesions at 1- and 3-days post laser injury, respectively. Significantly reduced CNV volumes were found in the eyes from scavenger receptor knockout mice compared with wild-type mice.
Conclusions.
The deficiency of scavenger receptors impairs the formation of CNV and immune cell recruitment. Our findings suggest a potential role for scavenger receptors in contributing to CNV formation and inflammation in AMD.
Keywords: AMD, CNV, macrophages, scavenger receptors
Class A scavenger receptor deficiency impairs immune cell recruitment and the formation of choroidal neovascularization (CNV). This suggests that scavenger receptors may contribute to CNV formation and inflammation seen in AMD.
Introduction
Age-related macular degeneration is the leading cause of irreversible central blindness in the elderly, worldwide. It is clinically characterized by degenerative changes in the macula, the region of the retina that permits fine central vision. One of the key pathologic features of AMD is the development of large drusen, extracellular deposits located in Bruch's membrane beneath the RPE. Their presence is a harbinger of further retinal change, the major risk factor for the development of advanced AMD, which can be classified into two subtypes: geographic atrophy (dry) and neovascular/exudative (wet).1 In wet AMD, new vessels from the choroid breach Bruch's membrane and grow into the subretinal space and retina. This process is also called choroidal neovascularization (CNV). It represents only 10% of all cases of advanced AMD, but about 90% of AMD patients experience severe vision loss resulting from this subtype. Various animal models attempt to mimic the clinical manifestations of AMD. Among them, a laser-induced murine CNV model is widely used to mimic the wet form of AMD by compromising Bruch's membrane to induce CNV formation in the subretinal region. This model allows for visualization and evaluation of the morphologic changes of experimental CNV and has been a valuable tool to study the diverse components of complex CNV lesions.2
The exact mechanisms that cause AMD are not clear. Recent information suggests that immune mechanisms play an important role in AMD.3–5 Complement dysregulation and the involvement of macrophages are thought to contribute to initiation and progression of the disease. Contradictory results have been reported regarding the role that macrophages play in CNV formation. Grossniklaus and colleagues suggested that macrophages may promote CNV since macrophages in the AMD lesions express VEGF.6 Ambati et al. showed that fewer macrophages were observed in Ccl2−/− and Ccr2−/− mice at the RPE and choroid region. These knockout animals developed histologic signs of AMD, including accumulation of lipofuscin and drusen beneath the RPE, photoreceptor atrophy, and CNV, suggesting that macrophages play a protective role in AMD.7 Cherepanoff showed in humans that activated macrophages were found more commonly in patients with CNV.8
Receptors on macrophages contribute to many cellular functions. For example, scavenger receptors, a diverse family of multifunctional receptor proteins expressed by macrophages and endothelial cells in lymphoreticular tissue, are associated with phagocytosis and uptake of waste products. More specifically, Class A scavenger receptors have been found to play a central role in regulating the inflammatory response in infectious models of disease.9 Two relevant subtypes of Class A scavenger receptors, scavenger receptor-A (SRA) and macrophage receptor with collagenous domain (MARCO), macrophage receptors with similar collagenous structures, are involved in homeostatic functions such as lipid metabolism and recognition and clearance of pathogens, bacteria, and microbial cell wall products. While SR-A is ubiquitously expressed by most tissue macrophages, MARCO expression is limited to a defined subset of macrophages in the steady state, although induced more widely on macrophages by infection.10 Whether scavenger receptors contribute to the infiltration of macrophages and formation of CNV in the eye is unknown.
In this study, we wished to see the effect of scavenger receptors in the mouse model for CNV. We showed that the eyes from SR-A−/−, MARCO−/−, and double knockout (DKO) mice have similar morphology compared with wild-type mice. The chemokine/receptor expression patterns between knockout mice and wild-type eyes were similar before laser administration. After laser administration, we found that wild-type eyes had higher global chemokine/receptor expression relative to knockout eyes. Consistently, we further showed that the eyes from knockout mice have reduced neutrophil and macrophage/microglia recruitment around laser lesions. Intriguingly, we observed significantly reduced CNV volumes in knockout mice as compared with wild type.
Materials and Methods
Animals
C57BL/6J animals were purchased from Taconic (Hudson, NY). SR-A−/−, MARCO−/−, and SR-A−/−-MARCO−/− DKO mouse strains on a C57BL/6J background were provided by the Sir William Dunn School of Pathology (Oxford, UK). The animals did not carry the RD8 mutation. Female animals between 7- to 10-weeks old were fed standard laboratory chow in an air conditioned room, and a 12 hour light/12 hour dark cycle was used. All animals were kept in specific pathogen-free conditions and procedures were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Laser-Induced CNV
Animals were anesthetized with intraperitoneal injections of ketamine (80 mg/kg) and xylazine (8 mg/kg). Topical 0.5% proparacaine was applied and pupils were dilated with a mixture of topical 0.5% tropicamide (Bausch & Lomb, Tampa, FL), 2.5% phenylephrine Alcon, Fort Worth, TX), and 1.0% atropine sulfate (Alcon). Four laser burns were made with an ND: YAG 532-nm laser (Alcon) using a 4.3-mm contact fundus laser lens (Ocular Instruments, Bellevue, WA) with a 50 μm, 100 mW of power, and 0.100 seconds of exposure time. Lesions with “bubble formation” were used for experiments since this ensures breakage of Bruch's membrane. The animals were then euthanized by CO2 exposure 6 hours, and 1, 3, 4, 7, and 14 days after laser injury.
Histopathology
Eyes were harvested and fixed in 10% formalin for at least 24 hours. Tissues were embedded in methacrylate and serially sectioned in the vertical pupillary-optic nerve plane. Eyes from each group were cut into six sections and stained with hematoxylin and eosin. If an ocular lesion was detected, another 6 to 12 sections would be made through the lesion. A histopathologic grading score was assigned and evaluated by a masked ocular pathologist (CCC) to each animal's eye from 0 to 4 based on the degree of inflammation, with 0 being no disease and 4 representing complete retinal destruction. The score of each eye was determined by comparing the severity of the retinal lesions to the mean percentage of the whole retina with degenerative lesions for each animal group. Slides were evaluated under a light microscope (×200).11
RNA Isolation and Microarray
Total RNA was extracted from the posterior cup of six eyes from six animals of wild-type, SR-A−/−, MARCO−/−, SR-A−/−-MARCO−/− DKO mouse groups before and 1 day after laser injury (RNeasy Kit; Qiagen, Valencia, CA). RNA from each animal group was pooled and microarray assay performed in single using Affymetrix mouse Gene ST 1.0 arrays (Affymetrix, Santa Clara, CA) and analyzed with GeneSpring GX software (Agilent Technologies, Inc., Santa Clara, CA). RNA samples with a 260/280 absorbance ratio between 1.8 and 2.0 measured by Nanodrop 2000 Spectrophotometer (Thermo Scientific, Wilmington, DE) were used. RNA quality was assessed by running the RNA samples on a denaturing agarose gel and the ratio of 28S rRNA:18S should be approximately 2:1. The microarray assay was repeated once. A 1.6-fold cutoff was used on the expression of chemokine/receptor in at least one paired comparison group.
Immunohistochemistry
Following enucleation, eyes were immediately fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS for 1 hour. Using a dissecting microscopy, the posterior eyecup was separated while also detaching the retina. The eyecups were washed three times with immunocytochemistry (ICC) buffer (0.5% BSA, 0.2% Tween 20, 0.05% sodium azide) for 15 minutes and once with PBS for 1 hour. For CNV volume staining, isolectin IB4 conjugated with Alexa Fluor 568 (1:100; Invitrogen-Molecular Probes, Eugene, OR) was prepared in ICC buffer. Isolectin IB4 has been used as an endothelial cell marker and allows for visualization of vascular tubes and CNV network.2 In addition, granulocyte receptor-1 (Gr-1) antibody conjugated with Alexa Fluor 488 (1:200; Biolegend, San Diego, CA) and 4′,6-diamidino-2-phenylindole (DAPI) were used for neutrophil quantification. Alternatively, F4/80 conjugated with Alexa Fluor 647 (1:200; Biolegend, San Diego, CA) was replaced for macrophage quantification. F4/80 has been established as one of the most specific cell surface markers for murine macrophages and is highly and constitutively expressed on most resident tissue macrophages.12–15 After overnight staining, eyecups were washed three times with ICC buffer for 15 minutes, and once with PBS for 1 hour. Flatmounts were then made with four radial incisions from the optic nerve and mounted in Fluorogel with Tris Buffer (Electron Microscopy Sciences, Hatfield, PA).
Confocal Microscopy
Images of whole mounts and lesions were visualized using the 40× objective of a scanning Zeiss LSM 700 Confocal Microscope (Carl Zeiss Microimaging, Inc., Thornwood, NY). After scanning whole mounts to examine immune cell distribution using the tile scan feature and Z-stack function of the Zen 2011 software (Carl Zeiss Microimaging GmbH, Jena, Germany), it was determined that the majority of immune cells were concentrated around a 5 × 5 tile scan area. Individual lesions were imaged using a 5 × 5 tile scan area with Z-stack settings of 0.5-μm sections and a number of sections that captured all immune cells, depending on each lesion. For three-dimensional (3D) reconstructions of each lesion, maximal intensity projection images for both whole mounts and individual lesions were generated and stitched. To obtain both immune cell quantitation and CNV volume, multiple z-series images were collected at 512 × 512 pixel resolution at a depth of 8 bits per channel. Voxel dimensions were 0.621 μm × 0.621 μm × 0.62 μm for the x, y, and z-axis.
Immune Cell Quantitation and Lesion Evaluation
Neutrophils and macrophages were quantified using the Zen 2011 software's region of interest (ROI) and number tools. Immune cells were quantified using maximal intensity projection images under a 5 × 5 tile scan area. An average of 10 eyes from each animal group was used at each time point. For CNV quantitation, confocal microscope z-series collected as TIFF images were analyzed with the image-analysis software, Volocity (Improvision, Inc., Lexington, MA). The image sequence was generated to build a 3D reconstruction of the CNV complex with the 3D opacity tool. CNV complexes were identified using the red channel (Isolectin IB4) and volumes measured in cubic micrometers at 14 days after laser injury. An average of 12 eyes was used from each animal group.
Flow Cytometry
Ten eyes from each wild-type, SR-A−/−, MARCO−/−, and DKO animal group were collectively cut in 10% complete medium containing 1 mg/mL collagenase D and 1 vial of DNase (0.9 mg/vial). The posterior eyecups were then incubated for 2 hours at 37°C. Gr-1 conjugated with Alexa Fluor 488 (Biolegend), was used to label neutrophils and F4/80 conjugated with Alexa Fluor 647 (Biolegend) to label macrophages. Digested tissue was collected and washed twice using complete medium. Four spleens were isolated from each animal group and used as a reference to gate immune cells of the eye. Data were acquired using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Inc., San Jose, CA).
Statistical Analysis
For immune cell quantification and CNV lesion evaluation, one-way ANOVA and Tukey's multiple comparisons tests were performed to test for significance between animal groups at selected time points using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA). Error bars represent SEM.
Results
The Eyes From Knockout Mice Demonstrate Similar Morphology as Compared With Wild-Type Eyes
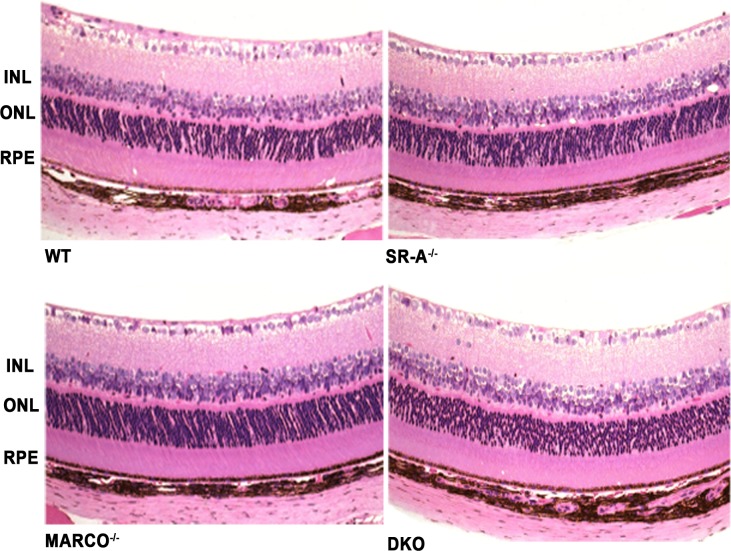
Histopathologic evaluation of the retina from wild-type, SR-A−/−, MARCO−/−, and SR-A−/−-MARCO−/− DKO mice was performed to compare scavenger receptor knockout and wild-type eyes. Before laser, scavenger receptor knockout eyes had a healthy retinal morphology identical to wild-type eyes (Fig. 1). Immune cell infiltration, retinal lesions, and retinal degeneration were not detected in all eyes of the four groups.
Figure 1.
The eyes from untreated knockout mice demonstrate similar morphology as wild-type eyes. Photomicrographs of the wild-type, SR-A−/−, MARCO−/−, and DKO retina before laser (n = 1 eye/group) show similar retinal morphology (hematoxylin and eosin, original magnifications: ×200).
Differential Expression of Chemokine and Chemokine Receptors Between Eyes From Wild-Type and Knockout Mice
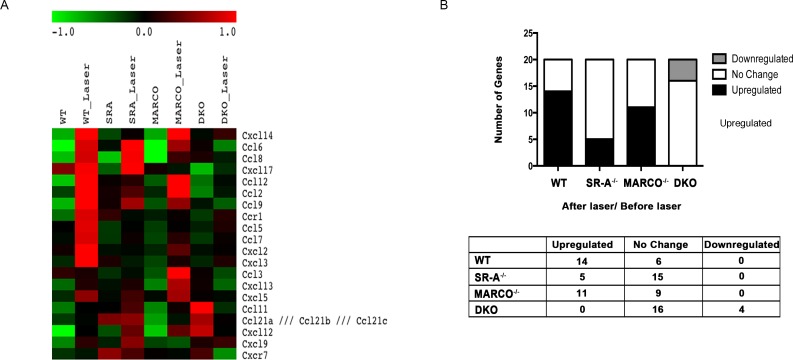
To study the gene expression pattern of wild type, SRA−/−, MARCO−/−, and DKO, we performed microarray analysis and identified 20 chemokine/receptor genes, up or downregulated with a 1.6-fold difference in at least one paired comparison among the different experimental groups (Table). Before laser injury, knockout eyes had similar expression of chemokines/receptors compared with wild-type eyes. After laser injury, wild-type eyes show higher chemokine/receptor expression compared with knockout eyes, particularly when compared with DKO eyes (Fig. 2A).
Table.
The Fold Change of 20 Chemokine/Receptor Genes After Laser Injury Compared With Untreated Eyes
|
Gene Symbol |
Gene Title |
WT_ Laser |
SR-A_ Laser |
MARCO_ Laser |
DKO_ Laser |
| Cxcl14 | Chemokine (C-X-C motif) ligand 14 | 4.9 | 1.2 | 5.3 | 1.2 |
| Ccl12 | Chemokine (C-C motif) ligand 12 | 4.1 | 1.1 | 3.1 | 1.3 |
| Ccl6 | Chemokine (C-C motif) ligand 6 | 4.0 | 2.7 | 3.1 | −1.5 |
| Ccl9 | Chemokine (C-C motif) ligand 9 | 3.5 | 1.5 | 1.9 | −1.2 |
| Cxcl3 | Chemokine (C-X-C motif) ligand 3 | 3.1 | −1.2 | 1.3 | 1.2 |
| Ccl8 | Chemokine (C-C motif) ligand 8 | 3.0 | 3.4 | 2.7 | −1.2 |
| Ccl2 | chemokine (C-C motif) ligand 2 | 2.5 | 1.2 | 2.4 | 1.3 |
| Ccr1 | Chemokine (C-C motif) receptor 1 | 2.5 | −1.2 | 1.1 | 1.3 |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | 2.1 | 1.0 | 1.4 | 1.0 |
| Cxcl12 | Chemokine (C-X-C motif) ligand 12 | 2.1 | 1.6 | 2.2 | -1.7 |
| Ccl7 | Chemokine (C-C motif) ligand 7 | 1.9 | 1.2 | 1.3 | 1.2 |
| Ccl5 | Chemokine (C-C motif) ligand 5 | 1.8 | 1.2 | 1.2 | 1.2 |
| Cxcl5 | Chemokine (C-X-C motif) ligand 5 | 1.6 | 1.2 | 1.6 | −1.1 |
| Cxcl9 | Chemokine (C-X-C motif) ligand 9 | 1.6 | 1.6 | −1.0 | 1.1 |
| Cxcl13 | Chemokine (C-X-C motif) ligand 13 | 1.5 | 1.1 | 2.3 | −1.4 |
| Cxcl17 | Chemokine (C-X-C motif) ligand 17 | 1.4 | 2.8 | −1.1 | 1.5 |
| Ccl11 | Chemokine (C-C motif) ligand 11 | 1.4 | 1.2 | 1.5 | -2.4 |
| Ccl21a,b,c | Chemokine (C-C motif) ligand 21A, B, C | 1.2 | 1.0 | 1.5 | -1.6 |
| Cxcr7 | Chemokine (C-X-C motif) receptor 7 | 1.1 | −1.2 | −1.0 | -1.8 |
| Ccl3 | Chemokine (C-C motif) ligand 3 | −1.1 | 1.1 | 2.8 | −1.3 |
Fold differences of 1.6 (our cutoff) and over are bolded.
Figure 2.
Reduced expression of chemokine and chemokine receptors in knockout eyes compared with the wild type. (A) Heat map of 20 chemokine/receptor genes differentially expressed between wild type, SR-A−/−, MARCO−/−, and DKO before, and after laser injury. Before laser injury, the expression profile is similar between wild-type and knockout eyes. After laser injury, wild-type laser had higher chemokine/receptor gene expression compared with knockout eyes. Red indicates an upregulation, black indicates no change, and green indicates downregulation. (B) When examining the chemokine/receptor expression after laser to before laser in the animal groups, the wild-type ratio had the highest number of upregulated genes and the least number of unchanged genes compared with knockout eyes.
The number of upregulated, unchanged, and downregulated genes in the ratio of wild-type, SR-A−/−, MARCO−/−, and DKO eyes after laser to before laser is shown in Figure 2B. Fourteen (70%), 6 (30%), and 0 genes were upregulated, unchanged, and downregulated in wild-type mice compared with 5 (25%), 15 (75%), and 0 in SR-A−/−, 11 (55%), 9 (45%), and 0 in MARCO−/−, and 0, 16 (80%), and 4 (20%) in SR-A−/−-MARCO−/− DKO mice, respectively. The Table lists fold differences of chemokine/receptor genes after laser compared with before laser treatment.
Knockout Eyes Have Reduced Neutrophil Recruitment
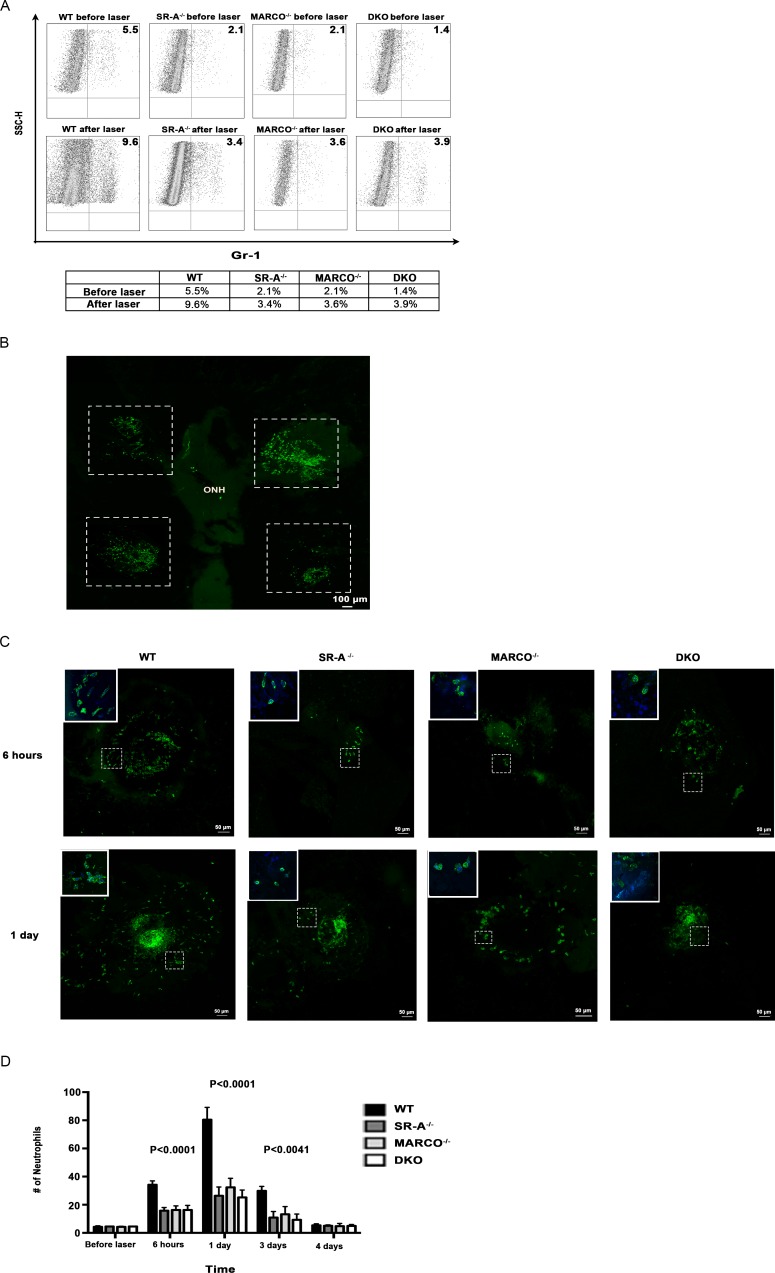
Neutrophils have been hypothesized to promote CNV through secretion of various angiogenic factors.16 Since we had observed that chemokine/receptor expression was reduced in knockout eyes, we examined neutrophil distribution across the groups by flow cytometry. Neutrophil recruitment was impaired in all scavenger receptor knockout eyes compared with wild-type eyes before laser injury (Fig. 3A). Neutrophil distribution after laser increased across all animal eyes compared with before laser treatment. Prelaser, SR-A−/−, MARCO−/−, and DKO had 1.4% to 2.1% of Gr-1 positive neutrophils, compared with 5.5% in the wild-type eyes. After laser injury, Gr-1 positive neutrophils increased to 9.6% in wild-type eyes, whereas scavenger receptor knockout eyes had only a slight increase in Gr-1 positive neutrophils, from 3.4% to 3.9% (Fig. 3A). Next, we examined immune cell recruitment through confocal microscopy. We firstly processed whole mounts of posterior eyecups 1-day post laser injury and observed that the majority of neutrophils were concentrated within and around lesions that fit a 5 × 5 tile scan area, as represented by the stippled white borders in Figure 3BA, representation of neutrophil distribution around individual laser lesions in a 5 × 5 tile scan area is shown in Figure 3C at 6 hours and 1 day post laser injury. The inserts show higher magnification of neutrophils labeled with Gr-1 (green) and DAPI (blue), which correspond to the lesion areas with white stippled borders. Results for confocal microscopy were consistent with neutrophil quantification obtained through flow cytometry. Neutrophil recruitment increased post laser injury for wild-type, SR-A−/−, MARCO−/−, and DKO eyes compared with baseline. Before laser, there was no difference in neutrophil distribution between scavenger receptor knockout and wild-type eyes as detected by Gr-1 staining. However, after laser injury, neutrophil recruitment was significantly increased in wild-type eyes compared with scavenger receptor knockout eyes, and this difference was most dramatic at 1 day after injury, a time point when neutrophil distribution peaked in all animal groups (P < 0.0001). This difference disappeared by 4-days post laser injury (Fig. 3D). Overall, the number of neutrophils in the scavenger receptor knockout eyes remained more stable over time compared with the wild-type eyes.
Figure 3.
Neutrophil distribution is reduced in knock out eyes. (A) Neutrophil recruitment is marginally impaired in knockout eyes before laser and becomes profoundly impaired after 1 day of laser injury, as detected by flow cytometry (n = 10 eyes/group). (B) A maximal intensity projection image of a wild-type whole mount 1-day post laser injury shows that the majority of neutrophils labeled with Gr-1 (green) are concentrated within and around the laser lesions and fit the 5 × 5 tile scan area used to image individual lesions, as represented by the stippled white borders. The periphery and optic nerve head (ONH) have a reduced amount of neutrophils. (C) Representations of laser lesions with neutrophil distribution labeled with Gr-1 (green) 6 hours and 1-day post laser injury show that knockout eyes have less neutrophil recruitment compared with wild-type eyes. Maximal intensity projections are used to show 3D reconstructions of lesions. The inserts show higher magnification of neutrophils labeled with Gr-1 (green) and DAPI (blue), which corresponds to the lesion areas with white stippled borders. (D) Knockout eyes have reduced neutrophil recruitment around laser lesions compared with wild-type eyes, most marked 1 day after laser injury when observed by confocal microscopy (P < 0.0001). While neutrophil recruitment in wild-type eyes changed substantially over 4 days, neutrophil distribution remained comparably stable over time in scavenger receptor knockout eyes (n = 10 eyes/group). One-way ANOVA and Tukey's multiple comparisons tests were performed to test for significance between animal groups at selected time points. Error bars, SEM.
Macrophage Recruitment Is Impaired in Scavenger Receptor Knockout Eyes Compared With the Wild Type
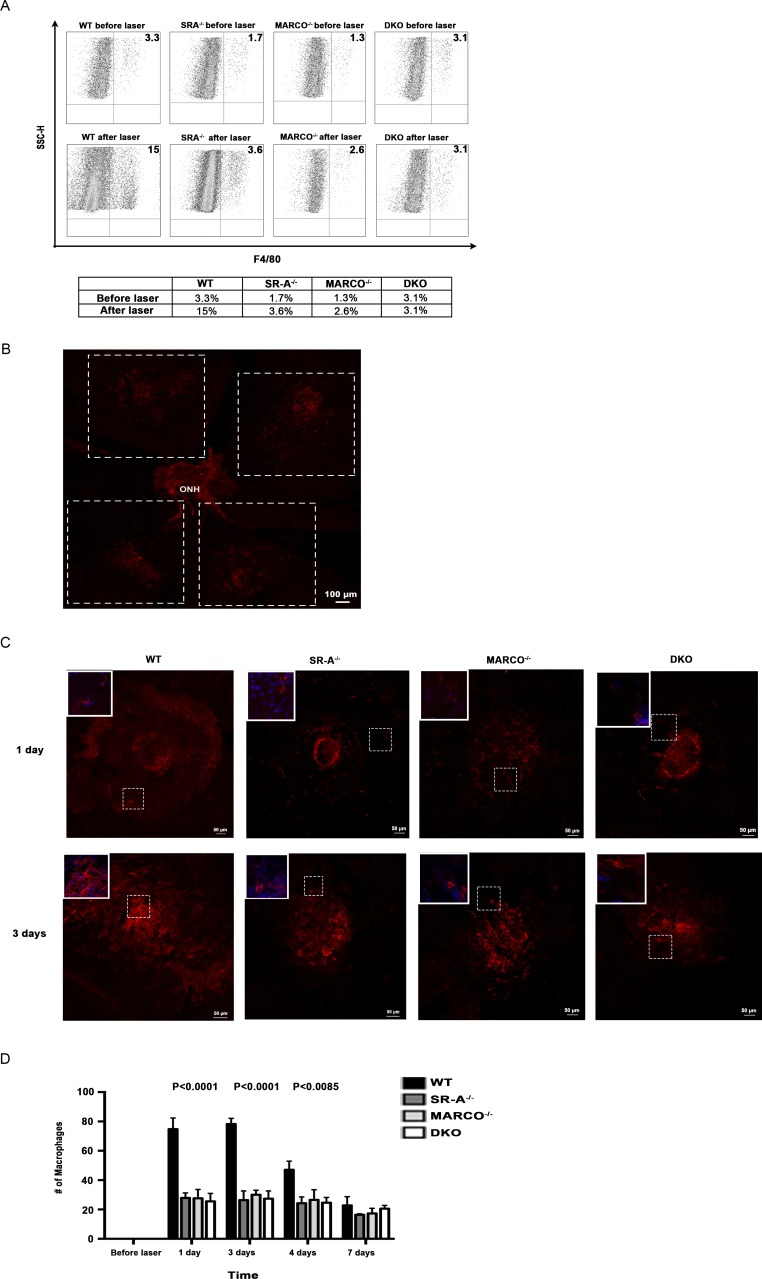
Macrophages have been postulated to contribute to the CNV and inflammation seen in AMD.17 We examined the distribution of macrophages across the groups before and after laser treatment by cytometry. F4/80 positive macrophages increased (from 3.3%–15%) in wild-type eyes, while remaining constant in knockout eyes post laser injury, compared with prelaser numbers (Fig. 4A). Confocal microscopy was performed to validate these findings. Figure 4B shows a wild-type whole mount 3-days post laser injury demonstrating that the majority of macrophages are concentrated within and around lesions that fit a 5 × 5 tile scan area, as represented by the stippled white borders. A representation of macrophage distribution around individual laser lesions in a 5 × 5 tile scan area is shown in Figure 4C at 1- and 3-days post laser injury. The inserts show higher magnification of macrophages labeled with F4/80 (red) and DAPI (blue), which correspond to the lesion areas with white stippled borders. Macrophage recruitment increased after laser injury across the groups in comparison to before laser injury. Before laser, macrophages were absent as determined by F4/80 staining in the eyes of all groups. Consistent with the flow cytometry findings, SR-A−/−, MARCO−/−, and DKO had statistically significantly lower numbers of macrophages at 1-, 3-, and 4-days post laser injury, compared with the wild-type eyes (P < 0.0001). Macrophage recruitment peaked around lesions in all animal groups at 3-days post laser and then declined afterwards, until day 7 (Fig. 4D).
Figure 4.
Macrophage distribution is reduced in knockout eyes. (A) Macrophage recruitment did not differ significantly between wild-type and scavenger receptor knockout eyes before laser injury, but the distribution is impaired in knockout eyes after 1 day of laser injury, as detected by flow cytometry (n = 10 eyes/group). (B) A maximal intensity projection image of a wild-type whole mount 3-days post laser injury shows that the majority of macrophages labeled with F4/80 (red) are concentrated within and around the lesions and fit the 5 × 5 tile scan area used to image individual lesions, as represented by the stippled white borders. The periphery and ONH have a reduced amount of macrophages. (C) Representation of laser lesions in a 5 × 5 tile scan area with macrophage distribution labeled with F4/80 (red) 1- and 3-days post laser injury shows that knockout eyes have reduced macrophage recruitment compared with wild-type eyes. Maximal intensity projections were used to depict 3D reconstructions of lesions. The inserts show higher magnification of macrophages labeled with F4/80 (red) and DAPI (blue), which corresponds to the lesion areas with white stippled borders. (D) Knockout eyes have reduced macrophage recruitment compared with wild-type eyes, most dramatically at 3 days after laser injury when observed by confocal microscopy (P < 0.0001). The difference between wild-type and scavenger receptor knockout eyes' macrophage distribution decreases over time (n = 12 eyes/group). One-way ANOVA and Tukey's multiple comparisons tests were performed to test for significance between animal groups at selected time points. Error bars, SEM.
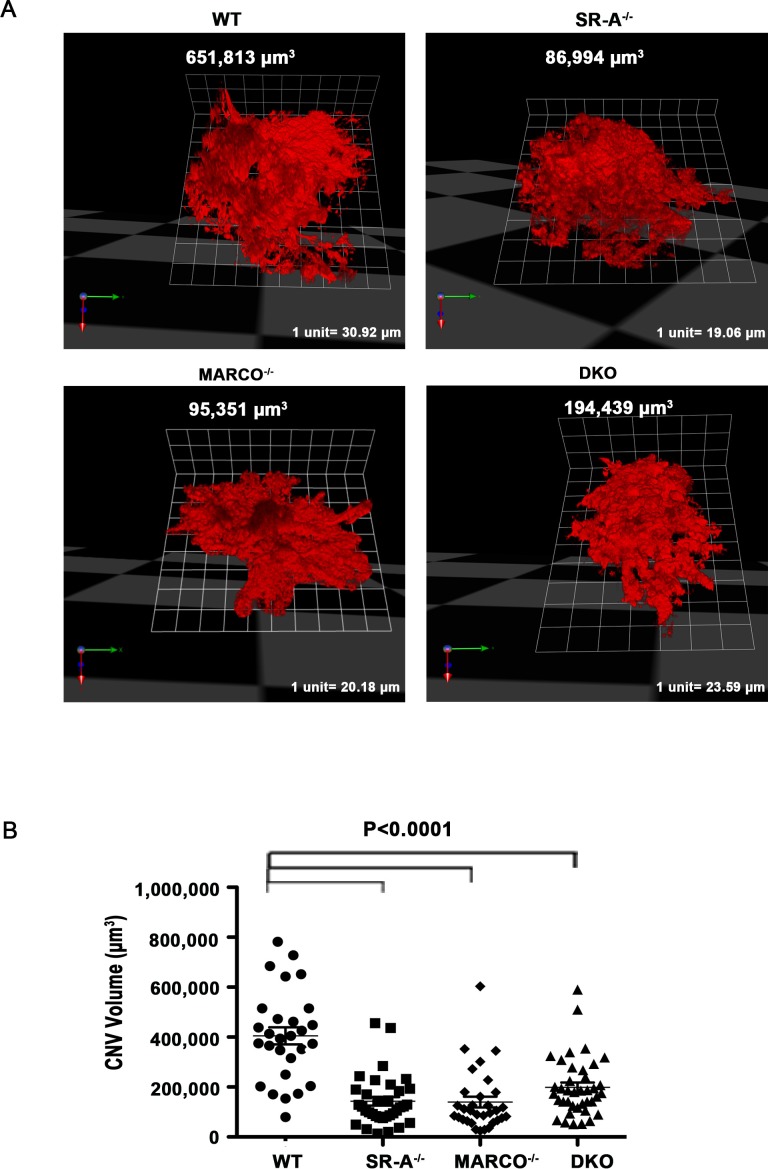
CNV Volumes in the Scavenger Receptor Knockout Eyes are Significantly Reduced Compared With Wild-Type Eyes
We examined the laser-induced CNV complexes through 3D reconstructions of lesions with Volocity in the wild-type, SR-A−/−, MARCO−/−, and DKO eyes 14-days post laser injury. A representative lesion from each animal group is shown in Figure 5A. As shown in Figure 5B, knockout eyes had statistically significantly smaller lesion volumes compared with wild-type eyes, 14 days after laser injury (P < 0.0001).
Figure 5.
Choroidal neovascularization volumes are reduced in knockout eyes after laser injury. (A) Typical examples of CNV lesions labeled with Isolectin IB4 (red) are shown for wild-type, SR-A−/−, MARCO−/−, and DKO eyes through the 3D opacity feature with Volocity. (B) Scavenger receptor knockout eyes have significantly smaller lesion volumes compared with wild-type eyes 14 days after laser injury (P < 0.0001, n = 12 eyes/group). One-way ANOVA and Tukey's multiple comparison tests were performed to test for significance between selected animal groups. Error bars, SEM.
Discussion
Our study demonstrated that class A scavenger receptor deficient eyes after laser injury exhibited defective chemokine and receptor expression compared with wild-type eyes (Fig. 2), which may account for the reduced recruitment of neutrophils and macrophages in knockout eyes (Figs. 3, 4). The roles SR-A and MARCO play in inflammation are complex and may involve multiple processes, including cell trafficking, chemokine secretion by both resident and infiltrated macrophages, cytoskeletal rearrangement, and damaged tissue remodeling.18 Consistent with our findings, Kuchibhotla et al. showed that CD36 and SR-A knockout mice displayed a decreased pro-inflammatory cytokine and chemokine expression profile, and reduced migration in experimental atherosclerosis.19 Along this line, a number of other studies also demonstrated that the absence of SR-A was protective in murine atherosclerosis models.20–23 Our current finding that scavenger receptors are pro-inflammatory in an ocular inflammation model is consistent with most previous reports; although, there are other studies that suggest an anti-inflammatory and antiatherogenic role for scavenger receptors.24–26
We demonstrated reduced neutrophil recruitment to the lesion areas in knockout eyes. Inhibiting SR-A leads to reduced interaction between macrophages and other immune cells including B cells and T cells suggesting that a scavenger receptor deficiency in macrophages may influence their local immune environment.27 Macrophages and neutrophils collaborate with each other in response to acute injury28; for instance, neutrophils produce oxidized phospholipids and can assist with the recognition and recruitment of macrophages through scavenger receptors.29 In atherosclerosis, recruited macrophages bearing scavenger receptors recognize and bind LDL from apoptotic cell populations including neutrophils, leading to the formation of foam cells.29,30 Cotena et al. reported that neutrophil recruitment was enhanced in sterile peritonitis and prolongs the inflammatory response in SR-A deficient mice.31 Other studies have reported that neutrophil recruitment was not affected in SR-A−/− mice.32,33 Therefore, the differences found in the inflammatory response with scavenger receptor deficiency may depend on the local inflammatory milieu and the type of inflammatory stimulus or injury.
We observed decreased laser induced CNV formation (Fig. 5) in scavenger receptor deficient animals. Our data suggest that the absence of scavenger receptors is protective in response to acute injury, possibly due to a reduced pro-inflammatory intraocular environment in knockout eyes. Studies investigating the role of immune cells in CNV are contradictory. The presence of inflammatory cells correlates with larger CNV lesions in AMD.6,34,35 Zhou et al. also demonstrated that in animals, neutrophil depletion is associated with decreased CNV and reduced VEGF expression.16 Furthermore, neutrophil depleted Ccr2 knockout mice after Gr-1 Ab treatment have a significantly decreased CNV compared with wild-type mice.36 Some reports show that macrophages provide a protective role by enhancing wound healing and controlling vessel growth during development.37,38 Furthermore, Apte et al. showed that Il10−/− mice have decreased CNV volume with increased macrophage infiltration compared with wild-type animals. This suggests that macrophage influx could play a protective role and inhibit CNV.39 Alternatively, other findings reported that macrophages can promote CNV development. Xie et al. reported that suppression of macrophage infiltration through Ccr-2 inhibited CNV formation, demonstrating that macrophages contribute to CNV development.40 Macrophage depletion was also found to decrease the severity of CNV by 50% in a mouse model of CNV.41
We attribute the pro-angiogenic effect of class A scavenger receptors to their pro-inflammatory role during inflammation. Similar to class A type receptors, CD36, a class B scavenger receptor, can also recognize and internalize oxidized forms of LDL, triggering pro-inflammatory reactions. However, CD36 functions as a negative regulator during angiogenesis. Apart from its pro-inflammatory function, CD36 is also known as a critical receptor for thrombospondin-1 (TSP-1). The CD36/ TSP-1 signal was thought to be essential for the inhibition of neovascularization. Febbraio et al. suggested TSP-1 can recruit nonreceptor protein tyrosine kinase fyn to CD36 membrane complex with activation of the mitogen-activated protein kinase (MAPKs) p38 and c-Jun N-terminal kinase (JNK), and caspase-3 to generate antiangiogenic signals that lead to apoptosis.20 Downstream induction of proapoptotic effector cell expression includes Fas ligand and TNF-α.42 In the eye, CD36 was reported to play a major role in the inhibition and regression of CNV.43 Our observation is the first to show that class A scavenger receptors are pro-angiogenic and establish their role in an ocular CNV model. The potential signal pathways involved in this process are unclear and warrant further investigation.
We observed that wild-type eyes had higher global chemokine/receptor expression compared with knockout eyes. From the chemokine/receptor genes upregulated in wild-type eyes, Cxcl14, Ccl12, Ccl6, Cxcl3, Ccl8, Ccl2, Ccr1, Cxcl12, Ccl7, Ccl5, Cxcl5, and Cxcl9 have been shown to be involved in monocyte/macrophage recruitment.44–59 Cxcl3, Ccr1, Cxcl2, Cxcl12, Ccl7, Ccl5, Cxcl5, and Cxcl9 have been reported to be chemotactic for neutrophils.59–67 Furthermore, Ccl12, Ccl2, Cxcl3, Ccr1, Cxcl12, Cxcl2, Ccl7, Ccl5, and Cxcl5 have been shown to play a role in angiogenesis.50,51,68–78 Therefore, chemokines/receptors upregulated in wild-type eyes may contribute to monocyte/macrophage and neutrophil recruitment and angiogenesis found in CNV lesions.
Recent genetic studies have highlighted the role of the complement system in the pathogenesis of AMD. Single nucleotide variants of complement factor H (CFH), complement component 3 (C3), complement factor B (BF), and complement component 2 (C2) are reported to be associated with AMD.79–83 Individuals with the risk allele “C” in a coding variant Y402H (rs1061170, T > C) of CFH exhibited an increased risk of AMD with odds ratios ranging from 2.4 to 4.6 for a single copy and 3.3 to 7.4 for two copies of the variant allele.3,5,84 Additionally, the evidence of histopathology and systemic complement activation also demonstrated the involvement of the complement system in AMD.85–89 There are very few publications discussing the relationship between scavenger receptors and complement components. Goh et al. reported that SR-A modulates macrophage activation through binding iC3b and suggested a novel property of SR-A complement receptor interaction able to elicit cell signaling.90 Fujita also reported that lectin-like oxidized LDL receptor 1 (LOX-1), a functional scavenger receptor, bound C-reactive protein (CRP), and was involved in CRP-induced complement activation.91 Based on the above publications, it may be reasonable to propose that the deficiencies of scavenger receptors may lead to reduced complement activation, and contribute to the attenuated inflammatory ocular environment observed in knockout eyes. More studies are needed to reveal how scavenger receptors interact with complement.
While our study aimed to provide insight into the role of scavenger receptors in AMD pathogenesis, limitations do exist with this acute injury model. The onset of inflammatory cell recruitment that occurs in AMD patients is insidious and does not occur abruptly after onset of macular disease. Mouse eyes do not have a macula, and the majority of the mouse RPE is binucleated, whereas HRPE is overwhelmingly mononucleated, suggesting important differences in the physiologic processes between human and mouse.92–94 Despite the limitations, our study shows that class A scavenger receptors are required for the recruitment of immune cells and play a role in CNV development and progression. Scavenger receptor subtypes, SR-A and MARCO, may have a redundant function in this regard and this may be why we do not observe any statistically significant differences between the SR-A−/−, MARCO−/−, and DKO groups. However, microarray results (Fig. 2) demonstrated that chemokine gene expression profile was not identical in SR-A−/− and MARCO−/− eyes suggesting different pro-inflammatory mechanisms may be involved.
In conclusion, our present study shows that the deficiency of scavenger receptors impairs immune cell recruitment, which correlates with decreased CNV volume. Our results suggest that class A scavenger receptors may contribute to CNV formation and inflammation seen in AMD and this line of investigation may help us understand AMD pathogenesis.
Acknowledgments
The authors thank Guangpu Shi and Igal Gery from the Laboratory of Experimental Immunology and De Fen Shen from the Laboratory of Immunopathology for providing us with material and advice.
Supported by grants from the Intramural Research Program of National Institutes of Health, National Eye Institute.
Disclosure: S. Jawad, None; B. Liu, None; Z. Li, None; R. Katamay, None; M. Campos, None; L. Wei, None; H.N. Sen, None; D. Ling, None; F. Martinez Estrada, None; J. Amaral, None; C.-C. Chan, None; R. Fariss, None; S. Gordon, None; R.B. Nussenblatt, None
References
- 1. Ferris FL III, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984; 102: 1640–1642 [DOI] [PubMed] [Google Scholar]
- 2. Campos M, Amaral J, Becerra SP, Fariss RN. A novel imaging technique for experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2006; 47: 5163–5170 [DOI] [PubMed] [Google Scholar]
- 3. Edwards AO, Ritter R III, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005; 308: 421–424 [DOI] [PubMed] [Google Scholar]
- 4. Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005; 102: 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308: 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grossniklaus HE, Miskala PH, Green WR, et al. Histopathologic and ultrastructural features of surgically excised subfoveal choroidal neovascular lesions: submacular surgery trials report no. 7. Arch Ophthalmol. 2005; 123: 914–921 [DOI] [PubMed] [Google Scholar]
- 7. Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003; 9: 1390–1397 [DOI] [PubMed] [Google Scholar]
- 8. Cherepanoff S, McMenamin P, Gillies MC, Kettle E, Sarks SH. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br J Ophthalmol. 2010; 94: 918–925 [DOI] [PubMed] [Google Scholar]
- 9. Ohnishi K, Komohara Y, Fujiwara Y, et al. Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem Biophys Res Comm. 2011; 411: 516–522 [DOI] [PubMed] [Google Scholar]
- 10. Areschoug T, Gordon S. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol. 2009; 11: 1160–1169 [DOI] [PubMed] [Google Scholar]
- 11. Tuo J, Ross RJ, Herzlich AA, et al. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009; 175: 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin HH, Faunce DE, Stacey M, et al. The macrophage F4/80 receptor is required for the induction of antigen-specific efferent regulatory T cells in peripheral tolerance. J Exp Med. 2005; 201: 1615–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981; 11: 805–815 [DOI] [PubMed] [Google Scholar]
- 14. Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991; 112: 517–526 [DOI] [PubMed] [Google Scholar]
- 15. McKnight AJ, Gordon S. The EGF-TM7 family: unusual structures at the leukocyte surface. J Leukoc Biol. 1998; 63: 271–280 [DOI] [PubMed] [Google Scholar]
- 16. Zhou J, Pham L, Zhang N, et al. Neutrophils promote experimental choroidal neovascularization. Mol Vis. 2005; 11: 414–424 [PubMed] [Google Scholar]
- 17. Patel M, Chan CC. Immunopathological aspects of age-related macular degeneration. Semin Immunopathol. 2008; 30: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mukhopadhyay S, Varin A, Chen Y, Liu B, Tryggvason K, Gordon S. SR-A/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell surface limits TLR4 response to pathogens. Blood. 2011; 117: 1319–1328 [DOI] [PubMed] [Google Scholar]
- 19. Kuchibhotla S, Vanegas D, Kennedy DJ, et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc Res. 2008; 78: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001; 108: 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem. 2002; 277: 49982–49988 [DOI] [PubMed] [Google Scholar]
- 22. de Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: a multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000; 20: 290–297 [DOI] [PubMed] [Google Scholar]
- 23. Segers FM, Yu H, Molenaar TJ, et al. Design and validation of a specific scavenger receptor class AI binding peptide for targeting the inflammatory atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2012; 32: 971–978 [DOI] [PubMed] [Google Scholar]
- 24. Ohnishi K, Komohara Y, Fujiwara Y, et al. Suppression of TLR4-mediated inflammatory response by macrophage class A scavenger receptor (CD204). Biochem Biophys Res Comm. 2011; 411: 516–522 [DOI] [PubMed] [Google Scholar]
- 25. Martin-Fuentes P, Civeira F, Recalde D, et al. Individual variation of scavenger receptor expression in human macrophages with oxidized low-density lipoprotein is associated with a differential inflammatory response. J Immunol. 2007; 179: 3242–3248 [DOI] [PubMed] [Google Scholar]
- 26. Moore KJ, Kunjathoor VV, Koehn SL, et al. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J Clin Invest. 2005; 115: 2192–2201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raycroft MT, Harvey BP, Bruck MJ, Mamula MJ. Inhibition of antigen trafficking through scavenger receptor A. J Biol Chem. 2012; 287: 5310–5316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Medeiros AI, Serezani CH, Lee SP, Peters-Golden M. Efferocytosis impairs pulmonary macrophage and lung antibacterial function via PGE2/EP2 signaling. J Exp Med. 2009; 206: 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greenberg ME, Sun M, Zhang R, Febbraio M, Silverstein R, Hazen SL. Oxidized phosphatidylserine-CD36 interactions play an essential role in macrophage-dependent phagocytosis of apoptotic cells. J Exp Med. 2006; 203: 2613–2625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009; 50 (suppl); S282–S286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokine production and the early infiltration of neutrophils in sterile peritonitis. J Immunol. 2004; 173: 6427–6432 [DOI] [PubMed] [Google Scholar]
- 32. Platt N, Suzuki H, Kurihara Y, Kodama T, Gordon S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc Natl Acad Sci U S A. 1996; 93: 12456–12460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomas CA, Li Y, Kodama T, Suzuki H, Silverstein SC, El Khoury J. Protection from lethal gram-positive infection by macrophage scavenger receptor-dependent phagocytosis. J Exp Med. 2000; 191: 147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bressler NM, Silva JC, Bressler SB, Fine SL, Green WR. Clinicopathologic correlation of drusen and retinal pigment epithelial abnormalities in age-related macular degeneration. Retina. 1994; 14: 130–142 [PubMed] [Google Scholar]
- 35. Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004; 122: 1013–1018 [DOI] [PubMed] [Google Scholar]
- 36. Tsutsumi-Miyahara C, Sonoda KH, Egashira K, et al. The relative contributions of each subset of ocular infiltrated cells in experimental choroidal neovascularisation. Br J Ophthalmol. 2004; 88: 1217–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004; 9: 283–289 [DOI] [PubMed] [Google Scholar]
- 38. Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am J Surg. 2004; 187: 11S–16S [DOI] [PubMed] [Google Scholar]
- 39. Apte RS, Richter J, Herndon J, Ferguson TA. Macrophages inhibit neovascularization in a murine model of age-related macular degeneration. PLoS Med. 2006; 3: e310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xie P, Kamei M, Suzuki M, et al. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS One. 2011; 6: e28933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, Cousins SW. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44: 3586–3592 [DOI] [PubMed] [Google Scholar]
- 42. Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009; 2: re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mwaikambo BR, Sennlaub F, Ong H, Chemtob S, Hardy P. Activation of CD36 inhibits and induces regression of inflammatory corneal neovascularization. Invest Ophthalmol Vis Sci. 2006; 47: 4356–4364 [DOI] [PubMed] [Google Scholar]
- 44. Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med. 2001; 194: 855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jia GQ, Gonzalo JA, Lloyd C, et al. Distinct expression and function of the novel mouse chemokine monocyte chemotactic protein-5 in lung allergic inflammation. J Exp Med. 1996; 184: 1939–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Asensio VC, Lassmann S, Pagenstecher A, Steffensen SC, Henriksen SJ, Campbell IL. C10 is a novel chemokine expressed in experimental inflammatory demyelinating disorders that promotes recruitment of macrophages to the central nervous system. Am J Pathol. 1999; 154: 1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berger MS, Taub DD, Orlofsky A, et al. The chemokine C10: immunological and functional analysis of the sequence encoded by the novel second exon. Cytokine. 1996; 8: 439–447 [DOI] [PubMed] [Google Scholar]
- 48. Smith DF, Galkina E, Ley K, Huo Y. GRO family chemokines are specialized for monocyte arrest from flow. Am J Physiol Heart Circ Physiol. 2005; 289: H1976–1984 [DOI] [PubMed] [Google Scholar]
- 49. Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2, −7, −8, and −13 antagonists: potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008; 112: 3455–3464 [DOI] [PubMed] [Google Scholar]
- 50. Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003; 9: 1390–1397 [DOI] [PubMed] [Google Scholar]
- 51. Luhmann UF, Robbie S, Munro PM, et al. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009; 50: 5934–5943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Grunin M, Burstyn-Cohen T, Hagbi-Levi S, Peled A, Chowers I. Chemokine receptor expression in peripheral blood monocytes from patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012; 53: 5292–5300 [DOI] [PubMed] [Google Scholar]
- 53. Tsou CL, Peters W, Si Y, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007; 117: 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jia T, Serbina NV, Brandl K, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008; 180: 6846–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder JM. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992; 176: 587–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. da Costa Martins P, van den Berk N, Ulfman LH, Koenderman L, Hordijk PL, Zwaginga JJ Platelet-monocyte complexes support monocyte adhesion to endothelium by enhancing secondary tethering and cluster formation. Arterioscler Thromb Vasc Biol. 2004; 24: 193–199 [DOI] [PubMed] [Google Scholar]
- 57. Balamayooran G, Batra S, Cai S, et al. Role of CXCL5 in leukocyte recruitment to the lungs during secondhand smoke exposure. Am J Respir Cell Mol Biol. 2012; 47: 104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Janatpour MJ, Hudak S, Sathe M, Sedgwick JD, McEvoy LM. Tumor necrosis factor-dependent segmental control of MIG expression by high endothelial venules in inflamed lymph nodes regulates monocyte recruitment. J Exp Med. 2001; 194: 1375–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Karin N. The multiple faces of CXCL12 (SDF-1alpha) in the regulation of immunity during health and disease. J Leukoc Biol. 2010; 88: 463–473 [DOI] [PubMed] [Google Scholar]
- 60. Reutershan J, Stockton R, Zarbock A, et al. Blocking p21-activated kinase reduces lipopolysaccharide-induced acute lung injury by preventing polymorphonuclear leukocyte infiltration. Am J Respir Crit Care Med. 2007; 175: 1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reichel CA, Khandoga A, Anders HJ, Schlondorff D, Luckow B, Krombach F. Chemokine receptors Ccr1, Ccr2, and Ccr5 mediate neutrophil migration to postischemic tissue. J Leukoc Biol. 2006; 79: 114–122 [DOI] [PubMed] [Google Scholar]
- 62. Wallace GR. John Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Progr Retin Eye Res. 2004; 23: 435–448 [DOI] [PubMed] [Google Scholar]
- 63. Struyf S, Gouwy M, Dillen C, Proost P, Opdenakker G, Van Damme J. Chemokines synergize in the recruitment of circulating neutrophils into inflamed tissue. EurJ Immunol. 2005; 35: 1583–1591 [DOI] [PubMed] [Google Scholar]
- 64. Di Stefano A, Caramori G, Gnemmi I, et al. Association of increased CCL5 and CXCL7 chemokine expression with neutrophil activation in severe stable COPD. Thorax. 2009; 64: 968–975 [DOI] [PubMed] [Google Scholar]
- 65. Persson T, Monsef N, Andersson P, et al. Expression of the neutrophil-activating CXC chemokine ENA-78/CXCL5 by human eosinophils. Clin Exp Allergy. 2003; 33: 531–537 [DOI] [PubMed] [Google Scholar]
- 66. Vilela MC, Lima GK, Rodrigues DH, et al. Absence of CCR5 increases neutrophil recruitment in severe herpetic encephalitis. BMC Neurosci. 2013; 14: 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xu LL, McVicar DW, Ben-Baruch A, et al. Monocyte chemotactic protein-3 (MCP3) interacts with multiple leukocyte receptors: binding and signaling of MCP3 through shared as well as unique receptors on monocytes and neutrophils. Eur J Immunol. 1995; 25: 2612–2617 [DOI] [PubMed] [Google Scholar]
- 68. Tsui P, Das A, Whitaker B, et al. Generation, characterization and biological activity of CCL2 (MCP-1/JE) and CCL12 (MCP-5) specific antibodies. Hum Antibodies. 2007; 16: 117–125 [PubMed] [Google Scholar]
- 69. Pearce WJ. Multifunctional angiogenic factors: add GnRH to the list. Focus on “Gonadotropin-releasing hormone-regulated chemokine expression in human placentation.” Am J Physiol Cell Physiol. 2009; 297: C4–5 [DOI] [PubMed] [Google Scholar]
- 70. Rodero MP, Auvynet C, Poupel L, Combadiere B, Combadiere C. Control of both myeloid cell infiltration and angiogenesis by CCR1 promotes liver cancer metastasis development in mice. Neoplasia. 2013; 15: 641–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Strieter RM, Kunkel SL, Elner VM, et al. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol. 1992; 141: 1279–1284 [PMC free article] [PubMed] [Google Scholar]
- 72. Lu P, Nakamoto Y, Nemoto-Sasaki Y, et al. Potential interaction between CCR1 and its ligand, CCL3, induced by endogenously produced interleukin-1 in human hepatomas. Am J Pathol. 2003; 162: 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Yang X, Lu P, Fujii C, et al. Essential contribution of a chemokine, CCL3, and its receptor, CCR1, to hepatocellular carcinoma progression. Int J Cancer. 2006; 118: 1869–1876 [DOI] [PubMed] [Google Scholar]
- 74. Li M, Ransohoff RM. The roles of chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin Cancer Biol. 2009; 19: 111–115 [DOI] [PubMed] [Google Scholar]
- 75. Esiri MM, Morris CS. Immunocytochemical study of macrophages and microglial cells and extracellular matrix components in human CNS disease. 2. Non-neoplastic diseases. J Neurol Sci. 1991; 101: 59–72 [DOI] [PubMed] [Google Scholar]
- 76. Bousquenaud M, Schwartz C, Leonard F, Rolland-Turner M, Wagner D, Devaux Y. Monocyte chemotactic protein 3 is a homing factor for circulating angiogenic cells. Cardiovasc Res. 2012; 94: 519–525 [DOI] [PubMed] [Google Scholar]
- 77. Suffee N, Richard B, Hlawaty H, Oudar O, Charnaux N, Sutton A. Angiogenic properties of the chemokine RANTES/CCL5. Biochemi Soc Trans. 2011; 39: 1649–1653 [DOI] [PubMed] [Google Scholar]
- 78. Begley LA, Kasina S, Mehra R, et al. CXCL5 promotes prostate cancer progression. Neoplasia. 2008; 10: 244–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gold B, Merriam JE, Zernant J, et al. Variation in factor B (BF) and complement component 2 (C2) genes is associated with age-related macular degeneration. Nat Genet. 2006; 38: 458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jakobsdottir J, Conley YP, Weeks DE, Ferrell RE, Gorin MB. C2 and CFB genes in age-related maculopathy and joint action with CFH and LOC387715 genes. PLoS One. 2008; 3: e2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Maller JB, Fagerness JA, Reynolds RC, Neale BM, Daly MJ, Seddon JM. Variation in complement factor 3 is associated with risk of age-related macular degeneration. Nat Genet. 2007; 39: 1200–1201 [DOI] [PubMed] [Google Scholar]
- 82. Spencer KL, Olson LM, Anderson BM, et al. C3 R102G polymorphism increases risk of age-related macular degeneration. Hum Mol Genet. 2008; 17: 1821–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yates JR, Sepp T, Matharu BK, et al. Complement C3 variant and the risk of age-related macular degeneration. New Engl J Med. 2007; 357: 553–561 [DOI] [PubMed] [Google Scholar]
- 84. Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005; 308: 419–421 [DOI] [PubMed] [Google Scholar]
- 85. Skerka C, Lauer N, Weinberger AA, et al. Defective complement control of factor H (Y402H) and FHL-1 in age-related macular degeneration. Mol Immunol. 2007; 44: 3398–3406 [DOI] [PubMed] [Google Scholar]
- 86. Laine M, Jarva H, Seitsonen S, et al. Y402H polymorphism of complement factor H affects binding affinity to C-reactive protein. J Immunol. 2007; 178: 3831–3836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hecker LA, Edwards AO, Ryu E, et al. Genetic control of the alternative pathway of complement in humans and age-related macular degeneration. Hum Mol Genet. 2010; 19: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Reynolds R, Hartnett ME, Atkinson JP, Giclas PC, Rosner B, Seddon JM. Plasma complement components and activation fragments: associations with age-related macular degeneration genotypes and phenotypes. Invest Ophthalmol Vis Sci. 2009; 50: 5818–5827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scholl HP, Issa Charbel P, Walier M, et al. Systemic complement activation in age-related macular degeneration. PLoS One. 2008; 3: e2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Goh JW, Tan YS, Dodds AW, Reid KB, Lu J. The class A macrophage scavenger receptor type I (SR-AI) recognizes complement iC3b and mediates NF-kappaB activation. Protein Cell. 2010; 1: 174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Fujita Y, Yamaguchi S, Kakino A, Iwamoto S, Yoshimoto R, Sawamura T. Lectin-like oxidized LDL receptor 1 is involved in CRP-mediated complement activation. Clin Chem. 2011; 57: 1398–1405 [DOI] [PubMed] [Google Scholar]
- 92. Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. II. Cell patterning in experimental chimaeras and mosaics. Dev Biol. 1987; 121: 205–219 [DOI] [PubMed] [Google Scholar]
- 93. Ershov AV, Stroeva OG. Post-natal pattern of cell-proliferation in retinal-pigment epithelium of mice studied with tritiated-thymidine autoradiography. Cell Differ Dev. 1989; 28: 173–177 [DOI] [PubMed] [Google Scholar]
- 94. Bodenstein L, Sidman RL. Growth and development of the mouse retinal pigment epithelium. I. Cell and tissue morphometrics and topography of mitotic activity. Dev Biol. 1987; 121: 192–204 [DOI] [PubMed] [Google Scholar]